
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 09 February 2021
Sec. Rheumatology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.600049
 Liping Si1†
Liping Si1† Kai Xuan2†
Kai Xuan2† Jingyu Zhong1
Jingyu Zhong1 Jiayu Huo2
Jiayu Huo2 Yue Xing1
Yue Xing1 Jia Geng3
Jia Geng3 Yangfan Hu4
Yangfan Hu4 Huan Zhang5
Huan Zhang5 Qian Wang2
Qian Wang2 Weiwu Yao1*
Weiwu Yao1*Background: It was difficult to distinguish the cartilage thinning of an entire knee joint and to track the evolution of cartilage morphology alongside ages in the general population, which was of great significance for studying osteoarthritis until big imaging data and artificial intelligence are fused. The purposes of our study are (1) to explore the cartilage thickness in anatomical regions of the knee joint among a large collection of healthy knees, and (2) to investigate the relationship between the thinning pattern of the cartilages and the increasing ages.
Methods: In this retrospective study, 2,481 healthy knees (subjects ranging from 15 to 64 years old, mean age: 35 ± 10 years) were recruited. With magnetic resonance images of knees acquired on a 3-T superconducting scanner, we automatically and precisely segmented the cartilage via deep learning and calculated the cartilage thickness in 14 anatomical regions. The thickness readings were compared using ANOVA by considering the factors of age, sex, and side. We further tracked the relationship between the thinning pattern of the cartilage thickness and the increasing ages by regression analysis.
Results: The cartilage thickness was always thicker in the femur than corresponding regions in the tibia (p < 0.05). Regression analysis suggested cartilage thinning alongside ages in all regions (p < 0.05) except for medial and lateral anterior tibia in both females and males (p > 0.05). The thinning speed of men was faster than women in medial anterior and lateral anterior femur, yet slower in the medial patella (p < 0.05).
Conclusion: We established the calculation method of cartilage thickness using big data and deep learning. We demonstrated that cartilage thickness differed across individual regions in the knee joint. Cartilage thinning alongside ages was identified, and the thinning pattern was consistent in the tibia while inconsistent in patellar and femoral between sexes. These findings provide a potential reference to detect cartilage anomaly.
Osteoarthritis (OA) is a chronic, disabling joint disease with a total incidence of 15% in populations (1, 2). OA causes prevalent damages to many parts of the knee joint, including articular cartilage and subchondral bone, which then seriously impair the quality of life of middle-aged and older people (3, 4). The osteoarthritic chondrocytes have undergone comprehensive degeneration and structural disorders (5). Pieces of evidence suggested that early degeneration in cartilage also impaired subchondral bone and incurred bone remodeling (6, 7). The current therapeutic approach for OA was largely palliative (8). Due to the unknown pathogenesis, there was no effective treatment other than pain relief (9) and joint replacement in the advanced stage of the disease (10).
Therefore, it is necessary to systematically analyze structural changes of knee cartilage by magnetic resonance (MR) imaging to better understand the development of OA and to focus on early detection and disease prevention. Previous studies reported cartilage/meniscus segmentation and quantification (11), evaluation of knee cartilage lesions (12), and measurement of femoral cartilage thickness (13). Several researchers inferred cartilage thickness using the joint space of plain films and small-scale femoral cartilage thickness (14). However, few efforts in the literature were devoted to systematically analyzing the cartilages of the whole joint and provided comprehensive baseline data on cartilage thickness of the individual regions-of-interest (ROIs) in the knee joint, which now became technically feasible by combining big imaging data and artificial intelligence, with the ubiquitous application of deep learning in medical imaging in the past years (15, 16).
Concerning the necessity to examine the critical role of articular cartilage in the whole knee joint, we conducted a comprehensive and in-depth big data study to track the thickness changes of knee cartilage in this work. Specifically, we quantified the cartilage morphology by automatic image segmentation powered by deep learning and standardize the knee cartilage thickness among a large Chinese population of healthy knees. We aim to compare the cartilage thickness among different age groups and associate the thickness readings with 14 individual ROIs in the knee joint according to the whole-organ magnetic resonance imaging score (WORMS) (17). In this way, our research could provide new insights into the cartilage thickness thinning pattern of normal knees and could potentially act as a reference to detect the anomaly and to track disease progress.
This research project was approved by the institutional review board, Tongren Hospital, Shanghai Jiao Tong University School of Medicine. Subjects were retrospectively selected from the hospital database with both plain films and MR images from January 2018 to March 2019. Figure 1 provided our flowchart to select the subjects. Inclusion criteria were knees without OA, i.e., having a Kellgren-Lawrence grade of 0 (18, 19), which was reviewed by two practicing board-certified musculoskeletal radiologists at a large academic practice (years in practice 10–20 years) independently. Finally, 2,481 subjects were included, and the ages of the subjects ranged from 15 to 64 years old, with mean age: 35 ± 10 years; male: 1,355 subjects (35 ± 10 years); female: 1,126 subjects (36±11 years); left knee: 1,228 subjects (36±11 years), right knee: 1,253 subjects (35 ± 10 years). The informed consent requirement was waived, as this study was a retrospective review of radiologic images without identification and health information of patients.
Images of the knees were acquired on a 3-T superconducting MR scanner (Achieva 3.0TX; Philips Healthcare, Best, Netherlands) with an eight-channel knee coil (Philips Healthcare). The knee flexion angle was kept naturally bent, and a dedicated holder was used to reduce motion artifacts at the time of acquisition. The MR protocol included four sequences (Table 1): (1) sagittal T1W sequence: repetition time/echo time (TR/TE) = 638/20 ms, field of view (FOV) = 160 mm, matrix = 260 × 208, section thickness = 3 mm, bandwidth = 289 KHz; (2) sagittal T2W fat-suppressed sequence: TR/TE = 3,004/62 ms, FOV = 100 mm, matrix = 292 × 188, section thickness = 3 mm, bandwidth = 218 KHz; (3) coronal proton density-weighted fat-suppressed sequence: TR/TE = 2,137/25 ms, FOV = 160 mm, matrix = 324 × 168, section thickness = 3 mm, bandwidth = 218 KHz; (4) transversal proton density-weighted fat-suppressed sequence: TR/TE = 2,351/25 ms, FOV = 160 mm, matrix = 324 × 168, section thickness = 3 mm, bandwidth = 218 KHz.
To facilitate region-based analysis of cartilage thickness, the knee MR image was divided into 14 structural ROIs, including anterior (A), central (C), and posterior (P) regions of the medial (M)/lateral (L) femur (F), tibia (T), and patella (P), with exemplar delineation in Figure 2. The above regions were defined in accordance with WORMS (17).
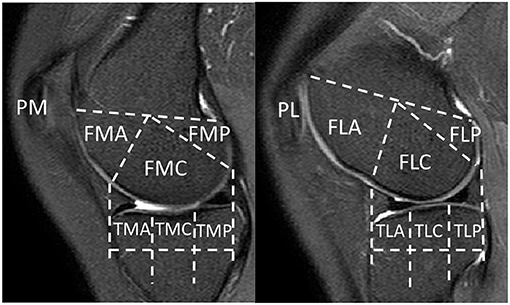
Figure 2. Knee MR images were divided into 14 structural regions-of-interest (ROIs). FMA, medial anterior femur; FMC, medial central femur; FMP, medial posterior femur; FLA, lateral anterior femur; FLC, lateral central femur; FLP, lateral posterior femur; TMA, medial anterior tibia; TMC, medial central tibia; TMP, medial posterior tibia; TLA, lateral anterior tibia; TLC, lateral central tibia; TLP, lateral posterior tibia; PM, medial patella; PL, lateral patella.
A neural network was used to segment knee joint from MR images automatically. Similar to Norman et al. (11), we chose the two-dimensional U-Net (20) architecture, a widely used method in medical image segmentation, to segment six regions (i.e., femur, tibia, patella, and corresponding cartilages) slice-to-slice. Considering our limited training data size, weight decay and data-augmentation techniques such as slice-wise spatial transformation of rotation, translation, and rescaling were applied to prevent overfitting. Also, subject-wise histogram-matching was adopted for better generalization. The neural network was implemented in PyTorch and trained on an Nvidia GeForce GTX 1080 Ti Graphics Card.
In this study, the Dice similarity coefficient (DSC) was used to evaluate the accuracy of segmentation. DSC was calculated with DSC(A, B) = 2|A∩B|/(|A|+|B|), where A and B were the reference segmentation (i.e., manual labeling) and automatic segmentation, respectively, whereas the operator |·| counts the number of elements in the set. The value of DSC ranges from 0 to 1, and the higher value indicates better segmentation performance. We used 27 subjects (570 slices) for training and 20 subjects (471 slices) for testing. The segmentation DSCs of femoral cartilage, tibial cartilage, and patellar cartilage were 0.87 ± 0.01, 0.82 ± 0.01, and 0.76 ± 0.04, respectively, which were comparable with manual labeling (see Table 2). The reproducibility experiment results of the segmentation model were shown in the Supplementary Materials (see Supplementary Figure 1.)
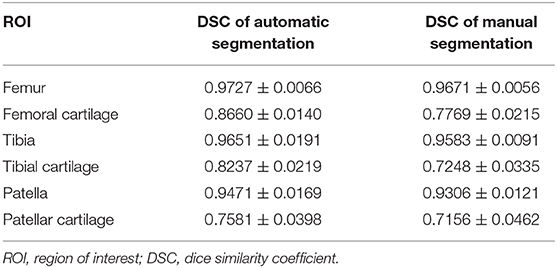
Table 2. Results of automatic and manual segmentation accuracy of knee joint in DSC (calculated based on individuals).
With the development and degeneration of normal human cartilage, the cartilage thickness at different regions of the knee joint changes gradually. On the basis of the automatic segmentation results described earlier, we could calculate the cartilage thickness (21) following the steps later. First, we used the femur as an example and generated the signed distance maps of femur and femur cartilage. The value in the distance map records the distance from the specific location to the nearest boundary point, with negative/positive signs for locations inside/outside the segmented region of femur or femur cartilage. Third, we calculated the first-order gradient maps from the corresponding signed distance maps. Fourth, by performing a dot-product operation on the two gradient maps, we determined inner cartilage boundary points (facing toward femur bone) if dot-products were negative or outer cartilage boundary points (away from femur) if positive. Finally, we used the outer boundary points and computed the distance map for the femur cartilage, from which the distances could be read for all inner boundary points.
Next, we used an atlas with predefined ROIs to complete the segmentation of individual regions per subject. The atlas was labeled to delineate all 14 ROIs. By deforming the atlas to align with each subject image via one-by-one registration, we acquired ROIs for all collected images. Then, for each subject MR image, we calculated the cartilage thickness and derived the average measure for each region (11). The cartilage thickness measures, corresponding to the 14 delineated ROIs, allowed us to compare across different age/sex groups and track the longitudinal thinning pattern accordingly. Error analysis of cartilage morphology and quality assessment of the quantitative analysis of the cartilage thickness was shown in the supplementary materials (see Supplementary Table 1, Supplementary Figures 2–15).
Cartilage thickness of different regions, ages, sexes, and sides were compared using ANOVA pairwise comparisons method Tukey. Tukey's method is used in ANOVA to create confidence intervals for all pairwise differences between factor level means while controlling the family error rate to a level specified. An alpha level of 0.05 was set for statistical significance, and all tests were two-tailed. In the Tukey simultaneous test results, the 95% simultaneous confidence level implies that all the confidence intervals contain the true differences. If an interval does not contain zero, the corresponding means are significantly different.
Regression analysis of cartilage thickness changes in 14 regions with sex/laterality was performed with a fit regression model. We created a categorical condition variable, i.e., sex/laterality. Then, we included the interaction term for input × condition, i.e., age × sex/laterality. We fit the regression model with input (continuous independent variable), condition (main effect), and input × condition (interaction effect). The coefficient of age × sex/laterality represents the difference between the coefficient for males and females. The p-value lower than 0.05 indicates that this difference is statistically significant. In other words, we could conclude that sex/laterality affects the relationship between input and output. The consistent (or inconsistent) change with age implies no significant (or significant) difference between sexes/lateralities. The analyses mentioned earlier were performed in Minitab Version 19.1, and the figures were drawn in Python 3.7.
The average thickness of each ROI over all subjects was plotted in Figure 3A. The comparisons among all 14 ROIs revealed prevalent thickness differences in the knee joint. Particularly, PM owned the highest cartilage thickness (2.18 ± 0.32 mm; for convenience, the “mm” unit would be skipped in the following thickness reading). When comparing corresponding A/C/P regions between femur and tibia (i.e., FMA vs. TMA, FMC vs. TMC, etc.), femur always led in the reading {FMA vs. TMA: 2.06 ± 0.20 vs. 1.48 ± 0.10 [difference of means 0.58 ± 0.01, 95% confidence interval (CI) 0.56–0.59], FMC vs. TMC: 1.75 ± 0.12 vs. 1.60 ± 0.12 (0.15 ± 0.01, 0.13–0.16); p < 0.05 in all above tests}. In the femur particularly, the cartilage thickness in the non-load-bearing areas (i.e., FMA and FLA) was greater than the load-bearing C areas (FMC and FLC) and P areas (FMP and FLP) (FMA vs. FMC/FMP: 2.06 ± 0.20 vs. 1.75 ± 0.12/1.59 ± 0.16, FLA vs. FLC/FLP: 2.15 ± 0.24 vs. 1.77 ± 0.13/1.61 ± 0.22, p < 0.05 in all tests mentioned earlier). On the contrary, for the tibia, the C areas of TMC and TLC led in the thickness measuring, compared with A and P areas (TMC vs. TMA/TMP: 1.60 ± 0.12 vs. 1.48 ± 0.10 mm/1.44 ± 0.08, TLC vs. TLA/TLP:1.66 ± 0.13 vs. 1.51 ± 0.11/1.40 ± 0.11; p < 0.05 in tests mentioned earlier).
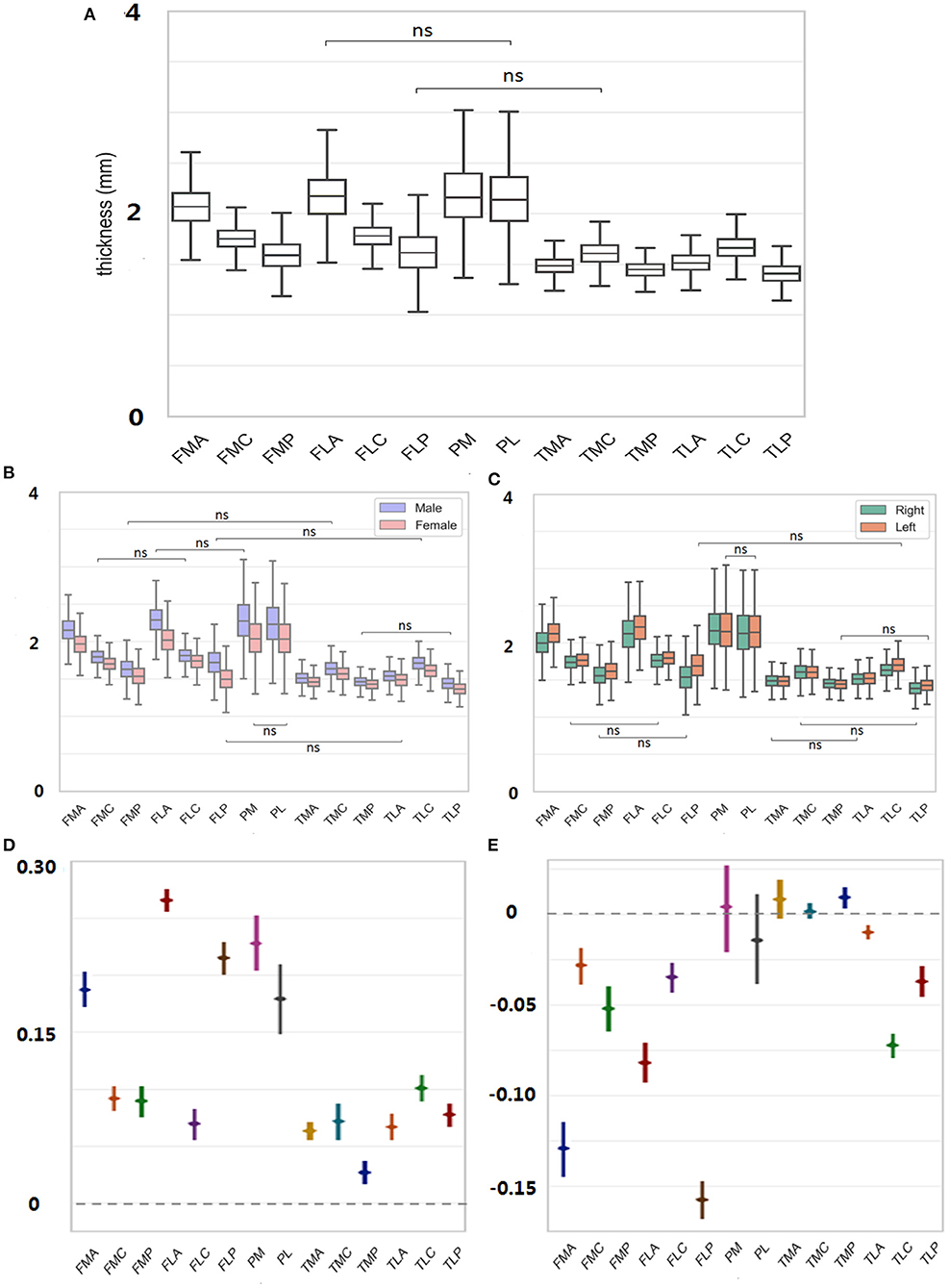
Figure 3. (A) Thickness of each ROI averaged was measured over all subjects. (B) For each ROI, thickness was further decomposed to male/female sex. (C) Thickness was further decomposed to left/right side. (D) Male to female difference in the cartilage thickness reading was provided for each region. (E) Right to left difference in cartilage thickness reading was provided for each region. ns = p > 0.05.
For each ROI, the thickness reading was further separated into male and female sexes and was compared in Figures 3B,D. The findings were consistent with those mentioned earlier without considering the sex factor. For both females and males, the cartilage thickness in the non-load-bearing areas was greater than the load-bearing C and P areas for the femur. Meanwhile, across the upper and lower corresponding A/C/P regions, the thickness in the femur was always greater than in the tibia. When referring to the sex difference, the cartilage thickness tended to be always thicker in men than in women (FMA, men vs. women, 2.16 ± 0.18 vs. 1.97 ± 0.16, FMC, 1.80 ± 0.11 vs. 1.70 ± 0.11, 0.10 ± 0.01; FMP, 1.63 ± 0.16 vs. 1.54 ± 0.15, 0.09 ± 0.01, p < 0.05 in all tests mentioned earlier). The largest margin for the sex-related cartilage thickness occurred in FLA (0.27 ± 0.01), contrary to the smallest difference in TMP (0.04 ± 0.01).
The thickness measured in Figure 3A was also separated into the left and right sides, and the result for the laterality is shown in Figures 3C,E. The cartilage thickness was thicker in left knees than in right knees, which was statistically significant in all femoral regions (FMA, left vs. right, 2.13 ± 0.18 vs. 2.00 ± 0.20; FMC, 1.77 ± 0.12 vs. 1.73 ± 0.12, p < 0.05 in tests mentioned earlier). In all TL regions, the same findings were revealed (TLA, left vs. right, 1.52 ± 0.11 vs. 1.50 ± 0.11; TLC, 1.70 ± 0.14 vs. 1.62 ± 0.12; TLP, 1.43 ± 0.10 vs. 1.38 ± 0.11; p < 0.05 in tests mentioned earlier). However, no significant difference between the left and right sides was detected in TMA, TMC, and the patella (p > 0.05).
For each ROI, the regression model of the cartilage thickness with respect to ages was derived for both men and women. The models for all 14 ROIs are shown in Figure 4. The tendency of cartilage thickness change with aging was consistent (i.e., no significant difference between females and males) from all tibial ROIs and PL, yet inconsistent (i.e., a significant difference between females and males) in PM and femoral ROIs except for FLC. Among the eight consistent regions, the cartilage thickness in the TMA and TLA regions did not change significantly with age (age coefficient > 0, p > 0.05), whereas others became thinner. Meanwhile, considering the inconsistent six regions, the cartilage thickness in FMP increased slightly alongside age for women (age coefficient > 0, p < 0.05) and in other ROIs decreased temporally (age coefficient < 0, p < 0.05). The thinning speed in the FMA area was faster in men than in women (p < 0.05 for all age × sex coefficients) and, in contrast, slower for men in the PM area (p < 0.05 for all age × sex coefficients). Additionally, the cartilage degenerated in the ROIs of FMC, FLA, and FLP for men (age coefficient <0, p < 0.05), whereas women's changes were not statistically significant (age coefficient <0, p > 0.05).
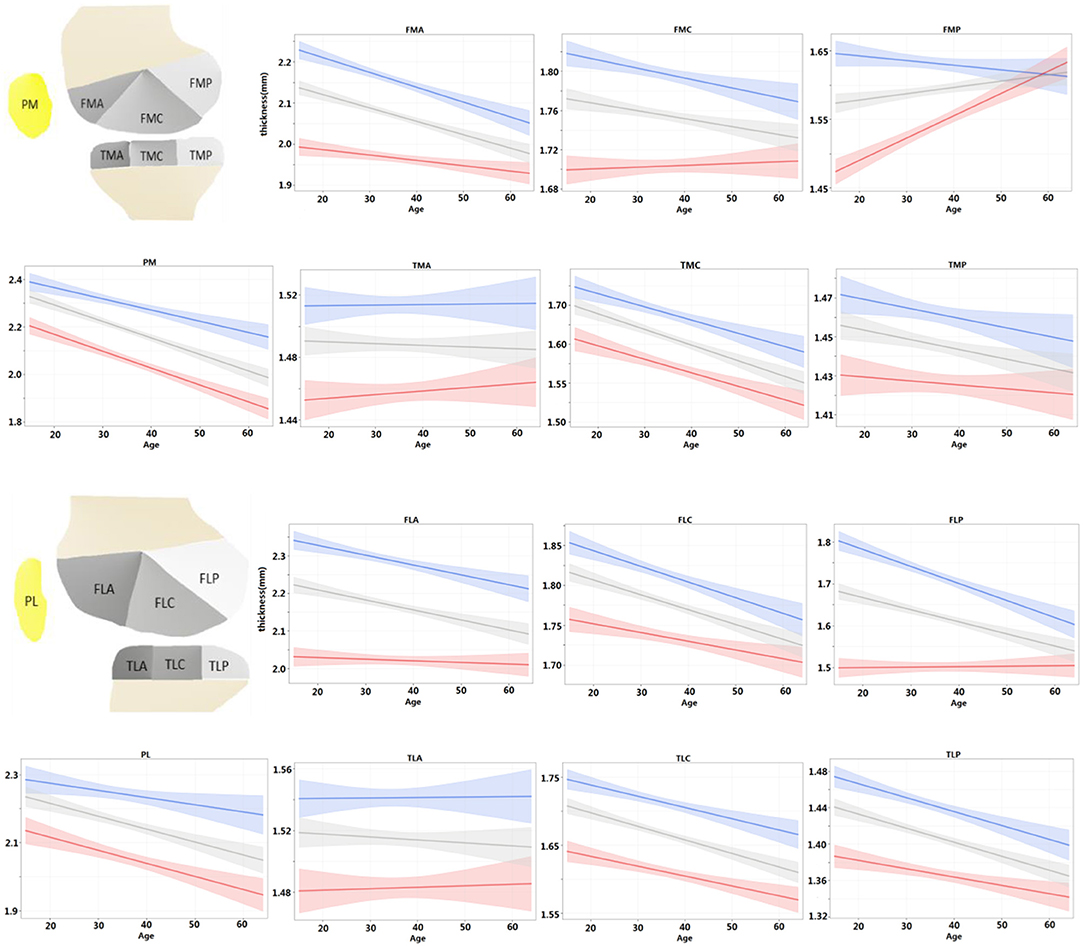
Figure 4. For each ROI, regression model of cartilage thickness with respect to ages was derived for both men and women and compared with population at same time. Shading represented 95% CIs.
Further, the regression models were fitted for the left and right knees and compared with the entire population in Figure 5. For the left and right knees, the temporal changes were consistent in all areas except for FMC (p > 0.05 for all age × laterality coefficients except for FMC). Among the consistent regions, the cartilage thickness in the TMA and TLA regions did not change significantly with age (age coefficient <0, p > 0.05), FMP increased with age (age coefficient > 0, p < 0.05), and others degenerated (age coefficient <0, p < 0.05). That is, both knees tended to degenerate in a synchronized manner.
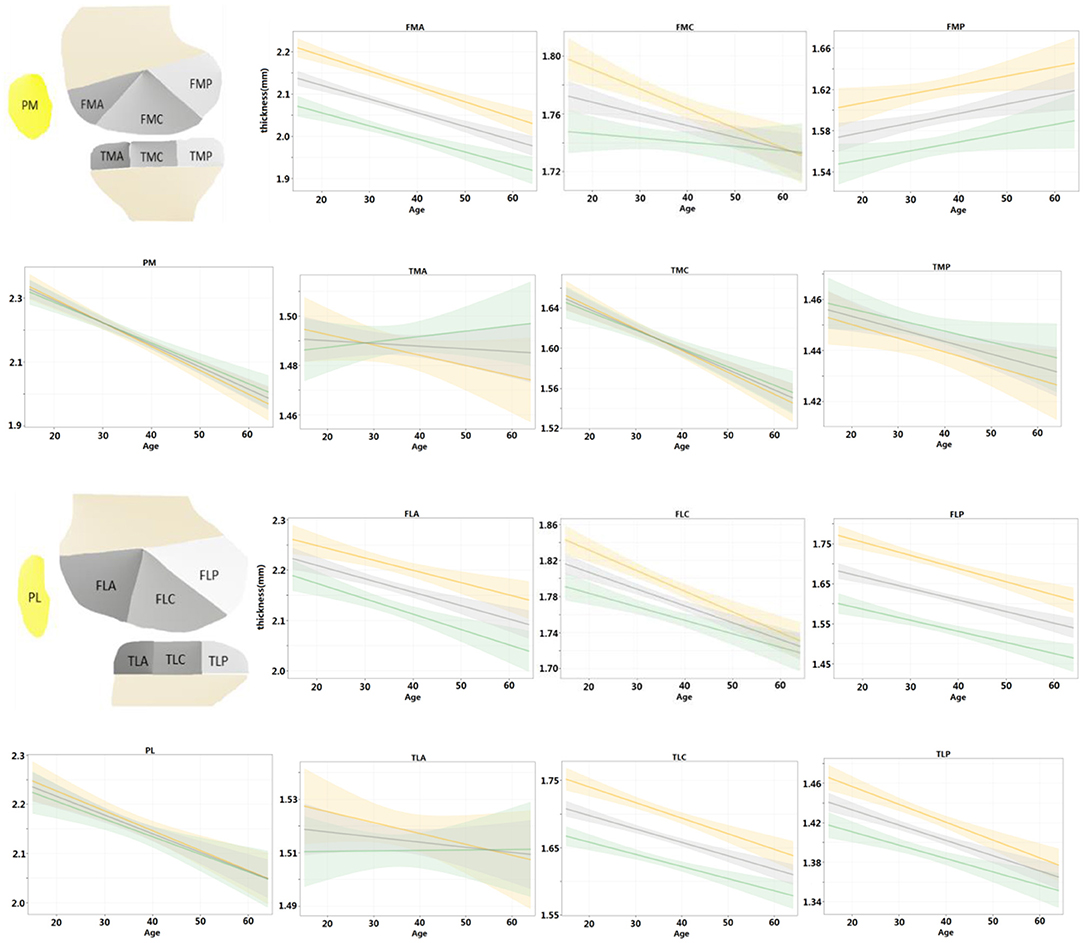
Figure 5. Regression models were fitted for left and right knees, respectively, and compared with the population at the same time. Shading represented 95% CIs.
We used the deep learning method to automatically segment the cartilage and calculated the cartilage thickness in 14 individual regions of the whole knee joint. For the first time, we specified the characteristics of cartilage thickness across 14 nonoverlapping ROIs of the knee joint and tracked their thinning alongside aging, which provided a standardized reference to reveal the pathogenesis of knee OA potentially. From our findings, male cartilage thickness was greater than female in all age groups across the knee joint. Furthermore, the thinning was inconsistent from PM and all femoral ROIs except for FLC between men and women. Age and sex do play a role in cartilage thinning despite the diversity in men and women. Differences in cartilage thickness may further suggest varying risks of developing knee OA between women and men (22, 23).
So far, only one study using deep learning to assess the thickness of femoral cartilage has been reported (24). Particularly, the segmentation model produced DSCs between 0.77 and 0.88 in the femoral cartilage compartments of several landmarks. Our model was applied to the whole knee joint in this work and produced comparable DSCs between 0.76 and 0.87 for the femoral, tibial, and patellar cartilage compartments. Moreover, compared with quantifying the cartilage thickness near the landmarks in medial and lateral femur only (24), we generalized to the whole knee joint, including femur, tibia, and patella. We made a comprehensive quantitative analysis of cartilage thickness in 14 regions according to WORMS (17), which was widely accepted for the accurate positioning of clinical assessment of cartilage thinning or defects. In addition, we further established a model of cartilage thickness variation alongside ages through robust statistical methods. Our findings further validated the previous work (24) upon the Chinese population and concluded that age and sex significantly impacted cartilage thickness.
Our study filled the vacancy with increasing ages in a relatively younger population (mean age: 36 years), comparing our results with another large population-based cross-sectional study, i.e., Framingham OA study investigating knee MR imaging in the middle-aged and elderly population (mean age: 62 years) (25–27). The results indicated that the medial posterior femoral region was more vulnerable in the whole knee joint. Specifically, the Framingham OA study (27) found that MR-detected cartilage damages were highly prevalent in the medial posterior tibiofemoral joints. Our findings, on the other hand, showed that the cartilage thickness in the non-load-bearing FMA area was greater than the load-bearing FMC and FMP areas in radiographically normal knees, which was possibly attributed to the factor that FMC and FMP had a higher chance of natural cartilage wear. Unfortunately, the thickness of the 14 individual regions considering body mass index information was not reported and needed further study. In addition, we found the cartilage thickness in the TMA and TLA areas did not change significantly with aging. It may be because these two regions do not have much contact or association with other areas during joint movement.
Based on the regression analysis of men and women, we found that the cartilage thickness in the FMP region of bilateral knees of women increased with age. Previous studies (28, 29) also reported that the cartilage thickness in the FMP area could increase in OA knees compared with non-OA knees without considering the sex differences. Therefore, we infer that the thickening of cartilage in the FMP area is a physiological change related to hormones. However, the biological mechanism underlying such a response is still unclear in the literature. Post-traumatic spontaneous reparation (30) and the measurement error should also be taken into account. We look forward to more studies in the future related to the histo-biological processes involved in this thickening area.
Given the regression results of left and right knees, we found that the cartilage thinning pattern was always consistent except for FMC only. Moreover, previous studies (31, 32) also reported that knees from subjects with bilaterally radiographically normal knees had lower cartilage thinning scores. It implies that the cartilage thinning is generally synchronized in the left and right knees. However, more information about the habitual use of limbs needs to be collected to get a more accurate interpretation.
There existed several limitations in our study. Firstly, our sample size was skewed to a certain extent, resulting in the relatively young age distribution (median age: 34 years; mean age: 36 years). Secondly, our segmentation protocol did not include high-resolution three-dimensional imaging, and we would further use the three-dimensional sequences of the knee joint to improve our segmentation model in the following research. Thirdly, because the assessment was blindly performed by two practicing board-certified musculoskeletal radiologists without identification and health information of patients, we did not assess more information such as body mass index and medical history such as common chronic diseases. Finally, we did not accomplish follow-up exams in this cohort to obtain and compare its cartilage information to make more accurate predictions, which would be a potential direction for future research.
In conclusion, prevalent cartilage thickness differences existed across individual regions in the knee joint, by considering the factors of age, sex, and side, with respect to a large population of 2,481 subjects and their 3-T MR images. Cartilage thinning alongside ages was identified for both men and women. The thinning pattern was consistent in the tibia while partially inconsistent from patellar and femoral cartilages between women and men. The findings earlier may provide references to detect cartilage anomaly.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Sixth People's Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
KX and QW: computer programming and manuscript editing. WY and HZ: guarantors of integrity of entire study and study concepts/study design. LS and KX: statistical analysis and experimental studies. LS and JH: data acquisition or data analysis/interpretation. LS, JZ, YX, JG, and YH: literature research and manuscript revision. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (81771790) and the Medicine and Engineering Combination Project of Shanghai Jiao Tong University (YG2019ZDB09). They have no role in the study design, data collection or analysis, decision to publish, or manuscript preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.600049/full#supplementary-material
ANOVA, analysis of variance; CI, confidence interval; F, female; FMA, medial anterior femur; FMC, medial central femur; FMP, medial posterior femur; FLA, lateral anterior femur; FLC, lateral central femur; FLP, lateral posterior femur; L, left; M, male; OA, osteoarthritis; PFJ, patellofemoral joint; PM, medial patella; PL, lateral patella; R, right; ROI, region of interest; TFJ, tibiofemoral joint TMA, medial anterior tibia; TMC, medial central tibia; TMP, medial posterior tibia; TLA, lateral anterior tibia; TLC, lateral central tibia; TLP, lateral posterior tibia; WORMS, Whole-Organ Magnetic Resonance Imaging Score.
1. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. (2013) 105:185–99. doi: 10.1093/bmb/lds038
2. Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. (2014) 73:1323–30. doi: 10.1136/annrheumdis-2013-204763
3. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers. (2016) 2:16072. doi: 10.1038/nrdp.2016.72
4. Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr Cart. (2020) 28:242–8. doi: 10.1016/j.joca.2020.01.002
5. Aigner T, Söder S, Gebhard PM, McAlinden A, Haag J. Mechanisms of disease: role of chondrocytes in the pathogenesis of osteoarthritis–structure, chaos and senescence. Nat Clin Pract Rheumatol. (2007) 3:391–9. doi: 10.1038/ncprheum0534
6. Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheum. (2012) 8:665–73. doi: 10.1038/nrrheum.2012.130
7. Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheum. (2011) 7:43–9. doi: 10.1038/nrrheum.2010.197
8. Hunter DJ. Pharmacologic therapy for osteoarthritis–the era of disease modification. Nat Rev Rheum. (2011) 7:13–22. doi: 10.1038/nrrheum.2010.178
9. Zeng C, Wei J, Persson MSM, Sarmanova A, Doherty M, Xie D, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. (2018) 52:642–50. doi: 10.1136/bjsports-2017-098043
10. Price AJ, Alvand A, Troelsen A, Katz JN, Hooper G, Gray A, et al. Knee replacement. Lancet. (2018) 392:1672–82. doi: 10.1016/S0140-6736(18)32344-4
11. Norman B, Pedoia V, Majumdar S. Use of 2D U-net convolutional neural networks for automated cartilage and meniscus segmentation of knee MR imaging data to determine relaxometry and morphometry. Radiology. (2018) 288:177–85. doi: 10.1148/radiol.2018172322
12. Liu F, Zhou Z, Samsonov A, Blankenbaker D, Larison W, Kanarek A, et al. Deep learning approach for evaluating knee MR images: achieving high diagnostic performance for cartilage lesion detection. Radiology. (2018) 289:160–9. doi: 10.1148/radiol.2018172986
13. Pitikakis M, Chincisan A, Magnenat-Thalmann N, Cesario L, Parascandolo P, Vosilla L, et al. Automatic measurement and visualization of focal femoral cartilage thickness in stress-based regions of interest using three-dimensional knee models. Int J Comp Assist Radiol Surg. (2016) 11:721–32. doi: 10.1007/s11548-015-1257-3
14. Eckstein F, Collins JE, Nevitt MC, Lynch JA, Kraus VB, Katz JN, et al. Brief report: cartilage thickness change as an imaging biomarker of knee osteoarthritis progression: data from the foundation for the national institutes of health osteoarthritis biomarkers consortium. Arthr Rheum. (2015) 67:3184–9. doi: 10.1002/art.39324
15. Wang Q, Shen D. A cybernetic eye for rare disease. Nat Biomed Eng. (2017) 1:32. doi: 10.1038/s41551-017-0032
16. Shen D, Wu G, Suk HI. Deep learning in medical image analysis. Annu Rev Biomed Eng. (2017) 19:221–48. doi: 10.1146/annurev-bioeng-071516-044442
17. Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cart. (2004) 12:177–90. doi: 10.1016/j.joca.2003.11.003
18. Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: kellgren-lawrence classification of osteoarthritis. Clin Orthopaed Rel Res. (2016) 474:1886–93. doi: 10.1007/s11999-016-4732-4
19. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. (1957) 16:494–502. doi: 10.1136/ard.16.4.494
20. Ronneberger O, Fischer P, Brox T, editors. U-Net: convolutional networks for biomedical image segmentation. In: Medical Image Computing and Computer-Assisted Intervention - MICCAI 2015. (2015). Cham: Springer International Publishing. doi: 10.1007/978-3-319-24574-4_28
21. Yezzi AJ Jr, Prince JL. An Eulerian PDE approach for computing tissue thickness. IEEE Trans Med Imaging. (2003) 22:1332–9. doi: 10.1109/TMI.2003.817775
22. Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin N Am. (2013) 39:1–19. doi: 10.1016/j.rdc.2012.10.004
23. Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr Cartil. (2005) 13:769–81. doi: 10.1016/j.joca.2005.04.014
24. Shah RF, Martinez AM, Pedoia V, Majumdar S, Vail TP, Bini SA. Variation in the thickness of knee cartilage. The use of a novel machine learning algorithm for cartilage segmentation of magnetic resonance images. J Arthropl. (2019) 34:2210–5. doi: 10.1016/j.arth.2019.07.022
25. Englund M, Guermazi A, Gale D, Hunter DJ, Aliabadi P, Clancy M, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. (2008) 359:1108–15. doi: 10.1056/NEJMoa0800777
26. Felson DT, Niu J, McClennan C, Sack B, Aliabadi P, Hunter DJ, et al. Knee buckling: prevalence, risk factors, and associated limitations in function. Ann Int Med. (2007) 147:534–40. doi: 10.7326/0003-4819-147-8-200710160-00005
27. Hayashi D, Felson DT, Niu J, Hunter DJ, Roemer FW, Aliabadi P, et al. Pre-radiographic osteoarthritic changes are highly prevalent in the medial patella and medial posterior femur in older persons: Framingham OA study. Osteoarthr Cart. (2014) 22:76–83. doi: 10.1016/j.joca.2013.10.007
28. Omoumi P, Babel H, Jolles BM, Favre J. Cartilage can be thicker in advanced osteoarthritic knees: a tridimensional quantitative analysis of cartilage thickness at posterior aspect of femoral condyles. Br J Radiol. (2018) 91:20170729. doi: 10.1259/bjr.20170729
29. Omoumi P, Michoux N, Roemer FW, Thienpont E, Vande Berg BC. Cartilage thickness at the posterior medial femoral condyle is increased in femorotibial knee osteoarthritis: a cross-sectional CT arthrography study (Part 2). Osteoarthr Cart. (2015) 23:224–31. doi: 10.1016/j.joca.2014.08.017
30. Dell'accio F, Vincent TL. Joint surface defects: clinical course and cellular response in spontaneous and experimental lesions. Eur Cells Mat. (2010) 20:210–7. doi: 10.22203/eCM.v020a17
31. Eckstein F, Maschek S, Roemer FW, Duda GN, Sharma L, Wirth W. Cartilage loss in radiographically normal knees depends on radiographic status of the contralateral knee - data from the osteoarthritis initiative. Osteoarthr Cartil. (2019) 27:273–7. doi: 10.1016/j.joca.2018.10.006
32. Wirth W, Maschek S, Roemer FW, Sharma L, Duda GN, Eckstein F. Radiographically normal knees with contralateral joint space narrowing display greater change in cartilage transverse relaxation time than those with normal contralateral knees: a model of early OA? - data from the Osteoarthritis Initiative (OAI). Osteoarthr Cartil. (2019). 27:1663–8. doi: 10.1016/j.joca.2019.06.013
Keywords: knee, cartilage, deep learning, MR, thickness
Citation: Si L, Xuan K, Zhong J, Huo J, Xing Y, Geng J, Hu Y, Zhang H, Wang Q and Yao W (2021) Knee Cartilage Thickness Differs Alongside Ages: A 3-T Magnetic Resonance Research Upon 2,481 Subjects via Deep Learning. Front. Med. 7:600049. doi: 10.3389/fmed.2020.600049
Received: 28 August 2020; Accepted: 30 December 2020;
Published: 09 February 2021.
Edited by:
Peter Mandl, Medical University of Vienna, AustriaReviewed by:
Jose Tamez-Peña, Escuela de Medicina y Ciencias de la Salud Tec Salud, Tecnológico de Monterrey, MexicoCopyright © 2021 Si, Xuan, Zhong, Huo, Xing, Geng, Hu, Zhang, Wang and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiwu Yao, eWFvd2Vpd3VodWFuQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.