
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 28 January 2021
Sec. Gastroenterology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.615858
This article is part of the Research Topic Game Changers in Inflammatory Bowel Diseases View all 12 articles
 Xiaojun Zhuang†
Xiaojun Zhuang† Zhenyi Tian†
Zhenyi Tian† Na Li
Na Li Ren Mao
Ren Mao Xiaozhi Li
Xiaozhi Li Min Zhao
Min Zhao Shanshan Xiong
Shanshan Xiong Zhirong Zeng
Zhirong Zeng Rui Feng*
Rui Feng* Minhu Chen*
Minhu Chen*Background and Aims: Gut microbiota recolonization after intestinal resection had been reported to be associated with post-operative recurrence in Crohn's disease (CD). However, the results of different studies are inconsistent and even contradictory. In addition, knowledge on the efficacy of microbial-based therapies in preventing post-operative recurrence of CD is limited. Therefore, the aim of this review was to investigate gut microbiota profiles in patients with CD before and after surgery and evaluate microbial-based therapies in preventing post-operative recurrence.
Methods: Electronic databases were searched from inception to 31 June 2020 using predefined terms. Studies that investigated gut microbiota pre- and post-intestinal resection, and microbial-based therapies in preventing post-operative recurrence, were eligible. Study quality was assessed using either the Newcastle–Ottawa scale or Jadad scoring system.
Results: Twelve studies investigating gut microbiota of CD patients suffering from operation, and other 12 studies evaluating the efficacy of antibiotics and probiotics, were included in our review. The mucosa-associated microbiota in surgical biopsy of CD patients is significantly distinct from that in normal mucosa from healthy subjects. Gut microbiota recolonization following surgery might be associated with post-operative recurrence in CD patients. Furthermore, CD patients with post-operative recurrence presented a gain in pro-inflammatory pathogenic bacteria and a loss in short-chain fatty acid-producing bacteria before and after surgery. However, no consistent bacteria or metabolites were found to predict the post-operative recurrence of CD. Additionally, microbial-based therapies are deficient and present restricted widespread clinical utility due to several deficiencies.
Conclusion: Recurrence-associated bacteria observed pre- and post- operation might be promising in preventing the post-operative recurrence of CD. Furthermore, potential microbe biomarkers for predicting subsequent disease recurrence should be validated with larger sample sizes using more rigorous and standardized methodologies.
Crohn's disease (CD) is a chronic relapsing inflammatory bowel disease (IBD) with multifactorial pathogenesis and is characterized by recurrent transmural inflammation (1). Eventually, the recurrent inflammation can lead to intestinal stricture and fistulae (often perianal) complications or the creation of abscesses (2). Surgical resection is required in ~70–80% of CD patients, owing to the penetrative nature of the disease, the development of structural changes, and the failure of medical therapy (3–5). However, operative management is not curative, and up to 75% of CD patients will experience post-operative disease recurrence (clinical, endoscopic, or surgical recurrence) over time (6–8). As a consequence, ~30% of patients require a second surgical resection within 5 years (9, 10). Given the significant recurrence risk after surgical resection in CD, elucidating the specific factors that predispose patients to post-operative CD recurrence is a high priority. Multiple clinical risk factors, including active smoking, perforating disorders, prior resection, myenteric plexitis, younger age of disease onset, short disease duration, CD behavior, histologic involvement of resection margins, remnant disease post-operation, and length of the resected segment, have been associated with post-operative CD recurrence; however, these factors are far from being adequate in predicting disease recurrence (11–13).
Gut microbiota alterations have been identified as key contributors to the pathogenesis of CD; the crucial link between gut microbiota dysbiosis and post-operative disease recurrence has been documented by numerous studies (14–16). However, the post-operative role of microbial communities in patients with CD remains unknown, largely owing to heterogeneous studies with highly diverse results. In order to facilitate the use of gut microbiota in improving the diagnosis and treatment of post-operative CD, it is imperative to elucidate the bacteria that are associated with disease recurrence or its absence and evaluate whether these microbial factors could predict the post-operative CD recurrence.
Although there is compelling evidence pointing to a critical role of gut microflora in the post-operative CD recurrence, microbial-based therapies for preventing CD recurrence following surgery remain limited (17). Antibiotics and probiotic supplements aimed at altering gut microbiota composition have both been studied in terms of their ability to prevent post-operative disease recurrence; however, there is currently no evidence-based consensus on the topic (18–20). Antibiotics may be the most cost-effective strategy to prevent post-operative disease recurrence in patients who can tolerate the treatment, but the long-term effects beyond antibiotic cessation are questionable (10). In addition, the prolonged administration of these antibiotics is not feasible, owing to a high rate of side effects, significant toxicity, and bacterial resistance (18). Accumulating evidence has implicated that manipulating gut microflora with probiotics is an appealing alternative in reducing the postsurgical CD relapse rate by counterbalancing harmful bacteria (21). However, there are currently design limitations in probiotic trials for the prevention of post-operative CD recurrence; these limitations include small sample sizes, short observation periods, or the co-administration of other drugs.
The aim of this review was to summarize the results of studies investigating gut microbiota alterations in CD individuals suffering from operative management and to evaluate whether specific gut microbiota variations are associated with the post-operative recurrence (PR) of the disease. Furthermore, we revisited previous randomized controlled trials and high-quality uncontrolled studies in an effort to better elucidate the role of microbial-based therapies in preventing the PR of CD.
The protocol for this systematic review was registered on the International Prospective Register of Systematic Reviews (PROSPERO) with the ID number CRD42020200956, and the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) checklist was used as a guideline. A comprehensive search was performed on public databases, including PubMed, Web of Science, Embase, Scopus, and the Cochrane Library (last search: 31 June, 2020), with no date or language restrictions. The MeSH term and free-text word combinations that we used were the following: “Crohn's disease,” “CD,” “post-operative,” “surgery,” “resection,” “recurrence,” “microbiota,” “microbiome,” “microflora,” “bacterial flora,” “antibiotic,” “probiotic.” Boolean operators (AND, OR, NOT) were used to widen and narrow the search results.
The articles were selected on the basis of certain criteria: observational studies that focused on gut microbiota profiles associated with the post-operative disease course in CD patients or clinical trials that evaluated the effect of microbial-based therapies (antibiotics and probiotics) on the prevention of the PR of CD. The microbial communities in these studies were assessed from either fecal or mucosal samples. Two independent investigators screened titles and abstracts from the databases according to the eligibility criteria. Subsequently, the included articles were subjected to whole-paper reading, and the accompanying references were checked to identify additional potentially eligible articles. Any discrepancies between the investigators were resolved through discussion until consensus was reached, and a third reviewer was involved if necessary.
For studies investigating gut microbiota profiles in the PR of CD, we extracted demographic, clinical, and bacterial richness and diversity and taxonomic bacterial composition (phylum, class, order, family, and genus), as well as information on the methodology applied to the microbiota analysis. For each of the studies that evaluated the effect of microbial-based therapies on preventing the PR of CD, we extracted data on the first author of the study, the year of publication, the population examined, intervention details, treatment duration, recurrence definition, follow-ups, and the outcomes.
The quality of the included studies was evaluated using the Newcastle–Ottawa Scale (NOS) for cohort studies (22). The NOS contains three criteria: selection (representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at start of study), comparability (comparability of cohorts on the basis of the design or analysis), and exposure (assessment of outcome, whether follow-up was long enough for outcomes to occur, adequacy of follow-up of cohorts). A quality score ranging from 0 to 9 was obtained by the use of a rating algorithm previously described: 0–3 (poor), 4–6 (moderate), and 7–9 (high).
The Jadad quality scoring system, which is based on randomization, blinding, and dropouts (withdrawals), was used to assess the quality of the randomized controlled trials (23). The quality scale ranges from 0 to 5 points, with a score of ≤2 indicating a low-quality report and a score of ≥3 indicating a high-quality report.
The initial database search yielded 3,712 records. After the automatic removal of duplicate, 3,346 unique abstracts were screened and 54 records were selected for full-text review. Finally, 24 original articles were included in this systematic review (Figure 1). These articles included 12 studies reporting gut microbiota profiles in post-operative, and five and seven studies evaluating the efficacy of antibiotics and probiotics, respectively, in preventing the PR of CD (24–47). The quality scores of the included studies were assessed and are reported in Supplementary Table 1.
The demographic and clinical characteristics of the patients from the included studies investigating gut microbiota are detailed in Table 1. The studies were performed in different geographical regions (including Sweden, Belgium, France, Canada, Australia, France, and the USA) from 2002 to 2020, and almost all of their subjects were adults. In patients with CD, either ileal or ileocecal resections were performed to remove diseased areas from the ileum and right colon with ileocolonic anastomosis. Approximately, 3–18 months after intestinal surgery, a post-operative colonoscopy was performed to assess the endoscopic recurrence based on the Rutgeerts score (48); and the PR was assigned a Rutgeerts score of ≥i2. In addition, the endoscopic recurrence rates were reported as ranging between 33.3 and 73.7% in the included studies.
Mucosal and/or fecal samples were collected at the time of surgery or at different time points in the follow-up period. The various differences in the handling and analysis of samples that were observed among the individual studies are shown in Supplementary Table 2. In the majority of the studies, the samples were preserved at either −20 or −80°C. In approximately half of the studies, DNA extraction was used for microbiota analysis, using reliable kits from different manufacturers, while other studies did not provide information on the methods they used. Most of the analyzed studies employed sequencing techniques based on the hypervariable regions of the 16S ribosomal ribonucleic acid (rRNA) gene for the gut microbiota analysis. However, two early studies analyzed specific bacterial strains using fluorescence in situ hybridization (FISH) and culture-based methods. In addition, taxonomic classification was assigned via multiple databases, with Greengenes and Silva being the most commonly used ones.
In order to characterize the mucosa-associated microbiota in CD patients, we compared the surgical biopsies of CD patients and the normal samples from healthy subjects in Table 2. With regard to the microbiota community diversity, the alpha-diversity of mucosa-associated microbiota from surgical specimens decreased significantly in CD patients. In addition, beta-diversity analysis revealed that the mucosa-associated microbiota in the surgical biopsies of CD patients deviated significantly from those of healthy controls.
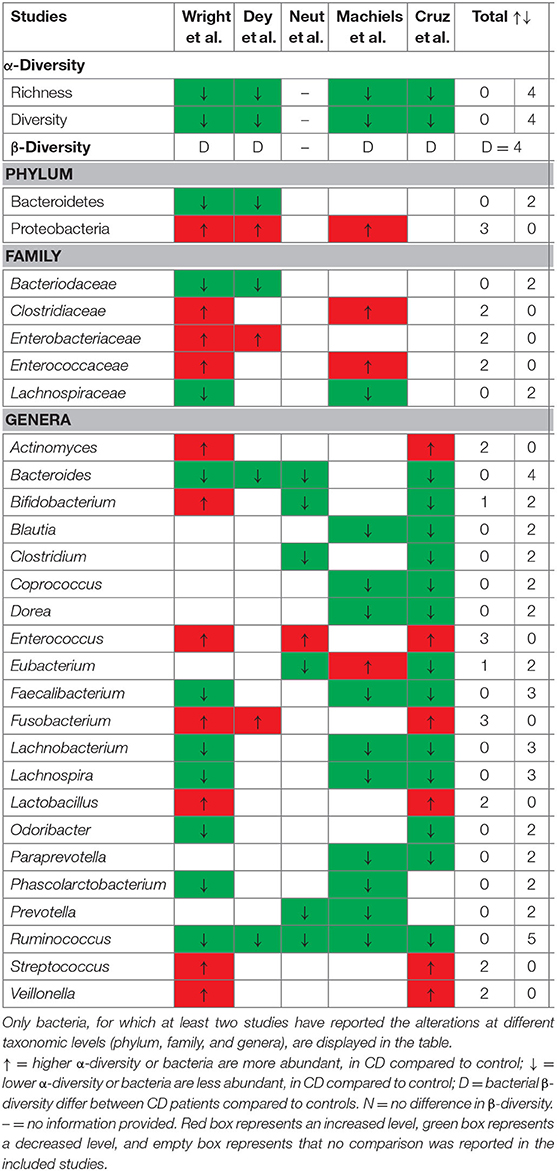
Table 2. Mucosa-associated microbiota of patients with CD from the resection specimen at the time of surgery compared to healthy controls.
A comparison of the relative bacterial abundance at different taxonomic levels revealed that the mucosal microbiome composition of CD patients and control cases differed in a manner of ways. At the phylum level, the relative abundance of Bacteroidetes and Firmicutes in surgical biopsies of CD patients at the time of resection decreased; on the contrary, the relative abundance of Proteobacteria and Fusobacteria increased. At the family level, surgical specimens from CD patients contained an increase in Clostridiaceae, Enterobacteriaceae, and Enterococcaceae populations and a decrease in Bacteroidaceae and Lachnospiraceae populations. Moreover, in CD patients who suffered from mucosal microbiota dysbiosis, which also showed mucosal microbiota dysbiosis at the deeper genus level, 21 bacterial genera were found in different abundances. More specifically, the Actinomyces, Enterococcus, Fusobacterium, Lactobacillus, Streptococcus, and Veillonella populations increased, while the Bacteroides, Blautia, Clostridium, Coprococcus, Dorea, Faecalibacterium, Lachnobacterium, Lachnospira, Odoribacter, Paraprevotella, Phascolarctobacterium, Prevotella, and Ruminococcus populations decreased. However, the changes in the Bifidobacterium and Eubacterium numbers reported from surgical biopsies are divergent and even contradictory in the included studies. A more comprehensive list of specific mucosa-associated microbiota alterations of CD patients at the phylum, class, order, family, and genus levels is presented in Supplementary Table 3.
Intestinal resection may play a crucial role in the gut microbiota recolonization process. Several studies have investigated temporal alterations of the mucosal microbiota community structure in CD patients before and after surgery (23–34). The taxonomic differences in the mucosa-associated microbiota of CD patients following surgery compared to baseline are presented in Table 3 and Supplementary Table 4. There were no significant alpha-diversity differences between the surgical samples and those obtained from the post-operative follow-up. In addition, it was observed that the beta-diversity of the gut microbiota of post-operative CD patents differed from that at the time of resection in three studies, whereas one study reported no significant differences.
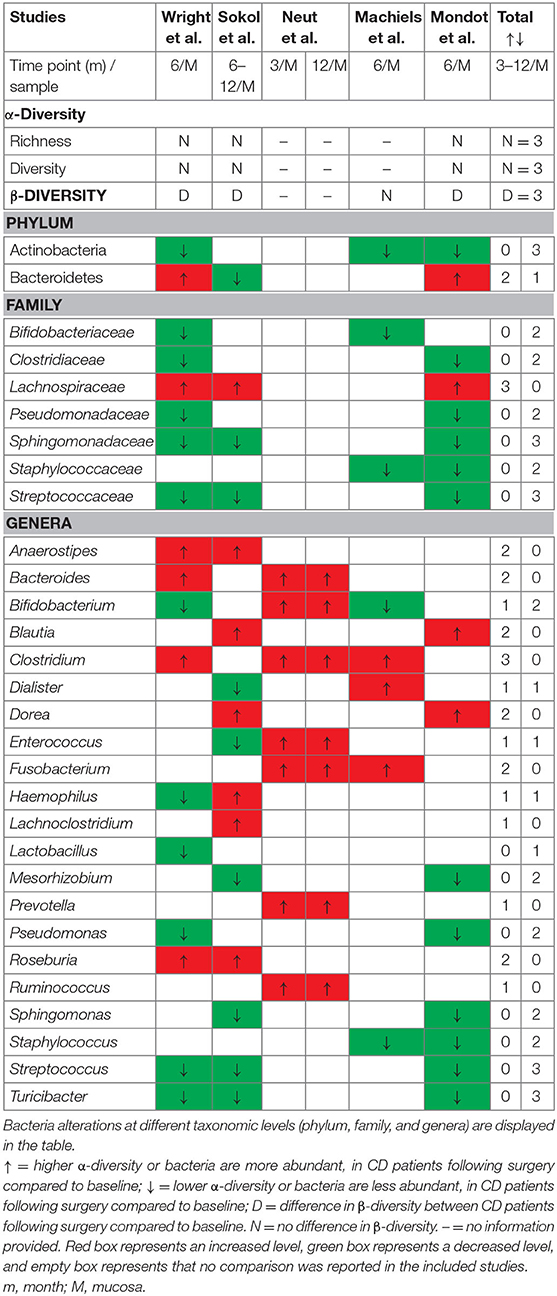
Table 3. Changes in mucosa-associated microbiota of patients with CD at different time points of follow-up compared to baseline.
At the phylum level, CD patients had reduced Actinobacteria and elevated Fusobacteria levels during the postsurgical disease course, while the Bacteroidetes levels were inconclusive in three studies. Following resection, the mucosa of CD patients was enriched with members of the Lachnospiraceae family; on the contrary, Bifidobacteriaceae, Clostridiaceae, Pseudomonadaceae, Sphingomonadaceae, Staphylococcaceae, and Streptococcaceae levels in these patients decreased. No clear overall conclusion could be drawn from the included studies in terms of the bacterial genera populations. Post-operative CD patients had higher relative abundances of the Anaerostipes, Bacteroides, Blautia, Clostridium, Dorea, Fusobacterium, Prevotella, Roseburia, Ruminococcus, and Lachnoclostridium genera and lower abundances of the Lactobacillus, Mesorhizobium, Pseudomonas, Sphingomonas, Staphylococcus, Streptococcus, and Turicibacter genera. Moreover, higher Enterococcaceae and Fusobacterium and lower Lachnospiraceae and Faecalibacterium were found in both post-operative mucosal and fecal microbiota in CD patients. Changes in Bifidobacterium, Dialister, Enterococcus, and Haemophilus were inconsistent among the included studies. A detailed description of gut microbiota alterations at the phylum, class, order, family, and genus levels in post-operative CD patients is provided in Supplementary Table 4. The fecal microbiota profiles of CD patients were assessed before and after surgery in two longitudinal studies. Strömbeck et al. found that the fecal microbiota composition at an early follow-up (3–10 weeks) after resection is similar to that at a 1-year follow-up (25), while Hamilton et al. demonstrated that fecal bacterial communities are associated with the protection from and the occurrence of CD recurrence after surgery (24).
The gut recolonization and details of the bacterial diversity and composition of CD patients that had undergone surgical intervention were compared with those of healthy control subjects at different time points during follow-up periods in three studies. Wright et al. reported that ileal specimens that were obtained from CD patients 6 and 18 months post-operatively had decreased alpha diversity compared to the control samples (30). In addition, the microbial composition of mucosal and fecal samples differed significantly between CD patients (at the post-operative follow-up) and healthy control subjects, which was reported in two studies.
A comparison of the gut microbial communities, including taxonomic changes and abundances in post-operative CD case and control subjects, is shown in Supplementary Table 5. The fecal microbiota composition in CD patients at the 1-year follow-up only had higher Ruminococcus gnavus, Shigella spp., and Escherichia spp. levels, while the majority of the bacterial taxa were less abundant than those in healthy subjects. Neut et al. identified and quantified specific bacterial populations in the mucosa-associated microbiota in CD patients that had undergone intestinal resection, using a traditional culture-based method; the authors demonstrated that Bifidobacterium, Eubacterium, and Ruminococcus populations were rarely encountered in the CD biopsy specimens collected after ileocolectomy at the 3-months and 1-year follow-ups, compared to the ileocolectomy controls (35). The authors of another study with a larger sample size reported that the mucosal biopsy samples that were obtained 6 and 18 months post-operatively from CD patients differed significantly from those of surgical controls; more specifically, they detected increased Fusobacteria, Bifidobacteriaceae, Enterococcaceae, Bifidobacterium, Fusobacterium, and Trabulsiella counts (30).
Numerous studies have indicated that distinct gut microbiota profiles at the time of surgery and during the post-operative follow-up are associated with disease recurrence and remission, respectively. As for post-operative endoscopic recurrence-related microbiota, no clear overall conclusion could be drawn from the included studies; however, a number of differences were observed between recurrence cases and those without recurrence when comparing the relative abundance of bacterial taxa (Table 4). Bacteria taxa from mucosal biopsies obtained at the time of resection and associated with PR risk were examined in seven studies, while feces-associated microbiota only examined in one study. The counts of the Bacteroidetes and Firmicutes phyla in CD patients with PR decreased, while those of the Actinobacteria, Fusobacteria, and Proteobacteria phyla increased. The counts of the Erysipelotrichaceae and Lachnospiraceae families in CD patients with PR were significantly lower, while those of the Enterobacteriaceae family increased. Several studies reported that the relative abundance of the Clostridium, Corynebacterium, Dialister, Enterococcus, Fusobacterium, Lactobacillus, Lactococcus, Proteus, Streptococcus, and Veillonella genera in CD patients with PR increased, while that of the Alistipes, Bacteroides, Bifidobacterium, Blautia, Coprobacillus, Dorea, Faecalibacterium, Gemella, Odoribacter, Parabacteroides, Paraprevotella, Ruminococcus, Subdoligranulum, and Turicibacter genera decreased. However, the disease recurrence-associated changes in the Actinomyces and Streptococcus counts were inconsistent among the included studies. Additionally, the microbial communities of fecal samples that were obtained before surgery revealed Atopobium, Corynebacterium, Gemella, and Rothia counts in CD patients that developed PR than in those that did not. Nine studies provided detailed microbiota profiles associated with recurrence and remission observed at a post-operative colonoscopy, and three studies investigated the fecal microbiota composition after surgery. The findings regarding the mucosa-associated microbiota at the time of surgery revealed that the patients with PR experienced increases in their Fusobacteria and Proteobacteria counts and decreases in their Bacteroidetes and Firmicutes counts. Moreover, CD patients experiencing PR had decreased Alistipes, Atopobium, Bacteroides, Bifidobacterium, Blautia, Dialister, Dorea, Faecalibacterium, Odoribacter, Parabacteroides, Paraprevotella, Ruminococcus, Subdoligranulum, and Turicibacter counts and increased Clostridium, Collinsella, Coprobacillus, Enterococcus, Fusobacterium, Proteus, and Streptococcus counts. However, there was no consensus among studies on how the Odoribacter and Oscillospira counts differed in CD patients with PR. Strömbeck et al. detected higher Actinobacteria and lower Alistipes counts in the fecal microbiota of the PR group at their 1-year follow-up; Alistipes was found to correlate negatively with the Rutgeerts score (25). In another study tracking the trends in the fecal microbiota changes in patients with endoscopic recurrence, the relative Fusobacterium and Bifidobacterium abundances increased and decreased, respectively, in 1, 3, and 6 months after surgery compared to that in patients in remission (26). In addition, Hamilton et al. found that bacterial clusters enriched with Enterobacteriaceae and Lachnospiraceae were associated with an increased risk of disease recurrence and the maintenance of remission, respectively (24).
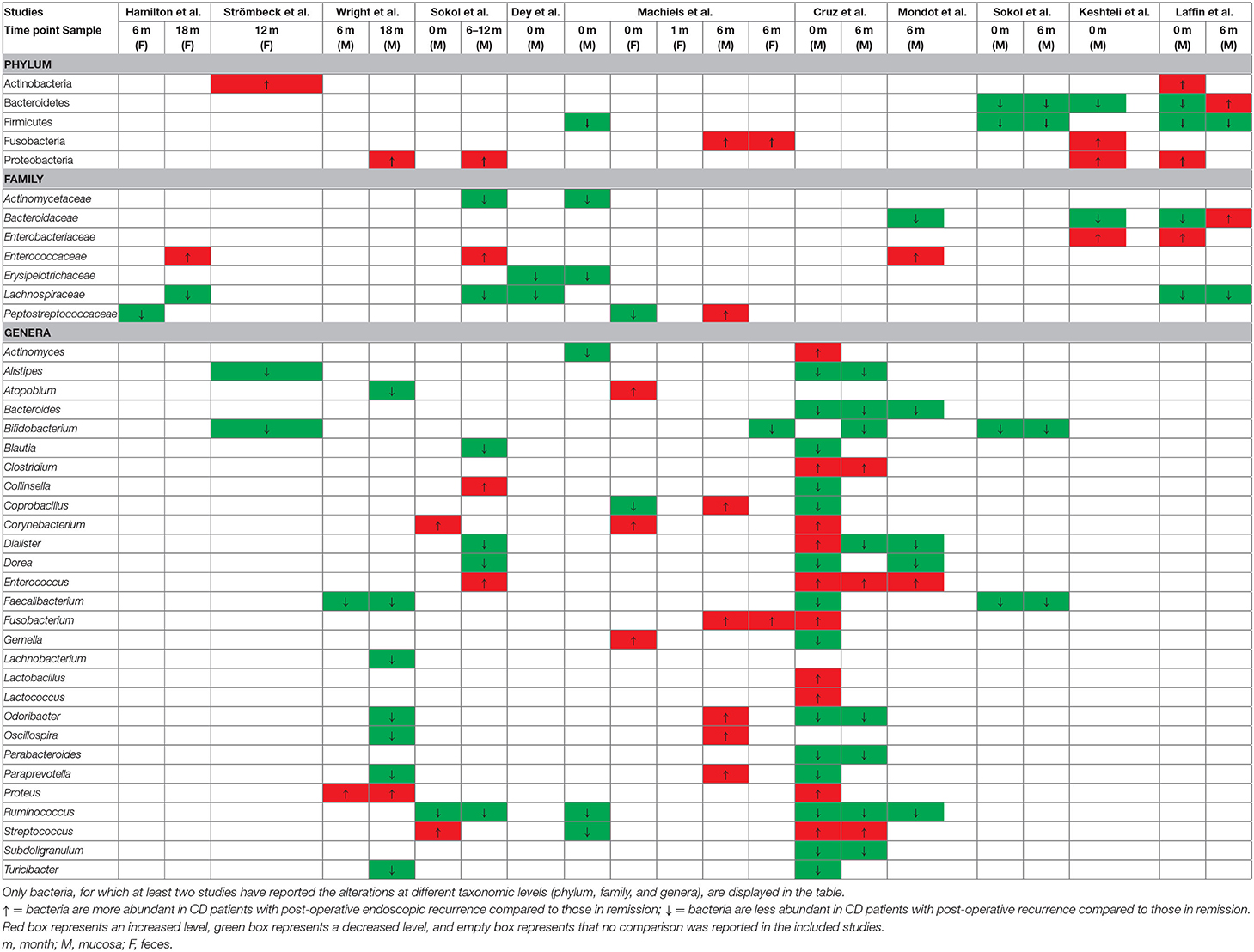
Table 4. Recurrence-associated fecal or mucosal microbiota at the time of resection and post-operative follow up.
The predictive potential of microbial factors at the time of surgery and at the time of post-operative endoscopic evaluation was further evaluated to guide disease diagnosis and treatment (Table 5). However, no consistent differences were detected in the counts of specific bacteria, which would allow their use in predicting endoscopic recurrence. Wright et al. reported that microbial analysis of the ileal mucosa, which takes into account the presence of Proteus, abundance of Faecalibacterium, and smoking status, at 6 and/or 18 months post-operatively, is moderately accurate in predicting endoscopic recurrence (30). In addition, increased Corynebacterium and decreased Ruminiclostridium 6 counts at baseline were identified as predictive factors of endoscopic recurrence for CD patients (27). Machiels et al. reported that the Ralstonia, Haemophilus, Gemella, and Phascolarctobacterium abundances in resected specimens were good predictors using C5.0 classification tree analyses or random forest models (26). However, the considerable predictive power of Coprobacillus, unidentified Lachnospiraceae, and Dorea obtained from fecal samples before surgery could not be confirmed after validation using the forest model. Furthermore, Keshteli et al. found that the concentrations of urinary 1,6-anhydro-beta-D-glucose, L-3,4-dihydroxyphenylalanine, propylene glycol, and ethylmalonate were related to CD recurrence after ileocolonic resection, with an associated area under the curve value of 0.91 (95% CI: 0.73–1.00) (28).
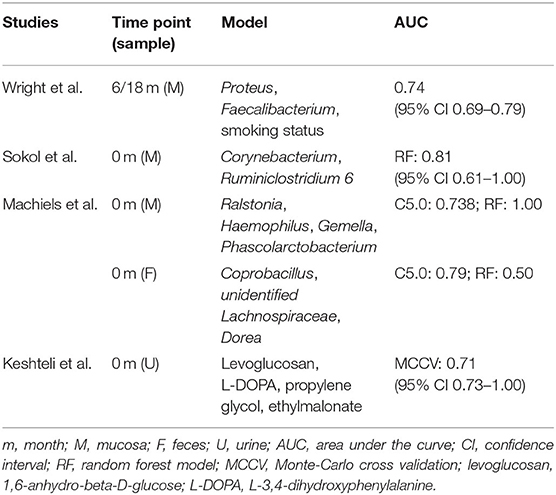
Table 5. Specific gut microbiota and metabolites as predictor of post-operative endoscopic recurrence at the time of resection and post-operative follow-up.
The recolonization of the intestinal tract by gut microbiota plays a critical role in determining whether there will be post-operative relapse at the resection site. This indicates that interventions that aim at manipulating the microbiome should, in theory, have an integral role in the prevention of PR for CD patients. However, there is a lack of evidence-based recommendations on this topic.
As is shown in Table 6, five studies (three comparing metronidazole with a placebo, one comparing ornidazole with a placebo, one comparing ciprofloxacin with a placebo) evaluated the efficacy of antibiotics in preventing post-operative endoscopic or clinical recurrence in CD patients. Based on the available data, nitroimidazole antibiotics (metronidazole, ornidazole) are effective in preventing the clinical and endoscopic PR of CD compared to placebo drugs, which may be the most cost-effective option. In the first study, Rutgeerts et al. demonstrated that 3-month-long metronidazole therapy significantly decreased the severe endoscopic recurrence incidences (13 vs. 43%, P = 0.02) in the neoterminal ileum. Additionally, this treatment seemed to delay symptomatic recurrence; however, it was associated with a high incidence of side effects (40). Nevertheless, there were no significant differences between the metronidazole treatment and the placebo treatment in terms of reducing the clinical PR at the 1-year follow-up. In a subsequent study, the same authors found that taking ornidazole at a dose of 1 g/day for a year is effective in preventing PR at the 3-months and 1-year follow-ups, while no significant differences in terms of clinical recurrence were observed at subsequent follow-ups (39). The results of another recent study showed that taking low doses of metronidazole (250 mg three times per day) for 3 months decreases significantly endoscopic PR rates in CD patients within 12 months and is well-tolerated (35). However, Mañosa et al. showed that the risk of endoscopic recurrence is not reduced significantly with the combined use of metronidazole and azathioprine compared to the sole use of azathioprine; however, the use of metronidazole does not worsen azathioprine's safety profile (37). Yet in spite of this, the results of another randomized controlled trial demonstrated that the long-term addition of azathioprine to a post-operative 3-months course of metronidazole is more effective than using metronidazole alone (49). Moreover, a 6-months course of ciprofloxacin is not more effective than using a placebo drug in terms of preventing PR in CD patients who underwent surgery, and a high proportion of patients discontinue their treatment because they cannot tolerate it well (38).
Another method of gut microbiota manipulation is by using a supplement of live and safe microbes that restore the beneficial intestinal microbial flora; in this case, the use of a probiotic formulation may be an appealing alternative. Seven studies examined the effect of probiotics on preventing the PR of CD; however, none of these studies detected a significant effect of probiotics on clinical or endoscopic recurrence (as is shown in Table 7). Among the included studies, three investigated the ability of VSL#3 (a mixture of eight different bacterial strains), two evaluated the efficacy of Lactobacillus johnsonii LA1, one examined the effect of Lactobacillus rhamnosus strain GG, and one observed the potency of Synbiotic 2000 (a cocktail of four probiotics and four prebiotics). Nevertheless, the combination of rifaximin and VSL#3 was efficient in preventing the severe endoscopic recurrence of CD after surgical resection, as reported by Campieri et al. (47).
There is limited consensus on the gut microbiota profiles of CD patients at the time of surgical resection and at the post-operative follow-up. Understanding the microbial communities associated with PR has the potential of improving the therapeutic options for CD patients that have undergone intestinal resection.
In this systematic review, we initially evaluated whether CD patients had a distinct microbiota composition at the time of surgery compared to healthy controls. As has been shown in previous studies, the majority of the studies included in this review suggested that the ileal mucosa-associated microbiota in CD patients exhibited reduced bacterial richness and diversity, while there was a clustering of samples with statistically significant differences between CD patients and healthy controls. Only one study from Machiels et al. simultaneously analyzed preoperative mucosal and fecal microbiota and found that CD patients had distinct characteristics of mucosal and fecal microbiota before surgery, which further confirmed that fecal and mucosal microbiota constitute different ecological environments (26). Although not even a single bacterial taxon had consistently altered counts across all included studies, we identified bacterial taxa obtained from surgical specimens that allowed us to discriminate between CD patients and healthy subjects, in several studies. Among the bacterial taxa reported to have altered relative abundances in CD cases, the Bacteroidetes and Firmicutes phyla were significantly less represented, while the Proteobacteria phylum was significantly more represented in mucosal microbiota, which corroborates the fecal microbiota findings. In addition, the expansion of Fusobacteria, a putative aggressive phylum, was observed in the surgical biopsies. A number of factors could influence the bacterial populations following ileocolonic resection, including substantial catabolic stress, retrograde flow of colonic contents, inflammatory changes involved in intestinal wound healing, and altered immune function (50, 51). At the time of post-operative endoscopy, the population of Actinobacteria, a proteolytic bacterial phylum with the capacity to invade and exacerbate inflammation, was depleted in patients with CD; however, the phylum Fusobacteria maintained its higher relative abundance, while the alterations in the phylum Bacteroidetes count were inconsistent. Even so, higher abundance of Enterococcaceae and Fusobacterium and lower abundance of Lachnospiraceae and Faecalibacterium existed in both post-operative mucosal and fecal microbiota in CD patients.
The counts of many known gut pathogens (Enterococcus, Escherichia, Fusobacterium, Streptococcus, Trabulsiella, and Veillonella) increased in the inflamed resection specimens, whereas the counts of essential types of butyrate and other short-chain fatty acid (SCFA)-producing bacteria were observed, such as Faecalibacterium, Blautia, Clostridium, Coprococcus, Lachnobacterium, Lachnospira, and Ruminococcus spp. In addition, baseline samples with enriched facultative anaerobic and oxygen-tolerant bacteria likely reflect active inflammation. The mechanisms through which these mucosa-associated bacteria that are involved in CD affect the intestinal permeability are by increasing the ability of the bacteria to adhere to the intestinal epithelium, inducing inflammatory responses by regulating the expression of inflammatory genes, restricting epithelial cell growth and differentiation by restricting energy sources, promoting invasion by pathogenic bacteria by destroying the intestinal mucus, weakening the anti-inflammatory functions by altering regulatory T cell differentiation, and influencing IBD-related genetic risk variants by altering the abundance of gut microbiota (52–58).
Numerous studies have documented that alterations in gut microbial profiles at the time of surgery or at the post-operative follow-up are linked to the post-operative disease course in CD patients. As fecal and mucosal-associated microbiota constitute different ecological environments, they have different alteration trends (59). Notably, the presence of mucosal bacterial genera, such as Bacteroides, Prevotella, and Parabacteroides, which are associated with saccharolytic metabolism, has been correlated with increased remission compared to the presence of bacterial genera, such as Enterococcus and Veillonella, which are associated with fermentation and lactic acid production. However, no specific bacterial taxa are consistently different between CD patients with or without PR in any of the included studies. Among the bacteria reported to have increased relative abundance in CD patients with PR, several genera have previously been associated with triggering host inflammatory responses (26, 60, 61). Conversely, several commensal bacteria with lower relative abundance are known to exert an anti-inflammatory effect by decreasing proinflammatory colonic pro-inflammatory cytokine synthesis and inducing anti-inflammatory cytokine secretion (33). In addition, patients with recurrent CD retain microbiota that favors proteolytic-fueled fermentation and lactic acid production, while CD patients in remission retain a predominantly saccharolytic and SCFA-producing microbiota (32). Moreover, altered bacteria involved in hydrogen sulfide production or a specific enzymatic machinery associated with the metabolism of bile acids are involved in the post-operative disease course of CD patients (31, 62, 63). Furthermore, changes in the ecology of depleted SCFA-producing bacteria may permit the expansion of pathogenic bacteria through luminal environmental perturbation (24). To date, no study has evaluated the role of the metabolomic profiling of gut microbiota at the time of surgery or at the post-operative follow-up in the identification of metabolites that may be associated with CD recurrence after intestinal resection.
As there were differences between CD patients with PR and those without in terms of their gut microbiota alterations, there may be a window of opportunity for microbial biomarkers to predict and monitor the post-operative disease outcome. Moreover, equipping clinicians with the prognostic biomarkers that will allow them to identify patients more likely to experience PR will reduce the duration of the drug treatment that is unlikely to be beneficial. However, the number of published studies investigating the involvement of gut microbiota both in monitoring the post-operative disease progression and in assessing the response of CD patients to a treatment is surprisingly low. In this review, we identified only four studies that provide information on the potential of gut microbiota to predict the PR of CD, but their results were too heterogeneous to allow us to reach confident conclusions regarding a microbial biomarker. Among the most discriminative features, the high abundance of bacteria from the Proteobacteria phylum (e.g., Proteus and Ralstonia) as well as in Ruminiclostridium gnavus (Gammaproteobacteria) and Corynebacterium, and the reduced abundance of several members of the Firmicutes phylum, particularly the Lachnospiraceae and the Ruminococcaceae families (e.g., Faecalibacterium, Gemella, Phascolarctobacterium, Coprobacillus, unidentified Lachnospiraceae, and Dorea), were predictive of endoscopic PR. Furthermore, adding the usual clinical risk factors (e.g., smoking status) in the prediction model may improve its diagnostic efficiency and accuracy regarding the gut microbiota of interest. As reported by Keshteli et al., distinctive urinary metabolomic profiling associated with Bacteroidales and Gammaproteobacteria has the potential to be used as a biomarker for the identification of CD patients who develop endoscopic disease recurrence after ileocolonic resection (28). The currently available studies are insufficient in elucidating the prevalence, diversity, distribution, and function of the identified bacteria associated with PR. Further research is required to confirm and define the sensitivity and specificity for recurrence or remission of such bacterial profiles using larger patient cohorts and more targeted bacterial analysis. Moreover, functional analyses are required to characterize the phylogenetic alterations at the gene expression level.
Various pharmaceutical treatments have been developed in an attempt to prevent or delay potential recurrence and subsequent surgery. However, the strategy directly targeted to gut microbiota disturbance is currently quite limited. While studies have attempted to utilize antibiotics or probiotics in an effort to prevent the PR of CD, their results are non-conclusive. Antibiotics can potentially ameliorate the microbial environment of patients suffering from IBD, both by decreasing the counts of pro-inflammatory bacteria and by increasing those of beneficial ones, throughout the intestinal lumen (64). Funayama et al. reported that antibacterial treatment was useful in post-operative CD patients whose assessments were complicated by bacterial overgrowth (65). Nitroimidazole antibiotics have been proven to be effective in preventing the clinical (RR: 0.23; 95% CI: 0.09–0.57; NNT: 4) and endoscopic (RR: 0.44; 95% CI: 0.26–0.74; NNT: 4) PR of CD, but the high rate of patients that are intolerant to them and their questionable long-term effects beyond the end of the treatment preclude their widespread use. Recent promising data on the activity of the non-absorbable antibiotic rifaximin (which is generally well-tolerated) in CD patients suggest that assessment of this agent in reducing post-operative recurrence is warranted. Probiotic supplements have been used successfully in the prevention of pouchitis and the maintenance of remission in active ulcerative colitis; nevertheless, the present review demonstrates that studies to date have failed to identify any benefit of probiotic supplement administration in preventing the PR of CD (66–69). Intriguingly, Campieri et al. reported that the combination of a non-absorbable antibiotic (rifaximin) and a highly bacterial concentrated probiotic (VSL#3) is efficient in preventing the severe endoscopic recurrence of CD (47). However, none of the studies examined in the present review investigated the therapeutic mechanisms of either antibiotics or probiotics on gut microbiota modification. Several mechanisms mediate the therapeutic action of antibiotics and probiotics, such as the inhibition of pathobionts, increase in beneficial bacteria, modification of bacterial metabolites, regulation of immunity, improvement in the mucosal barrier, or absorption of toxic substances (70–75). The outcomes of microbial-based treatments indicate that the possibility of using combination-based strategies, such as the early post-operative use of antibiotics to prevent pathogenic recolonization followed by maintenance with the use of probiotics to establish a durable anti-inflammatory post-operative microflora, may yield the greatest benefit with the least risk of disease recurrence in CD patients. Unfortunately, the efficacy of fecal microbiota transplants in preventing the PR of CD remains unknown.
There are some limitations to this review that warrant discussion. It is well-known that several factors may exert an influence on the composition of gut microbiota, including geographic, cultural, demographic, dietary, and preoperative and post-operative medications differences, which may explain some of the observed discrepancies among the different studies (76, 77). In addition, other risk factors associated with disease recurrence failed to be addressed in this review, which perhaps explain some of the discrepancies between the results. Furthermore, the included studies consisted of 10 retrospective studies and two prospective studies, which might be one possible reason for the inconsistencies. Lastly, differences in the methodology used, such as specimen type, sample storage methods, DNA extraction methods, primers targeting different regions, bioinformatic pipelines, and reference databases used, may explain the heterogeneous results observed in the examined studies.
In this systematic review, we characterized the mucosa-associated microbiota at the time of surgery and the profiles of the bacteria that recolonized the intestinal tract following resection. Additionally, we highlighted specific bacterial taxa, the counts of which either increased or decreased and that are associated with the endoscopic PR of CD. Although consistent recurrence-associated gut microbiota with predictive value could not be identified from the examined studies, a few microbial predictors were suggested. CD patients with PR tend to gain pathogenic bacteria with a pro-inflammatory effect and lose SCFA-producing bacteria. The gut microbiota manipulation through the administration of either antibiotics or probiotics may not offer a promising alternative in the prevention of PR in patients with CD. Future research should focus on investigating differences in the function and composition of the gut microbiota associated with PR and post-operative remission. Additionally, the use of larger patient cohorts is recommended to confirm the sensitivity and specificity of bacterial profiles with predictive value. Furthermore, effective microbial-based therapies based on an individual patient's microbial profile that are used to prevent PR and can be administered for prolonged time periods with acceptable side effects are urgently awaited.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
MC guarantor of the article. MC and XZ designed the study. XZ wrote the manuscript. ZT and XL collected the data. MZ, NL, and SX analyzed the data. RM, ZZ, and RF revised the manuscript. All authors approved the final version.
This work was supported by The Leona M. & Harry B. Helmsley Charitable Trust Grant (2019PG-CD018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.615858/full#supplementary-material
CD, Crohn's disease; IBD, Inflammatory bowel disease; PR, Post-operative recurrence; SCFA, Short chain fatty acid.
1. Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. (2019) 94:155–65. doi: 10.1016/j.mayocp.2018.09.013
2. Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. (2013) 10:585–95. doi: 10.1038/nrgastro.2013.117
3. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn's disease management after intestinal resection: a randomised trial. Lancet. (2015) 385:1406–17. doi: 10.1016/S0140-6736(14)61908-5
4. Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV Jr. Surgery in a population-based cohort of Crohn's disease from Olmsted County, Minnesota (1970-2004). Am J Gastroenterol. (2012). 107:1693–701. doi: 10.1038/ajg.2012.298
5. Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. (2005) 54:237–41. doi: 10.1136/gut.2004.045294
6. Landsend E, Johnson E, Johannessen HO, Carlsen E. Long-term outcome after intestinal resection for Crohn's disease. Scand J Gastroenterol. (2006) 41:1204–8. doi: 10.1080/00365520600731018
7. Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn's disease. Am J Gastroenterol. (2008) 103:196–205. doi: 10.1111/j.1572-0241.2007.01548.x
8. Ng SC, Kamm MA. Management of post-operative Crohn's disease. Am J Gastroenterol. (2008) 103:1029–35. doi: 10.1111/j.1572-0241.2008.01795.x
9. Larson DW, Pemberton JH. Current concepts and controversies in surgery for IBD. Gastroenterology. (2004) 126:1611–9. doi: 10.1053/j.gastro.2004.03.063
10. Ananthakrishnan AN, Hur C, Juillerat P, Korzenik JR. Strategies for the prevention of post-operative recurrence in Crohn's disease: results of a decision analysis. Am J Gastroenterol. (2011) 106:2009–17. doi: 10.1038/ajg.2011.237
11. Pascua M, Su C, Lewis JD, Brensinger C, Lichtenstein GR. Meta-analysis: factors predicting post-operative recurrence with placebo therapy in patients with Crohn's disease. Aliment Pharmacol Ther. (2008) 28:545–56. doi: 10.1111/j.1365-2036.2008.03774.x
12. Yang KM, Yu CS, Lee JL, Kim CW, Yoon YS, Park IJ, et al. Risk factors for post-operative recurrence after primary bowel resection in patients with Crohn's disease. World J Gastroenterol. (2017) 23:7016–24. doi: 10.3748/wjg.v23.i38.7016
13. Sachar DB, Wolfson DM, Greenstein AJ, Goldberg J, Styczynski R, Janowitz HD. Risk factors for post-operative recurrence of Crohn's disease. Gastroenterology. (1983) 85:917–21. doi: 10.1016/0016-5085(83)90444-4
14. Neurath MF. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:76–7. doi: 10.1038/s41575-019-0248-1
15. Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. (2019) 569:655–62. doi: 10.1038/s41586-019-1237-9
16. Di Sario A, Sassaroli P, Daretti L, Annulli G, Schiada L, Falcioni G, et al. post-operative recurrence of Crohn's disease: pathophysiology, diagnosis and treatment. Curr Pharm Biotechnol. (2017) 18:979–88. doi: 10.2174/1389201019666180216152805
17. Cohen LJ, Cho JH, Gevers D, Chu HT. Genetic factors and the intestinal microbiome guide development of microbe-based therapies for inflammatory bowel diseases. Gastroenterology. (2019) 156:2174–89. doi: 10.1053/j.gastro.2019.03.017
18. Doherty GA, Bennett GC, Cheifetz AS, Moss AC. Meta-analysis: targeting the intestinal microbiota in prophylaxis for post-operative Crohn's disease. Aliment Pharmacol Ther. (2010) 31:802–9. doi: 10.1111/j.1365-2036.2010.04231.x
19. Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post-operative recurrence of Crohn's disease. Cochrane Database Syst Rev. (2009) 4:CD006873. doi: 10.1002/14651858.CD006873.pub2
20. Ledder O, Turner D. Antibiotics in IBD: still a role in the biological era? Inflamm Bowel Dis. (2018) 24:1676–88. doi: 10.1093/ibd/izy067
21. Cottone M, Orlando A, Modesto I. Post-operative maintenance therapy for inflammatory bowel disease. Curr Opin Gastroenterol. (2006) 22:377–81. doi: 10.1097/01.mog.0000231811.95525.7c
22. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
23. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–2. doi: 10.1016/0197-2456(95)00134-4
24. Hamilton AL, Kamm MA, De Cruz P, Wright EK, Feng H, Wagner J, et al. Luminal microbiota related to Crohn's disease recurrence after surgery. Gut Microbes. (2020) 11:1713–28. doi: 10.1080/19490976.2020.1778262
25. Strömbeck A, Lasson A, Strid H, Sundin J, Stotzer PO, Simrén M, et al. Fecal microbiota composition is linked to the post-operative disease course in patients with Crohn's disease. BMC Gastroenterol. (2020) 20:130. doi: 10.1186/s12876-020-01281-4
26. Machiels K, Pozuelo Del Río M, Martinez-De la Torre A, Xie Z, Pascal Andreu V, Sabino J, et al. Early post-operative endoscopic recurrence in Crohn's disease is characterized by distinct microbiota recolonization. J Crohns Colitis. (2020) 14:1535–546. doi: 10.1093/ecco-jcc/jjaa081
27. Sokol H, Brot L, Stefanescu C, Auzolle C, Barnich N, Buisson A, et al. Prominence of ileal mucosa-associated microbiota to predict post-operative endoscopic recurrence in Crohn's disease. Gut. (2020) 69:462–72. doi: 10.1136/gutjnl-2019-318719
28. Keshteli AH, Tso R, Dieleman LA, Park H, Kroeker KI, Jovel J, et al. A distinctive urinary metabolomic fingerprint is linked with endoscopic post-operative disease recurrence in crohn's disease patients. Inflamm Bowel Dis. (2018) 24:861–70. doi: 10.1093/ibd/izx070
29. Laffin MR, Perry T, Park H, Gillevet P, Sikaroodi M, Kaplan GG, et al. Endospore forming bacteria may be associated with maintenance of surgically-induced remission in Crohn's disease. Sci Rep. (2018) 8:9734. doi: 10.1038/s41598-018-28071-z
30. Wright EK, Kamm MA, Wagner J, Teo SM, Cruz P, Hamilton AL, et al. Microbial factors associated with post-operative Crohn's disease recurrence. J Crohns Colitis. (2017) 11:191–203. doi: 10.1093/ecco-jcc/jjw136
31. Mondot S, Lepage P, Seksik P, Allez M, Tréton X, Bouhnik Y, et al. Structural robustness of the gut mucosal microbiota is associated with Crohn's disease remission after surgery. Gut. (2016) 65:954–62. doi: 10.1136/gutjnl-2015-309184
32. De Cruz P, Kang S, Wagner J, Buckley M, Sim WH, Prideaux L, et al. Association between specific mucosa-associated microbiota in Crohn's disease at the time of resection and subsequent disease recurrence: a pilot study. J Gastroenterol Hepatol. (2015) 30:268–78. doi: 10.1111/jgh.12694
33. Dey N, Soergel DA, Repo S, Brenner SE. Association of gut microbiota with post-operative clinical course in Crohn's disease. BMC Gastroenterol. (2013) 13:131. doi: 10.1186/1471-230X-13-131
34. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. (2008) 105:16731–6736. doi: 10.1073/pnas.0804812105
35. Neut C, Bulois P, Desreumaux P, Membré JM, Lederman E, Gambiez L, et al. Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn's disease. Am J Gastroenterol. (2002) 97:939–46. doi: 10.1111/j.1572-0241.2002.05613.x
36. Glick LR, Sossenheimer PH, Ollech JE, Cohen RD, Hyman NH, Hurst RD, et al. Low-dose metronidazole is associated with a decreased rate of endoscopic recurrence of Crohn's disease after ileal resection: a retrospective cohort study. J Crohns Colitis. (2019) 13:1158–62. doi: 10.1093/ecco-jcc/jjz047
37. Mañosa M, Cabré E, Bernal I, Esteve M, Garcia-Planella E, Ricart E, et al. Addition of metronidazole to azathioprine for the prevention of post-operative recurrence of crohn's disease: a randomized, double-blind, placebo-controlled trial. Inflamm Bowel Dis. (2013) 19:1889–95. doi: 10.1097/MIB.0b013e31828ef13f
38. Herfarth HH, Katz JA, Hanauer SB, Sandborn WJ, Loftus EV, Sands BE, et al. Ciprofloxacin for the prevention of post-operative recurrence in patients with Crohn's disease: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis. (2013) 19:1073–9. doi: 10.1097/01.MIB.0000428910.36091.10
39. Rutgeerts P, Van Assche G, Vermeire S, D'Haens G, Baert F, Noman M, et al. Ornidazole for prophylaxis of post-operative Crohn's disease recurrence: a randomized, double-blind, placebo-controlled trial. Gastroenterology. (2005) 128:856–61. doi: 10.1053/j.gastro.2005.01.010
40. Rutgeerts P, Hiele M, Geboes K, Peeters M, Penninckx F, Aerts R, et al. Controlled trial of metronidazole treatment for prevention of crohn's recurrence after ileal resection. Gastroenterology. (1995) 108:1617–21. doi: 10.1016/0016-5085(95)90121-3
41. Madsen K, Backer JL, Leddin D, Dieleman LA, Bitton A, Feagan B, et al. Randomized controlled trial of vsl#3 for the prevention of endoscopic recurrence following surgery for Crohn's disease. Gastroenterology. (1999) 134:M1207. doi: 10.1016/S0016-5085(08)61682-0
42. Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn's disease. Clin Gastroenterol Hepatol. (2015) 13:928–35. doi: 10.1016/j.cgh.2014.10.031
43. Chermesh I, Tamir A, Reshef R, Chowers Y, Suissa A, Katz D, et al. Failure of synbiotic 2000 to prevent post-operative recurrence of Crohn's disease. Dig Dis Sci. (2007) 52:385–9. doi: 10.1007/s10620-006-9549-7
44. Van Gossum A, Dewit O, Louis E, de Hertogh G, Baert F, Fontaine F, et al. Multicenter randomized-controlled clinical trial of probiotics (Lactobacillus johnsonii, LA1) on early endoscopic recurrence of Crohn's disease after ileo-caecal resection. Inflamm Bowel Dis. (2007) 13:135–42. doi: 10.1002/ibd.20063
45. Marteau P, Lémann M, Seksik P, Laharie D, Colombel JF, Bouhnik Y, et al. Ineffectiveness of Lactobacillus johnsonii LA1 for prophylaxis of post-operative recurrence in Crohn's disease: a randomised, double blind, placebo controlled GETAID trial. Gut. (2006) 55:842–7. doi: 10.1136/gut.2005.076604
46. Prantera C, Scribano ML, Falasco G, Andreoli A, Luzi C. Ineffectiveness of probiotics in preventing recurrence after curative resection for Crohn's disease: a randomised controlled trial with Lactobacillus GG. Gut. (2002) 51:405–9. doi: 10.1136/gut.51.3.405
47. Campieri M, Rizzello F, Venturi A, et al. Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn's disease: a randomized controlled study vs mesalamine. Gastroenterology. (2000) 118:A781. doi: 10.1016/S0016-5085(00)85267-1
48. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the post-operative course of Crohn's disease. Gastroenterology. (1990) 99:956–63. doi: 10.1016/0016-5085(90)90613-6
49. D'Haens GR, Vermeire S, Van Assche G, Noman M, Aerden I, Van Olmen G, et al. Therapy of metronidazole with azathioprine to prevent post-operative recurrence of Crohn's disease: A controlled randomized trial. Gastroenterology. (2008) 135:1123–9. doi: 10.1053/j.gastro.2008.07.010
50. Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. (2017) 14:43–54. doi: 10.1038/nrgastro.2016.139
51. Alazawi W, Pirmadjid N, Lahiri R, Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. (2016) 264:73–80. doi: 10.1097/SLA.0000000000001691
52. Imhann F, Vila AV, Bonder MJ, Fu JY, Gevers D, Visschedijk MC, et al. Interplay of host genetics and gut microbiota under lying the onset and clinical presentation of inflammatory bowel disease. Gut. (2018) 67:108–19. doi: 10.1136/gutjnl-2016-312135
53. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50. doi: 10.1038/nature12721
54. Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature. (2013) 504:451–5. doi: 10.1038/nature12726
55. Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. (2018) 11:1–10. doi: 10.1007/s12328-017-0813-5
56. Ahmed I, Roy BC, Khan SA, Septer S, Umar S. Microbiome, metabolome and inflammatory bowel disease. Microorganisms. (2016) 4:20. doi: 10.3390/microorganisms4020020
57. Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe. (2008) 3:417–427. doi: 10.1016/j.chom.2008.05.001
58. Sartor RB, Wu GD. Roles for intestinal bacteria, viruses, and fungi in pathogenesis of inflammatory bowel diseases and therapeutic approaches. Gastroenterology. (2017) 152:327–39. doi: 10.1053/j.gastro.2016.10.012
59. Ringel Y, Maharshak N, Ringel-Kulka T, Wolber EA, Sartor RB, Carroll IM. High throughput sequencing reveals distinct microbial populations within the mucosal and luminal niches in healthy individuals. Gut Microbes. (2015) 6:173–81. doi: 10.1080/19490976.2015.1044711
60. Neurath MF. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat Immunol. (2019) 20:970–9. doi: 10.1038/s41590-019-0415-0
61. Schirmer M, Smeekens SP, Vlamakis H, Jaeger M, Oosting M, Franzosa EA, et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell. (2016) 167:1125–36. doi: 10.1016/j.cell.2016.10.020
62. Ogilvie LA, Jones BV. Dysbiosis modulates capacity for bile acid modification in the gut microbiomes of patients with inflammatory bowel disease: a mechanism and marker of disease? Gut. (2012) 61:1642–3. doi: 10.1136/gutjnl-2012-302137
63. Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2020) 17:223–37. doi: 10.1038/s41575-019-0258-z
64. Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic opportunities in inflammatory bowel disease: mechanistic dissection of host-microbiome relationships. Cell. (2019) 178:1041–56. doi: 10.1016/j.cell.2019.07.045
65. Funayama Y, Sasaki I, Naito H, Fukushima K, Shibata C, Masuko T, et al. Monitoring and antibacterial treatment for post-operative bacterial overgrowth in Crohn's disease. Dis Colon Rectum. (1999) 42:1072–7. doi: 10.1007/BF02236706
66. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol. (2018) 233:2091–103. doi: 10.1002/jcp.25911
67. Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol. (2009) 104:437–43. doi: 10.1038/ajg.2008.118
68. Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial. Gastroenterology. (2000) 119:305–9. doi: 10.1053/gast.2000.9370
69. Derwa Y, Gracie DJ, Hamlin PJ, Ford AC. Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther. (2017) 46:389–400. doi: 10.1111/apt.14203
70. Dubinsky V, Reshef L, Bar N, Keizer D, Golan N, Rabinowitz K, et al. Predominantly antibiotic-resistant intestinal microbiome persists in patients with pouchitis who respond to antibiotic therapy. Gastroenterology. (2020) 158:610–24. doi: 10.1053/j.gastro.2019.10.001
71. Rafii F, Ruseler-Van Embden JGH, Van Lieshout LMC. Changes in bacterial enzymes and PCR profiles of fecal bacteria from a patient with ulcerative colitis before and after antimicrobial treatments. Dig Dis Sci. (1999) 44:637–42.
72. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. (2011) 469:543–7. doi: 10.1038/nature09646
73. Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. (2006) 55:833–41. doi: 10.1136/gut.2005.078303
74. Kühbacher T, Ott SJ, Helwig U, Mimura T, Rizzello F, Kleessen B, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. (2013) 504:446–50.
75. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. (2013) 341:569–73. doi: 10.1126/science.1241165
76. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. (2012) 489:220–30. doi: 10.1038/nature11550
Keywords: crohn's disease, mucosa-associated microbiota, feces-associated microbiota, post-operative recurrence, microbial-based therapies
Citation: Zhuang X, Tian Z, Li N, Mao R, Li X, Zhao M, Xiong S, Zeng Z, Feng R and Chen M (2021) Gut Microbiota Profiles and Microbial-Based Therapies in Post-operative Crohn's Disease: A Systematic Review. Front. Med. 7:615858. doi: 10.3389/fmed.2020.615858
Received: 10 October 2020; Accepted: 14 December 2020;
Published: 28 January 2021.
Edited by:
Uri Kopylov, Sheba Medical Center, IsraelReviewed by:
Nitsan Maharshak, Tel Aviv University, IsraelCopyright © 2021 Zhuang, Tian, Li, Mao, Li, Zhao, Xiong, Zeng, Feng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minhu Chen, Y2hlbm1pbmh1QG1haWwuc3lzdS5lZHUuY24=; Rui Feng, ZmVuZ3I3QG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.