- 1Department of Dermatology, The Third Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Dermatology and Allergy, Technical University of Munich, Munich, Germany
- 3Department of Cardiology, The People's Hospital of China Medical University, The People's Hospital of Liaoning Province, Shenyang, China
Background and Aims: Acute urticaria (AU) is the most frequently reported immediate hypersensitivity reaction in skin by administration of iodinated contrast media (ICM). We aimed to establish the pattern and identify the risk factors of AU among inpatients undergoing non-emergent coronary angiography (CAG) with prophylactic corticosteroids in China.
Methods: Medical records of 19,326 adult inpatients undergoing non-emergent CAG with prophylactic methylprednisolone in 2013–2019 were retrospectively investigated. AU was identified within 1 h post-ICM administration, and diffuse involvement was defined when wheals occur in two or more body parts, including the back, abdomen, chest, and extremities. Age- and sex-matched inpatients (1:4) without AU were randomly selected for assessment of risk factors.
Results: Approximately 0.8% of CAG inpatients had AU, including 101 diffuse and 64 limited form. The diffuse AU was more common in settings of non-diagnostic CAG, iohexol used, average ICM injection≥3 ml/min, recurrent CAG, and past history of immediate hypersensitivity to ICM. Inpatients with preexisting allergies, decreased evaluated glomerular filtration rate, and increased high sensitivity C reactive protein or neutrophil-to-lymphocyte ratio prior to CAG had a higher probability of AU (odds ratio >1, P < 0.05 for all variables). All AU inpatients complained of pruritus, and mild itching predominated. AU dissipated in several days under treatment of ebastine or levocetirizine 10 mg/daily, but ebastine showed superiority.
Conclusions: ICM-induced AU is not uncommon in non-emergent CAG inpatients with prophylactic methylprednisolone. Preexisting allergies, renal dysfunction, and mild inflammation are high-risk factors, and antihistamine monotherapy is a favorable candidate for ICM-related AU.
Introduction
Iodinated contrast media (ICM) is indispensable and invaluable for enhancing the quality of imaging by improving the visibility of specific organs, blood vessels, or tissues. With recent advancements in medical-imaging equipment and ICM-enhanced image-guided diagnostic requirements, the application of ICM is constantly increasing. Nowadays, it is estimated that over 100 million ICM-requiring procedures are performed annually worldwide and are increasing by 20% annually in China (1, 2). The increase in elderly population and incidence of cardiovascular diseases have also led to the widespread use of ICM through the intra-arterial route in coronary angiography (CAG) with or without percutaneous coronary intervention (PCI) (3).
Adverse reactions to ICM, primarily as hypersensitivity reactions (HSRs), “allergic-like” reactions, or anaphylaxis, have been recognized as an important issue (4). ICM-related HSRs comprise a broad range of clinical features with involvement of nearly all organ systems. They can be generally classified as immediate or delayed HSRs, occurring within 1 h or 1 h−2 weeks post-ICM administration, respectively (5). Immediate HSRs to ICM usually show cutaneous symptoms with acute limited or diffuse urticaria (6). ICM-induced acute urticaria (AU) is occasionally very severe or even lethal, especially diffuse AU with concurrent dyspnea and facial angioedema (7, 8).
Accurate incidence of ICM-induced AU cannot be acquired from reported literature since large-scale studies have grouped all HSRs (urticarial or non-urticarial, mild, or severe) caused by intra-arterially or intravenously administrated ICM together (9–12). In addition, ICM-induced AU has long been regarded as non-IgE-mediated pseudoallergic reaction involving direct complement activation (13), while accumulating evidence indicated that ICM-specific IgE-mediated AU (type I allergic reaction) may have been underreported in the past due to lack of pertinent allergy testing (14, 15). Without regard to IgE levels, excessive histamine or other biogenic amines released by basophils and tryptase by mast cells are also a characteristic of immediate HSRs (including AU) to ICM, which were partially evidenced by antihistamine treatments and blood tests (9). However, another disputable aspect that prevention of immediate HSRs by premedication with corticosteroids or antihistamines remains uncertain still challenges the role of IgE-mediated allergy in AU pathogenesis.
The occurrence of ICM-related AU is unpredictable and varies with underlying diseases, the routes of ICM administration, prophylaxis strategies, etc. (7, 8). For all we know, no studies focused on AU in patients who are exposed to ICM administered intra-arterially in China and abroad. In this retrospective study, we aimed to determine the prevalence, pattern, risk factors, and the management of AU in inpatients on corticosteroid prophylaxis undergoing non-emergent CAG with or without PCI in China. To some extent, the findings of the present study will help dermatologists and cardiologists to better understand and manage AU.
Patients and Methods
Study Population
Inpatients aged ≥18 years who received non-emergent CAG from January 2013 to December 2019 at the Department of Cardiology in the People's Hospital of China Medical University were included in the study. The exclusion criteria were as follows: current pregnancy; severe heart failure [New York Heart Association (NYHA) IV]; pulmonary edema; known allergy to antiplatelet aggregation agents including aspirin, clopidogrel, or ticagrelor; known allergy to proton pump inhibitors; patients on hemodialysis or those with estimated glomerular filtration rate (eGFR) <15 ml/min/1.73 m2; refusal to accept interventional therapy; and life expectancy <1 year. To assess the relevant risk factors in the same period of time, a control group was retrospectively selected among patients without AU after 1:4 matching for age and sex. The study was approved by the Ethics Committee of the People's Hospital of China Medical University. No patient consent was needed due to a retrospective study and analysis on the medical records of the recruited patients.
CAG Procedure
Three types of CAG were involved. The diagnostic referred to CAG without PCI, the coronary chronic total occlusion (CTO) interventional referred to CAG with PCI for treatment of CTO lesion, and the general interventional referred to CAG with PCI for treatment of partial occlusion lesion. A standard Judkins technique was used in all the studied cases. The CAG was performed via the radial or/and femoral artery approach. Bilateral CAG and PCI were performed when necessary. For CTO lesions, antegrade crossing techniques were preferably attempted, and retrograde crossing techniques were alternative if the former failed. The use of ICM (iohexol or iodixanol) was left to the operator's discretion. The prophylactic premedication to prevent HSRs included two doses of methylprednisolone 32 mg orally administered, 12 and 2 h before CAG, which is the standard of care in our institution. For prophylactic anticoagulation, a loading dose of aspirin 300 mg combined with clopidogrel 300 mg and heparin 100 U/kg were administered prior to and during the procedure, respectively. All patients were routinely administered with PPI for preventing the occurrence of stress ulcers. Withdrawal of potential nephrotoxic drugs and intravenous hydration conformed to the recommendations in the European Society of Cardiology guidelines. Dermatologists in our hospital were invited for cooperative consultation about the observed cutaneous symptoms or signs, and AU was diagnosed and recorded by dermatologists.
Data Collection
Demographic data (age, gender, occupation, education background, ethnicity, height, and weight), preexisting conditions (allergic or non-allergic comorbidities, pre-procedural medication, past history of ICM exposure, and previous immediate HSRs to ICM during CAG), eGFR, inflammatory biomarkers including high sensitivity C reactive protein (Hs-CRP) and neutrophil-to-lymphocyte ratio (NLR), CAG procedure (type of CAG, ICM used, procedural access, ICM volume in total, and time of procedure), and AU-related characteristics (type, onset time, distribution of wheal, degree of itching, concurrent facial angioedema and dyspnea, the antihistamine regimen, and its efficacy) were all obtained through retrospective analysis of the electronic medical records. Limited AU was defined by the occurrence of wheals in only one of the five parts (upper extremities, lower extremities, chest, abdomen, or back), while diffuse AU in two or more locations.
Statistical Analyses
Statistical analysis of the data compiled in an Excel databank was conducted using the SPSS/PC software (Version 19.0 for Windows; SPSS Inc., China). For descriptive analysis, the continuous and categorical variables were presented as mean (M) ± standard deviation (SD) and percentage (%), respectively. For the comparison of demographic information and clinical characteristics between groups, chi-square test or Student's t-test was used. Related covariates with significant associations with the occurrence of AU in the univariate analysis were resubjected to multivariate logistic regression analysis. For continuous variables, the receiver-operating characteristic (ROC) curve was depicted to calculate the cutoff value, sensitivity, and specificity of each predictor. A P < 0.05 was accepted as the cutoff for statistical significance.
Results
Incidence of AU
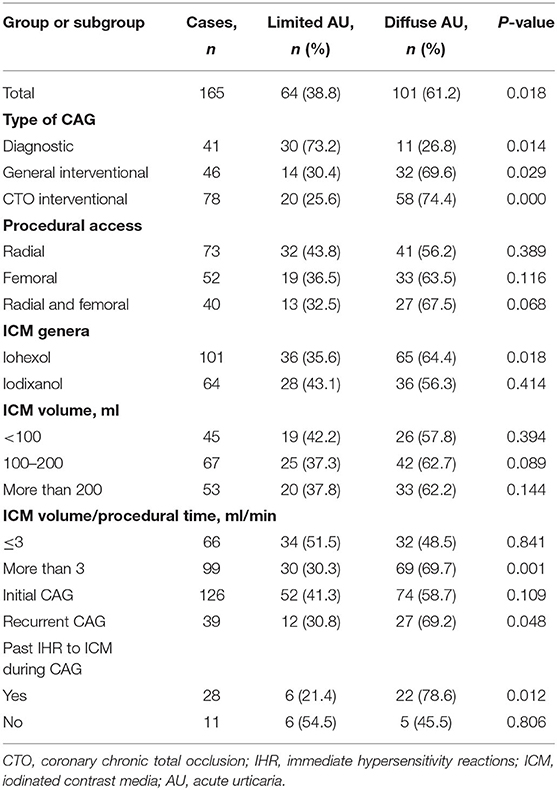
During the designated 7 years, a total of 24,058 non-emergent CAGs in 19,326 patients were conducted, while AU occurred in 0.8% (165/19,326) of the patients. Stratification of the AU according to ICM and procedure profiles was summarized in Table 1. Overall, more patients showed diffuse AU than limited AU (61.2 vs. 38.8%, P = 0.018), which was also observed in subgroups of general interventional CAG (P = 0.029), CTO interventional CAG (P < 0.001), application of iohexol (P = 0.018), average ICM injection speed more than 3 ml/min (P = 0.001), recurrent CAG (P = 0.048), and past history of immediate HSRs to ICM during CAG (P = 0.012). On the other hand, limited AU was more common only in patients undergoing diagnostic CAG (P = 0.014).
Clinical Characteristics of ICM-Related AU
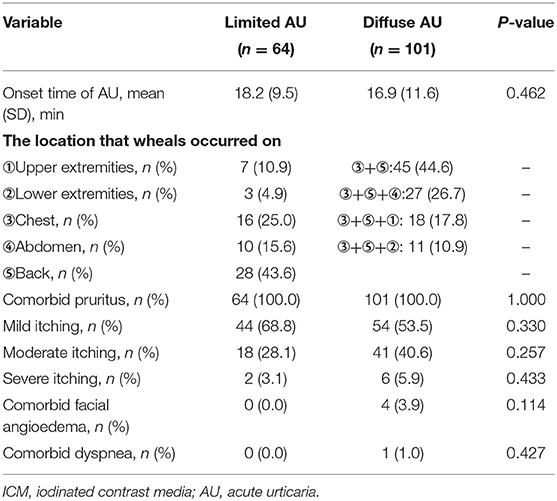
Between subgroups of limited AU and diffuse AU, there was no statistically significant difference in the onset time (P = 0.462). The wheals mostly occurred on the back (43.6%) in patients with limited AU and concomitantly on the chest and back in all diffuse AU patients. Itchiness was recorded in all AU patients. Mild pruritus was complained by 68.8% of the patients with limited and by 53.5% with diffuse AU, with no differences observed in the degree of pruritus between the two groups (P = 0.330, 0.257, and 0.433 for the mild, moderate, and severe, respectively). Only four and one diffuse AU cases concurrently experienced facial angioedema and dyspnea, respectively (Table 2).
Risk Factors for ICM-Induced AU
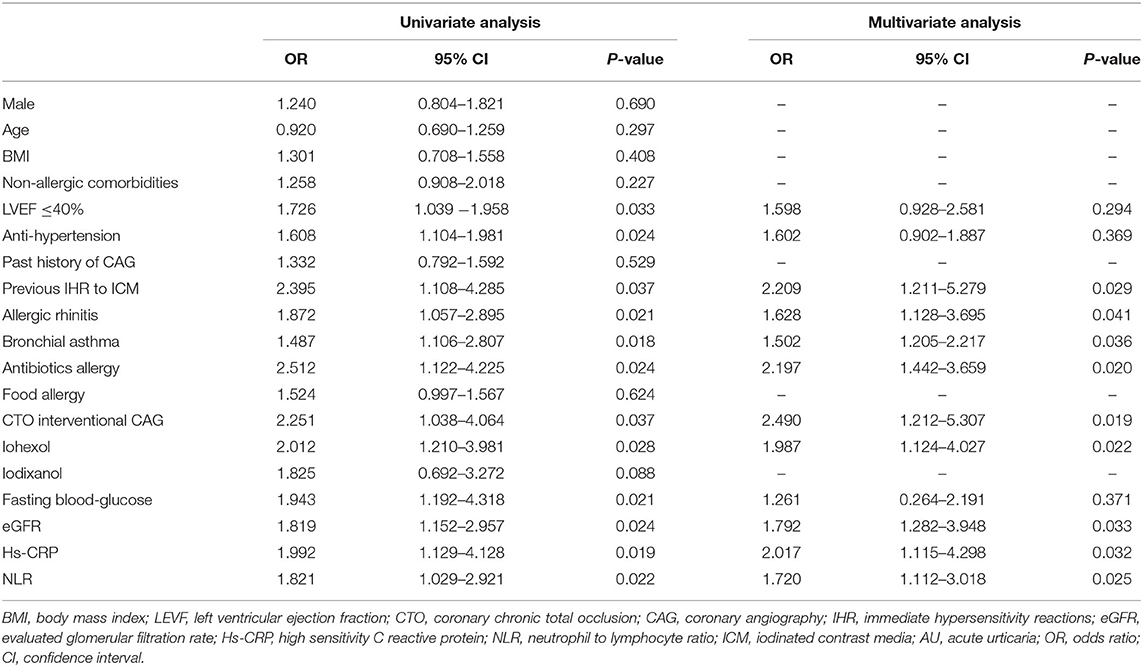
Men accounted for 70.9% (117 cases) of the 165 AU patients with mean age ± SD 65.2 ± 10.5 years. In the control group, a total of 660 age- and sex-matched cases were randomly selected from the remaining 19,161 non-AU patients. Univariate and multivariate analyses showed that preexisting allergic rhinitis, bronchial asthma, antibiotics allergy, past immediate HSRs to ICM during previous CAG, a lower level of eGFR as well as a higher level of Hs-CRP and NLR prior to CAG, CTO interventional CAG, and the application of iohexol are risk factors for the occurrence of AU (P < 0.05 for all variables, Table 3). eGFR ≤ 69 ml/min/1.73 mm2 (sensitivity: 85.71%, specificity: 73.23%), serum Hs-CRP >8.0 mmol/L (sensitivity: 67.35%, specificity: 84.32%), and NLR>2.8 (sensitivity: 76.2%, specificity: 89.0%) were demonstrated to be the predictors of AU in further ROC curve analyses (P < 0.05 for all variables).
Management of ICM-Induced AU
A total of 26 patients with limited AU and 42 with diffuse AU patients were treated with levocetirizine (10 mg daily), and the remaining AU patients were treated with ebastine (10 mg daily). As Table 4 shows, ebastine exerted better therapeutic effect regarding pruritus regression in the two types of AU (both P < 0.001) and wheals dissipation in the diffuse form (P < 0.001) compared with levocetirizine.
Discussion
The overall incidence of immediate HSRs to ICM ranges from 0.2 to 3% for mild-to-moderate cases and 0.004 to 0.04% for severe cases (16). Specifically, incidence associated with intravenously and intra-arterially administered ICM is 0.7–15 and 3.6%, respectively (17, 18). Although the overall safety profiles are well-understood, research on immediate ICM HSRs is still ongoing, and more results are greatly anticipated, especially those based on specific anaphylaxis or reaction.
This study was the first to focus on ICM-related AU in Asian inpatients undergoing non-emergent CAG with or without PCI and found that 0.8% (165/19,326) of the cases under prophylaxis with two doses of methylprednisolone (32 mg) experienced AU, which was roughly consistent with previous reports (14, 19). In addition, similar to what was observed in premedicated patients with exposure to intravenously administrated ICM or non-premedicated patients with exposure to intra-arterial ICM (2, 11, 18), a higher proportion of diffuse AU (61.2%) during CAG was found. Such results may further question the value of premedication with corticosteroids or antihistamines for prophylaxis of ICM HSRs including AU (20, 21). On one hand, premedications are not globally standardized. Different from that in our department of cardiology, high-risk patients in North America usually receive oral prednisone (50 mg) at 13, 7, and 1 h or diphenhydramine (1 mg/kg) at 1 h before ICM injection (22). On the other hand, premedication is not generally applied in Europe since breakthrough reactions of all severity grades have been observed in 1.2% up to 40% of pretreated patients, and pretreatment drugs themselves can occasionally induce adverse drug reactions including anaphylaxis (14, 19, 22, 23). Furthermore, a recent multidisciplinary consensus also opposed routine premedication, even in patients with prior HSRs to ICMs (24).
Although premedication may not always work, it seems that patients with preexisting high-risk factors usually urge clinicians to create more effective management approaches for ICM HSRs. In this regard, risk identification and related stratification in candidates for CAG are still critical for prophylaxis of ICM-associated AU or other immediate HSRs. Our results confirmed that atopy inpatients with immediate HSRs to ICM in previous CAG, preexisting allergic rhinitis, bronchial asthma, and antibiotics allergy are predisposed to the onset of AU during CAG, which were almost consistent with the findings from previous studies grouping all allergic reactions together in prophylactic and non-prophylactic patients (12, 18). Additionally, the present study found that decreased eGFR or inflammation with mildly elevated Hs-CRP and NLR were also risk factors for the occurrence of ICM-related AU, which has not been unmasked previously. It is well-proved that mild systemic inflammation positively correlates with ICM-caused acute renal insufficiency that is marked by gradually decreasing eGFR (25, 26). Dysfunction in kidney combined with longer and higher concentration of ICM exposure during CTO interventional CAG may further increase the risk of AU. Once the patients were suspected to be predisposed to ICM-related immediate HSRs before ICM administration, pretests including prick test with undiluted ICM and intradermal test with 1:10 dilution are recommended, which may potentially identify alternative ICM that could be well-tolerated (27, 28).
However, breakthrough reactions are sometimes unavoidable even after general premedication and careful screening. Thus, the more complicated problem is the application of remedy strategies, especially for patients with comorbidities such as renal dysfunction. Various modalities including second-generation non-sedating H1 antihistamines with or without corticosteroids are available for first-line treatment of ICM or non-ICM associated AU. Corticosteroids are not highly recommended due to their controversial benefit, whereas antihistamines are necessary (29). In our center, both ebastine 10 mg daily and levocetirizine 10 mg daily could control the symptoms of ICM-induced AU within several days, and ebastine showed superior overall efficacy. In contrast to this, ebastine 10 mg daily was found to be inferior to levocetirizine 5 mg for symptomatic relief of non-ICM related AU (30). The discrepancy may be reasonable as many variables must be considered, such as study types, gender ratio, ethnic difference, subjects with comorbidities, and concomitant medications other than antihistamines. Although efficacy comparisons among other antihistamines in ICM-related AU are lacking, the above data collectively imply the preference of ebastine or levocetirizine monotherapy for AU-affected patients with prophylactic methylprednisolone.
There are many limitations in the present study. The evidence on prophylaxis and treatment of AU from retrospective analysis are usually less stringent compared with prospective and randomized controlled trials. The incidence of AU may be underreported as some self-limited AU might be unnoticed. Furthermore, we could not rule out the effect of other drugs consumed, especially heparin, on the incidence of AU during CAG, but related drug hypersensitivity could be negligible judging from the rate of HSRs during computed tomography procedures without application of these drugs (1, 10, 12). A scientifically proven explanation for the difference between limited and diffuse AU could not be provided. On the whole, the current study only involved Asian patients, and hence, the evidence we provided may not be generalized to non-Asian population.
Conculusion
Collectedly, the first large-scale systematic analysis in China demonstrated that ICM-induced AU is not uncommon in non-emergent CAG inpatients with prophylactic methylprednisolone. Preexisting allergies, renal dysfunction, and mild inflammation are high-risk factors, and antihistamine monotherapy is a favorable candidate for ICM-related AU. Future prospective investigations are needed to develop a better personalized prophylaxis and management strategy to tackle ICM-associated AU or other HSRs in high-risk patients, especially the patients who are undergoing ICM administration through the intra-arterial route.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study was approved by the Ethics Committee of the People's Hospital of China Medical University. No patient consent was needed due to a retrospective study and analysis on the medical records of the recruited patients.
Author Contributions
YW and FH designed the research study. FY performed the analysis. BC and YW collected data and wrote the manuscript. WC made revisions to the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No.82003337), China Postdoctoral Science Foundation (No.2020M683268), Chongqing Natural Science Foundation (No. cstc2020jcyj-bshX0023), Postdoctoral Foundation of Chongqing Medical University (No. R9001), and Funding for Key Disciplines of Third Affiliated Hospital of Chongqing Medical University (No. ZK201902).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Li X, Chen J, Zhang L, Liu H, Wang S, Chen X, et al. Clinical observation of the adverse drug reactions caused by non-ionic iodinated contrast media: results from 109,255 cases who underwent enhanced CT examination in Chongqing, China. Br J Radiol. (2015) 88:20140491. doi: 10.1259/bjr.20140491
2. Chen QL, Zhao XY, Wang XM, Lv N, Zhu LL, Xu HM, et al. Retrospective analysis of non-laboratory-based adverse drug reactions induced by intravenous radiocontrast agents in a Joint Commission International-accredited academic medical center hospital in China. Ther Clin Risk Manag. (2017) 13:565–73. doi: 10.2147/TCRM.S134265
3. Khan MR, Kayani WT, Ahmad W, Manan M, Hira RS, Hamzeh I, et al. Effect of increasing age on percutaneous coronary intervention vs coronary artery bypass grafting in older adults with unprotected left main coronary artery disease: a meta-analysis and meta-regression. Clin Cardiol. (2019) 42:1071–8. doi: 10.1002/clc.23253
4. Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, Moya Quesada MC, García-Avilés C, García Nuñez I, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. (2016) 26:144–55. doi: 10.18176/jiaci.0058
5. Macy EM. Current epidemiology and management of radiocontrast-associated acute- and delayed-onset hypersensitivity: a review of the literature. Perm J. (2018) 22:17–72. doi: 10.7812/TPP/17-072
6. Tasker F, Fleming H, McNeill G, Creamer D, Walsh S. Contrast media and cutaneous reactions. Part 1. immediate hypersensitivity reactions to contrast media and gadolinium deposition. Clin Exp Dermatol. (2019) 44:839–43. doi: 10.1111/ced.13990
7. Losappio L, Heffler E, Bussolino C, Cannito CD, Carpentiere R, Raie A, et al. Acute urticaria presenting in the emergency room of a general hospital. Eur J Intern Med. (2014) 25:147–50. doi: 10.1016/j.ejim.2013.11.003
8. Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA2LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. (2018) 73:1393–414. doi: 10.1111/all.13397
9. Endrikat J, Michel A, Kölbach R, Lengsfeld P, Vogtländer K. Risk of hypersensitivity reactions to iopromide after intra-arterial versus intravenous administration: a nested case-control analysis of 133,331 patients. Invest Radiol. (2020) 55:38–44. doi: 10.1097/RLI.0000000000000611
10. Lee SY, Kang DY, Kim JY, Yoon SH, Choi YH, Lee W, et al. Incidence and risk factors of immediate hypersensitivity reactions associated with low-osmolar iodinated contrast media: a longitudinal study based on a real-time monitoring system. J Investig Allergol Clin Immunol. (2019) 29:444–50. doi: 10.18176/jiaci.0374
11. Hsu Blatman KS, Sánchez-Borges M, Greenberger PA. Anaphylaxis in the radiology suite. J Allergy Clin Immunol Pract. (2020) 8:1203–9. doi: 10.1016/j.jaip.2020.01.014
12. Cha MJ, Kang DY, Lee W, Yoon SH, Choi YH, Byun JS, et al. Hypersensitivity reactions to iodinated contrast media: a multicenter study of 196 081 patients. Radiology. (2019) 293:117–24. doi: 10.1148/radiol.2019190485
13. Szebeni J. Complement activation-related pseudoallergy caused by liposomes, micellar carriers of intravenous drugs, and radiocontrast agents. Crit Rev Ther Drug Carrier Syst. (2001) 18:567–606. doi: 10.1615/CritRevTherDrugCarrierSyst.v18.i6.50
14. Böhm I, Morelli J, Nairz K, Silva Hasembank Keller P, Heverhagen JT. Myths and misconceptions concerning contrast media-induced anaphylaxis: a narrative review. Postgrad Med. (2017) 129:259–66. doi: 10.1080/00325481.2017.1282296
15. Trcka J, Schmidt C, Seitz CS, Bröcker EB, Gross GE, Trautmann A. Anaphylaxis to iodinated contrast material: nonallergic hypersensitivity or IgE-mediated allergy? AJR Am J Roentgenol. (2008) 190:666–70. doi: 10.2214/AJR.07.2872
16. Kim TH, Yoon SH, Lee SY, Choi YH, Park CM, Kang HR, et al. Biphasic and protracted anaphylaxis to iodinated contrast media. Eur Radiol. (2018) 28:1242–52. doi: 10.1007/s00330-017-5052-0
17. Park HJ, Park JW, Yang MS, Kim MY, Kim SH, Jang GC, et al. Re-exposure to low osmolar iodinated contrast media in patients with prior moderate-to-severe hypersensitivity reactions: a multicentre retrospective cohort study. Eur Radiol. (2017) 27:2886–93. doi: 10.1007/s00330-016-4682-y
18. Sohn KH, Kim GW, Lee SY, Kim HS, Cho SH, Han JK, et al. Immediate and delayed hypersensitivity after intra-arterial injection of iodinated contrast media: a prospective study in patients with coronary angiography. Eur Radiol. (2020) 30:3596–7. doi: 10.1007/s00330-019-06550-9
19. Mervak BM, Davenport MS, Ellis JH, Cohan RH. Rates of breakthrough reactions in inpatients at high risk receiving premedication before contrast-enhanced CT. AJR Am J Roentgenol. (2015) 205:77–84. doi: 10.2214/AJR.14.13810
20. Sánchez-Borges M, Aberer W, Brockow K, Celik GE, Cernadas J, Greenberger PA, et al. Controversies in drug allergy: radiographic contrast media. J Allergy Clin Immunol Pract. (2019) 7:61–5. doi: 10.1016/j.jaip.2018.06.030
21. Kolbe AB, Hartman RP, Hoskin TL, Carter RE, Maddox DE, Hunt CH, et al. Premedication of patients for prior urticarial reaction to iodinated contrast medium. Abdom Imaging. (2014) 39:432–7. doi: 10.1007/s00261-013-0058-9
22. Greenberger PA, Patterson R. The prevention of immediate generalized reactions to radiocontrast media in high-risk patients. J Allergy Clin Immunol. (1991) 87:867–72. doi: 10.1016/0091-6749(91)90135-B
23. Dillman JR, Ellis JH, Cohan RH, Strouse PJ, Jan SC. Allergic-like breakthrough reactions to gadolinium contrast agents after corticosteroid and antihistamine premedication. AJR Am J Roentgenol. (2008) 190:187–90. doi: 10.2214/AJR.07.2718
24. Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL, et al. Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. (2020) 145:1082–123. doi: 10.1016/j.jaci.2020.01.017
25. Satilmis S, Karabulut A. Value of C-Reactive protein/albumin ratio in predicting the development of contrast-induced nephropathy in patients with Non-ST elevation myocardial infarction. Angiology. (2020) 71:366–71. doi: 10.1177/0003319719898057
26. Zorlu C, Koseoglu C. Comparison of the relationship between inflammatory markers and contrast-induced nephropathy in patients with acute coronary syndrome after coronary angiography. Angiology. (2020) 71:249–55. doi: 10.1177/0003319719892160
27. Schrijvers R, Breynaert C, Ahmedali Y, Bourrain JL, Demoly P, Chiriac AM. Skin testing for suspected iodinated contrast media hypersensitivity. J Allergy Clin Immunol Pract. (2018) 6:1246–54. doi: 10.1016/j.jaip.2017.10.040
28. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International Consensus on drug allergy. Allergy. (2014) 69:420–37. doi: 10.1111/all.12350
29. Barniol C, Dehours E, Mallet J, Houze-Cerfon CH, Lauque D, Charpentier S. Levocetirizine and prednisone are not superior to levocetirizine alone for the treatment of acute urticaria: a randomized double-blind clinical trial. Ann Emerg Med. (2018) 71:125–31. doi: 10.1016/j.annemergmed.2017.03.006
Keywords: acute urticaria, iodinated contrast media, coronary angiography, premedication, risk factors
Citation: Chen B, Yu F, Chen W, Wang Y and Hao F (2021) Acute Urticaria in Inpatients Undergoing Non-emergent Coronary Angiography With Corticosteroid Prophylaxis: A Retrospective Study. Front. Med. 8:616015. doi: 10.3389/fmed.2021.616015
Received: 10 October 2020; Accepted: 29 April 2021;
Published: 10 June 2021.
Edited by:
Robert Gniadecki, University of Alberta, CanadaReviewed by:
Elena Netchiporouk, McGill University, CanadaYunsheng Xu, Sun Yat-sen University, China
Copyright © 2021 Chen, Yu, Chen, Wang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Wang, ZHJ3YW5neW9uZzIwMTZAMTYzLmNvbQ==; Fei Hao, aGFvZmVpNjJAbWVkbWFpbC5jb20uY24=
†These authors have contributed equally to this work
 Bangtao Chen
Bangtao Chen Fubing Yu
Fubing Yu WenChieh Chen2
WenChieh Chen2 Yong Wang
Yong Wang Fei Hao
Fei Hao