
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Med. , 09 March 2021
Sec. Regulatory Science
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.595797
This article is part of the Research Topic Insights in Regulatory Science: 2021 View all 25 articles
Introduction: Gene therapies are innovative therapies that are increasingly being developed. However, health technology assessment (HTA) and payer decision making on these therapies is impeded by uncertainties, especially regarding long-term outcomes. Through measuring patient preferences regarding gene therapies, the importance of unique elements that go beyond health gain can be quantified and inform value assessments. We designed a study, namely the Patient preferences to Assess Value IN Gene therapies (PAVING) study, that can inform HTA and payers by investigating trade-offs that adult Belgian hemophilia A and B patients are willing to make when asked to choose between a standard of care and gene therapy.
Methods and Analysis: An eight-step approach was taken to establish the protocol for this study: (1) stated preference method selection, (2) initial attributes identification, (3) stakeholder (HTA and payer) needs identification, (4) patient relevant attributes and information needs identification, (5) level identification and choice task construction, (6) educational tool design, (7) survey integration, and (8) piloting and pretesting. In the end, a threshold technique survey was designed using the attributes “Annual bleeding rate,” “Chance to stop prophylaxis,” “Time that side effects have been studied,” and “Quality of Life.”
Ethics and Dissemination: The Medical Ethics Committee of UZ KU Leuven/Research approved the study. Results from the study will be presented to stakeholders and patients at conferences and in peer-reviewed journals. We hope that results from the PAVING study can inform decision makers on the acceptability of uncertainties and the value of gene therapies to patients.
The pharmaceutical sector is shifting from a focus on classic chemical and first-generation biological medicines to the development of more complex biological therapies like gene therapy. Gene therapies are high-cost treatments, but may come with the promise of permanent benefits or even a cure. First efforts to market European Medicine Agency (EMA) approved gene therapies showed that obtaining market access is difficult (1). One of the main challenges is that uncertainty on magnitude and duration of effect may limit value perceived by HTA and payers (1, 2). In this context, uncertainty regarding long-term efficacy and safety is caused by limited comparative data and lack of long-term evidence (1). With the rise of therapies that have the potential to create permanent effects in patients, decision-making on the macro (marketing authorization), meso (pricing and reimbursement), and micro (shared-decision making) level will increasingly have to deal with uncertainty regarding long-term efficacy and safety.
With regard to value assessments of therapies potentially offering a cure, it has been argued that Quality Adjusted Life Years (QALYs) may not be appropriate for use, may be insensitive and may not cover all aspects of gene therapies relevant to patients; possibly resulting in a misjudgment on the value of such therapies (3–5). Gutknecht et al. (4) stated that QALYs only reflect outcomes that have a direct impact on Quality of Life (QoL) and/or survival, and suggested that through measuring patient preferences also other treatment features (e.g., mode of administration and cost) can be considered.
Performing patient preference studies in the context of gene therapies will not take away the uncertainty regarding long-term outcomes that can only be resolved by life-long follow-up of these patients, and will most likely not replace use of QALYs as this measure allows for comparison across diseases. However, performing patient preference studies in this context can inform decision-making by providing (1) additional insights on the acceptability of uncertainties to patients, (2) insights on the value of these therapies to patients, and (3) a pathway for the patient to weigh in on decision-making regarding gene therapies.
One of the rare diseases for which gene therapies are in development is hemophilia (A and B) (6–8). Current hemophilia treatment consists of regular intravenous administration of factor replacement therapy. In hemophilia, unmet medical needs result from the invasiveness of current treatment, the fluctuations of achieved factor levels making patients more prone to bleeds and joint damage, and the development of antibodies against current therapies in some patients (9–12). In hemophilia, gene therapy comes with the promise that one infusion could potentially replace lifelong administration of other high-cost drugs. To date, no research has been conducted regarding the preferences of hemophilia patients regarding gene therapy (13).
Therefore, we decided to initiate the Patient preferences to Assess Value IN Gene therapies (PAVING) study, to investigate trade-offs that adult Belgian hemophilia A and B patients are willing to make when asked to choose between a standard of care and gene therapy; the protocol of which is reported in this manuscript. The survey established through this protocol will allow for exploration of preference heterogeneity and serves to meet the needs of HTA and payers. In the design of the protocol, special attention was given to the innovative nature and potential lack of knowledge of patients regarding gene therapies.
The main objectives of the PAVING study are:
- To understand the trade-offs that patients make when they are asked to choose between gene therapy and a standard of care.
- To explore preference heterogeneity by investigating the impact of patient characteristics on preferences.
Protocol development for the PAVING survey was undertaken in sequential steps (Figure 1). Overall, a transparent and systematic approach was taken to develop the protocol, covering steps in the organization, design and conduct of a patient preference study as described by van Overbeeke et al. (14). Patients were involved as advisors (15) in protocol development (steps 3–8), and included in the stakeholder advisory board of the study, that further consisted of hematologists, HTA and payer decision-making experts, industry market access experts, rare disease experts, patient education (EUPATI) experts and caregivers. Moreover, patients steered the selection of attributes through participation in interviews (Step 4).
A number of stated-preference (elicitation) methods exists, but guidance is lacking on when to choose what method. Method selection started from the nine elicitation methods identified by Whichello et al. (16) as most promising in meeting decision-makers' needs in the medical product lifecycle (MPLC): DCE, Threshold Technique, Standard Gamble, Time trade-off, Swing-Weighting, Visual Analog Scale, Analytical Hierarchy Process, Best-Worst Scaling type 1, and Best-Worst Scaling type 2. The match of the method to the research question, patient population and decision-making context influences the value of patient preference studies for decision making (17). Therefore, in selecting our method we used criteria based on the research questions, patient population (rare disease), decision-making context, as well as validity requirements and budget. The criteria used and the thresholds used for this selection were informed by the work of Whichello et al. (16) and discussion with method experts further informed our choice of method. Ideally, we wanted the method to: (1) estimate weights of attributes, (2) estimate trade-offs between attributes, (3) quantify preference heterogeneity, (4) incorporate internal validity measures, (5) not have technical issues, (6) have a low minimal necessary sample size, and (7) allow for incorporation in an unsupervised survey.
While sample sizes of fewer than 100 participants may be sufficient when there is a limited number of attributes and levels (e.g., four attributes each with 2 levels) (18), DCEs typically include more than 100 participants and may require sample sizes >250 if there are 6–8 attributes each with 3–4 levels (16, 19). DCEs were excluded as a method due to our estimation that it will be challenging to recruit 100 patients (see section on sample) (Table 1). Moreover, as described under Steps 4 and 5, we wanted to include four attributes with a maximum of 7 levels in our design. From the nine promising methods, experts initially believed that the threshold technique and swing-weighting showed the most potential to meet study needs. In the end, swing-weighting was excluded based on concerns regarding the need to provide support for participants (i.e., through interviews or workshops) due to complex choice tasks with high cognitive burden, and the threshold technique was chosen.
In a threshold technique survey, participants are presented with multiple choice tasks in which they have to choose between two labeled profiles (e.g., prophylactic factor replacement therapy and gene therapy). The level of one attribute in the target profile (gene therapy) is varied systematically until the respondent switches from his/her preferred alternative. The level of this attribute is made systematically better (more attractive) if the reference profile is chosen, or the level of the key attribute is made systematically worse (less attractive) if the target treatment is chosen. The responses to these questions are then used to define an interval per respondent within which their threshold lies. This threshold represents the maximum acceptable risk (MAR) or minimal accepted benefit (MAB) for that switch (25).
A literature review was conducted on gene therapy clinical trials and previous initiatives investigating patients' preferences and needs in hemophilia to identify attributes. Clinical trials were identified in PubMed using the search terms “gene therapy” AND “hemophilia” and filters “Clinical Trial” and “Human.” Aditionally, the worldwide clinical trial gene therapy database (26) and clinicaltrials.gov were consulted. Results were cross-checked with the review on hemophilia gene therapy clinical trials of Batty and Pasi (27). Publications reporting results of trials were identified and included if published after 2005 and if intravenous administration of liver-targeting vectors was used. Patient preference studies and public patient meetings were identified in the literature. An initial list of attributes was generated based upon clinical outcomes identified in these clinical trials, and patient relevant outcomes identified in the patient preference studies and public patient meetings.
In total, 18 publications reporting on results from 21 clinical trials were retrieved (Supplementary Material I). Four publications published before 2005 and another publication demonstrating intramuscular application of gene therapy were excluded. In addition, we identified 19 patient preference studies and public patient meetings (Supplementary Material II). Patient preference studies only investigated preferences for treatment attributes of factor replacement therapy, blood transfusion or treatments no longer under development (28). Public meetings of the FDA investigated attitudes of hemophilia patients toward their current therapy and gene therapy (29). From these 13 clinical trials and 19 patient preference studies/public patient meetings, eight attribute classes comprising 22 attributes were identified (Table 2).
To identify classes of attributes important to decision-makers, consultations were held with the advisory board. Attributes identified in Step 2 and value assessment criteria (according to the Belgian Royal Decree of 1 February 2018) were presented and discussed to explore their relevance. Stakeholders confirmed the importance of the presented value assessment criteria and identified the following attribute classes: benefits, risks, administration, level of unmet need, cost and budget impact, applicability, and burden of disease. A consensus among the advisory board was reached on the need to investigate attributes related to benefits (including clinical endpoints and QoL), risks, and administration in the preference study, and to exclude other attribute classes (Supplementary Material III).
To identify attributes to be included in the survey design, relevance of attributes was investigated in interviews with 20 Belgian hemophilia A and B patients. An interview guide for semi-structured interviews with Belgian hemophilia patients was designed. The interview guide was created in Dutch, translated to English and French by a certified translator and checked by one of the researchers (EvO). Patients participated in their native language (Dutch or French). Prior to any questions about gene therapy, patients received information (based on the literature retrieved in Step 2, validated by three hematologists and piloted with two patients) regarding the disease, standard of care and gene therapy (Supplementary Material IV). Overall, patients found the provided information comprehensible. Some patients requested more information on inhibitors against factor replacement therapy, viral vectors, development of light inflammation of the liver, antibodies against vectors, and re-administration of gene therapy if benefits are not maintained in the long-term. Moreover, several patients suggested to use illustrations to visualize difficult concepts and ensure comprehension by other patients.
A ranking exercise was performed during interviews to prioritize attributes according to their importance to patients; using attributes identified through a mixed top-down and bottom-up approach. Top-down attributes included attributes identified in Step 2, except those belonging to classes of attributes excluded in Step 3. Attributes were listed per class and defined (Supplementary Material V). Definitions were validated by three hematologists and pilot tested with two patients. Bottom-up attributes were identified by asking patients to name the top three elements influencing their choice between standard of care and gene therapy before disclosing the top-down attributes. Patients ranked their top six attributes among the top-down and bottom-up identified attributes. This ranking was transformed for each participant so that a score between 1 and 6 was assigned to each of the attributes in the top six, with six points being assigned to the most important attribute. Sum totals of the scores were calculated per attribute. The ranking exercise revealed that the five attributes most important to patients were: annual bleeding rate (ABR), factor level, uncertainty of long-term risks, impact on daily life, and probability that prophylaxis can be stopped (Table 3). Full details on methods and results (on general gene therapy perception) of the interviews have been reported elsewhere (58), according to the guidelines of Hollin et al. (59). In a second consultation with the advisory board the interview results were presented. A consensus was reached to include attributes in the survey that were most important to patients, with emphasis on including a QoL-related attribute.
To keep the threshold technique survey of manageable length, it was decided to include four attributes. As the meaning of “Factor level” is different for factor replacement therapy (fluctuating factor levels) compared to gene therapy (stable factor levels), and as “Annual bleeding rate” is dependent on “factor level,” the researchers decided to exclude “Factor level” and include “Annual bleeding rate.” “Probability that prophylaxis can be stopped” was rephrased to “Chance to stop prophylaxis” as this was found to be more comprehensible to patients. “Uncertainty regarding long-term risks” was rephrased to “Time that side effects have been studied” as current uncertainty in long-term risks of gene therapy is caused by limited follow-up in a relative small number of patients (60); a similar attribute has been used by Mohamed et al. (61). In addition, a “Quality of life” attribute similar to Tomlinson et al. (62) was chosen as a substitute for “Impact on daily life,” as no hemophilia-specific impact on daily life instrument exists. The final selection of attributes thus included three benefits: “Annual bleeding rate” (ABR), “Chance to stop prophylaxis” (STOP) and “Quality of Life” (QOL); and one risk: “Time that side effects have been studied” (TIME). Attributes were further defined, and definitions were validated by three hematologists.
Three threshold series comprising up to three choice tasks and a drop-down question were designed to identify threshold intervals, one for each benefit (“Annual bleeding rate,” “Chance to stop prophylaxis” and “Quality of Life”). We opted to ask up to three choice questions per threshold series to each individual participant as shown in Figure 2 as this is often the number of questions used in threshold technique surveys to identify individual thresholds. As demonstrated in Figure 2, seven levels (levels A-G) were required to complete the design. Attribute levels were identified through literature gathered in Step 2, hematologist consultation and additional literature on QoL scores in hemophilia patients (63–67).
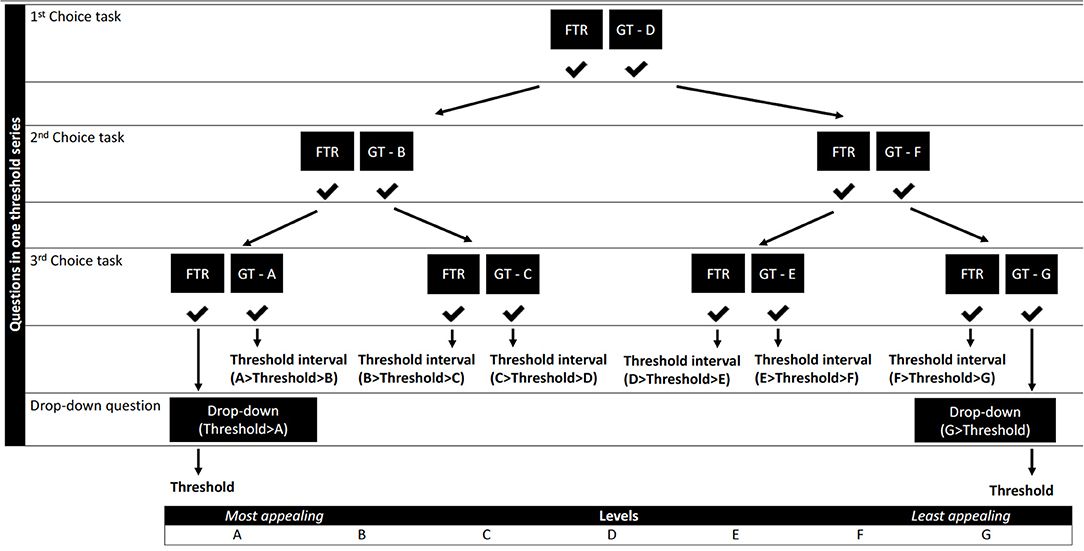
Figure 2. Flow of the levels throughout the questions of one threshold technique series. GT - A-G, gene therapy levels A-G (Table 4); FTR, factor replacement therapy level.
The range of attribute levels was based on the best available clinical data at the time this protocol was designed. From the 18 publications identified in Step 2 that reported on results from Phase I/II gene therapy trials in hemophilia (Supplementary Material I), one publication (68) was excluded as it described an intramuscular application of gene therapy and four other publications were withheld as they were published before 2005 and therefor found to be outdated (69–72). From the remaining 13 publications, lower and upper bounds of levels were identified and a range was set for all attributes using the lowest and highest value identified across publications (30–42). As QoL was not yet studied in these trials, we hypothesized that gene therapy would at least not reduce QoL and current QoL levels were identified using five additional studies (63–67). The ranges of the levels were discussed with hematologists (n = 3) and the range of the TIME attribute was slightly adapted based on their input to reflect the number of years of available evidence at that time (Table 4).
A threshold technique response logic was created using levels within the identified ranges (Table 4 and Figure 2). Spacing of these levels was established by setting the most extreme values of these ranges as cut-offs. We aimed to obtain even spacing between levels, with a maximum spacing of five units between levels. The threshold technique requires one attribute to be fixed as a comparator (25). It was decided to keep the risk attribute “Time that side effects have been studied” constant at level D throughout the threshold questions, to enable estimation of patient preferences for all benefit attributes. Levels D of all attributes represent the gene therapy profile in the initial threshold question. These levels represent the baseline scenario on which all threshold estimates are contingent. The levels for PFRT and the gene therapy baseline scenario were also fixed in discussion with hematologists to reflect a conservative scenario, where gene therapy would not provide additional ABR and QoL benefits, that still fit within the identified level ranges (Table 4).
The levels of only one benefit will change throughout a threshold series to identify an individual's threshold for that benefit. With a total of three series and with the initial question (levels D) representing the first choice task of all threshold series, participants need to answer seven choice questions in total to obtain a threshold interval for the three benefits within which their individual thresholds will lie. If participants end up at the extreme ends of Figure 2, no threshold interval can be identified and participants will be asked an additional drop-down question to elicit their exact threshold.
To ensure comprehension of the attributes and the gene therapy context by participants, an educational tool was designed. The information presented in the educational tool comprised hemophilia, current therapies and gene therapy and covered information needs of patients as identified in Step 4 (Supplementary Material VI). The original English script was translated to Dutch and French translations by a researcher (EvO) and validated by a certified translator. Voice-overs were recorded and Mindbytes BVBA developed the educational tool with visuals according to their standards (73).
The content and visuals of the educational tool were reviewed by three hematologists, two patients and a patient education expert. Necessary changes to the tool were made, and the Dutch and French versions were piloted with 10 additional patients. Patients were asked how comprehensible the tool was to them (user comprehensibility) and how comprehensible it would be to other patients. User comprehensibility of all modules was rated between “Very comprehensible” and “Totally comprehensible;” except the side effects module that was rated as “Comprehensible” by one patient. Comprehensibility to other patients was rated between “Comprehensible” and “Very comprehensible” across all modules. Ease of navigation was rated by all patients between “Very easy” and “Easy”. In addition, six patients reported that no changes needed to be made to the educational tool, two mentioned minor navigation changes and two requested additional information (on antibodies and gene therapy re-administration). Overall, the tool was very well-received by patients. Therefore, no additional changes were made.
The final survey was designed to include (1) a consent form and information sheet, (2) questions on patient characteristics including demographics, health literacy Chew et al. (74) and QoL (EQ5D5L), (3) the educational tool established in Step 6, (4) the choice tasks using the threshold technique as designed in Step 5, and (5) survey evaluation questions. Questions on demographics (e.g., age, disease severity, number of damaged joints) and on QoL (EQ5D5L) were included to identify factors that may influence preferences of patients. Health literacy questions were included to identify patients that may have difficulties with understanding medical information. To evaluate the validity of the study, validity checks were built into the survey to identify respondents whose responses appear to “fail” these validity checks based on expected norms. Validity checks included evaluation of a comprehension question similar to that of Mansfield et al. (75), time to complete the survey, and choice consistency (the initial threshold question was repeated after the first threshold series). Dutch and French translations of the English survey were made by a certified translator and reviewed by a researcher (EvO), excluding QoL questions for which validated translations were used. The survey was programmed by Qualtrics and thoroughly reviewed by the researchers.
The full survey was piloted and pretested with patients. Four patients (including two bilingual patient representatives) participated in a paper-based pilot that evaluated comprehensibility of Dutch and French choice questions and choice behavior in think aloud interviews (76, 77). During this pilot no major issues were found and only minor text edits were made to a definition of one attribute and one question.
Online unsupervised pretesting evaluated comprehensibility and length of the survey, functioning of the response logic, and ability to identify thresholds and trade-offs. Of 14 invited patients, 12 completed the online pretest. The majority of pretesting participants found the choice questions to be “Very easy” or “Easy” to understand and answer. Some found it “Not easy nor difficult,” and none found it “Difficult” or “Very difficult”. Participants found the survey length “Just right” (n = 3), “Manageable” (n = 7), or “Too long” (n = 2). However, seven participants took over 40 min to complete the survey. Two of these participants had paused the survey and others might have taken a longer time than expected as they were also asked to evaluate the survey. Participants reported no other issues besides one textual error in the consent form and two in demographics questions. Therefore, the textual errors were corrected and three demographics questions were excluded to reduce the length of the survey. Inspection of the data sheet confirmed correct functioning of the response logic and ability to identify thresholds and trade-offs. The final survey can be found in Supplemental Material VII.
No specific power calculation method exists to determine sample sizes for threshold technique studies. Most threshold technique studies are conducted with 100 or fewer respondents (successful small studies include between 20 and 42 respondents) (18, 21–23). The threshold technique allows for elicitation of individual preferences (n = 1) and the method can therefore be used in very low sample sizes. The significance of the estimates will be greater and standard deviations will be smaller when the sample size increases. Hemophilia is a rare disease but relatively common compared to other rare diseases. The number of people affected by hemophilia A and B in Belgium was 1 258 in 2018 (78). Based on this number we estimate that we will be able to include around 100 patients in Belgium, and a method expert confirmed that the method can be performed with this limited proposed sample size.
Patients will be considered eligible if they are diagnosed with moderate or severe hemophilia A or B, are 18 years or older, and live in Belgium. Patients will be recruited through national hemophilia reference centers and the national patient organization. These recruiting parties will send an invitation via mail or newsletters containing a link to the online survey. Recruiting parties will keep a record of the number of eligible patients they sent an invitation to so that response rates can be calculated.
Analysis of thresholds and trade-offs will be done through interval regression and plotting of thresholds. Threshold intervals will be analyzed per benefit attribute (ABR, STOP, and QOL) using two interval regression (Tobit) models: (1) a constant-only model to identify the mean threshold (MAB) across the sample, and (2) a covariate-adjusted model to explore whether and how patient characteristics influence the MAB for each benefit (i.e., to explore preference heterogeneity). A separate Tobit model will thus be run for each benefit attribute.
A number of patient characteristics will be tested for inclusion in the covariate-adjusted model which may explain some of the observed preference heterogeneity. These include sociodemographic characteristics (e.g., age, residence, employment status), medical characteristics (e.g., hemophilia type, disease severity, and self-reported ABR and QoL), and survey behavior characteristics (e.g., time spent on the educational tool). The final selection of patient characteristics to be included in the covariate-adjusted model will be based on results from correlation tests between these covariates.
This research resulted in the development of the PAVING protocol to investigate trade-offs that adult Belgian hemophilia A and B patients are willing to make between standard of care and gene therapy. To the authors knowledge, this is the first patient preference study protocol that has been designed in the context of market access of gene therapies.
A transparent and systematic approach was taken to develop the PAVING protocol. While protocols of preference studies explaining the choice of attributes are increasingly being published (79–82), it is not standard practice to justify the choice of the preference method and the choice is often DCE (83). However, depending on the research question, researchers may prefer other methods over DCEs in case of very small sample sizes. The current research resulted in a transparent selection of a preference method (i.e., the threshold technique), attributes and levels. The protocol adheres to the five considerations of van Overbeeke et al. (17) to ensure value of a preference study for decision making: (1) investigate preferred treatment attributes, and trade-offs between attributes, (2) have a design that matches the research question and patient population, (3) include a patient sample and method that matches the MPLC phase, (4) be conducted in collaboration with different stakeholders, and (5) allow for sharing of results with relevant stakeholders.
The researchers believe that by taking a patient-centered approach (i.e., involving patient throughout protocol development and conducting interviews with patients) attributes were included that are relevant and comprehensible to patients (15). The research resulted in the inclusion of the attributes “Annual bleeding rate,” “Chance to stop prophylaxis,” “Quality of Life,” and “Time that side effects have been studied”. While “Quality of Life” may not be a usual attribute to include in a preference study, our QoL attribute is reliable as it will visually be presented as the EQ5D visual analog scale (VAS) that ranges from 0 (worst possible QoL) to 100 (best possible QoL). The researchers also believe that this VAS scale (reflecting patients' own valuation of their health) is easier to understand to patients and that results using this scale are easier to interpret than when using the utility scale that goes from 0 (death) to 1 (full health), as these utilities can go below 0 and the scale reflects a societal valuation of health states. Moreover, the QoL attribute is described according to the five dimensions of EQ5D5L [a reliable tool to measure QoL (84)] to make the attribute concrete. Potential concerns regarding ambiguity of QoL reflect the limitations of its current use as a generic measure of value in decision-making. While QoL may not be fully independent from ABR, bleedings do not occur on a daily basis, and patients can have different QoLs with the same ABR and also have the same QoL with different ABRs; to the extent of realistic ABR and QoL levels. As the threshold technique allows for the use of realistic levels within labeled profiles, the researchers argue that QoL and ABR can both be included as attributes. In contrast, simultaneous use of these two attributes in a DCE may not be possible as hypothetical scenarios may for example present unrealistic high ABR in combination with high QoL, possibly leading to rejection by patients. As demographics and QoL of patients will be investigated, clinical independence between the two variables, and the relation between current QoL and preferences can be investigated.
An important limitation of our design is that interactions between attributes cannot be assessed. Potential effects of uncertainty in risks (time that side effect have been studied) on interpretation of benefits can thus not be studied. Anchoring effects are always a possible limitation in any survey in which one value is changed systematically until switching or indifference is achieved. This is true for time tradeoff and standard gamble, modified swing weighting, and the threshold technique. However, to the extent that the baseline level to which the decision is anchored represents reality “in that it is based on data or on a value that would be expected even if data do not exist, then the starting point reflects the true decision context and will reflect bias inherent in that decision context” (25). In our case, the levels of each attribute in the initial (i.e., baseline) question, represent levels likely to be associated with the relevant alternatives (factor replacement and gene therapy) according to the clinical evidence available at time protocol design, and therefor may reflect a real-world decision context. However, as Phase III trial data still has to become available and uncertainties about the outcomes of gene therapy in hemophilia exist, the relevance of the baseline scenario may be affected by new clinical data becoming available. Therefore, the results of this study should always be interpreted relative to the latest available clinical data (85).
Comprehension of the survey by participants will be ensured through use of the educational tool that was designed. Vass et al. (86) showed in their study that the use of an animated educational tool did not change preferences of respondents, but improved choice consistency. The information presented in the educational tool developed in the current research covers information needs of patients and was validated by hematologists and piloted with patients. Moreover, the tool also covers different aspects highlighted in the work of Barber et al. (60), including but not limited to uncertainty in long-term safety and efficacy, eligibility criteria, variability in achieved outcomes, and current absence of major safety issues.
It should be acknowledged that the data presented in this paper, and that informed protocol development, was elicited from a small sample of stakeholders and patients. While this approach is appropriate for development of stated preference protocols and is supported by an extensive literature review, a larger sample would be required to reach representativeness of results.
Ethics approval was sought and granted by the Medical Ethics Committee of UZ KU Leuven/Research in Belgium for both the interviews (S62670) that informed this protocol, as well as the conduct of the PAVING survey (S63686). In addition, the ethics committee also approved the analysis plan and data management plan of the study. Prior to the interviews, all interviewees provided written informed consent. Survey participants will be informed that their participation is anonymous and that, to ensure anonymity, they will not be able to view, edit or remove responses once submitted. They will then be asked to provide electronic informed consent before they can answer any questions in the survey. An open text question included at the end of the survey will allow participants to raise any concerns.
Results of the study will be communicated to stakeholders through publications. Results will also be disseminated at clinical and health economic conferences, and will be presented to the advisory board of the study. Moreover, the researchers plan to write a lay language summary of the results to be distributed to patients via the recruiting parties.
Learnings gained through the development of this protocol and the results of the PAVING study may:
- Inform Belgian HTA and payer (and potentially also regulatory) decision-making on gene therapies, by providing insights on the elements of these therapies that patients value, and the acceptability of long-term safety uncertainties. Moreover, the results from the PAVING survey can demonstrate what gene therapy profiles will be acceptable to patients, while also showing the potential existence of preference heterogeneity.
- Lead to the design of similar studies in hemophilia to inform decision making in other countries. While this protocol was setup to specifically meet needs of the Belgian market access setting, the included attributes may also be relevant for HTA/payers in other countries. Before this protocol can be used in other countries, it should be investigated if HTA representatives and payers in other countries believe that attribute classes excluded in this study should be explored in a preference study. Moreover, we advise researchers interested in using this protocol in another country, to perform interviews with patients similar to our interviews to confirm whether the selected attributes are also important to patients in their country of interest.
- Inspire other researchers to conduct similar gene therapy patient preference studies in different disease areas. This protocol describes how the unique features of gene therapies can be transformed to attributes and included in preference studies. While some of the attributes described in this protocol are specific to hemophilia, the researchers would like to encourage others to apply the PAVING approach and use similar attributes in other disease areas where gene therapies are in development.
The studies involving human participants were reviewed and approved by Medical Ethics Committee of UZ KU Leuven/Research. The patients/participants provided their written informed consent to participate in this study.
EO, BH, SM, MG, SS, and IH were involved in the development of the protocol. In Step 1–3, EO performed literature reviews, held meetings with methods experts and the advisory board to inform method and attribute selection. In Step 4, EO and SM designed study materials, conducted interviews with patients, and analyzed them. In Step 5, EO designed the choice tasks and response logic. In Step 6, EO and SM designed the educational tool in collaboration with Mindbytes BVBA. In Step 7 and 8, EO was responsible for the integration of the survey, coordination with Qualtrics, and conduct of the pilot and pretest. Along all steps BH, MG, SS, and IH participated in meetings and reviewed materials. EO produced the first draft of the manuscript, which was subsequently revised and finalized with all authors. All authors contributed to the article and approved the submitted version.
This study was funded by the Patient Preferences in Benefit-Risk Assessments during the Drug Life Cycle (PREFER) project. The PREFER project has received funding from the Innovative Medicines Initiative (IMI) 2 Joint Undertaking under grant agreement No 115966. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and the European Federation of Pharmaceutical Industries and Associations (EFPIA). This text and its contents reflect the PREFER project's view and not the view of IMI, the European Union or EFPIA.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Nigel Cook (Novartis), Juhaeri Juhaeri (Sanofi), Ami Patel (CSL Behring), and the other members of the extended team for their review of the protocol, and all members of the PREFER project for their support in the development of this protocol. In addition, we thank Guildhawk and Oneliner Translations for their translation services, Mindbytes BVBA for their collaboration on the educational tool, and Qualtrics for the programming of the survey. Special thanks to Patrick De Smet (the Belgian hemophilia association, AHVH), Noémie Colasuonno (AHVH), Kathelijne Peerlinck (UZ Leuven), Cedric Hermans (St-Luc University Hospital), Catherine Lambert (St-Luc University Hospital), Nancy Thiry (Belgian Health Care Knowledge Centre, KCE), Irina Cleemput (KCE), Wim Goettsch (National Health Care Institute/University of Utrecht), Rene Westhovens (UZ Leuven), Mitchell Silva (the Belgian European Patients Academy on Therapeutic Innovation, EUPATI BE), and the other members of the advisory board for their support of this study. We would also like to thank all patients that participated in the pilot and pretest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.595797/full#supplementary-material
1. Getting Ready: Recommendations for Timely Access to Advanced Therapy Medicinal Products (ATMPs) in Europe. (2019). Available online at: http://alliancerm.org/wp-content/uploads/2019/07/ARM-Market-Access-Report-FINAL.pdf
2. Hanna E, Rémuzat C, Auquier P, Toumi M. Gene therapies development: slow progress and promising prospect. J Mark Access Health Policy. (2017) 5:1265293. doi: 10.1080/20016689.2017.1265293
3. Marsden G, Towse A, Pearson S, Dreitlein B, Henshall C. Gene Therapy: Understanding the Science, Assessing the Evidence, and Paying for Value. A Report From the 2016 ICER Membership Policy Summit. Institute for Clinical and Economic Review (ICER) (2017).
4. Gutknecht M, Schaarschmidt ML, Herrlein O, Augustin M. A systematic review on methods used to evaluate patient preferences in psoriasis treatments. J Eur Acad Dermatol Venereol. (2016) 30:1454–64. doi: 10.1111/jdv.13749
5. van Overbeeke E, Michelsen S, Toumi M, Stevens H, Trusheim M, Huys I, et al. Market access of gene therapies across Europe, USA, and Canada: challenges, trends, and solutions. Drug Discov Today. (2020). doi: 10.1016/j.drudis.2020.11.024
6. UniQure. UniQure Enrolls First Patient in Phase III HOPE-B Pivotal Study of AMT-061 in Patients with Hemophilia B. (2018). Available online at: https://tools.eurolandir.com/tools/Pressreleases/GetPressRelease/?ID=3479015&lang=en-GB&companycode=nl-qure&v= (accessed June 28, 2018).
7. Pfizer. Pfizer Initiates Pivotal Phase 3 Program for Investigational Hemophilia B Gene Therapy. (2018). Available online at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_initiates_pivotal_phase_3_program_for_investigational_hemophilia_b_gene_therapy-0 (accessed July 16, 2018).
8. BioMarin. BioMarin Provides 2 Years of Clinical Data in 6e13 vg/kg Dose from Ongoing Phase 1/2 Study in Valoctocogene Roxaparvovec Gene Therapy for Severe Hemophilia A at World Federation of Hemophilia 2018 World Congress. (2018). Available online at: https://investors.biomarin.com/2018-05-22-BioMarin-Provides-2-Years-of-Clinical-Data-in-6e13-vg-kg-Dose-from-Ongoing-Phase-1-2-Study-in-Valoctocogene-Roxaparvovec-Gene-Therapy-for-Severe-Hemophilia-A-at-World-Federation-of-Hemophilia-2018-World-Congress (accessed May 22, 2018).
9. Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. (2012) 379:1447–56. doi: 10.1016/S0140-6736(11)61139-2
10. Berntorp E. Joint outcomes in patients with haemophilia: the importance of adherence to preventive regimens. Haemophilia. (2009) 15:1219–27. doi: 10.1111/j.1365-2516.2009.02077.x
11. Bonanad S, Schulz M, Gordo A, Spurden D, Cicchetti M, Cappelleri JC, et al. HaemoPREF: further evaluation of patient perception and preference for treatment in a real world setting. Haemophilia. (2017) 23:884–93. doi: 10.1111/hae.13321
12. Hacker MR, Geraghty S, Manco-Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia. (2001) 7:392–6. doi: 10.1046/j.1365-2516.2001.00534.x
13. Chaugule SS, Hay JW, Young G. Understanding patient preferences and willingness to pay for hemophilia therapies. Patient Prefer Adherence. (2015) 9:1623–30. doi: 10.2147/PPA.S92985
14. van Overbeeke E, Whichello C, Janssens R, Veldwijk J, Cleemput I, Simoens S, et al. Factors and situations influencing the value of patient preference studies along the medical product lifecycle: a literature review. Drug Discov Today. (2019) 24:57–68. doi: 10.1016/j.drudis.2018.09.015
15. van Overbeeke E, Vanbinst I, Jimenez-Moreno AC, Huys I. Patient centricity in patient preference studies: the patient perspective. Front Med. (2020) 7:93. doi: 10.3389/fmed.2020.00093
16. Whichello C, Levitan B, Juhaeri J, Patadia V, Disantostefano R, Pinto CA, et al. Appraising patient preference methods for decision-making in the medical product lifecycle: an empirical comparison. BMC Med Inform Decis Mak. (2020) 20. doi: 10.1186/s12911-020-01142-w
17. van Overbeeke E, Janssens R, Whichello C, Schölin Bywall K, Sharpe J, Nikolenko N, et al. Design, conduct, and use of patient preference studies in the medical product life cycle: a multi-method study. Front Pharmacol. (2019) 10:1395. doi: 10.3389/fphar.2019.01395
18. de Bekker-Grob EW, Donkers B, Jonker MF, Stolk EA. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. (2015) 8:373–84. doi: 10.1007/s40271-015-0118-z
19. Yang J-C, Johnson FR, Kilambi V, Mohamed AF. Sample size and utility-difference precision in discrete-choice experiments: a meta-simulation approach. J Choice Model. (2015) 16:50–7. doi: 10.1016/j.jocm.2015.09.001
20. Gafni A. The standard gamble method: what is being measured and how it is interpreted. Health Serv Res. (1994) 29:207–24.
21. Steures P, Berkhout JC, Hompes PG, van der Steeg JW, Bossuyt PM, van der Veen F, et al. Patients' preferences in deciding between intrauterine insemination and expectant management. Hum Reprod. (2005) 20:752–5. doi: 10.1093/humrep/deh673
22. Sung L, Feldman BM, Schwamborn G, Paczesny D, Cochrane A, Greenberg ML, et al. Inpatient versus outpatient management of low-risk pediatric febrile neutropenia: measuring parents' and healthcare professionals' preferences. J Clin Oncol. (2004) 22:3922–9. doi: 10.1200/JCO.2004.01.077
23. Dales RE, O'Connor A, Hebert P, Sullivan K, McKim D, Llewellyn-Thomas H. Intubation and mechanical ventilation for COPD: development of an instrument to elicit patient preferences. Chest. (1999) 116:792–800. doi: 10.1378/chest.116.3.792
24. Tervonen T, Gelhorn H, Sri Bhashyam S, Poon JL, Gries KS, Rentz A, et al. MCDA swing weighting and discrete choice experiments for elicitation of patient benefit-risk preferences: a critical assessment. Pharmacoepidemiol Drug Saf. (2017) 26:1483–91. doi: 10.1002/pds.4255
25. Hauber B, Coulter J. Using the threshold technique to elicit patient preferences: an introduction to the method and an overview of existing empirical applications. Appl Health Econ Health Policy. (2020) 18:31–46. doi: 10.1007/s40258-019-00521-3
26. Gene Therapy Clinical Trials Worldwide Database. (2018). Available online at: https://a873679.fmphost.com/fmi/webd/GTCT (accessed December 8, 2018).
27. Batty P, Pasi KJ. Gene therapy trials for haemophilia: a step closer to a cure? Expert Rev Precis Med Drug Develop. (2019) 4:259–62. doi: 10.1080/23808993.2019.1632704
28. Costea I, Isasi R, Knoppers BM, Lillicrap D. Haemophilia gene therapy: the patients' perspective. Haemophilia. (2009) 15:1159–61. doi: 10.1111/j.1365-2516.2009.02065.x
29. United States. Food and Drug Administration. Gene Therapy as a Treatment Modality for Hemophilia. Available online at: https://www.fda.gov/media/124436/download (accessed February 12, 2019).
30. Nathwani AC, Reiss UM, Tuddenham EGD, Rosales C, Chowdary P, McIntosh J, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. (2014) 371:1994–2004. doi: 10.1056/NEJMoa1407309
31. Nathwani AC, Tuddenham EGD, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. (2011) 365:2357–65. doi: 10.1056/NEJMoa1108046
32. Sullivan SK, Giermasz A, Samelson-Jones BJ, Ducore JM, Teitel JM, Cuker A, et al. Investigational SPK-9001: adeno-associated virus-mediated gene transfer for hemophilia B - persistent, stable factor IX activity at one year independent of downstream purification method. Haemophilia. (2018) 24:209–18.
33. George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. (2017) 377:2215–27. doi: 10.1056/NEJMoa1708538
34. Miesbach W, Meijer K, Coppens M, Kampmann P, Klamroth R, Schutgens R, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. (2018) 131:1022–32. doi: 10.1182/blood-2017-09-804419
35. Leebeek F, Meijer K, Coppens M, Kampmann P, Klamroth, Schutgens R, et al. Reduction in annualized bleeding and factor IX consumption up to 2.5 years in adults with severe or moderate-severe haemophilia B treated with AMT-060 (AAV5-hFIX) gene therapy. Blood. (2018) 132:92–5856. doi: 10.1182/blood-2018-99-109995
36. Chowdary P, Shapiro S, Davidoff AM, Reiss U, Alade R, Brooks G, et al. A single intravenous infusion of FLT180a results in factor IX activity levels of more than 40% and has the potential to provide a functional cure for patients with haemophilia B. Blood. (2018) 132. doi: 10.1182/blood-2018-99-118050
37. Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. (2006) 12:342–7. doi: 10.1038/nm1358
38. Rangarajan S, Kim B, Lester W, Symington E, Madan B, Laffan M, et al. Achievement of normal factor VIII activity following gene transfer with valoctocogene roxaparvovec (BMN 270): long-term efficacy and safety results in patients with severe hemophilia A. Haemophilia. (2018) 24:65.
39. Pasi KJ, Rangarajan S, Kim B, Lester W, Perry D, Madan B, et al. Achievement of normal circulating factor VIII activity following Bmn 270 AAV5-FVIII gene transfer: interim, long-term efficacy and safety results from a phase 1/2 study in patients with severe hemophilia A. Blood. (2017) 130:603.
40. Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5–Factor VIII gene transfer in severe hemophilia A. N Engl J Med. (2017) 377:2519–30. doi: 10.1056/NEJMoa1708483
41. High KA, George LA, Eyster E, Sullivan SK, Ragni MV, Croteau SE, et al. A phase 1/2 trial of investigational Spk-8011 in hemophilia a demonstrates durable expression and prevention of bleeds. Blood. (2018) 132(Suppl_1):487. doi: 10.1182/blood-2018-99-115495
42. Nathwani AC, Tuddenham EGD, Chowdary P, McIntosh J, Lee D, Rosales C, et al. GO-8: preliminary results of a phase I/II dose escalation trial of gene therapy for haemophilia a using a novel human factor VIII variant. Blood. (2018) 132(Suppl. 1):489. doi: 10.1182/blood-2018-99-118256
43. Teal S, Brohan E, Hettema Y, Humphrey L, Willgoss T, Hudgens S, et al. Development and psychometric evaluation of a novel tool for assessing patient perception and preference for haemophilia treatment (HaemoPREF). Haemophilia. (2014) 20:666–73. doi: 10.1111/hae.12432
44. Brown TM, Pashos CL, Joshi AV, Lee WC. The perspective of patients with haemophilia with inhibitors and their care givers: preferences for treatment characteristics. Haemophilia. (2011) 17:476–82. doi: 10.1111/j.1365-2516.2010.02401.x
45. Steen Carlsson K, Andersson E, Berntorp E. Preference-based valuation of treatment attributes in haemophilia a using web survey. Haemophilia. (2017) 23:894–903. doi: 10.1111/hae.13322
46. Furlan R, Krishnan S, Vietri J. Patient and parent preferences for characteristics of prophylactic treatment in hemophilia. Patient Prefer Adherence. (2015) 9:1687–94. doi: 10.2147/PPA.S92520
47. Mantovani LG, Monzini MS, Mannucci PM, Scalone L, Villa M, Gringeri A, et al. Differences between patients', physicians' and pharmacists' preferences for treatment products in haemophilia: a discrete choice experiment. Haemophilia. (2005) 11:589–97. doi: 10.1111/j.1365-2516.2005.01159.x
48. Mohamed AF, Epstein JD, Li-McLeod JM. Patient and parent preferences for haemophilia a treatments. Haemophilia. (2011) 17:209–14. doi: 10.1111/j.1365-2516.2010.02411.x
49. Scalone L, Mantovani LG, Borghetti F, Von Mackensen S, Gringeri A. Patients', physicians', and pharmacists' preferences towards coagulation factor concentrates to treat haemophilia with inhibitors: results from the COHIBA study. Haemophilia. (2009) 15:473–86. doi: 10.1111/j.1365-2516.2008.01926.x
50. DiBenedetti DB, Coles TM, Sharma T, Pericleous L, Kulkarni R. Assessing patients' and caregivers' perspectives on stability of factor VIII products for haemophilia A: a web-based study in the United States and Canada. Haemophilia. (2014) 20:e296–303. doi: 10.1111/hae.12459
51. Lock J, de Bekker-Grob EW, Urhan G, Peters M, Meijer K, Brons P, et al. Facilitating the implementation of pharmacokinetic-guided dosing of prophylaxis in haemophilia care by discrete choice experiment. Haemophilia. (2016) 22:e1–10. doi: 10.1111/hae.12851
52. Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. (2000) 288:669–72. doi: 10.1126/science.288.5466.669
53. Musso R, Santoro R, Coppola A, Marcucci M, Sottilotta G, Targhetta R, et al. Patient preference for needleless factor VIII reconstitution device: the Italian experience. Int J Gen Med. (2010) 3:203–8. doi: 10.2147/IJGM.S12096
54. Moia M, Mantovani LG, Carpenedo M, Scalone L, Monzini MS, Cesana G, et al. Patient preferences and willingness to pay for different options of anticoagulant therapy. Intern Emerg Med. (2013) 8:237–43. doi: 10.1007/s11739-012-0844-3
55. Wasserman J, Aday LA, Begley CE, Ahn C, Lairson DR. Measuring health state preferences for hemophilia: development of a disease-specific utility instrument. Haemophilia. (2005) 11:49–57. doi: 10.1111/j.1365-2516.2005.01054.x
56. Barlow JH, Stapley J, Ellard DR. Living with haemophilia and von Willebrand's: a descriptive qualitative study. Patient Educ Couns. (2007) 68:235–42. doi: 10.1016/j.pec.2007.06.006
57. Arnold E, Heddle N, Lane S, Sek J, Almonte T, Walker I. Handheld computers and paper diaries for documenting the use of factor concentrates used in haemophilia home therapy: a qualitative study. Haemophilia. (2005) 11:216–26. doi: 10.1111/j.1365-2516.2005.01095.x
58. van Overbeeke E, Michelsen S, Hauber B, Peerlinck K, Hermans C, Lambert C, et al. Patient perspectives regarding gene therapy in haemophilia: interviews from the PAVING study. Haemophilia. (2020) 27:129–36. doi: 10.1111/hae.14190
59. Hollin IL, Craig BM, Coast J, Beusterien K, Vass C, DiSantostefano R, et al. Reporting formative qualitative research to support the development of quantitative preference study protocols and corresponding survey instruments: guidelines for authors and reviewers. Patient. (2020) 13:121–36. doi: 10.1007/s40271-019-00401-x
60. Miesbach W, O'Mahony B, Key NS, Makris M. How to discuss gene therapy for haemophilia? A patient and physician perspective. Haemophilia. (2019) 25:545–57. doi: 10.1111/hae.13769
61. Mohamed AF, Johnson FR, Hauber AB, Lescrauwaet B, Masterson A. Physicians' stated trade-off preferences for chronic hepatitis B treatment outcomes in Germany, France, Spain, Turkey, and Italy. Eur J Gastroenterol Hepatol. (2012) 24:419–26. doi: 10.1097/MEG.0b013e328350914c
62. Tomlinson D, Bartels U, Gammon J, Hinds PS, Volpe J, Bouffet E, et al. Chemotherapy versus supportive care alone in pediatric palliative care for cancer: comparing the preferences of parents and health care professionals. Cmaj. (2011) 183:E1252–8. doi: 10.1503/cmaj.110392
63. den Uijl I, Biesma D, Grobbee D, Fischer K. Turning severe into moderate haemophilia by prophylaxis: are we reaching our goal? Blood Transfus. (2013) 11:364–9. doi: 10.2450/2012.0092-12
64. Carroll L, Benson G, Lambert J, Benmedjahed K, Zak M, Lee XY. Real-world utilities and health-related quality-of-life data in hemophilia patients in France and the United Kingdom. Patient Prefer Adherence. (2019) 13:941–57. doi: 10.2147/PPA.S202773
65. Grosse SD, Chaugule SS, Hay JW. Estimates of utility weights in hemophilia: implications for cost-utility analysis of clotting factor prophylaxis. Expert Rev Pharmacoecon Outcomes Res. (2015) 15:267–83. doi: 10.1586/14737167.2015.1001372
66. O'Hara J, Walsh S, Camp C, Mazza G, Carroll L, Hoxer C, et al. The impact of severe haemophilia and the presence of target joints on health-related quality-of-life. Health Qual Life Outcomes. (2018) 16:84. doi: 10.1186/s12955-018-0908-9
67. Forsyth AL, Witkop M, Lambing A, Garrido C, Dunn S, Cooper DL, et al. Associations of quality of life, pain, and self-reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: results in adults with hemophilia in the HERO study. Patient Prefer Adherence. (2015) 9:1549–60. doi: 10.2147/PPA.S87659
68. Jiang H, Pierce GF, Ozelo MC, de Paula EV, Vargas JA, Smith P, et al. Evidence of multiyear factor IX expression by AAV-mediated gene transfer to skeletal muscle in an individual with severe hemophilia B. Mol Ther. (2006) 14:452–5. doi: 10.1016/j.ymthe.2006.05.004
69. Fabb SA, Dickson JG. Technology evaluation: AAV factor IX gene therapy, Avigen Inc. Curr Opin Mol Ther. (2000) 2:601–6.
70. Lu DR, Zhou JM, Zheng B, Qiu XF, Xue JL, Wang JM, et al. Stage I clinical trial of gene therapy for hemophilia B. Sci China B. (1993) 36:1342–51.
71. Powell JS, Ragni MV, White GC III, Lusher JM, Hillman-Wiseman C, Moon TE, et al. Phase 1 trial of FVIII gene transfer for severe hemophilia a using a retroviral construct administered by peripheral intravenous infusion. Blood. (2003) 102:2038–45. doi: 10.1182/blood-2003-01-0167
72. Roth DA, Tawa NE III, O'Brien JM, Treco DA, Selden RF. Nonviral transfer of the gene encoding coagulation factor VIII in patients with severe hemophilia A. N Engl J Med. (2001) 344:1735–42. doi: 10.1056/NEJM200106073442301
73. Verschueren S, Buffel C, Vander Stichele G. Developing theory-driven, evidence-based serious games for health: framework based on research community insights. JMIR Serious Games. (2019) 7:e11565. doi: 10.2196/11565
74. Chew LD, Griffin JM, Partin MR, Noorbaloochi S, Grill JP, Snyder A, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. (2008) 23:561–6. doi: 10.1007/s11606-008-0520-5
75. Mansfield C, Poulos C, Boeri M, Hauber B. Pmu129 performance of a comprehension question in discrete-choice experiment surveyS (DCE). Value Health. (2019) 22:S730–S1. doi: 10.1016/j.jval.2019.09.1747
76. Ryan K, Gannon-Slater N, Culbertson MJ. Improving survey methods with cognitive interviews in small- and medium-scale evaluations. Am J Eval. (2012) 33:414–30. doi: 10.1177/1098214012441499
77. Eccles DW, Arsal G. The think aloud method: what is it and how do I use it? Qual Res Sport Exerc Health. (2017) 9:514–31. doi: 10.1080/2159676X.2017.1331501
78. World Federation of Hemophilia. Report on the Annual Global Survey 2018. (2019). Available online at: http://www1.wfh.org/publications/files/pdf-1731.pdf (accessed July 08, 2020).
79. Shanahan M, Larance B, Nielsen S, Cohen M, Schaffer M, Campbell G. A protocol for a discrete choice experiment: understanding patient medicine preferences for managing chronic non-cancer pain. BMJ Open. (2019) 9:e027153. doi: 10.1136/bmjopen-2018-027153
80. Barber S, Bekker H, Marti J, Pavitt S, Khambay B, Meads D. Development of a discrete-choice experiment (DCE) to elicit adolescent and parent preferences for hypodontia treatment. Patient. (2019) 12:137–48. doi: 10.1007/s40271-018-0338-0
81. Asbell P, Messmer E, Chan C, Johnson G, Sloesen B, Cook N. Defining the needs and preferences of patients with dry eye disease. BMJ Open Ophthalmol. (2019) 4:e000315. doi: 10.1136/bmjophth-2019-000315
82. Patalano F, Gutzwiller FS, Shah B, Kumari C, Cook NS. Gathering structured patient insight to drive the PRO strategy in COPD: patient-centric drug development from theory to practice. Adv Ther. (2020) 37:17–26. doi: 10.1007/s12325-019-01134-x
83. Soekhai V, Whichello C, Levitan B, Veldwijk J, Pinto CA, Donkers B, et al. Methods for exploring and eliciting patient preferences in the medical product lifecycle: a literature review. Drug Discovery Today. (2019) 24:1324–31. doi: 10.1016/j.drudis.2019.05.001
84. Janssen MF, Pickard AS, Golicki D, Gudex C, Niewada M, Scalone L, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. (2013) 22:1717–27. doi: 10.1007/s11136-012-0322-4
85. Arruda VR, Doshi BS. Gene therapy for hemophilia: facts and quandaries in the 21st century. Mediterr J Hematol Infect Dis. (2020) 12:e2020069. doi: 10.4084/mjhid.2020.069
Keywords: preference, instrument design, hemophilia, interviews, survey, gene therapy
Citation: van Overbeeke E, Hauber B, Michelsen S, Goldman M, Simoens S and Huys I (2021) Patient Preferences to Assess Value IN Gene Therapies: Protocol Development for the PAVING Study in Hemophilia. Front. Med. 8:595797. doi: 10.3389/fmed.2021.595797
Received: 17 August 2020; Accepted: 15 February 2021;
Published: 09 March 2021.
Edited by:
Steffen Thirstrup, NDA Advisory Services Ltd., United KingdomReviewed by:
Juan Marcos Gonzalez, Duke University, United StatesCopyright © 2021 van Overbeeke, Hauber, Michelsen, Goldman, Simoens and Huys. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eline van Overbeeke, ZWxpbmUudmFub3ZlcmJlZWtlQHBmaXplci5jb20= orcid.org/0000-0003-0073-9350
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.