
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 23 November 2020
Sec. Dermatology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.598903
This article is part of the Research Topic Progress and Prospects on Skin Imaging Technology, Teledermatology and Artificial Intelligence in Dermatology View all 22 articles
Teledermatology has been proving to be of great help for delivering healthcare, especially now, during the SARS-CoV-2 pandemic. It is crucial to assess how accurate this method can be for evaluating different dermatoses. Such knowledge can contribute to the dermatologists' decision of whether to adhere to teledermatology or not. Our objective was to determine the accuracy of teledermatology in the 10 most frequent skin neoplasms in our population, comparing telediagnosis to histopathological report and in-person dermatologists' diagnosis. A retrospective cohort study was conducted in São Paulo, Brazil, where a store-and-forward teledermatology project was implemented under primary-care attention to triage surgical, more complex, or severe dermatoses. A total of 30,976 patients presenting 55,012 lesions took part in the project. Thirteen teledermatologists who participated in the project had three options to refer the patients: send them directly to biopsy, to the in-person dermatologist, or back to the general physician with the most probable diagnosis and management. In the groups referred to the in-person dermatologist and biopsy, we looked for the 10 most frequent International Statistical Classification of Diseases and Related Health Problems-10 (ICD-10) of skin neoplasms, which resulted in 289 histopathologic reports and 803 in-person dermatologists' diagnosis. We were able to compare the ICD-10 codes filled by teledermatologists, in-person dermatologists, and from histopathological reports. The proportion of complete, partial, and no agreement rates between the in-person dermatologist's, histopathologic report, and the teledermatologist's diagnosis was assessed. We also calculated Cohen's kappa, for complete and complete plus partial agreement. The mean complete agreement rate comparing telediagnosis to histopathological report was 54% (157/289; kappa = 0.087), being the highest for malign lesions; to in-person dermatologists was 61% (487/803; kappa = 0.213), highest for benign lesions. When accuracy of telediagnosis for either malign or benign lesions was evaluated, the agreement rate with histopathology was 70% (kappa = 0.529) and with in-person dermatologist, 81% (kappa = 0.582). This study supports that teledermatology for skin neoplasms has moderate accuracy. This result reassures that it can be a proper option for patient care, especially when the goal is to differentiate benign from malign lesions.
As we face this pandemic time around the world, telemedicine has been proving to be of great help for delivering healthcare. Any medical specialty that is based on image analysis, such as dermatology, is especially suitable for this method of care. Teledermatology has the potential to improve access to subspecialty expertise, reduce healthcare costs, and improve the overall quality of care. The three main teledermatology delivery platforms are: synchronous (RT: real-time teledermatology), asynchronous (SF-TD: store-and-forward teledermatology), and hybrid (both synchronous and asynchronous forms). Synchronous teledermatology uses live video conferencing between the patient and the dermatologist. Asynchronous teledermatology is a method whereby clinical or dermoscopy dermatologic images are obtained and sent to the responding dermatologist who can review them at a later time. Although it provides high-resolution dermatologic images and promotes an efficient practice, this modality is limited by the ability of the teledermatologist to obtain additional clinical history while evaluating the case (1). Teledermoscopy involves the use of dermoscopic images for remote consultation and decision-making in skin cancer screening. Its addition significantly improved the results of an internet-based skin cancer screening system, compared with clinical images alone (2).
The majority of studies in teledermatology have found rates of accuracy to be in the range of 75–80%, comparable to those with in-person care (1). Many of the articles were focused on skin neoplasms, especially skin cancer and pigmented lesions (3–6), or on general dermatology (7–11). A recent systematic review concluded that robust implementation studies of teledermatology are needed, with attention to reducing the risk of bias when assessing diagnostic accuracy (3). For this purpose, we performed research with the primary goal of determining the accuracy of teledermatology for skin neoplasms in a robust number of cases, assessing the agreement rate between the histopathological report, in-person dermatologists', and the teledermatologists' diagnoses. The secondary aim was to analyze the differential diagnosis of the lesions.
This was a retrospective cohort study designed to assess concordance between diagnoses made by in-person dermatologists and teledermatologists, and by histopathological reports and teledermatologists, approved by the Ethics Committee of Hospital Israelita Albert Einstein (CAAE: 97126618.6.0000.0071). We analyzed the reports of 30,976 patients included in a teledermatology triage project conducted in the city of São Paulo, Brazil, from July 2017 to July 2018.
A Teledermatology project implemented from July 2017 to July 2018, in São Paulo. There were 57,832 individuals waiting for a consultation with a dermatologist in the public health system in July 2017. To reduce the waiting list and accelerate the flow of patients with the most severe, complex or surgical dermatoses to in-person dermatologists, the municipal health government requested Hospital Israelita Albert Einstein, a large private hospital in the city with expertise in Telemedicine, to develop a project using Telemedicine to reach these goals. Therefore, an online platform and a mobile app were designed to take photographs with phone cameras and directly upload them to the app and a short clinical history and data of the patient, which were meant to be used by for health technicians or nurses. The photographs and collected information were uploaded to a platform using a secure online process and were accessed only by the 13 Brazilian Board Certified dermatologists associated with this project, for whom the patients were randomly assigned. These 13 dermatologists will be called teledermatologists in this study. All the patients waiting for dermatologist consultations were called consecutively, via phone, by the municipal health care service and scheduled for an appointment in one of the three public city hospitals enabled to carry out the project, depending on the location of the individual's residence. Once there, a short history of the complaint (history of bleeding, pruritus, time from the onset, and location) and demographic data (sex and age) as well as three photographs in different angles and distances from each lesion were taken by a trained health technician: first one at medium distance (50 cm away), second one in close-up (15 cm away) and last one of a lateral view, aiming to evaluate the volume of the lesion. After accessing the patients' pictures and clinical history of the patients, the dermatologists first decided whether the photographs of the lesions were satisfactory for diagnostic purposes. If not, they categorized it under “bad photo,” and the patient was referred to a dermatologist for an in-person appointment. If the photo quality was good enough, they formulated the most probable diagnostic hypothesis. They chose one of three options for each lesion assessed: (1) a direct referral for a biopsy (after which the patient would return for an in-person dermatologist appointment), (2) a referral for an in-person dermatologist (IPD) visit, or (3) a referral to go back to the primary care physician (GP) with the most probable diagnosis, treatment and/or recommendation on how to proceed with the investigation and/or management of the lesion. A schematic for this process is shown in Figure 1. If the same patient had more than one lesion but different referrals, they were referred to the most specialized one and that would be his/her last referral in terms of statistical accounting. For instance, a biopsy would prevail over a dermatologist visit, which would in turn prevail over general physician referral. This teledermatology project followed the American Medical Association telemedicine policy, adapted for the needs of the Brazilian public health care system (12).
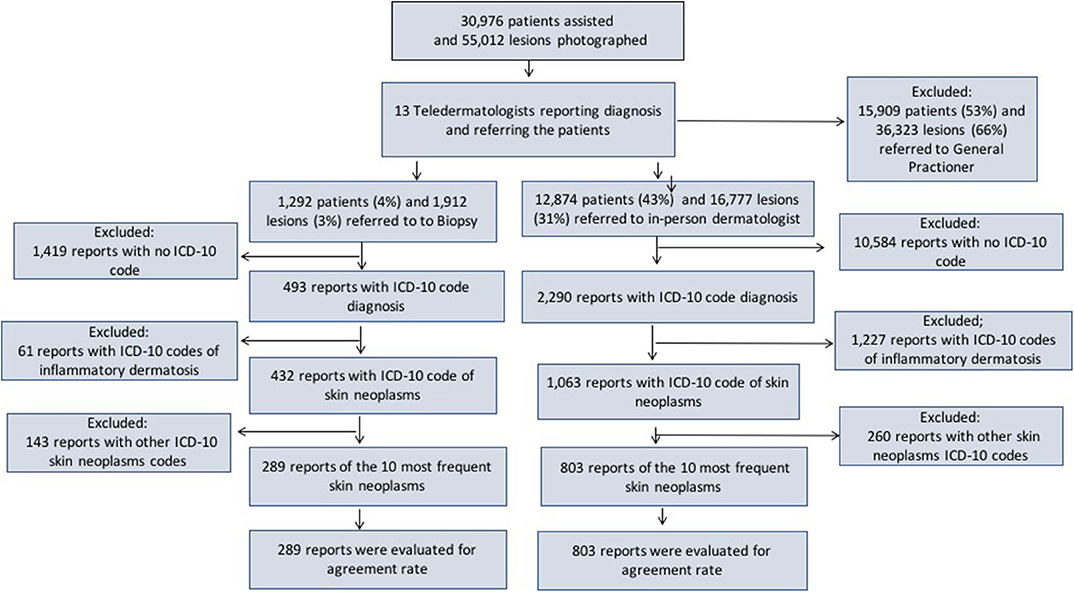
Figure 1. Frequency of patients included, photographed lesions and referrals made by the teledermatologists, along with the flow used to assess the diagnosis accuracy for the 10 most frequent skin neoplasms.
We selected the groups referred to IPD (12,874 patients/16,777 lesions) and biopsy (1,292 patients/1,912 lesions). Then, we assessed the reports that had the International Statistical Classification of Diseases and Related Health Problems 10th version (ICD-10 code) (13) diagnoses filled by IPD (2,290) and histopathological reports (HP: 493). Next, we separated the reports filled with ICD-10 codes of skin neoplasms sent to IPD (1,063) and from histopathological reports (432). Afterward, we looked for the 10 most frequent dermatoses in each group, 803 in IPD and 289 to include in our study (803 for IPD and 289 in HP Figure 1).
We classified the rate of agreement as follows: (1) complete agreement when the ICD-10 code used in both reports were the same, (2) partial agreement when the ICD-10 code used in both reports were different, but in the same group of disease (malign or benign neoplasms), posing as a probable differential diagnosis, and (3) no agreement when both reports did not fill the previous two conditions. Basal cell carcinoma (BCC), squamous cell carcinoma (SCC), melanoma, and actinic keratosis (AK) were considered the malign group. Nevus, seborrheic keratosis (SK), acrochrodon/other benign neoplasms, cyst, lipoma, and wart were the benign group. If the cases did not belong to one of those 10 classes, it was classified as “other.” As the gold standard diagnosis is the histopathological diagnosis, the agreement was stated in this research as accurate.
Rates of concordance were expressed using percentages and Cohen's kappa coefficient, which was used to compare between groups of inter-rater observers (Graph Pad Prism 6.0). The guidelines used to characterize kappa values were created by Landis and Koch (14), as follows: kappa <0: no agreement, 0.00–0.20: slight agreement, 0.21–0.40: fair agreement, 0.41–0.60: moderate agreement, 0.61–0.8: substantial agreement, and 0.81–1.00: almost perfect agreement.
Table 1 shows the 10 most frequent skin neoplasms diagnosed by teledermatology according to the frequency of lesions, sex, age, and referral distribution. The total amount represents 31% (17,233/55,012) of the lesions diagnosed in the entire teledermatology project. The female and male proportion was 72 and 28%, respectively, although the female population accounts for 52.6% in the city of São Paulo (2010) (15). Lesions diagnosed as benign were 90% (15,496/17,233) and as malign, 10% (1,727/17,233). Among benign neoplasms, 63% (9,849/15,496) were sent to GP for follow-up, 34% (5,242/15,496) were sent to IPD and only 3% (405/15,496) were sent to biopsy. For malign lesions, 3% (4/1,727) was referred to GP, 51% (880/1,727) to IPD and 46% (803/1,727) to biopsy.
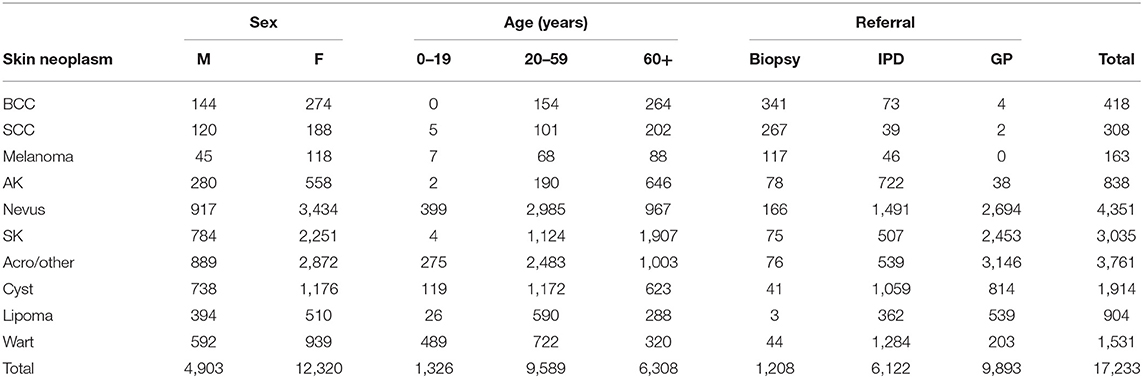
Table 1. Patient's demographics and referrals for the 10 most frequent skin neoplasms diagnosed in the Teledermatology Project in São Paulo from July 2017–2018.
Figures 2, 3 demonstrate the comparison between teledermatologists' diagnosis to the histopathological reports and to in-person dermatologists, respectively.
For instance, in Figure 2, among the 65 histopathological reports of BCC in this study, the teledermatologists had diagnosed 49 correctly (complete agreement in yellow), 6 as SCC, 2 as melanoma, 3 as AK (all considered partial agreement, in green), 2 as nevus, 2 as SK, and 1 as other (all considered no agreement, in red).
Following the same path, in Figure 3, among 40 telediagnosis of BCC, 26 were in agreement with IPD (complete agreement, in yellow), 5 were SCC, 3 were AK (partial agreement, in green) and 1 nevus, 2 SK, 1 acro/other, 1 wart, and 1 other (no agreement, in red).
Table 2 reveals the percentage of accuracy for complete, partial, and no agreement and Cohen's kappa calculations for complete and complete plus partial agreement, comparing the telediagnosis to histopathological reports (upper part) and in-person dermatologists' diagnosis (bottom part). In the upper part, the mean frequency of complete agreement was 54% for all 10 dermatoses tested (157/289) and its kappa coefficient was 0.087), which is considered a slight agreement. Three malign skin tumors had the highest rates of accuracy: melanoma (7/8), BCC (49/65), and SCC (22/31), respectively with 88, 75, and 71% and kappas = 0.060, 0.326, and 0,117. Among benign neoplasms, cyst, and nevus were the most accurate diagnoses, respectively with 64 and 60% and kappas = 0.335 and 0.298 (fair agreement). Warts, SK and lipomas showed an intermediate rate, around 50%, and acrochordon and other benign neoplasms had the lowest rate (17%; kappa = −0.078). AK had only 30% of agreement, being the least accurate in the malign group (kappa = −0.146). Partial agreement was verified in 22% of all cases (64/289), ranging from 5% in nevus up to 60% in AK. No agreement was found in 24% (68/289); CBC, SCC, and lipoma had zero no agreements while nevus, SK, warts, and cysts had around 30% of no agreement.

Table 2. Agreement between teledermatologists' diagnosis and histopathological reports (upper part) and in-person dermatologists (bottom part).
Comparing teledermatologists' to in-person dermatologists' diagnosis accuracy, we found a mean frequency of complete agreement in 61% of all 10 skin neoplasms (487/803) and its kappa coefficient was 0.213, which is considered a fair agreement. Benign skin tumors had the highest rates of accuracy: lipoma (7/8), warts (83/116), and nevus (160/230), with 87.5, 72, and 70% and kappas = 0.369, 0.490, and 0.446, respectively. Among malign neoplasms, all four of them were between 56% (AK) and 65% (CBC). The lowest rate was achieved by SK (41%; kappa = 0.068) and acro/other benign (44%; kappa = −0.117). Partial agreement was verified in 20% of all cases (159/803), ranging from 7% in melanoma up to 34% in SK. No agreement was found in 19% (156/803); lipoma had zero no agreements while melanoma, AK, and acro/other benign had around 36, 35, and 29%, respectively.
Analyzing Table 1, we can see that most of the benign cases of skin neoplasms in this cohort were not sent to biopsy and in-person dermatologists; they were sent back to the GP to follow-up and, therefore, could not be compared with the teledermatologists' diagnosis. This fact is very relevant when discussing accuracy in this study. As the teledermatology triage project prioritized the severe, more complex, or surgical cases for biopsy and in-person dermatologists, trying to manage the mild cases under the primary-care attention along with the GP, skin neoplasms diagnosed by histopathological analysis or in-person dermatologists have a bias of being the most challenging/difficult cases. Typical or “regular” skin neoplasms were most probably diagnosed and referred back to the GP. In this manner, our results in the rate of agreement would be probably much higher if more typical cases were analyzed.
In terms of health delivery, choosing the proper management of the skin neoplasms is even more important than elaborating the right diagnosis itself. In this way, what is more important than complete agreement (ICD-10 code) is to analyze rates of complete plus partial agreement (benign vs. malign). If the diagnosis fits within the benign neoplasms, an expectant conduct is acceptable, while within the malign neoplasms, a biopsy, or surgery ought to be expected.
Analyzing teledermatogists' diagnosis and histopathological reports' agreement, we showed a mean agreement rate of 70% (54% total + 16% partial agreement rates) and kappa = 0.529 (moderate). Considering studies that compared TD diagnosis and histopathological reports, Silveira et al. (16) included 364 suspected cases of skin malignancy by TD to be confirmed by the biopsy. The majority of the lesions were BCCs with 286 cases, followed by 59 SCCs and 5 melanomas. Two oncologists showed an overall accuracy of 85.3% for the first and 87.3% for the other in categorizing benign x malign lesions. Our results for malign and benign lesions showed 94% (126/134) and 61% (95/155) of agreement, respectively. In another study, 144 pigmented lesions diagnosed via TD were examined histologically, and 63 (43%) showed complete agreement between the clinical and histological diagnoses (17). Comparatively, our study showed 60 and 88% complete agreement for nevus and melanoma respectively. In another article, 201 biopsies were requested to rule out malignancy via TD, which was confirmed in 45.3% (18). A survey among the teledermatologists participating in the project, after its conclusion, showed that the use of dermoscopy would significantly improve the decision to refer nevus and SK to in-person dermatologists, GP or biopsy and, most probably, the agreement rate too. They felt that many cases referred to biopsy or IPD could have been referred to GP if dermoscopic images were available (19). Melanoma had a fair agreement rate. We believe that happened because, when in doubt, one would rather excise it and send it to histopathological than wait to see how it develops. In this project, with no access to dermoscopic images, there were 112 lesions suspected of melanoma and 8 melanomas confirmed (14 benign lesions:1 melanoma). Other studies show that the number of nevus needed to diagnose one melanoma can vary from 4.5 to 22 (20). Acrochordon/other benign neoplasms had slight agreement rate kappa, probably because of the reasons explained before. Only “weird looking” acrochordons would be sent to biopsy, confirming that they were, in fact, not acrochordons.
The results of our study showed a slightly better agreement rate between diagnoses made by teledermatologists and in-person dermatologists in general. If we summed up complete (61%) and partial (20%) agreement rates, we have reached 81% (kappa = 0.582-moderate), but there was an inversion in the accuracy for benign and malign lesions. The agreement rate for benign lesions was 82% and for malign, 73%. Nevus and acrochordons/other benign neoplasms in this referral were probably more typical than the ones sent to biopsy, and that could explain why the agreement rate for benign skin neoplasms had an improvement compared to the previous one. On the other hand, BCC, SCC, and AK showed lower agreement rate to IPD than histopathological reports. The worst agreement rate was for melanoma. Cohen's kappa coefficient showed no agreement (−0.050). This may reflect the same reasons stated before, the absence of dermoscopy images and also the teledermatologists' fear of letting a melanoma diagnosis go undetected. When compared to the previous studies, Kroemer et al. (21) included 104 pigmented lesions and found a kappa = 0.84. Moreno-Ramirez et al. (22) showed a kappa = 0.81 in 890 cases of skin cancer triage.
According to the latest Cochrane review (2018) about teledermatology in skin cancers, using a more widely defined threshold to identify “possibly” malignant cases or lesions that should be considered for excision is likely to appropriately triage those lesions requeiring face-to-face assessment by a specialist. Despite the increasing use of teledermatology on an international level, the evidence base to support its ability to accurately diagnose lesions and to triage lesions from primary to secondary care is lacking and further prospective and pragmatic evaluation is needed (5).
Although this was a retrospective study and much data was missing, we believe this was one of the studies with the largest number of skin neoplasms included in the literature, comparing teledermatologists' diagnosis both to IPD and histopathological reports. Pathologists were responsible for histopathological reports. The study was performed in two centers and different dermatologists performed the tele and in-person examinations. This is a limitation because there is interoperator variability and the technical skills in the groups may be different. Moreover, teledermatologists could diagnose the lesions differently if they were face-to-face. The profile of 12 out of the 13 teledermatologists involved in the project was shown in a previously study (19). Ten had either subspecialty in dermoscopy or skin cancer, which we thought was very suitable for this study. Six had more than 10 years as Board Certified Dermatologists. Five of them integrated the Skin Oncology outpatient unity at University of São Paulo Dermatology Department. Unfortunately, we do not have the profile of the IPD. The risk of false negative cases, which we could not assess, exists and it is another limitation of this research. Nonetheless, during the project, there was always a physician, either a GP or an IPD, responsible for monitoring the patients.
Our study, performed in a large number of patients presenting the 10 most common skin neoplasms, showed moderate accuracy between teledermatology and histopathological reports, and moderate agreement rate between teledermatologists and in-person dermatologists' diagnosis. Accuracy may differ among skin neoplasms. This reassures that store-and-forward teledermatology can be an option for triage skin neoplasms in primary-care attention.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Hospital Israelita Albert Einstein (CAAE: 97126618.6.0000.0071). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
MG-B and EC: study design. MG-B, RS, MA, and EC: data collection and review of the article. MG-B and MA: data analysis. MG-B and RS: writing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
TD, teledermatology; RT, real time; SF-TD, store and forward; IPD, in-person dermatologist; GP, general physician; ICD-10 code, international code of diseases 10; BCC, basal cell carcinoma; SCC, squamous cell carcinoma; AK, actinic keratosis; SK, seborrheic keratosis; Acro/other benign, acrochordon/other benign neoplasms.
1. Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. (2018) 19:253–60. doi: 10.1007/s40257-017-0317-6
2. Ferrándiz L, Ojeda-Vila T, Corrales A, Martín-Gutiérrez FJ, Ruíz-de-Casas A, Galdeano R, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. (2017) 76:676–82. doi: 10.1016/j.jaad.2016.10.041
3. Finnane A, Dallest K, Janda M, Soyer HP. Teledermatology for the diagnosis and management of skin cancer: a systematic review. JAMA Dermatol. (2017) 153:319–27. doi: 10.1001/jamadermatol.2016.4361
4. Warshaw EM, Lederle FA, Grill JP, Gravely AA, Bangerter AK, Frontier LA, et al. Accuracy of teledermatology for pigmented neoplasms. J Am Acad Dermatol. (2009) 61:753–65. doi: 10.1016/j.jaad.2009.04.032
5. Chuchu N, Dinnes J, Takwoingi Y, Martin RN, Bayliss SE, Davenport C, et al. Teledermatology for diagnosing skin cancer in adults. Cochrane Database Syst Rev. (2018) 12:CD013193. doi: 10.1002/14651858.CD013193
6. Markun S, Scherz N, Rosemann T, Tandjung R, Braun RP. Mobile teledermatology for skin cancer screening: a diagnostic accuracy study. Medicine (Baltimore). (2017) 96:e6278. doi: 10.1097/MD.0000000000006278
7. Warshaw EM, Hillman YJ, Greer NL, Hagel EM, MacDonald R, Rutks IR, et al. Teledermatology for diagnosis and management of skin conditions: a systematic review. J Am Acad Dermatol. (2011) 64:759–72. doi: 10.1016/j.jaad.2010.08.026
8. Lasierra N, Alesanco A, Gilaberte Y, Magallón R, García J. Lessons learned after a three-year store and forward teledermatology experience using internet: strengths and limitations. Int J Med Inform. (2012) 81:332–43. doi: 10.1016/j.ijmedinf.2012.02.008
9. Lim AC, Egerton IB, See A, Shumack SP. Accuracy and reliability of store-and-forward teledermatology: preliminary results from the St. George Teledermatology Project. Australas J Dermatol. (2001) 42:247–51. doi: 10.1046/j.1440-0960.2001.00529.x
10. Weingast J, Scheibböck C, Wurm EM, Ranharter E, Porkert S, Dreiseitl S, et al. A prospective study of mobile phones for dermatology in a clinical setting. J Telemed Telecare. (2013) 19:213–8. doi: 10.1177/1357633x13490890
11. O'Connor DM, Jew OS, Perman MJ, Castelo-Soccio LA, Winston FK, McMahon PJ. Diagnostic accuracy of pediatric teledermatology using parent-submitted photographs: a randomized clinical trial. JAMA Dermatol. (2017) 153:1243–8. doi: 10.1001/jamadermatol.2017.4280
12. American Medical Association. AMA Telemedicine Policy. (2016). Available online at: www.ama-assn.org (accessed october 10, 2020).
13. DATASUS. CID-10. (2008). Available online at: http://www.datasus.gov.br/cid10/v2008/webhelp/cid10.htm (accessed May 11, 2017).
14. Landis J, Koch G. The measurement of observer agreement for categorical categorical data. Biometrics. (1977) 33:159–74. doi: 10.2307/2529310
15. IBGE. Censo 2010, São Paulo: IBGE. (2013). Available online at: https://cidades.ibge.gov.br/brasil/sp/sao-paulo/pesquisa/23/27652 (accessed June 12, 2020).
16. Silveira CE, Silva TB, Fregnani JH, da Costa Vieria RA, Haikel RL Jr, Syrjanen K, et al. Digital photography in skin cancer screening by mobile units in remote areas of Brazil. BMC Dermatol. (2014) 14:19. doi: 10.1186/s12895-014-0019-1
17. Jolliffe VM, Harris DW, Whittaker SJ. Can we safely diagnose pigmented lesions from stored video images? A diagnostic comparison between clinical examination and stored video images of pigmented lesions removed for histology. Clin Exp Dermatol. (2001) 26:84–7. doi: 10.1046/j.1365-2230.2001.00767.x
18. Tandjung R, Badertscher N, Kleiner N, Wensing M, Rosemann T, Braun RP, et al. Feasibility and diagnostic accuracy of teledermatology in Swiss primary care: process analysis of a randomized controlled trial. J Eval Clin Pract. (2015) 21:326–31. doi: 10.1111/jep.12323
19. Giavina Bianchi M, Santos A, Cordioli E. Dermatologists' perceptions on the utility and limitations of teledermatology after examining 55,000 lesions. J Telemed Telecare. (2019):1357633X19864829. doi: 10.1177/1357633X19864829
20. Elliott TM, Whiteman DC, Olsen CM, Gordon LG. Estimated healthcare costs of melanoma in Australia over 3 years post-diagnosis. Appl Health Econ Health Policy. (2017) 15:805–16. doi: 10.1007/s40258-017-0341-y
21. Kroemer S, Frühauf J, Campbell TM, Massone C, Schwantzer G, Soyer HP, et al. Mobile teledermatology for skin tumour screening: diagnostic accuracy of clinical and dermoscopic image tele-evaluation using cellular phones. Br J Dermatol. (2011) 164:973–9. doi: 10.1111/j.1365-2133.2011.10208.x
Keywords: teledermatology, telemedicine, accuracy: skin neoplasms, skin cancer, benign skin lesions, malign skin lesions, tele-health
Citation: Giavina-Bianchi M, Azevedo MFD, Sousa RM and Cordioli E (2020) Part II: Accuracy of Teledermatology in Skin Neoplasms. Front. Med. 7:598903. doi: 10.3389/fmed.2020.598903
Received: 25 August 2020; Accepted: 28 October 2020;
Published: 23 November 2020.
Edited by:
Paola Pasquali, Pius Hospital de Valls, SpainReviewed by:
Amanda Oakley, Waikato District Health Board, New ZealandCopyright © 2020 Giavina-Bianchi, Azevedo, Sousa and Cordioli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mara Giavina-Bianchi, bWFyYWhnYmlhbmNoaUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.