- 1Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2School of Public Health, Fujian Medical University, Fuzhou, China
- 3Department of Neurology, The Second Affiliated Hospital, Shandong Academy of Medical Sciences, Shandong First Medical University, Taian, China
- 4The Institute of Neuroscience, Soochow University, Suzhou, China
Background and Purpose: Neutrophil to lymphocyte ratio (NLR) is positively associated with poor prognosis in patients with cerebral infarction. The goal of this prospective study is to explore the predictive value of NLR in patients with acute ischemic stroke (AIS) caused by cervicocranial arterial dissection (CCAD).
Methods: Ninety-nine patients with AIS caused by CCAD met criteria for inclusion and exclusion were selected for this study. We collected baseline data on the admission including NLR. The primary poor outcome was major disability (modified Rankin Scale score ≥ 3) or death at 3 months after AIS.
Results: A total of 20 (20.2%) patients had a poor outcome at 3 months after AIS. According to the 3-month outcome, the patients were divided into two groups and univariate and multivariable analyses were conducted. Among the risk factors, elevated NLR levels were independently associated with 3-month poor outcomes. Further, we made the ROC curve to evaluate the predictive value of NLR level on prognosis. The area under the curve was 0.79 and a cut-off value of NLR was 2.97 for differentiating the poor outcome. We divided patients into groups according to the cut-off value. Patients with high NLR have a higher risk of poor outcome than those with low NLR (P < 0.05).
Conclusion: As an inflammatory marker, elevated NLR levels were associated with 3-month poor outcome in AIS caused by CCAD.
Introduction
Arterial dissection is the angiopathy that blood flow enters the artery wall and causes vascular wall tissue dissect. Arterial dissection leads to various pathological changes, such as the stenosis or occlusion of the lumen and dissecting aneurysm of the artery (1). With the development of imaging techniques and clinical knowledge, incidence of cervicocranial arterial dissection (CCAD) is increasing. It has become a common cause of stroke in young patients, in whom it accounts for 10–25% (2). The etiology of CCAD is still unclear. Trauma and connective tissue diseases can only explain partial dissection (3). Inflammation is known to be involved in the development of a variety of vascular diseases (4–7). Recently, infections have been reported to be associated with the occurrence and pathogenesis of CCAD (8). In previous studies on AIS, inflammation and immune response are also important parts of pathophysiology of stroke (9–11). Therefore, we speculated inflammatory response is important in AIS by CCAD.
The neutrophil to lymphocyte ratio (NLR), as a simple parameter of innate (neutrophil), and adaptive (lymphocyte) immune response, is easy to obtain from peripheral blood (12). Recent studies show NLR can predict the prognosis and mortality of patients with aortic dissection or cerebral infarction (5, 13, 14). Qun et al. (15) demonstrated that high NLR was highly correlated with the 3-month poor outcome of AIS. Clinical values of NLR in prognoses of patients with AIS caused by CCAD have not been fully explored. Therefore, we conducted a comprehensive analysis of those patients to explore the prognostic value of NLR on the 3-month outcome.
Materials and Methods
Study Population
Patients with AIS by CCAD in the First Affiliated Hospital of Soochow University from April 2014 to October 2019 were selected as the research subjects. Data were collected from electronic patient records and administrative databases used for quality improvement.
CCAD was initially diagnosed by computed tomography angiography (CTA), and was further confirmed by digital subtraction angiography (DSA) or high-resolution magnetic resonance imaging (HR-MRI); in addition, thickened vascular intima and atherosclerotic plaque formation were excluded (16). All cases of AIS were confirmed by MRI. The diagnosis of CCAD was made according the previous description (17). The diagnostic criteria included the following factors: clinical symptoms such as neck pain, edema, and signs of Horner's syndrome; the disruption of normal arterial wall on CTA or DSA imaging including stenosis, intimal flap, false lumen, mural thrombus, and pseudoaneurysm; the exclusion of vessel hypoplasia, pseudodissection, and the signs of atherosclerosis such as vessel calcification (17). We confirmed the relationship between CCAD and AIS that the dissected artery was the only responsible vessel and the only cause of AIS by HR-MRI or CTA imaging. In addition, cardiogenic stroke was excluded in all patients by cardiac examination.
Exclusion criteria were as follows: (1) Patients with AIS admitted more than 48 h; (2) Patients had a history of infection within 2 weeks before admission that was defined as fever (T ≥ 38°C) and at least one other typical symptoms (cough, rhinitis, hoarseness, sneezing, or vomiting); (3) Patients had a history of cancer, chronic inflammation, hematological diseases, autoimmune diseases, or treatment with immunosuppressive agents; (4) Patients had a stroke history within 6 months or the modified Rankin scale (mRS) > 0 before the onset; (5) Patients did not complete a blood count within 24 h of admission; (6) There was no evidence of AIS at this admission; (7) patients with iatrogenic and traumatic dissections. The diagnosis of CCAD was made by two senior imaging doctors.
Clinical Information Collection
We collected baseline data including gender, age, history of trauma, history of head and neck pain, cerebral vascular risk factors such as hypertension, and diabetes. Peripheral venous blood samples were collected on the morning of the second day after admission with an overnight fasting.
Evaluation of 3-Month Outcome
Modified Rankin Scale (mRS) was used to evaluate the 3-month outcome after the onset of AIS. The primary outcome was death or major disability at 3 months after AIS. other outcomes were stroke recurrence and hemodynamics of the diseased vessels. The poor outcome was defined as the mRS ≥ 3.
Statistical Analysis
Continuous variables were analyzed as mean and standard deviation or the median and interquartile range while categorical variables were analyzed as frequency and percentage, properly. The differences among continuous variables were analyzed by the Student's t-test or the Mann-Whitney U-test while differences among categorical variables were assessed by the Chi-square test. Logistic regression analysis was used to find risk factors associated with poor prognosis in patients with AIS caused by CCAD after adjusting for other variables selected from univariate analyses. Receiver operating curves (ROC) were used to evaluate the predictive value of NLR level and to establish optimal cut-off values of NLR correlated with poor outcome. Statistical analysis was performed in SPSS 25.0. A value of P < 0.05 was considered statistically significant.
Results
Study Population and Baseline Characteristics
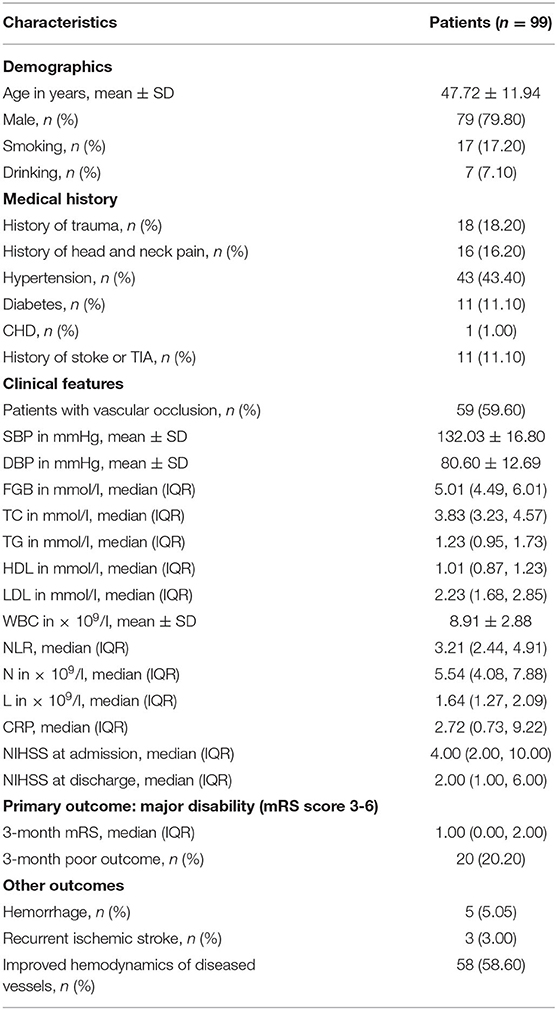
A total of 168 patients with CCAD were admitted between April 2014 and October 2019 in the First Affiliated Hospital of Soochow University (Suzhou city, China). The CCAD mainly showed dual-chamber sign, line-like sign, endometrial flap sign, bead sign, or rat tail sign in CTA or DSA examination. HR-MRI revealed signs of hematoma, aneurysm-like dilatation, or double cavity with true cavity stenosis in the dissection. Among these patients, 69 patients were excluded according to the exclusion criteria, and 99 patients met the study criteria. Patient baseline characteristics were shown in Table 1. The average age of all patients was 47.72 ± 11.94; 79 (79.8%) were male and the ratio of male to female was about 4:1. 17 (17.2%) patients had a smoking history; 43 (43.4%) had a hypertension history; 11 (11.1%) had type 2 diabetes; 11 (11.1%) had a stroke history or TIA; and 18 (18.2%) had a history of trauma. White blood cell (WBC) was 8.91 ± 2.88 × 109 /L; neutrophil count was 5.54 (4.08, 7.88) × 109/L, and lymphocyte count was 1.64 (1.27, 2.09) × 109 /L; NLR was 3.21 (2.44, 4.91). All of our patients received anticoagulant or antiplatelet therapy and five patients (5.05%) stopped medication after intracranial/gastrointestinal hemorrhage during treatment. Among them, 20 (20.2%) patients had a poor outcome at 3 months after AIS; 3 (3%) patients had recurrent ischemic stroke, and 58 (58.6%) patients of ultrasound showed improved hemodynamics of diseased vessels.
Risk Factors Associated With Poor Outcome in Patients With AIS Caused CCAD
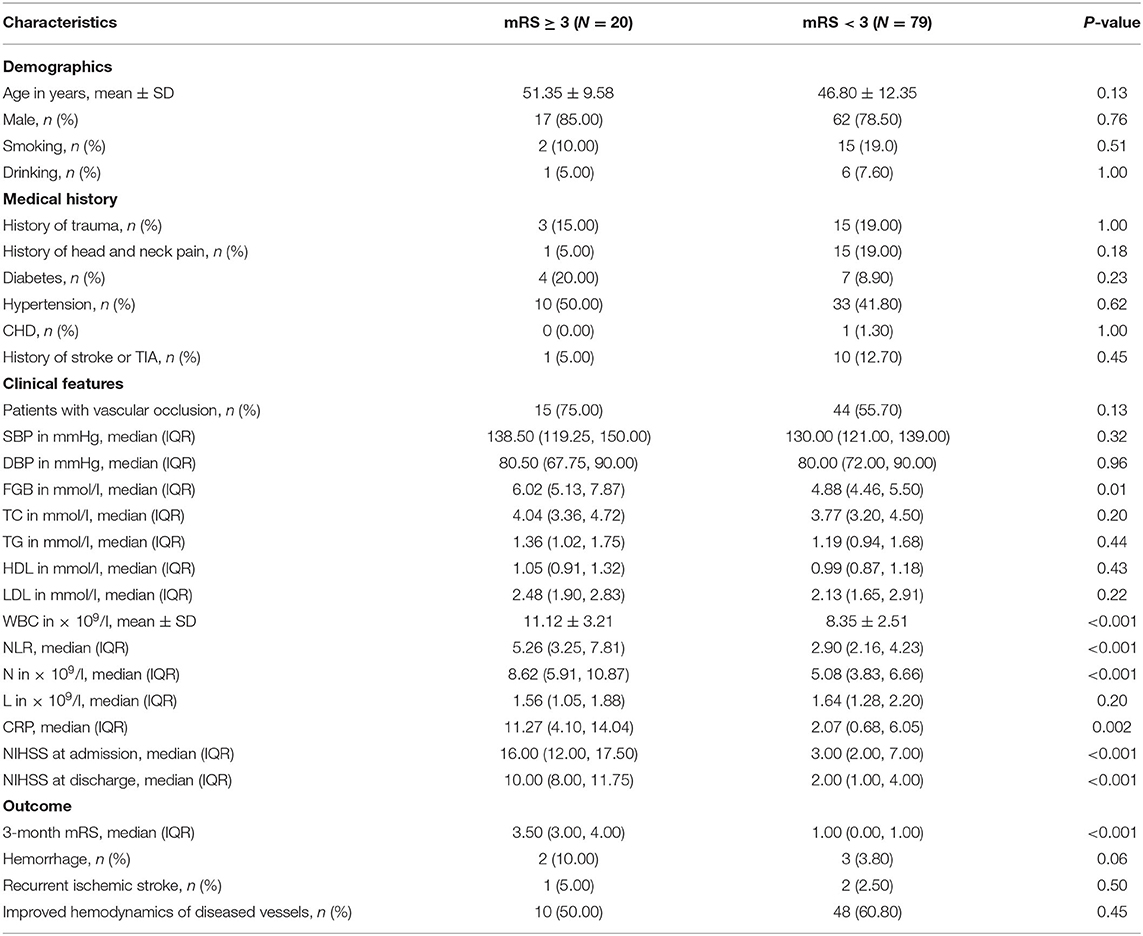
According to the outcome after a 3-month follow-up, the patients were divided into two groups: the good outcome group with 79 patients (mRS < 3) and the poor outcome group with 20 patients (mRS ≥ 3). Statistical analysis indicated that there were significant differences on FGB, WBC, NLR, neutrophil count, C-reactive protein, NIHSS at admission and NIHSS score at discharge, 3-month mRS between two groups (P < 0.05); However, there was no difference on age, gender, history of trauma, hypertension, diabetes, CHD, smoking, or drinking, TC, TG, HDL, LDL, neutrophil count, recurrent ischemic stroke, improved hemodynamics of diseased vessels, hemorrhage, and other factors between two groups (P > 0.05, Table 2).
Binary logistic regression analysis was used to determine factors that were significantly associated with poor outcome at 3 months after AIS. After the factors that might potentially affect the outcome were adjusted, our results indicated that NLR (adjusted OR, 2.457; 95%CI, 1.096–5.508; P = 0.03), TG (adjusted OR, 10.015; 95%CI, 1.143–87.736; P = 0.04), WBC (adjusted OR, 1.794; 95%CI, 1.056–3.049; P = 0.03), age (adjusted OR, 1.258; 95%CI, 1.015–1.559; P = 0.04), ANC (adjusted OR, 2.919; 95%CI, 1.198–7.111; P = 0.02), and NIHSS (adjusted OR, 1.767; 95%CI, 1.234–2.529; P = 0.002) at admission were associated with 3-month poor outcome in the study. However, history of trauma, history of head and neck pain, smoking, type 2 diabetes, SBP, FGB, LDL, history of stroke or TIA and ALC showed no association with poor outcome (P > 0.05, Table 3).

Table 3. Binary logistic regression analysis predicting the poor outcome in patients with AIS caused by CCAD.
NLR Was Associated With 3-Month Poor Outcome
Since NLR is a simple and convenient biomarker to obtain and was validated in patients with AIS, we examined whether NLR was a more specific biomarker for 3-month outcome in patients with AIS caused by CCAD. According to the NLR value, the study population was divided into three tertiles, each containing 33 people. In the first tertile (NLR 2.05, 1.24–2.86), the poor outcome rate was 3%; in the second tertile (NLR 3.21, 2.42–4.00), the poor outcome rate was 15.2%; in the third tertile (NLR 6.15, 2.96–9.34), the poor outcome rate was 42.4% (Figure 1). We used the Jonckheere-Terpstra test to evaluate the relationship of the poor outcome rate and NLR and found that the difference on the poor outcome rate between each tertile was statistically significant (P < 0.001), indicating that high NLR level was associated with 3-month poor outcome.
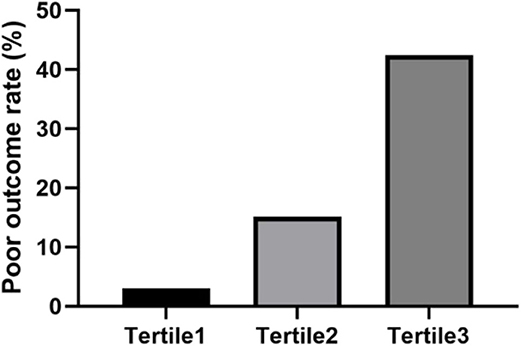
Figure 1. The percentage of patients with poor outcome was stratified by the tertile of NLR. Patients were divided into three groups according to the tertiles of NLR (NLR ≤ 2.59, 2.59 < NLR ≤ 4.23, NLR > 4.23). The poor outcome rate was calculated. The difference between each tertile was assessed by Jonckheere-Terpstra test (P < 0.001).
Further, we made the ROC curve to evaluate the predictive value of NLR level on prognosis. An NLR value of 2.97 was calculated as an optimal cut-off value to discriminate between good and poor outcome of patients with AIS caused by CCAD. The area under the curve was 0.79 (95%CI, 0.69–0.89). An NLR value of 2.97 as a cut-off value for differentiating the poor outcome with a sensitivity of 95% and a specificity of 53% (Figure 2).
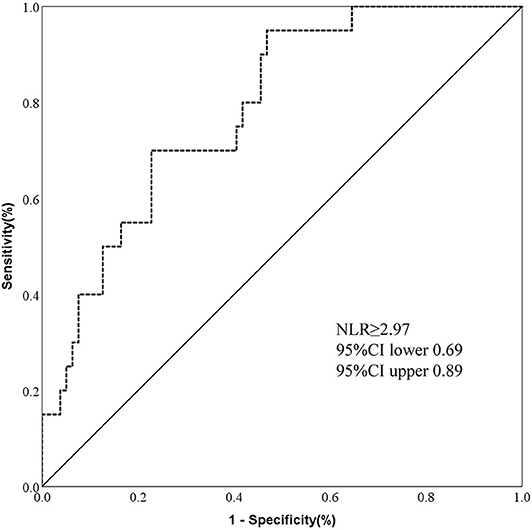
Figure 2. ROC showed predictive value of NLR for 3-month poor outcome in AIS by CCAD. [n = 99; sensitivity = 0.95; specificity = 0.53; NLR = 2.97; area under curve (AUC) = 0.79].
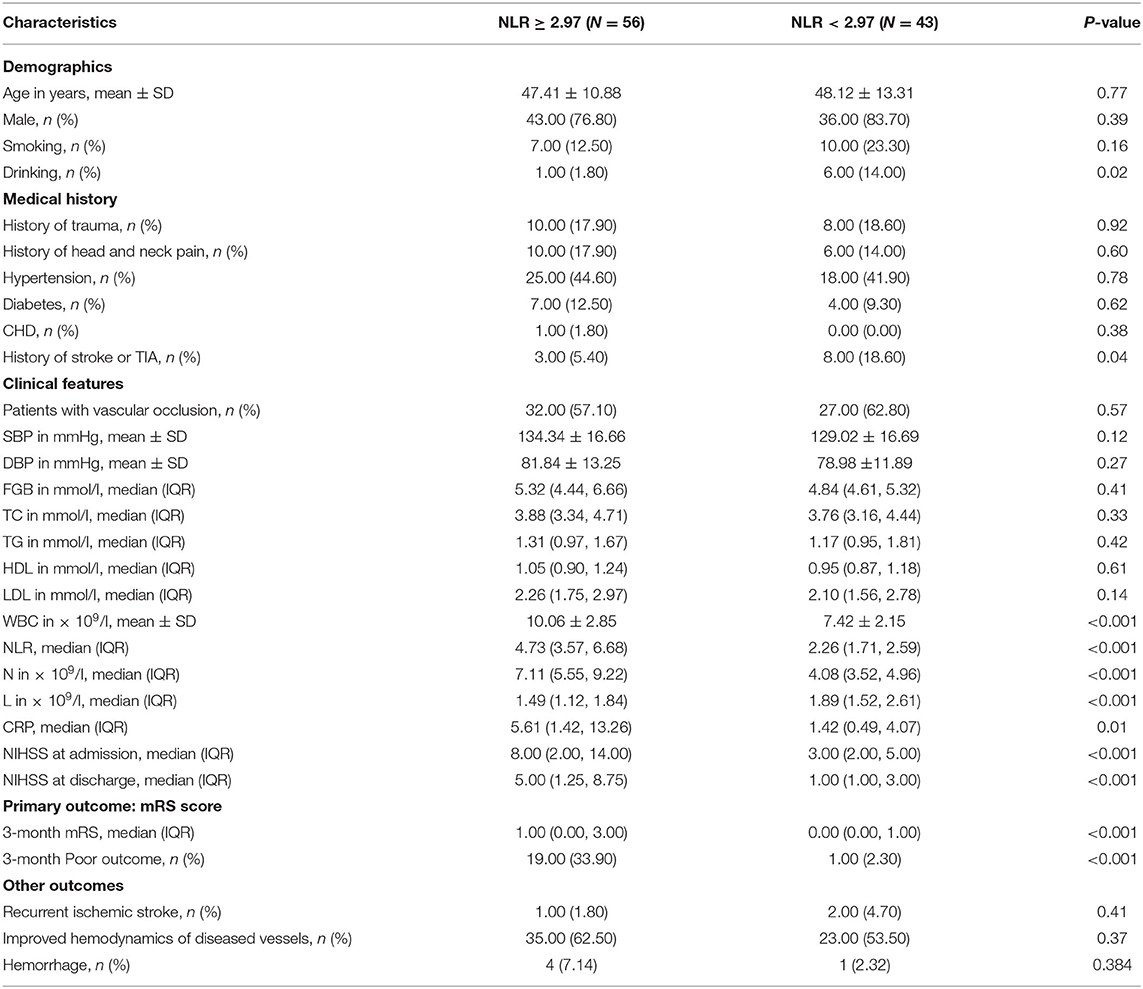
Patients With High NLR Have an Increased Risk of Poor Outcome
According to the cut-off point, the patients were divided into a high NLR group with 56 patients (≥3) and a low NLR group with 43 patients (<3). We found statistically significant differences on drinking, history of stroke or TIA, WBC, neutrophil count, lymphocyte count, C-reactive protein levels, NIHSS at admission, NIHSS score at discharge, and 3-month mRS between the two groups (P < 0.05). However, there was no statistically significant difference in other factors between the two groups (P > 0.05, Table 4). In the high NLR group, 19 patients (33.9%) had 3-month poor outcome, whereas in the low NLR group, 1 patient (2.3%) had 3-month poor outcome (Table 4). Compared with patients in the low NLR group, patients in the high NLR group had higher poor outcome rate (P < 0.01).
Discussion
NLR is a composite marker of absolute peripheral neutrophil and lymphocyte counts and reflects the burden of inflammation (12). Previous studies showed that inflammation played an important role in the pathogenesis of arterial dissection and stroke (7, 18–20). In this study, we showed that high NLR was an independent predictor of 3-month poor outcome for AIS caused by CCAD. It was a convenient and simple indicator of inflammatory response after CCAD-induced AIS. To our knowledge, this study was the first time to analyze the relationship between NLR and 3-month outcome in CCAD-induced AIS patients.
Previous study showed that inflammation played an important role in the initiation and progression of ischemic cerebrovascular diseases (20). Therefore, NLR, the marker of inflammation, may also reflect the progress and prognosis of ischemic cerebrovascular diseases. Oz et al. (7) found that NLR at the time of admission was a predictor of the short-term outcome of patients with Stanford type aortic dissection. Kocaturk et al. (21) and Qun et al. (15) also confirmed that NLR can predict the 3-month prognosis of AIS. Considering that AIS caused by CCAD may have different inflammatory changes from AIS, we expanded the previous study by screening AIS patients with CCAD. We recruited 99 patients with AIS by CCAD as the research objects; however, we still found that NLR is a good predictor of poor outcome at 3 months. Interestingly, 79.8% of patients were male, showing a strong gender predisposition. In addition, for our total CCAD patients (n = 168 with or without AIS), male patients accounted for about 66.0%. In previous CCAD population-based studies, it seemed a slight gender predisposition favoring males (53–57%) (22). This may be due to the selection bias caused by small sample size in our study. However, male gender predisposition may be further examined in CCAD patients in future studies.
The increase in NLR indicates the suppression of lymphocytes or the excessive activation of neutrophils (12). After the occurrence of acute aortic dissection and AIS, systemic immune suppression may occur due to the brain's immune response (23–25), especially for T cells and natural killer cells in lymphocytes (26). The function of lymphocyte in ischemic brain injury and ischemic vascular endothelial is still controversial at present, but certain specific subtypes have been shown to play a protective role in the pathophysiology of cerebral ischemia (24, 26). Some studies suggested that lymphopenia was an early feature of stroke, which is a sign of persistent brain damage, stress response, and the greater possibility of infection (26). Acute stroke can trigger the reduction of regulatory T cells that suppress the inflammatory response to increase tissue damage (23, 24, 26, 27). We didn't find a correlation between low lymphocyte and 3-month poor outcome.
Considerable studies have demonstrated the damaging effects of neutrophils on vascular endothelial cells in arterial dissection and ischemic brain tissues (20, 28–32). Due to the similar pathogenesis, we speculate that neutrophils play an important role in the occurrence and development of AIS caused by CCAD. Infiltration of inflammatory cells can usually be found in the ischemic arterial walls of patients with CCAD and AIS (18, 28, 33). Peripheral neutrophils penetrate into the blood vessel walls and release vasoactive or cytotoxic media, including reactive oxygen species, proteases, matrix metalloproteinase (MMP), and cytokines, which may lead to the destruction of the extracellular matrix and the collapse of the vessel walls (32, 34). Our study found higher neutrophil count and higher leukocyte count baselines both correlate with 3-month poor outcome in the binary logistic regression analysis.
However, NLR reflects the balance of neutrophil and lymphocyte levels, which can comprehensively reflect the immune status, and the ratio may be more stable than a single parameter. In this study, we also used NIHSS at admission to analyze CCAD patients and found that the scale may not adequately capture all forms of functional change. The NIHSS has many advantages; however, it may miss some functional changes when used in place of neurological examination or blood parameters to measure improvement stroke (35, 36). Therefore, we collected both NIHSS score at admission and blood biochemical parameters to analyze their correlation with the prognosis of patients. Although statistical analysis showed that many factors, including NIHSS, were associated with prognosis, NLR was still independently associated with 3-month prognosis in binary regression analysis after adjusting for confounding factors. We took NLR = 2.97 as the cutoff value. The proportion of 3-month poor outcome in patients with high NLR was significantly higher than that in patients with low NLR, which was consistent with previous studies of a single ischemic stroke with a larger size of samples. In addition, we also found that the age and triglyceride level were associated with 3-month outcome. This may be related to the pathogenesis of CCAD. However, there was no significant difference between two groups in the improvement of the hemodynamics of the diseased vessels and the risk of recurrent ischemic stroke at 3 months.
A higher NLR level may indicate devascularization, early neurological deterioration and systemic immune dysfunction. These events increase the risk of death and poor outcome in AIS caused by CCAD. Generally, as an available clinical indicator, NLR has strong practicability for acute clinical decision-making and prognosis judgment.
There are also some limitations in our research. First, the number of cases of AIS caused by CCAD is relatively small. The insufficient sample size leads to weakened statistical strength of conclusions. Second, the study is observational in nature. We can't prove the causal relationship between NLR and 3-month adverse outcome. Third, our study included patients with anterior and posterior circulation dissections and did not explore the relationship between cerebral infarct volume and poor prognosis.
Summary
The NLR level is related to the 3-month poor outcome of patients with AIS caused by CCAD. When NLR ≥ 2.97, the risk of poor outcome increases. This reliable and easy-to-use predictor could contribute to clinical treatment strategy design in patients with CCAD. Further studies should be performed to expand the sample size and investigate the relevant inflammation and immune pathways.
Data Availability Statement
The original contributions generated for the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
This study involving human participants were reviewed and approved by the Institutional Review Board of the First Affiliated Hospital of Soochow University. All patients provided their written informed consent.
Consent for Publication
All the authors approved the manuscript and are consent for the submission.
Author Contributions
GS, YY, ZC, and XX designed the study. GS, YY, ZC, SD, SH, YiqW, and YitW evaluated the subjects and collected the data. LY, BS, and YY analyzed the data. GS, YY, XY, and XX wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the grants from National Key R&D Program of China (2017YFE0103700), the National Natural Science Foundation of China (81120108011 and 81771454), Shandong Provincial Natural Science Foundation (ZR2019ZD32), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. (2005) 81:383–8. doi: 10.1136/pgmj.2003.016774
2. Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. (2009) 8:668–78. doi: 10.1016/S1474-4422(09)70084-5
3. Park KW, Park JS, Hwang SC, Im SB, Shin WH, Kim BT. Vertebral artery dissection: natural history, clinical features and therapeutic considerations. J Korean Neurosurg Soc. (2008) 44:109–15. doi: 10.3340/jkns.2008.44.3.109
4. Sullivan GW, Sarembock IJ, Linden J. The role of inflammation in vascular diseases. J Leukocyte Biol. (2000) 67:591–602. doi: 10.1002/jlb.67.5.591
5. Kalkan ME, Kalkan AK, Gündeş A, Yanartaş M, Oztürk S, Gurbuz AS, et al. Neutrophil to lymphocyte ratio: a novel marker for predicting hospital mortality of patients with acute type A aortic dissection. Perfusion. (2017) 32:321–7. doi: 10.1177/0267659115590625
6. Li C, Zhang F, Shen Y, Xu R, Chen Z, Dai Y, et al. Impact of neutrophil to lymphocyte ratio (NLR) index and its periprocedural change (NLRΔ) for percutaneous coronary intervention in patients with chronic total occlusion. Angiology. (2017) 68:640–6. doi: 10.1177/0003319716649112
7. Oz K, Iyigun T, Karaman Z, Çelik Ö, Akbay E, Akinc O, et al. Prognostic value of neutrophil to lymphocyte ratio and risk factors for mortality in patients with stanford type a aortic dissection. Heart Surg Forum. (2017) 20:E119–23. doi: 10.1532/hsf.1736
8. Grau AJ, Brandt T, Buggle F, Orberk E, Mytilineos J, Werle E, et al. Association of cervical artery dissection with recent infection. Arch Neurol. (1999) 56:851–6. doi: 10.1001/archneur.56.7.851
9. Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, et al. Mechanisms contributing to cerebral infarct size after stroke: gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. J Cereb Blood Flow Metab. (2010) 30:1306–17. doi: 10.1038/jcbfm.2010.14
10. Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, et al. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. (2009) 158:1184–93. doi: 10.1016/j.neuroscience.2008.07.044
11. Zhou J, Wu J, Zhang J, Xu T, Zhang H, Zhang Y, et al. Association of stroke clinical outcomes with coexistence of hyperglycemia and biomarkers of inflammation. J Stroke Cereb Dis. (2015) 24:1250–5. doi: 10.1016/j.jstrokecerebrovasdis.2015.01.028
12. Song SY, Zhao XX, Rajah G, Hua C, Kang RJ, Han YP, et al. Clinical significance of baseline neutrophil-to-lymphocyte ratio in patients with ischemic stroke or hemorrhagic stroke: an updated meta-analysis. Front Neurol. (2019) 10:1032. doi: 10.3389/fneur.2019.01032
13. Brooks SD, Spears C, Cummings C, VanGilder RL, Stinehart KR, Gutmann L, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg. (2014) 6:578–83. doi: 10.1136/neurintsurg-2013-010780
14. Yang Y, Sun G, Diao S, Yang L, Dong W. Diagnostic performances of neutrophil to lymphocyte ratio and lymphocyte to monocyte ratio in acute ischemic stroke caused by cervicocranial arterial dissection. J Clin Lab Anal. (2020) e23515. doi: 10.1002/jcla.23515
15. Qun S, Tang Y, Sun J, Liu Z, Wu J, Zhang J, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotox Res. (2017) 31:444–52. doi: 10.1007/s12640-017-9707-z
16. Markus HS, Levi C, King A, Madigan J, Norris J. Antiplatelet therapy vs anticoagulation therapy in cervical artery dissection: the cervical artery dissection in stroke study (CADISS) randomized clinical trial final results. JAMA Neurol. (2019) 76:657–64. doi: 10.1001/jamaneurol.2019.0072
17. Hassan AE, Jadhav V, Zacharatos H, Chaudhry SA, Rodriguez GJ, Mohammad YM, et al. Determinants of neurologic deterioration and stroke-free survival after spontaneous cervicocranial dissections: a multicenter study. J Stroke Cereb Dis. (2013) 22:389–96. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.017
18. Chen WT, Chang FC, Huang HC, Tsai JY, Chung CP. Total and differential leukocyte counts in ischemic stroke caused by vertebrobasilar artery dissection. J Neurol Sci. (2019) 404:101–5. doi: 10.1016/j.jns.2019.07.022
19. Yan J, Read SJ, Henderson RD, Hull R, O'Sullivan JD, McCombe PA, et al. Frequency and function of regulatory T cells after ischaemic stroke in humans. J Neuroimmunol. (2012) 243:89–94. doi: 10.1016/j.jneuroim.2011.12.019
20. Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. (2016) 15:869–81. doi: 10.1016/S1474-4422(16)00114-9
21. Kocaturk O, Besli F, Gungoren F, Kocaturk M, Tanriverdi Z. The relationship among neutrophil to lymphocyte ratio, stroke territory, and 3-month mortality in patients with acute ischemic stroke. Neurol Sci. (2019) 40:139–46. doi: 10.1007/s10072-018-3604-y
22. Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci. (2015) 2:26670. doi: 10.5812/archneurosci.26670
23. Papp V, Molnár T, Bánáti M, Illés Z. [Immune responses and neuroimmune modulation in the pathogenesis of acute ischemic stroke and poststroke infections]. Ideggyogy Sz. (2010) 63:232–46.
24. Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. (2011) 10:471–80. doi: 10.1016/S1474-4422(11)70066-7
25. del Porto F, Proietta M, Tritapepe L, Miraldi F, Koverech A, Cardelli P, et al. Inflammation and immune response in acute aortic dissection. Ann Med. (2010) 42:622–9. doi: 10.3109/07853890.2010.518156
26. Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. (2009) 158:1174–83. doi: 10.1016/j.neuroscience.2008.06.014
27. Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. (2005) 6:775–86. doi: 10.1038/nrn1765
28. Justicia C, Panés J, Solé S, Cervera A, Deulofeu R, Chamorro A, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cereb Blood Flow Metab. (2003) 23:1430–40. doi: 10.1097/01.WCB.0000090680.07515.C8
29. Soubhye J, Aldib I, Delporte C, Prévost M, Dufrasne F, Antwerpen PV. Myeloperoxidase as a target for the treatment of inflammatory syndromes: mechanisms and structure activity relationships of inhibitors. Curr Med Chem. (2016) 23:3975–4008. doi: 10.2174/0929867323666160607111806
30. Trentini A, Rosta V, Spadaro S, Bellini T, Rizzo P, Vieceli Dalla Sega F, et al. Development, optimization and validation of an absolute specific assay for active myeloperoxidase (MPO) and its application in a clinical context: role of MPO specific activity in coronary artery disease. Clin Chem Lab Med. (2020) 58:1749–58. doi: 10.1515/cclm-2019-0817
31. Siegel RJ, Koponen M. Spontaneous coronary artery dissection causing sudden death. Mechanical arterial failure or primary vasculitis? Arch Pathol Lab Med. (1994) 118:196–8.
32. Li D, Liu S, Teng F, Yang W, Zhang L, Deng Y, et al. Temporal change of leukocytes and chemokines in aortic dissection patient: relationship to regional lesion on aorta. Int J Cardiol. (2013) 168:3065–6. doi: 10.1016/j.ijcard.2013.04.094
33. Eskenasy-Cottier AC, Leu HJ, Bassetti C, Bogousslavsky J, Regli F, Janzer RC. A case of dissection of intracranial cerebral arteries with segmental mediolytic “arteritis”. Clin Neuropathol. (1994) 13:329–37.
34. Gautier S, Ouk T, Tagzirt M, Lefebvre C, Laprais M, Pétrault O, et al. Impact of the neutrophil response to granulocyte colony-stimulating factor on the risk of hemorrhage when used in combination with tissue plasminogen activator during the acute phase of experimental stroke. J Neuroinflamm. (2014) 11:96. doi: 10.1186/1742-2094-11-96
35. Marsh EB, Lawrence E, Gottesman RF, Llinas RH. The NIH stroke scale has limited utility in accurate daily monitoring of neurologic status. Neurohospitalist. (2016) 6:97–101. doi: 10.1177/1941874415619964
Keywords: cervicocranial arterial dissection, acute ischemic stroke, inflammation, neutrophil to lymphocyte ratio, 3-month outcome
Citation: Sun G, Yang Y, Chen Z, Yang L, Diao S, Huang S, Wang Y, Wang Y, Sun B, Yuan X and Xu X (2020) Neutrophil to Lymphocyte Ratio Predicts Outcome of Stroke by Cervicocranial Arterial Dissection. Front. Med. 7:598055. doi: 10.3389/fmed.2020.598055
Received: 23 August 2020; Accepted: 03 November 2020;
Published: 27 November 2020.
Edited by:
Ti-Fei Yuan, Shanghai Jiao Tong University, ChinaReviewed by:
Qing Liu, Peking Union Medical College Hospital (CAMS), ChinaFang Zhang, Mass General Research Institute, United States
Yujiao Lu, Augusta University, United States
Copyright © 2020 Sun, Yang, Chen, Yang, Diao, Huang, Wang, Wang, Sun, Yuan and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Yuan, eXgyMDc3QHNpbmEuY29t; Xingshun Xu, eGluZ3NodW54dUBzdWRhLmVkdS5jbg==
†These authors have contributed equally to this work
 Guangbi Sun
Guangbi Sun Yi Yang
Yi Yang Zhiguo Chen
Zhiguo Chen Le Yang2
Le Yang2 Xingshun Xu
Xingshun Xu