
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 14 January 2021
Sec. Gastroenterology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.600095
This article is part of the Research Topic Capsule Endoscopy Procedure for the Diagnosis of Gastrointestinal Diseases View all 7 articles
Background and Aims: There is little agreement on the nomenclature and description of Crohn's disease (CD) lesions that can be found in the small and large bowel using capsule endoscopy (CE). We performed a systematic review to identify mucosal lesions that have been described using CE in CD, in both the small bowel and colon, with the aim to make propositions to homogenize such descriptions.
Methods: A systematic literature search was conducted using Embase, Medline (OvidSP), and Cochrane Central on August 6, 2019. Clinical studies providing nomenclature and descriptions for small bowel and colonic inflammatory lesions using CE in CD were selected for data collection.
Results: In total, 851 articles were included for abstract screening out of which 219 were analyzed for full-text review. Twenty-two articles were selected for data extraction. Seven items, accompanied by clear descriptions, were found for the small bowel: i.e., ulcer, erosion, aphthoid lesion, edema, fissure, cobblestone appearance, and villous atrophy. No studies were found describing inflammatory items using CE in colonic CD.
Conclusions: The most frequently described CD lesions using CE were ulcers and erosions. Subjective interpretation of CE inflammatory findings plays an important role. Based on our findings, a range of suggestions regarding items and descriptions is made that might form the basis of a pan-enteric CE activity index.
Capsule endoscopy (CE) provides a reliable and non-invasive method to visualize the entire gastrointestinal tract. CE has a diagnostic yield of 50% to detect mucosal lesions in the small bowel of Crohn's disease (CD) patients (1). However, little agreement exists on how to describe such mucosal lesions using CE in CD. Capsule Endoscopy Structured Terminology (CEST) has been designed and published by international societies trying to seek consensus in interpreting and reporting small bowel CE examinations (2). Despite standardized terminology, descriptions vary considerably throughout different studies, and also interpretation of these findings varies widely among different observers.
Furthermore, criteria for small bowel CE-based activity assessment in CD vary considerably between different studies. The two available and validated endoscopic activity indices to assess small bowel CD activity [Capsule Endoscopy Crohn's Disease Activity Index (CECDAI) (3) and Lewis Score (LS) (4)] both rely on three parameters: inflammatory lesions, disease extension, and presence of strictures. In the LS, differentiation of ulcers from mucosal breaks, erosions and aphthoid lesions have been eliminated in order to develop a more user-friendly activity index. Moreover, this score provides an accurate description of inflammatory items (villous edema and ulcer). In contrast, the CECDAI does not provide item descriptions but grades findings from mild to severe and separates mucosal breaks into different sizes. Both activity scores contain inflammatory lesions with clinical significance according to a CE consensus meeting. However, only the LS provides clear descriptions for each item. Nevertheless, proposed descriptions were not consensually agreed upon.
On the other hand, validated scoring systems to assess colonic CD activity with CE are lacking. Hence, CE cannot be recommended yet to replace conventional colonoscopy in CD (5). In the past years, several studies showed that CE, using the second-generation colon capsule, is a safe and feasible tool to assess inflammatory activity in the small and large bowel (6–10). However, one study demonstrated an underestimation of the total ulcerated surface in the colon mainly because of insufficient bowel cleansing techniques compared to conventional endoscopy (6). Moreover, evidence suggests that CE is a useful technique to monitor post-operative CD patients (11). Nevertheless, all available CE studies used endoscopic activity indices, and no specific terminology for colonic findings has been proposed using CE.
In the treat-to-target era, where mucosal healing is an important treatment goal in CD, development of an activity index to assess the entire gastrointestinal tract, including the colon, is warranted. Recently, a pan-enteric index (CECDAIic) has been created demonstrating its usefulness in CD (12, 13). This particular CE activity score is based on the CECDAI and gives a comprehensive view of the entire intestinal tract but does not provide a clear description of inflammatory lesions.
Hence, validated scoring systems for pan-enteric CE that are accompanied by clear descriptions of items are lacking. As a first step, all CD-related mucosal lesions that have been reported in the literature should be characterized. Next, a consensus meeting consisting of CE experts should find agreement in the nomenclature and how to describe these items. As a final step, this might result in the development of a pan-enteric CE activity index. The development of such an index will likely decrease intra- and inter-observer disagreement.
Currently, scarce consensus exists on how to describe CD lesions detected by CE. In that regard, a Delphi consensus meeting took place consisting of small bowel CE experts. This working group proposed a set of items, together with clear descriptions, on how to describe small bowel CD lesions using CE (14), based on the LS and CECDAI.
With the aim of contributing to a uniform report of mucosal lesions, we performed a systematic review to identify all descriptions for mucosal inflammatory lesions using CE in the small and large bowels in CD patients, in order to make recommendations that might form the basis of a pan-enteric CE activity index.
A systematic literature search was performed using the following databases on August 6, 2019: MEDLINE, EMBASE, and Cochrane library. The search strategy can be found as Supplementary Material. Two authors (MM and AS) independently screened all the articles by title and abstract, and when included by at least one of the authors for full-text revision the full article was analyzed. Any disagreements were resolved by discussion with a third author (ML) followed by a consensus meeting (ML, MM, and AS).
Study selection was carried out according to PICOS criteria for including and excluding studies. The inclusion criteria were as follows: description of CE items in the small bowel and/or the colon in patients with known or suspected CD who underwent CE for different indications [i.e., diagnosis (suspicion of CD) or staging, assessment of disease activity, or mucosal healing (in patients with known CD)]. Exclusion criteria were as follows: (1) provide CE items without description; (2) sample size <10 CD patients; (3) editorials, letters, review articles, meta-analyses, guidelines, meeting abstracts, and non-fully published data; (4) duplicated studies; and (5) other than English language. After the full-text selection, every CE item with its description was collected. Finally, we checked if the terminology was in line with international recommendations (2). We decided to exclude articles with <10 CD patients as a cut-off to assume the experience in CE in inflammatory bowel diseases. In that same line, we also checked if the study provided data regarding inter- and intra-observer agreement of each described item between CE observers.
The literature search identified 1,285 records. Three additional records were identified through other sources. After removing duplicates, a total of 854 records were screened for inclusion. After screening titles and abstracts, 219 reports were selected for full-text review. After full-text review, 22 studies were included in the data collection process. A flowchart of the selection process is shown in Figure 1.
The selected studies provided an item description of the mucosal lesions with assumed clinical relevance in CD. All papers provided a priori specified item description before study performance. These definitions are shown in Table 1.
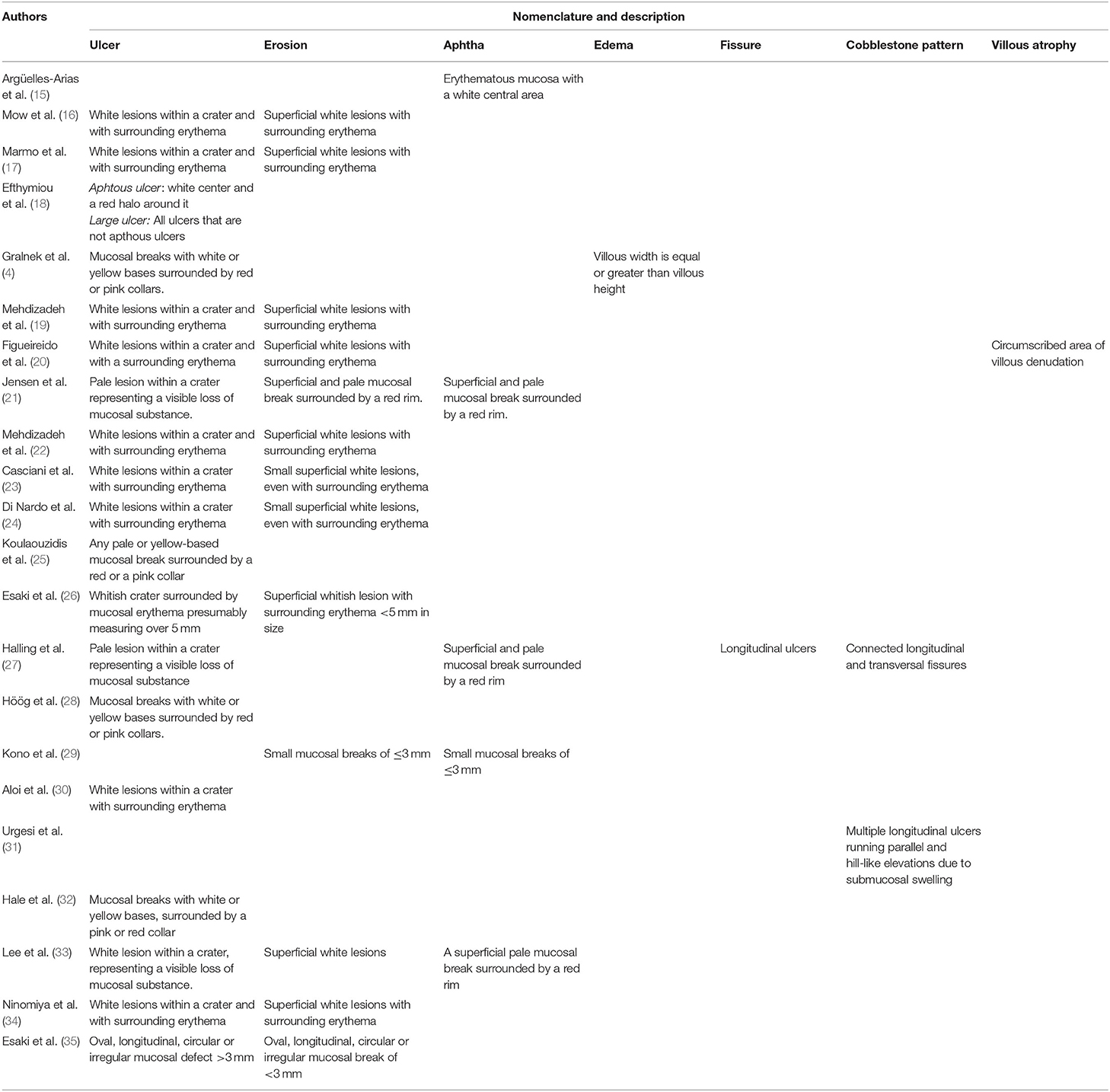
Table 1. Nomenclature used to specify inflammatory-related findings for CE in small bowel CD and the different descriptions given to each of them.
We found in total seven mucosal lesions with different descriptions. All items referred to small bowel CD lesions. The most frequently described items were ulcer (4, 16–28, 30, 32–35) and erosion (16, 17, 19–24, 26, 29, 33–35). Both definitions are characterized by a central mucosal defect with surrounding focal erythema and are distinguished from each other based on their size and depth of the defect. Different terminology has been used concerning each feature: crater, white lesion, mucosal break, pale lesion, white/yellow base, loss of mucosal substance, red/pink collar, or red rim. Esaki and colleagues (35) also distinguished ulcers and erosions according to shape (i.e., oval, circular, longitudinal, and irregular).
For aphthoid lesion, three different definitions were found (15, 21, 27, 29, 33). These are comparable to erosion descriptions, specifying its superficiality or small size. The validation study of the well-known LS provided the description for the item edema, characterized by equal or greater villous width when compared to villous height (4). To describe cobblestone pattern, two definitions have been used that take into account the presence and disposition of longitudinal ulcers (27, 31). One single definition has been provided for fissure (27) and villous atrophy (20). We did not identify any description regarding colonic lesions. With reference to the CEST standardized nomenclature, all terms are included in these international recommendations except for fissure and cobblestone pattern.
Two studies assessed agreement between CE observers regarding lesion identification and description (4, 35). These results are summarized in Table 2. Kappa statistics (k) were used to measure inter-rater reliability, with values ranging from 0 (absence of agreement) to 1 (perfect agreement). They interpreted values <0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as good, and >0.81 as excellent agreement.
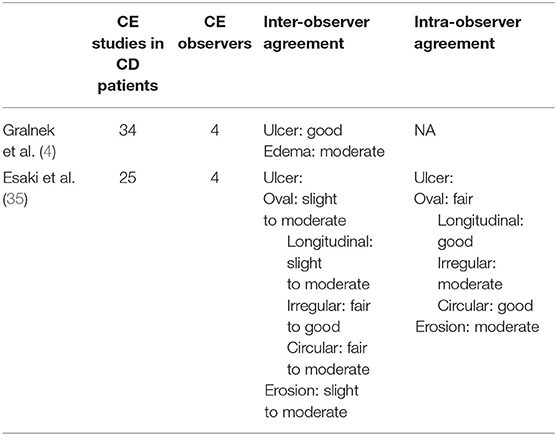
Table 2. Studies assessing CE readers' agreement in relation to particular small bowel lesions and the grade of agreement obtained for each of them.
Esaki and collaborators (35) distinguished ulcers and erosions according to four shapes. They evaluated the inter-observer agreement between one expert capsule endoscopist and three observers, analyzing small bowel CE results obtained from 25 CD patients. A slight to moderate agreement was found for oval and longitudinal ulcers, fair to good agreement for irregular ulcers, and fair to moderate agreement for circular ulcers. The authors detected slight to moderate agreement for overall erosion forms. The intra-observer agreement for the expert capsule endoscopist was fair, good, moderate, and good for oval, longitudinal, irregular, and circular ulcers, respectively. Intra-observer agreement was moderate for all erosion shapes.
Gralnek and colleagues (4) found moderate agreement for villous edema and good agreement for ulcer detection between four CE observers who analyzed 34 CE studies from CD patients.
Furthermore, four studies assessed observer agreement with regard to global small bowel inflammation by means of different small bowel CD scoring but not for any particular lesion (4, 17, 21, 31).
Currently, there is no consensus on how to describe small bowel and colonic lesions that can be found with CE in CD patients. We aimed to identify all mucosal lesions that have been described, both in the small bowel and in the colon, seeking common points in terms and descriptions to homogenize definition of mucosal lesions. Studies that lacked a clear description of mucosal lesions were excluded.
The most frequently described items related to inflammation in the small bowel of CD patients were ulcers and erosions. Both items are distinguished based on size or depth of the lesion, expressed by the presence of a crater or mucosal break. We propose to avoid this distinction based on size estimation, because objective measurement tools in CE are lacking and CE layout in relation to lesions when assessing a video may determine its interpretation. It should also be noted that the term mucosal break could lead to confusion, since it is generally used as a way to describe loss of mucosal substance, but some authors use it as a way to establish a deeper mucosal defect. We encourage to avoid this description of deep mucosal defect and propose to use other descriptors in that regard, such as crater. Moreover, the shape-based description approach by Esaki and coworkers could be counter-productive, because this is a subjective evaluation that might result in intra- and inter-observer disagreement.
With conventional endoscopy, an aphthoid lesion has been traditionally considered different from an erosion, since an aphthoid lesion has been seen as a flat or elevated lesion and an erosion as an excavated lesion covered by fibrin material. However, an aphthoid lesion is not included in the recommendations for endoscopy terminology of the World Endoscopy Organization (36). In the CEST, these two terms are classified separately, and the term aphthoid lesion is also considered an excavated lesion. Moreover, the CECDAI includes both terms with no attribute assessment. Therefore, we propose that these two terms could be used indistinctly.
The term edema is included in the CEST as a mucosal feature and is used in the two available validated activity indices, but only the LS validation study provides a description, testing its reproducibility with moderate inter-observer agreement but with no intra-observer agreement evaluation. Since the LS has been widely accepted for small bowel assessment in CD, few authors have insisted on changing this item description.
Fissure and cobblestone pattern are not included in the CEST. Halling et al. use the term fissure to describe longitudinal ulcers, and, due to its redundancy, we think it should be avoided. The cobblestone pattern definition provided by Urgesi and colleagues seems to be more accurate than the one provided by Halling and coworkers, since the Japanese criteria for CD diagnosis proposes the definition only by the presence of longitudinal ulcers when diagnosing CD (37), for both ileal and colonic diseases. Otherwise, it has always been related to a severe affectation, and it rarely appears in other inflammatory bowel diseases. As for colonoscopy, well-known activity indices (SES-CD and CDEIS) do not include cobblestone pattern as a diagnostic criteria by itself; hence, we think it should be avoided for inclusion in small bowel disease CE index.
We found one single definition of villous atrophy. The CEST uses the term atrophy when referring to mucosal aspect, not to villi, and applies the labels of shape and color concerning villi appearance. A Delphi consensus meeting (14) proposed to describe the absence of villi with the term denudation, with no reference to mucosal atrophy. Nevertheless, the group describing the LS eliminated denuded mucosa, because it was considered an item unable to be judged objectively and with perceived lack of clinical significance.
No studies were found that described colonic lesions with CE in CD. Most of these studies used endoscopic activity indices to score disease activity. The CECDAIic pan-enteric score validation study extrapolated to colon the inflammatory indicator used in the CECDAI for small bowel disease (12), with no colonic lesions description. Future studies are warranted using colon CE and pan-enteric CE in CD patients in order to better characterize mucosal lesions that can be found in the whole intestine. It will be of great importance to reach consensus between experts in characterizing mucosal lesions to improve agreement between readers using CE in CD patients. Additionally, these will help in better CE training, optimization of the learning curve, and broad implementation of CE in clinical practice.
Here, we mainly focus on inflammatory item description, working toward a more objective nomenclature and description. Of note, we did not analyze items that were related to stenosis, because its general definition implies a delay or withholding of CE rather than describing the mucosal pattern, even if it may be related with deep ulcers or edematous tissue. Likewise, we did not investigate studies focusing on the clinical relevance of mucosal lesions that can be detected by CE. Theoretically, the lesions to be considered suitable for scoring should contribute to clinical symptoms, correlate with endoscopic activity scores and with biological markers, and have a good rate of responsiveness on treatment outcomes. Beyond this point, the general line in the practice is to describe clinical relevance once different items have been included in a global score. The LS and the CECDAI both demonstrated its usefulness in diagnosing CD (38, 39) as well as staging (40) and monitoring CD patients (41, 42). Moreover, correlations between these scores and inflammatory biomarkers (fecal calprotectin and C-reactive protein) have been shown (43, 44). Likewise, the above-cited expert Delphi consensus about nomenclature and description of small bowel lesions only took into account small bowel items that were part of the LS and CECDAI. As stated by the authors, this consensus meeting did not assess the clinical relevance of such lesions. We are also aware that we may have left behind information regarding clinical relevance in studies not providing a clear description of mucosal lesions.
In conclusion, this robust systematic review identifies mucosal lesions that have been described using CE in CD in the small and large bowels. Personal interpretation plays an important role in describing these mucosal lesions. Here, we make suggestions to homogenize description of mucosal lesions detected by CE in CD. We suggest that to avoid ulcerative lesion distinction based on size or shape, the terms erosion and aphtoid lesion may be used indistinctly, the term mucosal crater should be avoided when describing an ulcer since it may be confusing, and the items fissure and cobblestone pattern might be of unnecessary redundancy and should be avoided. This manuscript may serve as a starting point to reach consensus between experts and might contribute to the development of a pan-enteric CE activity index.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MM, GD'H, and ML developed the study design. MM and AS carried out the search process and data extraction and wrote the draft. All co-authors have reviewed and corrected the draft. ML gave the final approval for the submission. All authors have contributed to and agreed on the content of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.600095/full#supplementary-material
1. Klein A, Cellier C, Despott E, Eliakim R, Sidhu R, Saurin J, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. (2015) 47:352–86. doi: 10.1055/s-0034-1391855
2. Korman LY, Delvaux M, Gay G, Hagenmuller F, Keuchel M, Friedman S, et al. Capsule Endoscopy Structured Terminology (CEST): proposal of a standardized and structured terminology for reporting capsule endoscopy procedures. Endoscopy. (2005) 37:951–9. doi: 10.1055/s-2005-870329
3. Gal E, Geller A, Fraser G, Levi Z, Niv Y. Assessment and validation of the new capsule endoscopy Crohn's disease activity index (CECDAI). Dig Dis Sci. (2008) 53:1933–7. doi: 10.1007/s10620-007-0084-y
4. Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. (2008) 27:146–54. doi: 10.1111/j.1365-2036.2007.03556.x
5. Enns RA, Hookey L, Armstrong D, Bernstein CN, Heitman SJ, Teshima C, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology. (2017) 152:497–514. doi: 10.1053/j.gastro.2016.12.032
6. D'Haens G, Löwenberg M, Samaan MA, Franchimont D, Ponsioen C, van den Brink GR, et al. Safety and feasibility of using the second-generation Pillcam colon capsule to assess active colonic crohn's disease. Clin Gastroenterol Hepatol. (2015) 13:1480–6.e3. doi: 10.1016/j.cgh.2015.01.031
7. Negreanu L, Smarandache G, Mateescu RB. Role of capsule endoscopy Pillcam COLON 2 in patients with known or suspected Crohn's disease who refused colonoscopy or underwent incomplete colonoscopic exam: a case series. Tech Coloproctol. (2014) 18:277–83. doi: 10.1007/s10151-013-1054-3
8. Tjandra D, Kheslat HH, Amico F, MacRae F. Colon capsule endoscopy: looking beyond the colon in Crohn's disease. Inflamm Bowel Dis. (2017) 23:E43–4. doi: 10.1097/MIB.0000000000001224
9. Hall B, Holleran G, Mcnamara D. PillCam COLON 2 © as a pan-enteroscopic test in Crohn's disease. World J Gastrointest Endosc. (2015) 7:1230–2. doi: 10.4253/wjge.v7.i16.1230
10. Carvalho PB, Rosa B, Dias De Castro F, Moreira MJ, Cotter J. PillCam COLON 2© in Crohn's disease: a new concept of pan-enteric mucosal healing assessment. World J Gastroenterol. (2015) 21:7233–41. doi: 10.3748/wjg.v21.i23.7233
11. Yung DE, Har-Noy O, Yuen †, Tham S, Ben-Horin S, Eliakim R, et al. Capsule endoscopy, magnetic resonance enterography, and small bowel ultrasound for evaluation of postoperative recurrence in Crohn's disease: systematic review and meta-analysis. Inflamm Bowel Dis. (2017) 24:93–100. doi: 10.1093/ibd/izx027
12. Niv Y, Gal E, Gabovitz V, Hershkovitz M, Lichtenstein L, Avni I. Capsule endoscopy Crohn's disease activity index (CECDAIic or Niv Score) for the small bowel and colon. J Clin Gastroenterol. (2017) 52:45–9. doi: 10.1097/MCG.0000000000000720
13. Arieira C, Magalhães R, Dias de Castro F, Boal Carvalho P, Rosa B, Moreira MJ, et al. CECDAIic–a new useful tool in pan-intestinal evaluation of Crohn's disease patients in the era of mucosal healing. Scand J Gastroenterol. (2019) 54:1326–30. doi: 10.1080/00365521.2019.1681499
14. Leenhardt R, Buisson A, Bourreille A, Marteau P, Koulaouzidis A, Li C, et al. Nomenclature and semantic descriptions of ulcerative and inflammatory lesions seen in Crohn's disease in small bowel capsule endoscopy: an international Delphi consensus statement. United Eur Gastroenterol J. (2020) 8:99–107. doi: 10.1055/s-0040-1704486
15. Argüelles-Arias F, Caunedo A, Romero J, Sánchez A, Rodríguez-Téllez M, Pellicer FJ, et al. The value of capsule endoscopy in pediatric patients with a suspicion of Crohn's disease. Endoscopy. (2004) 36:869–73. doi: 10.1055/s-2004-825854
16. Mow WS, Lo SK, Targan SR, Dubinsky MC, Treyzon L, Abreu-Martin MT, et al. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Diagnostic yield of wireless capsule enteroscopy in comparison with computed tomography enteroclysis. Clin Gastroenterol Hepatol. (2004) 2:31–40. doi: 10.1016/S1542-3565(03)00289-1
17. Marmo R, Rotondano G, Piscopo R, Bianco MA, Siani A, Catalano O, et al. Capsule endoscopy versus enteroclysis in the detection of small-bowel involvement in Crohn's disease: a prospective trial. Clin Gastroenterol Hepatol. (2005) 3:772–6. doi: 10.1016/S1542-3565(05)00483-0
18. Efthymiou A, Viazis N, Mantzaris G, Papadimitriou N, Tzourmakliotis D, Raptis S, et al. Does clinical response correlate with mucosal healing in patients with Crohn's disease of the small bowel? A prospective, case-series study using wireless capsule endoscopy. Inflamm Bowel Dis. (2008) 14:1542–7. doi: 10.1002/ibd.20509
19. Mehdlzadeh S, Chen G, Enayati PJ, Cheng DW, Han NJ, Shaye OA, et al. Diagnostic yield of capsule endoscopy in ulcerative colitis and inflammatory bowel disease of unclassified type (IBDU). Endoscopy. (2008) 40:30–5. doi: 10.1055/s-2007-995359
20. Figueiredo P, Almeida N, Lopes S, Duque G, Freire P, Lérias C, et al. Small-bowel capsule endoscopy in patients with suspected Crohn's disease -diagnostic value and complications. Diagn Ther Endosc. (2010) 2010:101284. doi: 10.1155/2010/101284
21. Jensen MD, Nathan T, Kjeldsen J. Inter-observer agreement for detection of small bowel Crohn's disease with capsule endoscopy. Scand J Gastroenterol. (2010) 45:878–84. doi: 10.3109/00365521.2010.483014
22. Mehdizadeh S, Chen GC, Barkodar L, Enayati PJ, Pirouz S, Yadegari M, et al. Capsule endoscopy in patients with Crohn's disease: diagnostic yield and safety. Gastrointest Endosc. (2010) 71:121–7. doi: 10.1016/j.gie.2009.06.034
23. Casciani E, Masselli G, Di Nardo G, Polettini E, Bertini L, Oliva S, et al. MR enterography versus capsule endoscopy in paediatric patients with suspected Crohn's disease. Eur Radiol. (2011) 21:823–31. doi: 10.1007/s00330-010-1976-3
24. Di Nardo G, Oliva S, Ferrari F, Riccioni ME, Staiano A, Lombardi G, et al. Usefulness of wireless capsule endoscopy in paediatric inflammatory bowel disease. Dig Liver Dis. (2011) 43:220–4. doi: 10.1016/j.dld.2010.10.004
25. Koulaouzidis A, Smirnidis A, Douglas S, Plevris JN. QuickView in small-bowel capsule endoscopy is useful in certain clinical settings, but QuickView with Blue Mode is of no additional benefit. Eur J Gastroenterol Hepatol. (2012) 24:1099–104. doi: 10.1097/MEG.0b013e32835563ab
26. Esaki M, Matsumoto T, Watanabe K, Arakawa T, Naito Y, Matsuura M, et al. Use of capsule endoscopy in patients with Crohn's disease in Japan: a multicenter survey. J Gastroenterol Hepatol. (2014) 29:96–101. doi: 10.1111/jgh.12411
27. Halling ML, Nathan T, Kjeldsen J, Jensen MD. High sensitivity of quick view capsule endoscopy for detection of small bowel Crohn's disease. J Gastroenterol Hepatol. (2014) 29:992–6. doi: 10.1111/jgh.12488
28. Höög CM, Bark LA, Broström O, Sjöqvist U. Capsule endoscopic findings correlate with fecal calprotectin and C-reactive protein in patients with suspected small-bowel Crohn's disease. Scand J Gastroenterol. (2014) 49:1084–90. doi: 10.3109/00365521.2014.920915
29. Kono T. Prospective postsurgical capsule endoscopy in patients with Crohn's disease. World J Gastrointest Endosc. (2014) 6:88. doi: 10.4253/wjge.v6.i3.88
30. Aloi M, Di Nardo G, Romano G, Casciani E, Civitelli F, Oliva S, et al. Magnetic resonance enterography, small-intestine contrast US, and capsule endoscopy to evaluate the small bowel in pediatric Crohn's disease: a prospective, blinded, comparison study. Gastrointest Endosc. (2015) 81:420–7. doi: 10.1016/j.gie.2014.07.009
31. Urgesi R, Cianci R, Marmo C, Costamagna G, Riccioni M. E. But how many misunderstood Crohn's disease are revealed “by chance” using Capsule Endoscopy in Chronic Recurrent OGIB? Experience of a Single Italian Center and long term follow-up. Eur Rev Med Pharmacol Sci. (2015) 19:4553–7. Available online at: https://www.europeanreview.org/article/9939
32. Hale MF, Drew K, McAlindon ME, Sidhu R. The diagnostic accuracy of faecal calprotectin and small bowel capsule endoscopy and their correlation in suspected isolated small bowel Crohn's disease. Eur J Gastroenterol Hepatol. (2016) 28:1145–50. doi: 10.1097/MEG.0000000000000696
33. Lee HS, Lim YJ, Shim KN, Moon CM, Song HJ, Kim JO, et al. Diagnostic value of small bowel capsule endoscopy in isolated ileitis: a CAPENTRY study. Dig Dis Sci. (2017) 62:180–7. doi: 10.1007/s10620-016-4387-8
34. Ninomiya K, Hisabe T, Okado Y, Takada Y, Yamaoka R, Sato Y, et al. Comparison of small bowel lesions using capsule endoscopy in ulcerative colitis and crohn's disease: a single-center retrospective analysis. Digestion. (2018) 98:119–26. doi: 10.1159/000487796
35. Esaki M, Matsumoto T, Ohmiya N, Washio E, Morishita T, Sakamoto K, et al. Capsule endoscopy findings for the diagnosis of Crohn's disease: a nationwide case–control study. J Gastroenterol. (2019) 54:249–60. doi: 10.1007/s00535-018-1507-6
36. Aabakken L, Rembacken B, Olivier LeMoine U, Konstantin Kuznetsov B, Jean-Francois Rey R, Thomas Rösch F. Minimal standard terminology for gastrointestinal endoscopy – MST 3.0. Endoscopy. (2009) 41:727–8. doi: 10.1055/s-0029-1214949
37. Yao T, Matsui T, Hiwatashi N. Crohn's disease in Japan: diagnostic criteria and epidemiology. Dis Colon Rectum. (2000) 43:S85–93. doi: 10.1007/BF02237231
38. Monteiro S, Boal Carvalho P, Dias De Castro F, Magalhães J, MacHado F, Moreira MJ, et al. Capsule endoscopy: diagnostic accuracy of lewis score in patients with suspected Crohn's disease. Inflamm Bowel Dis. (2015) 21:2241–6. doi: 10.1097/MIB.0000000000000517
39. Niv Y, Ilani S, Levi Z, Hershkowitz M, O'Morain CA, O'Donnell S, et al. Validation of capsule endoscopy Crohn's disease activity score (CECDAI or Niv Score) – a multi center prospective study. Gastroenterology. (2018) 140:S-768. doi: 10.1016/S0016-5085(11)63186-7
40. Hansel SL, McCurdy JD, Barlow JM, Fidler J, Fletcher JG, Becker B, et al. Clinical benefit of capsule endoscopy in Crohn's disease: impact on patient management and prevalence of proximal small bowel involvement. Inflamm Bowel Dis. (2018) 24:1582–8. doi: 10.1093/ibd/izy050
41. Melmed GY, Dubinsky MC, Rubin DT, Fleisher M, Pasha SF, Sakuraba A, et al. Utility of video capsule endoscopy for longitudinal monitoring of Crohn's disease activity in the small bowel: a prospective study. Gastrointest Endosc. (2018) 88:947–55.e2. doi: 10.1016/j.gie.2018.07.035
42. Tsibouris P, Periklis A, Chrissostomos K, Antonios Z, Panagiota M, Erasmia V, et al. When Crohn's disease is in remission, more patients complete capsule endoscopy study but less lesions are identified. Saudi J Gastroenterol. (2013) 19:63–8. doi: 10.4103/1319-3767.108468
43. Arieira C, Dias-de-Castro F, Rosa B, Moreira MJ, Firmino-Machado J, Cotter J. Can we rely on inflammatory biomarkers for the diagnosis and monitoring crohn's disease activity? Rev Esp Enfermedades Dig. (2017) 109:828–33. doi: 10.17235/reed.2017.5126/2017
44. Yablecovitch D, Lahat A, Neuman S, Levhar N, Ben-Horin S, IBD on behalf of the I (IIRN) RN. The Lewis score or the capsule endoscopy Crohn's disease activity index: which one is better for the assessment of small bowel inflammation in established Crohn's disease? Therap Adv Gastroenterol. (2018) 11:1756283X17747780. doi: 10.1177/1756283X17747780
Keywords: inflammatory bowel diseases, Crohn's disease, capsule endoscopy, intestine, small/diagnostic imaging, intestine, small/pathology
Citation: Marquès Camí M, Serracarbasa A, D'Haens G and Löwenberg M (2021) Characterization of Mucosal Lesions in Crohn's Disease Scored With Capsule Endoscopy: A Systematic Review. Front. Med. 7:600095. doi: 10.3389/fmed.2020.600095
Received: 28 August 2020; Accepted: 26 November 2020;
Published: 14 January 2021.
Edited by:
Fernando Gomollón, University of Zaragoza, SpainReviewed by:
Maria Moreira, Hospital da Senhora da Oliveira Guimarães, PortugalCopyright © 2021 Marquès Camí, Serracarbasa, D'Haens and Löwenberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miquel Marquès Camí, bWltYXJxdWVzLmxsZWlkYS5pY3NAZ2VuY2F0LmNhdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.