
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 21 January 2021
Sec. Obstetrics and Gynecological Surgery
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.596405
Background: Preeclampsia is a significant cause of maternal and perinatal mortality worldwide. Oxidative stress plays a key role in its pathophysiology, hence antioxidants such as tocotrienol may be preventive against preeclampsia. In 2018, the ISSHP revised the definition of preeclampsia. In accordance with the new definition, we report a secondary data analysis from a clinical trial comparing palm oil vitamin E in the form of tocotrienol-rich fraction (TRF) against placebo, in preventing preeclampsia.
Method: A randomized double-blind controlled trial was conducted in 2002–2005 to assess the benefits of TRF in preeclampsia prevention. A total of 299 primigravidae were recruited. The intervention group was supplemented with TRF 100 mg daily in super-olein capsules, whereas the placebo group was prescribed super-olein capsules without TRF, beginning from 12 to 16 gestational weeks until delivery. The primary outcome measure was incidence of preeclampsia.
Results: The total incidence of pregnancy induced hypertension (PIH) was 5%, whereas the incidence of preeclampsia was 2.3%. The odds of developing PIH (adjusted OR 0.254; 95% CI: 0.07–0.93; p–value 0.038) and preeclampsia (adjusted OR 0.030; 95% CI: 0.001–0.65; p-value 0.025) were significantly lower in the TRF arm compared to the placebo arm.
Conclusion: Antenatal supplementation with palm oil vitamin E in the form of TRF is associated with significant reductions in the incidence of preeclampsia and PIH in a single urban tertiary hospital. Palm oil vitamin E deserves further scrutiny as a potential public health preventive measure against preeclampsia and PIH.
Hypertensive disorders of pregnancy (HDP) was reported as one of the main risk factors leading to bad outcome of pregnancies (1). Urbanization has driven the human population to practice unhealthy lifestyles that lead to increasing trends of non-communicable diseases (2).
The prevalence of HDP is 5–10% (3) with preeclampsia affecting 3–5% of pregnancies (1). Preeclampsia was traditionally diagnosed by the combined presentation of high blood pressure and proteinuria. New definitions include maternal organ dysfunction, such as renal insufficiency, liver involvement, neurological or hematological complications, and uteroplacental dysfunction as evidenced by fetal growth restriction (FGR).
In year 2001 the International Society for the Study of Hypertension in Pregnancy (ISSHP) updated the HDP classification (4), followed by another revision in 2014 (5) and subsequently in 2018 (6). The revised classification reflected new perspectives and understanding that influenced management of this disease.
Approximately 287,000 preventable maternal deaths occur annually worldwide and HDP is listed as one of the major contributing factors (7). Other than early diagnosis and prompt appropriate treatment (8, 9), screening and prevention is vital in order to reduce morbidity and mortality especially in low resource settings where accessibility to healthcare is inadequate (10, 11). Currently calcium and aspirin (6, 12–14) have established, albeit small, roles in the primary prevention of preeclampsia.
Oxidative stress in the placenta leads to systemic maternal inflammatory response causing maternal vascular endothelial dysfunction in preeclampsia (15, 16). The significant evidence of oxidative stress playing a key role in the development of preeclampsia directed to the hypothesis that antioxidant supplementation may have a role in preventing the disease. The potential of antioxidants such as vitamins C, E, selenium and lycopene (17) in the prevention of conditions associated with oxidative stress like preeclampsia, is supported by evidence-based studies (18–21).
Vitamin E, specifically tocotrienol, is a powerful antioxidant that has been shown to reduce oxidative stress and prevent propagation of free radical reactions in female reproductive health (22, 23). The two major forms of vitamin E are tocopherol and tocotrienol (24). Tocotrienol is 40–60 times more potent as an antioxidant, with superior anti-inflammatory properties compared to tocopherol (24).
Palm oil Vitamin E in the form of tocotrienol-rich fraction (TRF) has been shown to increase the antioxidant levels of older adults (22). However, there is limited clinical trials on the effects of palm oil vitamin E in women of reproductive age (25).
We have published the outcome of our randomized controlled trial (RCT) on the role of tocotrienol in prevention of preeclampsia (25) using the old definition (4). With recent revision of the classification of HDP, we decided to review our results and add more perspectives into the data analyses.
The present study used secondary data from a previous randomized double-blind controlled trial (Grant Number: 06-02-02-0136) (25), where the intervention group was supplemented with palm oil vitamin E (TRF 100 mg daily in super olein) capsules and the placebo group was prescribed super olein capsules, beginning from 12 to 16 gestational weeks until delivery. The project was carried out at a single tertiary hospital in an urban setting in Malaysia from June 2002 to March 2005 by recruiting primigravidae from the antenatal clinic as stated in the published article (25). Sample size calculation, inclusion and exclusion criteria, method of double blinding and randomization, and other details have been described previously (25).
The secondary data covered a total of 299 samples in four different excel files, comprising the subjects' sociodemographic profiles, obstetric profiles, and admission and delivery details. The validity and completeness were verified during data collection and analysis by the previous research team.
All 299 samples were included in this secondary data analysis. Preeclampsia was redefined according to the new definition by ISSHP (6). Fetal growth (clinical and sonographic) data and laboratory findings such as full blood count, renal profile, and liver function test were reviewed for criteria fulfilling the updated diagnosis of preeclampsia.
The incidence of preeclampsia is the primary outcome measure in this secondary data analysis. The revised definition of preeclampsia according to the ISSHP in 2018 (6) was used in this study, and applied to secondary data from the previously published RCT. The incidence of preeclampsia was re-determined in fulfillment of the new ISSHP criteria. In 2001, besides a clinical definition of preeclampsia, the ISSHP suggested a research definition that must include significant proteinuria (4), and this was adopted as the definition of preeclampsia in our original study (25). However, in the revised classification and diagnosis in 2018, this research definition was no longer included in the ISSHP recommendations (6).
The birthweight data was recoded into status of fetal growth restriction either yes or no (according to WHO growth chart and weeks of gestation at delivery). Urine creatinine was recoded into more than 90 and <90 μmol/l for determination of status of renal function. Platelet count was recoded into <150 and more than 150/μl for presence of thrombocytopenia. The diagnosis of preeclampsia was subsequently re-determined based on the recoded criteria.
This is defined as the incidence of (1) pregnancy induced hypertension (PIH, defined as gestational hypertension and preeclampsia); and (2) maternal and fetal outcomes, including onset of labor (either spontaneous or induced), mode of delivery (either vaginal or cesarean section), gestation at delivery and baby's birth weight.
The independent variables in this study comprise sociodemographic characteristics (age, race, occupation, household income), gestation at recruitment, TRF supplement status and other nutritional supplements.
Age was defined in years at recruitment based on date of birth. Race was categorized and recoded into Malay, Chinese, Indian and others according to major ethnicities in Malaysia. Occupation was categorized and recoded into health care worker group (doctor, nurse, medical laboratory technician etc.); educator group (teacher, lecturer, tutor, etc.); “others” (defined as occupations other than medical and educator groups); and, unemployed (defined as home maker or student with no income nor contributing toward household income). Household income was categorized into three groups based on monthly family income (1) more than MYR 5,000; (2) within MYR 5,000–3,000; and (3) < MYR 3,000.
Gestation at recruitment was defined as period of gestation (in weeks) at recruitment, either based on the date of the last menstrual period or on ultrasonography.
TRF was defined as Yes if the participant received TRF (intervention group) or No if the participant received placebo (control group). Other nutritional supplements was defined as supplements either prescribed by medical personnel [such as folic acid, ferrous fumarate, vitamin C, tablet ObiminTM (Unam Pharmaceuticals), tablet IberetTM (Abbott Pharmaceuticals)] or other nutritional supplements taken by the participant on their own accord.
Data from the previous RCT was recorded in Excel spreadsheets and coded according to operational definition of study variables. The data was then transferred to IBM Statistical Package for Social Sciences (SPSS) version 21 software for further statistical analysis. Univariate descriptive analysis was performed by using frequency (n) and percentage (%) for categorical data, and using mean and standard deviation (SD) for normally distributed continuous data, or median and interquartile range for data that is not normally distributed. Data exploration was performed to assess the normality of quantitative data distribution by using Kolmogorov–Smirnov test, mean, median, mode, skewness and kurtosis values.
The bivariate analysis was performed using chi-square test for categorical variables to compare participant/maternal characteristics including sociodemographic profile, gestation at recruitment, other supplements taken by participants, and pregnancy outcome such as the occurrence of HDP, preeclampsia, onset of labor, mode of delivery (vaginal or cesarean section), preterm delivery, and low birth weight between the intervention group and the control group.
Multivariate analysis was performed by using multiple logistic regression to control for confounders in order to assess the role of TRF in preventing PIH and/or preeclampsia. Intention-to-treat analysis was not performed as data was unavailable from 20 participants who did not complete the study.
The total number of participants who completed the RCT was 299, with 151 participants in the TRF arm and 148 in the control arm. The incidence of PIH was 5% and the incidence of preeclampsia 2.3% according to the latest definitions and classification published by ISSHP (6). The incidence of PIH was no different compared to the primary data analysis (25). After re-categorization according to the new classification, the incidence of preeclampsia remained the same in the TRF arm, but increased in the placebo arm (from 3.4 to 4.1%), compared to the primary analysis. Total incidence of preeclampsia was therefore increased.
The differences in relative risk (RR) of PIH and preeclampsia (TRF vs. control arm) between the old and new classification/definition is shown in Table 1. The risk of developing either PIH or preeclampsia is lower in the TRF arm compared to the control arm. The differences in the risks of developing preeclampsia and PIH between the two arms were not statistically significant in bivariate analysis, although the p-value in the case of RR for preeclampsia approaches significance (p = 0.052) following ISSHP reclassification of preeclampsia as shown in Table 1.
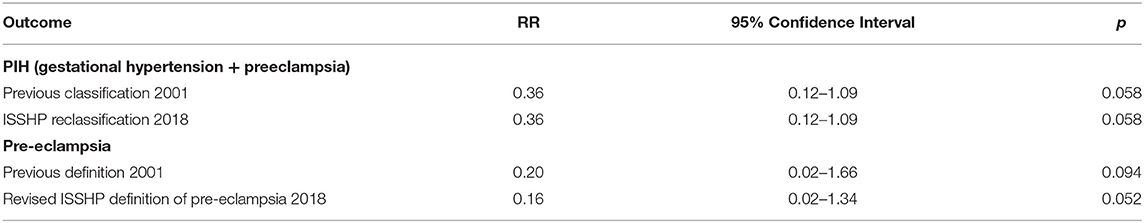
Table 1. The RR of PIH and preeclampsia in the TRF arm compared to the control arm, when defined according to the latest ISSHP classification of HDP (6) compared to the older classification (4).
Comparison of maternal characteristics between the TRF and control arms is shown in Table 2, whereas comparison of pregnancy outcome between the two arms is shown in Table 3. There are no significant differences in secondary outcome measures between the two arms.
The risk of disease, absolute risk difference (ARD), RR reduction, and number needed to treat (NNT) between the two arms, preeclampsia and PIH, are shown in Table 4. TRF is seen to have significant negative associations with the incidence of PIH and preeclampsia after control of other confounders such as age, maternal weight, race, occupation, household income and gestational age in multivariate logistic regression analysis. The odds of developing PIH and preeclampsia were lower in the TRF arm compared to the control arm. Factors associated with the incidence of PIH and preeclampsia are presented in Tables 5,6. The magnitudes of reduction in the incidence of PIH and preeclampsia with TRF supplementation are 64 and 83% respectively.
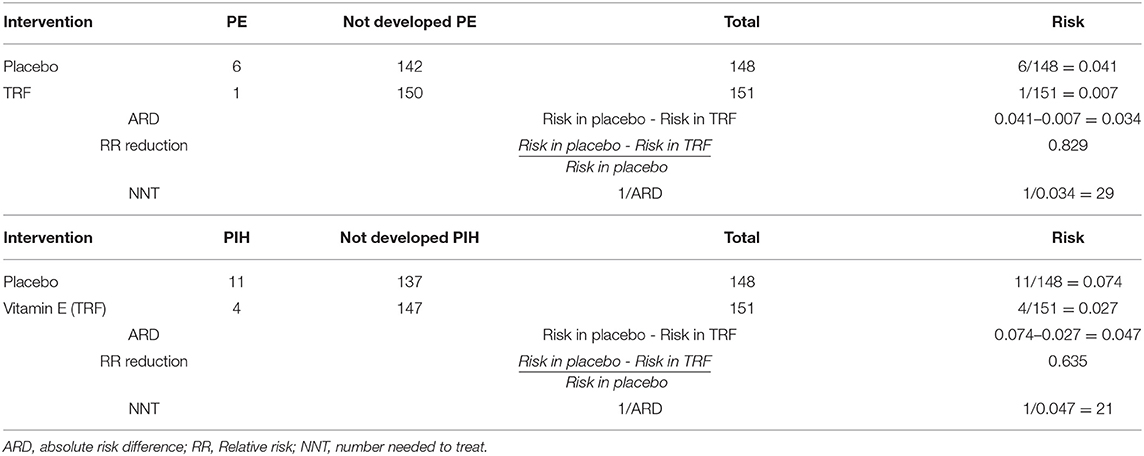
Table 4. Risk, ARD, RR reduction and NNT in the TRF arm compared to the control arm for both preeclampsia (PE) and PIH.
The reclassification of HDP by ISSHP signifies an important change in the perception of the disease and acknowledges the wider spectrum of disease manifestation compared to previous schools of thought. In line with this the outcome of studies in recent years should be reviewed to take into consideration this new rationale in definition and classification.
This secondary analysis of a double blind RCT on TRF vs. placebo in prevention of PIH and preeclampsia uncovered interesting differences when preeclampsia was redefined according to the latest ISSHP classification of HDP. We found an increase in the incidence of preeclampsia to 2.3%, compared to 2.0% when calculated on the basis of the older definition by ISSHP in 2001 (4). The incidence of PIH remained 5%, as in the primary analysis. In view of the presence of significant confounders, multivariate analysis was performed, demonstrating that the odds of PIH and preeclampsia were significantly lower among women who were prescribed palm oil vitamin E intervention in the form of TRF compared to those who received placebo.
Bivariate analysis only summarizes the relationship between two variables which are one predictor variable and one outcome variable, whereas multivariate analysis simultaneously analyses more than one predictors and have an ability to control the confounding effects for preeclampsia such as maternal weight and socioeconomic status (26). In multivariate analysis after controlling other variables (such as maternal age, weight, race, occupation, household income and intake of other supplements), TRF significantly reduced the risk of PIH and preeclampsia. The adjusted odds of developing PIH is 0.254 [(95% CI; 0.07–0.93), p-value: 0.038], which means the risk is lower in the TRF arm, compared to the control arm. Similarly, the adjusted odds of developing preeclampsia is 0.030 [(95% CI: 0.001; 0.65), p-value: 0.025] in the TRF arm compared to the placebo arm.
The calculated absolute risk reduction was 4.7% for PIH and 3.4% for preeclampsia. Thus, TRF may reduce the risk of a patient developing PIH by 4.7% and preeclampsia by 3.4%. The NNT were 21 for PIH and 29 for preeclampsia. This implies that, 21 pregnant women should be treated prophylactically with TRF in order to prevent one occurrence of PIH, and 29 in the case of preeclampsia. These findings support public health measures on primary prevention of PIH and preeclampsia using palm oil vitamin E in the form of TRF, in addition to the currently available prophylaxis of aspirin and calcium.
Another important, albeit unsurprising finding, is that maternal weight was significantly associated with PIH, hence healthy lifestyle and eating habits should continue to be promoted and emphasized. Interestingly, household income of more than RM 5,000/month is significantly associated with preeclampsia. However, this finding should be interpreted cautiously and should not be generalized to other populations (especially in rural settings), since this study was conducted in a single urban tertiary hospital.
The protective effect of a strong antioxidant agent against preeclampsia supports the belief that oxidative stress plays a vital role in the pathophysiology of the disease. Therefore, TRF with its substantial antioxidant capacity (16) has good potential for use as one of the preventive measures against preeclampsia, especially in mothers with low baseline levels of antioxidant (27).
To the best of our knowledge, this is the only study so far where vitamin E, in the form of TRF from palm oil, demonstrated protection against PIH and preeclampsia. Other studies do not support the role of vitamin E in preventing either preeclampsia or PIH (18, 27–34). However, the other studies used vitamin E in the form of the much weaker antioxidant tocopherol, either as a monotherapy or in combination with vitamin C.
The safety and efficacy of tocotrienols have been clearly demonstrated in various research and reviews, both in human and animal studies (35–38).
In summary, palm oil vitamin E in the form of TRF 100 mg daily prescribed from before 16 weeks gestation appears to have potential impact in reducing the risk of developing preeclampsia and PIH among primigravidae.
This study was performed in a single urban tertiary hospital in Malaysia, involving only primigravidae. The numbers were too small to conduct a sub-analysis on early-onset vs. late-onset preeclampsia.
Future research with a larger sample that covers a variety of populations will shed more light on this prophylaxis against preeclampsia. Very importantly, the impact of early onset preeclampsia should be looked into.
Antenatal supplementation with palm oil vitamin E in the form of TRF is associated with a significant reduction in the incidence of preeclampsia and PIH among primigravidae in a single urban tertiary hospital in Malaysia. Palm oil vitamin E deserves further scrutiny as a potential public health preventive measure against preeclampsia and PIH. A more robust extrinsic validity study is indicated in future in order to consolidate the findings of this study.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by UKM Research and Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
NAA, ZAM, and RS designed the secondary data analysis and wrote the manuscript. NAA and RS analyzed and interpreted the results. All authors contributed to the article and approved the submitted version.
The primary RCT received funding through the IRPA Grant (06-02-02-0136) from the Ministry of Science, Technology and Environment under RMK-7, and contribution of TRF supplements and placebo capsules from Golden Hope Bioganics Sdn Bhd.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to acknowledge the contribution of all clinicians, scientists, technologists, and patients who were involved in the primary research.
ARD, absolute risk difference; FGR, fetal growth restriction; HDP, hypertensive disorders of pregnancy; ISSHP, International Society for the Study of Hypertension in Pregnancy; MYR, Malaysian Ringgit; NNT, numbers needed to treat; PIH, pregnancy induced hypertension; RCT, randomized controlled trial; RR, relative risk; SD, standard deviation; TRF, tocotrienol-rich fraction.
1. Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. The Lancet. (2016) 387:999–1011. doi: 10.1016/S0140-6736(15)00070-7
2. Goryakin Y, Rocco L, Suhrcke M. The contribution of urbanization to non-communicable diseases: evidence from 173 countries from 1980 to 2008. Econ Human Biol. (2017) 26:151–63. doi: 10.1016/j.ehb.2017.03.004
3. Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. (2017) 40:213–20. doi: 10.1038/hr.2016.126
4. Brown MA, Lindheimer MD, de Swiet M, Assche AV, Moutquin J-M. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. (2001) 20:IX-XVI. doi: 10.1081/PRG-100104165
5. Tranquilli A, Dekker G, Magee L, Roberts J, Sibai B, Steyn W, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregn Hypertens. (2014) 4:97. doi: 10.1016/j.preghy.2014.02.001
6. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. (2018) 72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803
7. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller A-B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health. (2014) 2:e323–33. doi: 10.1016/S2214-109X(14)70227-X
8. Danso KA, Opare-Addo HS. Challenges associated with hypertensive disease during pregnancy in low-income countries. Int J Gynecol Obstet. (2010) 110:78–81. doi: 10.1016/j.ijgo.2010.01.026
9. Bhutta ZA, Das JK, Bahl R, Lawn JE, Salam RA, Paul VK, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. (2014) 384:347–70. doi: 10.1016/S0140-6736(14)60792-3
10. Meher S, Duley L. Nitric oxide for preventing pre-eclampsia and its complications. Cochr Database Syst Rev. (2007). doi: 10.1002/14651858.CD006490
11. Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochr Database Syst Rev. (2014) CD001059. doi: 10.1002/14651858.CD001059.pub4
12. Browne JL, Klipstein-Grobusch K, Franx A, Grobbee DE. Prevention of hypertensive disorders of pregnancy: a novel application of the polypill concept. Curr Cardiol Rep. (2016) 18:59. doi: 10.1007/s11886-016-0725-x
13. Ronsmans C, Campbell O. Quantifying the fall in mortality associated with interventions related to hypertensive diseases of pregnancy. BMC Public Health. (2011) 11 (Suppl. 3):S8. doi: 10.1186/1471-2458-11-S3-S8
14. Hofmeyr GJ, Lawrie TA, Atallah Á, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochr Database Syst Rev. (2018) 10:CD001059. doi: 10.1002/14651858.CD001059.pub5
15. Williamson RD, McCarthy C, McCarthy FP, Kenny LC. Oxidative stress in pre-eclampsia; have we been looking in the wrong place? Pregn Hypertens. (2017) 8:1–5. doi: 10.1016/j.preghy.2017.01.004
16. Cohen JM, Kramer MS, Platt RW, Basso O, Evans RW, Kahn SR. The association between maternal antioxidant levels in midpregnancy and preeclampsia. Am J Obstet Gynecol. (2015) 213:695.e1-13. doi: 10.1016/j.ajog.2015.07.027
17. Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia. Cochr Database Syst Rev. (2008) CD004227. doi: 10.1002/14651858.CD004227.pub3
18. Guan Z, Li HF, Guo LL, Yang X. Effects of vitamin C, vitamin E, and molecular hydrogen on the placental function in trophoblast cells. Arch Gynecol Obstet. (2015) 292:337–42. doi: 10.1007/s00404-015-3647-8
19. Mercer BM, Abdelrahim A, Moore RM, Novak J, Kumar D, Mansour JM, et al. The impact of vitamin C supplementation in pregnancy and in vitro upon fetal membrane strength and remodeling. Reprod Sci. (2010) 17:685–95. doi: 10.1177/1933719110368870
20. Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. (1999) 354:810–6. doi: 10.1016/S0140-6736(99)80010-5
21. Omar M, Borg HM. Assessment of oxidative stress markers and level of antioxidant in preeclampsia. Ind J Obstet Gynecol Res. (2019) 6:268–75. doi: 10.18231/j.ijogr.2019.062
22. Nor Azman NHE, Goon JA, Abdul Ghani SM, Hamid Z, Wan Ngah WZ. Comparing palm oil, tocotrienol-rich fraction and alpha-tocopherol supplementation on the antioxidant levels of older adults. Antioxidants. (2018) 7:74. doi: 10.3390/antiox7060074
23. Mohd Mutalip SS, Ab-Rahim S, Rajikin MH. Vitamin E as an antioxidant in female reproductive health. Antioxidants. (2018) 7:22. doi: 10.3390/antiox7020022
24. Peh HY, Tan WS, Liao W, Wong WS. Vitamin E therapy beyond cancer: tocopherol vs. tocotrienol. Pharmacol Ther. (2016) 162:152–69. doi: 10.1016/j.pharmthera.2015.12.003
25. Mahdy ZA, Siraj HH, Khaza'ai H, Mutalib MSA, Azwar MH, Wahab MA, et al. Does palm oil vitamin E reduce the risk of pregnancy induced hypertension. Acta Med. (2013) 56:104–9. doi: 10.14712/18059694.2014.17
26. Mrema D, Lie RT, Østbye T, Mahande MJ, Daltveit AK. The association between pre pregnancy body mass index and risk of preeclampsia: a registry based study from Tanzania. BMC Pregn Childb. (2018) 18:56. doi: 10.1186/s12884-018-1687-3
27. McCance DR, Holmes VA, Maresh MJ, Patterson CC, Walker JD, Pearson DW, et al. Vitamins C and E for prevention of pre-eclampsia in women with type 1 diabetes (DAPIT): a randomised placebo-controlled trial. Lancet. (2010) 376:259–66. doi: 10.1016/S0140-6736(10)60630-7
28. Abramovici A, Gandley RE, Clifton RG, Leveno KJ, Myatt L, Wapner RJ, et al. Prenatal vitamin C and E supplementation in smokers is associated with reduced placental abruption and preterm birth: a secondary analysis. BJOG. (2015) 122:1740–7. doi: 10.1111/1471-0528.13201
29. Weissgerber TL, Gandley RE, Roberts JM, Patterson CC, Holmes VA, Young IS, et al. Haptoglobin phenotype, pre-eclampsia, and response to supplementation with vitamins C and E in pregnant women with type-1 diabetes. BJOG. (2013) 120:1192–9. doi: 10.1111/1471-0528.12288
30. Conde-Agudelo A, Romero R, Kusanovic JP, Hassan SS. Supplementation with vitamins C and E during pregnancy for the prevention of preeclampsia and other adverse maternal and perinatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. (2011) 204:503.e1-12. doi: 10.1016/j.ajog.2011.02.020
31. Rossi AC, Mullin PM. Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: a systematic review with meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2011) 158:9–16. doi: 10.1016/j.ejogrb.2011.04.010
32. Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. New Engl J Med. (2010) 362:1282–91. doi: 10.1056/NEJMoa0908056
33. Wang Z, Wang C, Qiu J, Ni Y, Chai S, Zhou L, et al. The association between dietary Vitamin C/E and gestational hypertensive disorder: a case-control study. J Nutr Sci Vitaminol. (2018) 64:454–65. doi: 10.3177/jnsv.64.454
34. Xu H, Perez-Cuevas R, Xiong X, Reyes H, Roy C, Julien P, et al. An international trial of antioxidants in the prevention of preeclampsia (INTAPP). Am J Obstet Gynecol. (2010) 202:239.e1-.e10. doi: 10.1016/j.ajog.2010.01.050
35. Meganathan P, Fu J-Y. Biological properties of tocotrienols: evidence in human studies. Int J Mol Sci. (2016) 17:1682. doi: 10.3390/ijms17111682
36. Mahipal A, Klapman J, Vignesh S, Yang CS, Neuger A, Chen D-T, et al. Phamacokinetics and safety of vitamin E d-tocotrienol after single and multiple doses in healthy subjects with measurement of vitamin E metabolites. Cancer Chemother Pharmacol. (2016) 78:157–65. doi: 10.1007/s00280-016-3048-0
37. Fiume MM, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC, et al. Safety assessment of tocopherols and tocotrienols as used in cosmetics. Int J Toxicol. (2018) 37 (Suppl. 2):61S−94S. doi: 10.1177/1091581818794455
Keywords: preeclampsia prevention, antioxidant, palm oil vitamin E, tocotrienol-rich fraction, pregnancy induced hypertension
Citation: Aminuddin NA, Sutan R and Mahdy ZA (2021) Role of Palm Oil Vitamin E in Preventing Pre-eclampsia: A Secondary Analysis of a Randomized Clinical Trial Following ISSHP Reclassification. Front. Med. 7:596405. doi: 10.3389/fmed.2020.596405
Received: 27 August 2020; Accepted: 30 December 2020;
Published: 21 January 2021.
Edited by:
Musalmah Mazlan, Universiti Teknologi MARA, MalaysiaReviewed by:
Harbindarjeet Singh, Universiti Teknologi MARA, MalaysiaCopyright © 2021 Aminuddin, Sutan and Mahdy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zaleha Abdullah Mahdy, emFsZWhhQHBwdWttLnVrbS5lZHUubXk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.