- 1Department of Nursing, Zhongshan Hospital, Fudan University, Shanghai, China
- 2Nursing School, Fudan University, Shanghai, China
- 3Faculty of Health: Medicine, Dentistry and Human Sciences, School of Nursing and Midwifery, University of Plymouth, Plymouth, United Kingdom
- 4Department of Biostatistics, Zhongshan Hospital, Fudan University, Shanghai, China
- 5Department of Cardiac Surgical Intensive Care Unit, Zhongshan Hospital, Fudan University, Shanghai, China
- 6Department of Cardiac Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
Objectives: The aim of this study was to investigate the prevalence and explore the predictors and early outcomes of post-operative delirium (POD) in patients with type A aortic dissection (AAD) during intensive care unit (ICU) stays.
Methods: We retrospectively reviewed the records of 301 patients with AAD who underwent surgical treatment in our institution from January 2017 to December 2018.
Results: Delirium developed in 73 patients (24.25%) during the ICU stay. Patients with lower estimated glomerular filtration rates [odds ratio (OR) 0.84, 95% CI 0.74–0.94, p = 0.003], post-operative midazolam use (OR 2.37, 95% CI 1.33–4.23, p = 0.004), and post-operative morphine use (OR 1.87, 95% CI 1.07–3.29, p = 0.029) were more susceptible to developing POD. Patients who developed POD had a longer ICU stay (11.52 vs. 7.22 days, p < 0.001) and hospital stay (23.99 vs. 18.91, p = 0.007) with higher hospitalization costs (48.82 vs. 37.66 thousand dollars, p < 0.001) than those without POD. The in-hospital mortality rate was higher in the delirium group, but the difference was not significant (6.85 vs. 4.82%, p = 0.502).
Conclusions: The incidence of POD in patients with AAD was high and was associated with renal dysfunction and the use of midazolam and morphine. POD was associated with poor early outcomes, suggesting the importance of early screening, such as for renal dysfunction, and prevention by using sedation scales to minimize the use of midazolam and morphine in these patients.
Introduction
Post-operative delirium (POD) is common in patients undergoing elective cardiac surgery, including aortic valve replacement and coronary artery bypass grafting (CABG). The reported incidence of elective cardiac surgery varies between 3 and 17.3% (1–4). Type A aortic dissection (AAD) is a life-threatening condition requiring surgical intervention (5, 6). As both the disease itself and intraoperative deep hypothermic circulatory arrest might cause ischemia to the cerebral circulation and nervous system, patients with AAD might have a greater risk of developing neuropsychiatric complications than patients undergoing other cardiac surgeries (7, 8). Due to the stress of surgery and the typical environment, post-operative patients in intensive care units (ICUs) have higher incidence rates of POD than other patients (9). Although diagnostics and surgical techniques have significantly improved, the post-operative neurological morbidity rate in AAD patients remains high (10). POD is associated with several negative outcomes, including elevated morbidity and mortality, prolonged ICU stay, and extra medical expenses (11–13).
POD is a common neuropsychiatric disorder in AAD patients, but studies focusing on the potential effects of POD among these patients are sparse. Several studies have demonstrated a high prevalence of POD among these patients and raised concerns about delirium prevention in this population. However, the risk factors reported was diverse, and these studies are limited by small sample size (10, 14, 15). Due to the particularity of disease and surgery, the characteristics of POD in AAD patients might be quite different from other patients of cardiac surgery. The predictors and prognosis still need to be further explored to offer evidence for POD prevention, and help find out the perniciousness of POD in these patients. In this study, we aimed to explore the prevalence, predictors, and early outcomes of POD in patients with AAD during the ICU stay.
Materials and Methods
Design
This is a retrospective cohort study. The study has been reported following the STROBE guidelines for observational studies (16). Ethical approval was obtained from the institutional review board. The requirement for informed consent was waived because of the retrospective nature of the study.
Setting
This study was conducted in a 39-bed cardiac surgery ICU of a tertiary hospital. The cardiac surgery ICU admits ~4,800 post-operative patients per year, including 150–200 patients with AAD.
Study Population
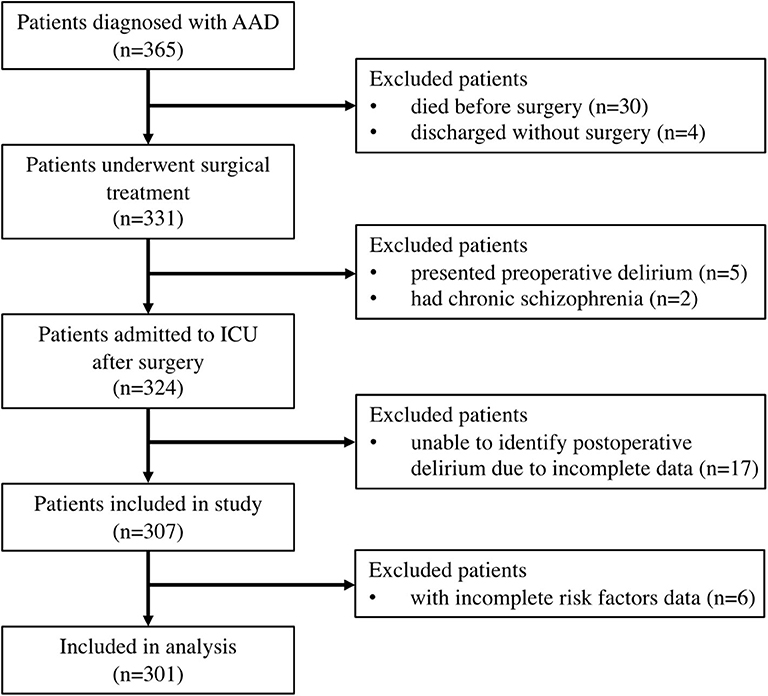
We retrospectively reviewed patients who underwent surgical treatment for AAD from January 2017 to December 2018. The diagnosis of AAD was determined according to CT angiography. We excluded patients who had delirium or chronic schizophrenia before surgery. We also excluded patients who were unable to be diagnosed with POD due to missing data. Finally, 301 patients were included in this study (Figure 1).
Data Collection
The primary outcome was POD, which was defined as a minimum of one positive assessment using the Confusion Assessment Method–ICU (CAM-ICU) tool during the ICU stay. The CAM-ICU defines delirium if at least two features of the CAM-ICU are present (Features 1 and 2 OR 3 and 4) (17, 18). In addition, we also used treatment with olanzapine as a proxy for the diagnosis of delirium because in the ICU of our hospital, we used olanzapine only to treat delirium.
The pre-operative data were retrospectively reviewed and analyzed. Surgery within 24 h from the onset of symptoms was considered emergent surgery. In-hospital mortality was defined as death occurring during the post-operative hospital stay. The estimated glomerular filtration rate (eGFR) was used to assess renal function after surgery. The eGFR was classified into four groups: normal (eGFR >90 ml/min/1.73 m2), mild (eGFR 60–90 ml/min/1.73 m2), moderate (eGFR 30–60 ml/min/1.73 m2), and severe (eGFR <30 ml/min/1.73 m2).
Operative Technique
All operations were performed through a mid-sternotomy. Total arch replacement combined with stented elephant trunk (SET) implantation was the primary surgical strategy in our hospital. Other techniques used along or in combination were the Bentall procedure, David I procedure, or CABG. All patients received standard transcutaneous cerebral oximetry monitoring and transesophageal echocardiography during surgery.
Statistical Analysis
The primary end point is the incidence of POD. The rule of thumb in logistic modeling is that a minimum of ten events per predictor variable should be achieved based on simulation studies (19). We used the lowest incidence 34% (14) and ten valuables in logistic model to estimate the sample size of 294.
Data are presented as the mean and SD or median (interquartile range) for continuous variables and as percentages for categorical variables. We log transformed the N-terminal prohormone of brain natriuretic peptide (NT-proBNP) level because it had a right-skewed distribution. Normally distributed continuous variables were compared using one-way ANOVA. The Pearson χ2-test was applied to all categorical variables. Logistic regression models were used to investigate univariable and multivariable risk factors for POD. In the logistic regression analysis, potential predictors of POD were tested in a univariable fashion, and variables with p-values < 0.1 were included in the multivariable analysis. In the multivariable analysis, a backwards stepwise model was used. The results of the logistic regression analysis are presented as odds ratios (ORs) with the corresponding 95% CIs. The analyses were performed using SPSS version 22.0 (IBM, New York, NY) and R statistical software (R, version 3.5.1; R Project).
Results
Patient Characteristics
A total of 365 cases have been reviewed and after exclusion 34 patients who did not receive surgery, seven patients who presented with pre-operative delirium or had chronic schizophrenia, 17 patients with incomplete data to identify POD, and six patients with incomplete risk factor data. Data from 301 cases were analyzed (Figure 1).
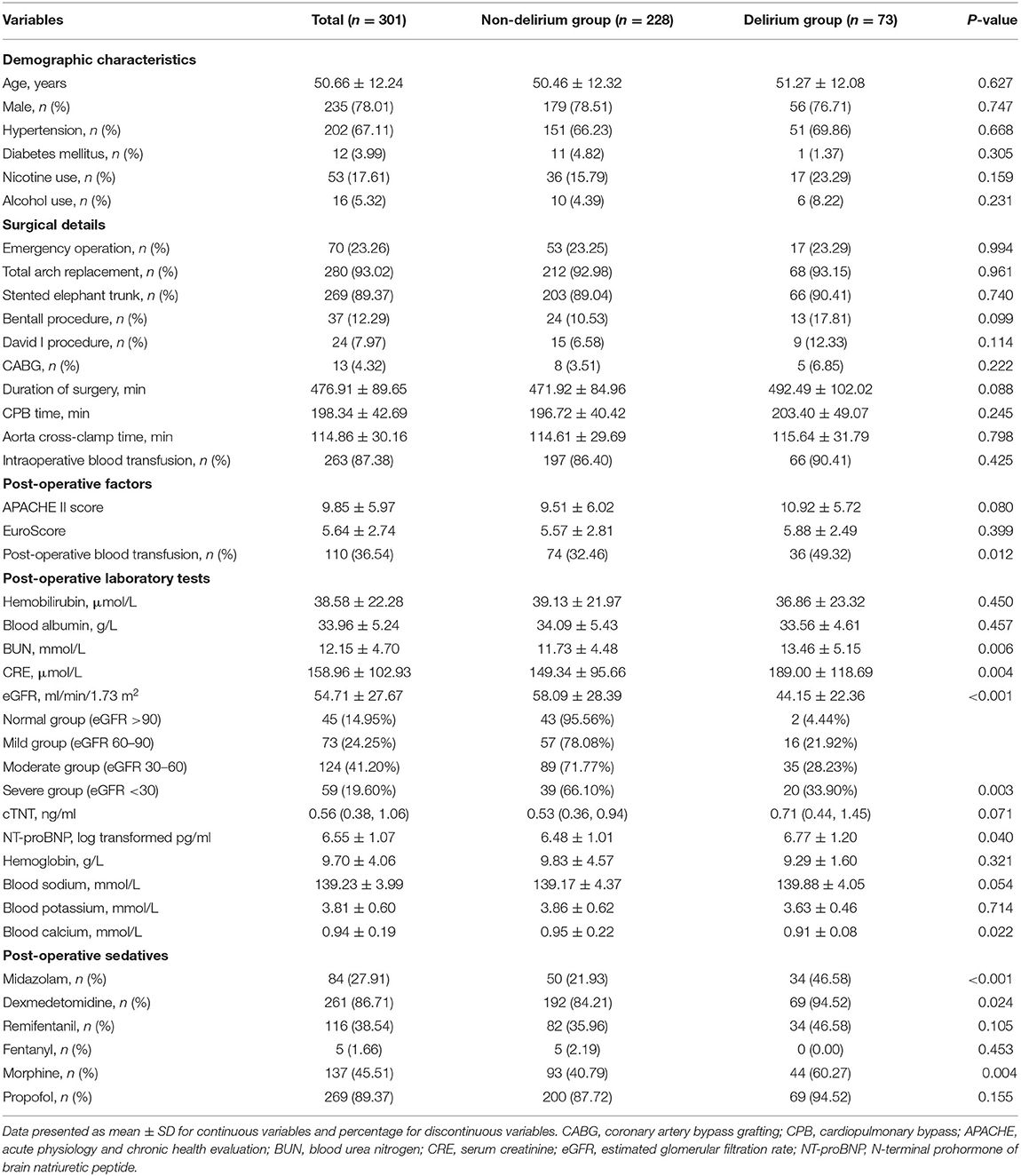
The patients were divided into two groups according to whether they developed POD during the ICU stay. The demographic characteristics and perioperative factors of the non-delirium group and delirium group are shown in Table 1. The mean participant age was 50.66 (SD 12.24), and 79.40% were males. The incidence of POD was 24.25% in awake AAD patients after surgery. The incidence of POD was 23.83% (56/235) in males, whereas in females, the incidence was 25.76% (17/66). Patients with delirium had more post-operative blood transfusions, higher eGFRs, and higher blood urea nitrogen, NT-proBNP, and blood calcium levels than those without delirium. In addition, more patients in the delirium group received midazolam, dexmedetomidine, and morphine post-operatively than those in the non-delirium group (Table 1).
Predictors of POD
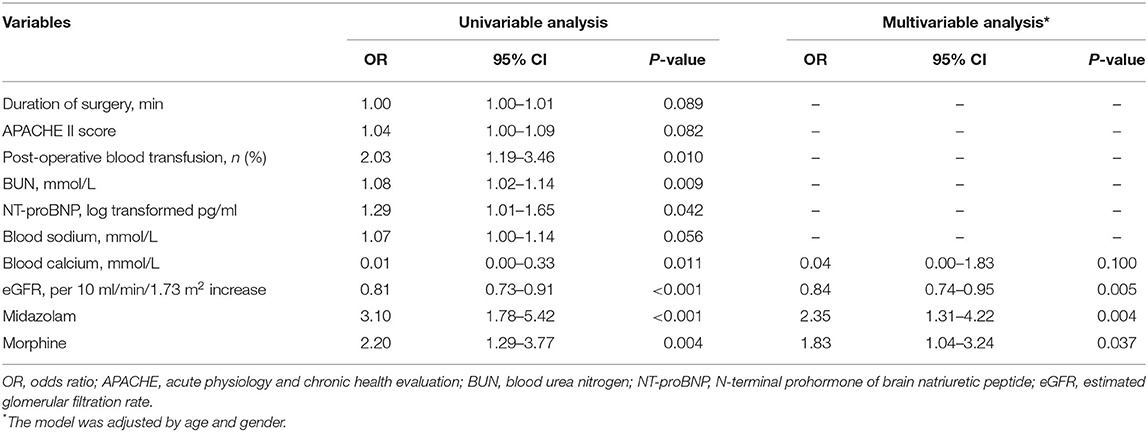
The results of the univariate and multivariate logistic regression analysis for the predictors of POD are presented in Table 2. In the univariate analysis, variables with a p-value < 0.1 were included in the multivariable analysis. In the multivariate analysis, a lower eGFR (OR 0.84, 95% CI 0.74–0.94, p = 0.003), post-operative use of midazolam (OR 2.37, 95% CI 1.33–4.23, p = 0.004), and post-operative use of morphine (OR 1.87, 95% CI 1.07–3.29, p = 0.029) were independent predictors of the development of POD among AAD patients. Figure 2 shows the incidence of POD in different groups divided by the independent predictors. We found that patients who used morphine after surgery presented a higher incidence of POD than those who did not use morphine (32.12 vs. 17.68%, p = 0.004). In addition, patients who received midazolam had a higher incidence of POD than those who did not (40.48 vs. 17.97%, p < 0.001). Patients were classified into four groups according to eGFR to assess renal function after surgery. The incidence of POD showed a significant increase as renal function declined (p for trend is <0.001).
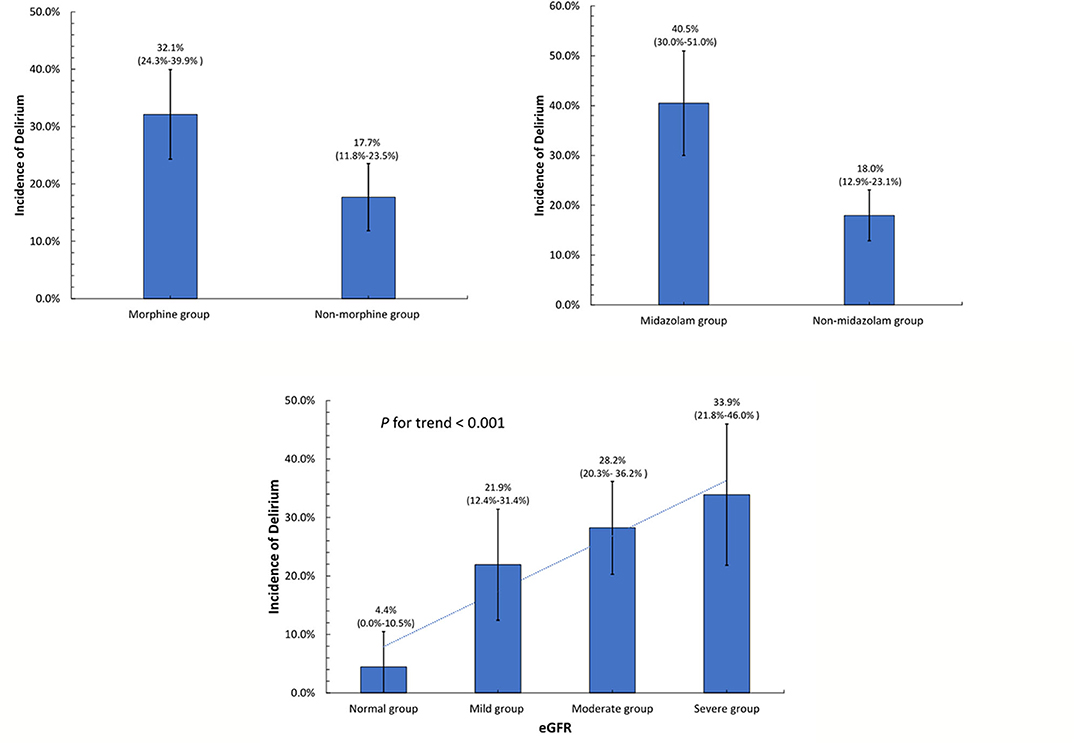
Figure 2. Incidence of post-operative delirium in different groups divided by the independent determinants.
Early Outcomes
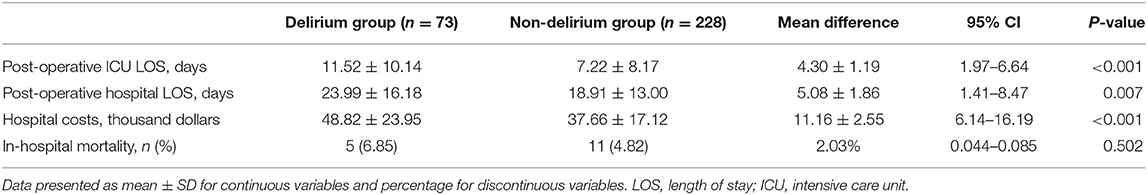
The comparison of early outcomes during hospitalization between the delirium group and the non-delirium group is shown in Table 3. Patients with POD had a longer ICU stay (11.52 vs. 7.22 days, p < 0.001) and hospital stay (23.99 vs. 18.91, p = 0.007) than those without POD. In addition, patients with POD had higher medical expenses than those without POD (48.82 vs. 37.66 thousand dollars, p < 0.001). The in-hospital mortality rate was higher in the delirium group than in the non-delirium group, but the difference was not statistically significant (6.85 vs. 4.82%, p = 0.502).
Discussion
In this study, we found that 24.25% of patients with AAD developed POD during the ICU stay. A lower eGFR and the use of midazolam and morphine after surgery were independent predictors of POD. As a result, patients with POD had longer ICU stays and hospital stays and higher medical expenses than those without POD.
Because AAD is a very emergent and life-threatening disease, most surgeons pay attention to mortality while ignoring post-operative neurological symptoms. However, with improvements in surgical techniques, an increasing number of patients survive after surgery. Thus, post-operative morbidity should be emphasized, as it may directly influence patient outcomes and quality of life. As one of the most severe and complex surgical complications of cardiac vascular disease, POD was frequently observed in our clinical practice. However, although several studies have reported the incidence and risk factors for POD after cardiac surgeries, few have focused on AAD patients. In previous studies, the reported incidence of POD after cardiac surgery varied between 3 and 17.3% (1–4, 20). Gaul et al. found that POD is the most common neurological complication in patients with AAD, accounting for 31.9% of all neurological complication events (10). Liu et al. (14) also reported that the incidence of POD in 100 AAD patients reached 34%. Shi et al. (15) stated an incidence of 45.95% in 148 AAD patients. These studies indicated a higher incidence of POD among AAD patients than among other patients. However, these investigations were limited by small sample sizes. Our study enrolled more than 300 AAD patients and collected detailed data to show that POD affected up to 24.25% of AAD patients after surgery and could help raise awareness of POD management for AAD patients in clinical practice.
Because the mechanism of delirium is unknown, an effective treatment is still lacking at present. Many studies have attempted to identify modifiable and non-modifiable risk factors to detect and prevent the development of delirium early. Clinical practice guidelines describe that benzodiazepine use and blood transfusions are the only two modifiable risk factors for delirium in critically ill adults with strong evidence support (21). In our study, we only found that post-operative blood transfusion was associated with POD in the univariate analysis, but it was not significant in the multivariate analysis. However, avoiding blood transfusions and receiving lower levels of hemoglobin is still controversial (22). In addition, we also found that the use of midazolam and morphine were independent risk factors for POD in AAD patients. Our results also confirmed the associations between delirium and benzodiazepine and opioid use in AAD patients. Furthermore, we identified that a lower eGFR was an independent risk factor for POD in AAD patients. A systematic review including 34 articles demonstrated that the association between renal dysfunction and delirium after on-pump cardiac surgery was inconclusive (23). Our study showed a positive relationship between post-operative renal dysfunction and the development of POD among the AAD population. The incidence of POD significantly increased as the degree of renal function declined. In contrast to previous studies involving patients with other diseases, we did not find any relationship between older age and delirium in AAD patients (23). Furthermore, two studies that included AAD patients showed that age is not an independent risk factor for POD, which was consistent with our results (14, 24).
Many studies have documented that POD is associated with several negative clinical consequences, including major post-operative complications, longer hospital stays with increased costs, and higher mortality (12, 13, 25). However, despite the emergent nature of AAD and high mortality in patients with AAD, only a few studies have focused on POD in these patients. In our study, we further compared the early outcomes of patients with and without POD. The results showed that patients with POD had a longer ICU length of stay and longer hospital length of stay than those without POD. As a consequence, the hospital costs for patients with POD are much higher than those for patients without POD. Contrary to previous studies on other populations, our study showed that the mortality rate was higher in the delirium group than in the non-delirium group, but the difference was not statistically significant. This result might be influenced by the exclusion of patients who were deeply sedated or in a coma, who were the most severe patients, which may underestimate the reported mortality rate. Although we did not find a positive association between POD and post-operative hospital mortality in this study, the longer hospital stay and higher medical costs showed that POD burdens patients and hospitals to some extent. Further prospective studies and follow-up evaluations are required to assess mid-term and long-term patient outcomes.
We acknowledge the potential limitations of our study. First, this study was limited by its observational and retrospective nature. Second, our participants were from a single hospital, so the generalizability of the findings remains to be confirmed. Third, the incidence of delirium might be underestimated as a result of missed diagnoses because delirium status changes over time. However, we also used olanzapine as a proxy for the diagnosis of delirium. Further studies are required to conduct a prospective and multicenter investigation to elucidate the prevalence and clinical outcomes of POD among AAD patients.
Conclusion
In summary, our findings showed that patients with AAD had a higher incidence of POD during the ICU stay than those without AAD. Patients with lower eGFRs who used midazolam and morphine after surgery were more susceptible to developing POD. Patients who developed POD had a longer ICU stay and hospital stay with higher hospitalization costs than those without POD. More attention should be paid to POD in patients with AAD. Early screening, such as for renal dysfunction, and prevention by using nurse-led sedation scales and protocols to minimize the use of sedative medication might contribute to improving the clinical outcomes of these patients during and after the ICU stay.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Zhongshan hospital, Fudan University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
YZ and SC initiated the study. YZ, SC, XZ, WP, ZL, and CW contributed to the design of the study. WP, JZheng, and JZhong contributed to the data collection. JG, ML, and SC contributed to the data analysis and interpretation. YZ, SC, and JL drafted the first manuscript. All authors contributed to manuscript revisions, read and approved the final version of the manuscript. All authors agree to be accountable for the content of the work.
Funding
This study was supported by the Clinical Research grant from Zhongshan Hospital of Fudan University (2018ZSLC08), by Fudan University Double First-Class Discipline Construction (2018-40-22), by Shanghai Municipal Health Commission Clinical Research grant (20194Y0137), by Zhongshan Hospital of Fudan University the Youth Fund grant (2018ZSQN22), and by Zhongshan Hospital of Fudan University the Development Fund grant (2018ZSFZ022).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Katznelson R, Djaiani G, Tait G, Wasowicz M, Sutherland AM, Styra R, et al. Hospital administrative database underestimates delirium rate after cardiac surgery. Can J Anaesth. (2010) 57:898–902. doi: 10.1007/s12630-010-9355-8
2. Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Prediction of delirium after cardiac surgery and the use of a risk checklist. Eur J Cardiovasc Nurs. (2013) 12:284–92. doi: 10.1177/1474515112450244
3. Mariscalco G, Cottini M, Zanobini M, Salis S, Dominici C, Banach M, et al. Preoperative statin therapy is not associated with a decrease in the incidence of delirium after cardiac operations. Ann Thorac Surg. (2012) 93:1439–47. doi: 10.1016/j.athoracsur.2012.02.012
4. Norkiene I, Ringaitiene D, Kuzminskaite V, Sipylaite J. Incidence and risk factors of early delirium after cardiac surgery. Biomed Res Int. (2013) 2013:323491. doi: 10.1155/2013/323491
5. Guo T, Zhou X, Zhu A, Peng W, Zhong Y, Chai X. The role of serum tenascin-C in predicting in-hospital death in acute aortic dissection. Int Heart J. (2019) 60:919–23. doi: 10.1536/ihj.18-462
6. Ji Q, Lai H, Sun Y, Luo Z, Liu L, Liu C, et al. Impact of presurgical mild acute respiratory distress syndrome on surgical mortality after surgical repair of acute type A aortic dissection. Int Heart J. (2017) 58:739–45. doi: 10.1536/ihj.16-306
7. Di Eusanio M, Schepens MA, Morshuis WJ, Dossche KM, Di Bartolomeo R, Pacini D, et al. Brain protection using antegrade selective cerebral perfusion: a multicenter study. Ann Thorac Surg. (2003) 76:1181–8. doi: 10.1016/S0003-4975(03)00824-5
8. Hagl C, Ergin MA, Galla JD, Lansman SL, McCullough JN, Spielvogel D, et al. Neurologic outcome after ascending aorta-aortic arch operations: effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg. (2001) 121:1107–21. doi: 10.1067/mtc.2001.113179
9. McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. (2003) 51:591–8. doi: 10.1034/j.1600-0579.2003.00201.x
10. Gaul C, Dietrich W, Friedrich I, Sirch J, Erbguth FJ. Neurological symptoms in type A aortic dissections. Stroke. (2007) 38:292–7. doi: 10.1161/01.STR.0000254594.33408.b1
11. Bellelli G, Mazzola P, Morandi A, Bruni A, Carnevali L, Corsi M, et al. Duration of postoperative delirium is an independent predictor of 6-month mortality in older adults after hip fracture. J Am Geriatr Soc. (2014) 62:1335–40. doi: 10.1111/jgs.12885
12. Krzych LJ, Wybraniec MT, Krupka-Matuszczyk I, Skrzypek M, Bolkowska A, Wilczynski M, et al. Detailed insight into the impact of postoperative neuropsychiatric complications on mortality in a cohort of cardiac surgery subjects: a 23,000-patient-year analysis. J Cardiothorac Vasc Anesth. (2014) 28:448–57. doi: 10.1053/j.jvca.2013.05.005
13. Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. (2010) 304:443–51. doi: 10.1001/jama.2010.1013
14. Liu Z, Pang X, Zhang X, Cao G, Fang C, Wu S. Incidence and risk factors of delirium in patients after type-A aortic dissection surgery. J Cardiothorac Vasc Anesth. (2017) 31:1996–9. doi: 10.1053/j.jvca.2016.11.011
15. Shi Q, Mu X, Zhang C, Wang S, Hong L, Chen X. Risk factors for postoperative delirium in type A aortic dissection patients: a retrospective study. Med Sci Monit. (2019) 25:3692–9. doi: 10.12659/MSM.913774
16. von Elm E AD, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. (2007) 335:806–8. doi: 10.1136/bmj.39335.541782.AD
17. Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, et al. Evaluation of delirium in critically ill patients: validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit Care Med. (2001) 29:1370–9. doi: 10.1097/00003246-200107000-00012
18. Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. (1999) 340:669–76. doi: 10.1056/NEJM199903043400901
19. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol. (2007) 165:710–8. doi: 10.1093/aje/kwk052
20. Cai S, Latour JM, Lin Y, Pan W, Zheng J, Xue Y, et al. Preoperative cardiac function parameters as valuable predictors for nurses to recognise delirium after cardiac surgery: a prospective cohort study. Eur J Cardiovasc Nurs. (2019) 19:310–9. doi: 10.1177/1474515119886155
21. Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73. doi: 10.1097/CCM.0000000000003299
22. Koster S, Hensens AG, Schuurmans MJ, van der Palen J. Risk factors of delirium after cardiac surgery: a systematic review. Eur J Cardiovasc Nurs. (2011) 10:197–204. doi: 10.1016/j.ejcnurse.2010.09.001
23. Gosselt AN, Slooter AJ, Boere PR, Zaal IJ. Risk factors for delirium after on-pump cardiac surgery: a systematic review. Crit Care. (2015) 19:346. doi: 10.1186/s13054-015-1060-0
24. Kawatani Y, Nakamura Y, Hayashi Y, Taneichi T, Ito Y, Kurobe H, et al. Development of delirium in the intensive care unit in patients after endovascular aortic repair: a retrospective evaluation of the prevalence and risk factors. Crit Care Res Pract. (2015) 2015:405817. doi: 10.1155/2015/405817
Keywords: type A aortic dissection, delirium, incidence, risk factors, early outcomes
Citation: Cai S, Zhang X, Pan W, Latour JM, Zheng J, Zhong J, Gao J, Lv M, Luo Z, Wang C and Zhang Y (2020) Prevalence, Predictors, and Early Outcomes of Post-operative Delirium in Patients With Type A Aortic Dissection During Intensive Care Unit Stay. Front. Med. 7:572581. doi: 10.3389/fmed.2020.572581
Received: 15 June 2020; Accepted: 14 August 2020;
Published: 25 September 2020.
Edited by:
Marcelo Arruda Nakazone, Faculty of Medicine of São José do Rio Preto, BrazilReviewed by:
Federico Bilotta, Sapienza University of Rome, ItalyNan Lin, Regeneron Genetic Center, United States
Copyright © 2020 Cai, Zhang, Pan, Latour, Zheng, Zhong, Gao, Lv, Luo, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxia Zhang, emhhbmcueXV4aWFAenMtaG9zcGl0YWwuc2guY24=
†These authors have contributed equally to this work and share first authorship
 Shining Cai1†
Shining Cai1† Xiaomin Zhang
Xiaomin Zhang Jos M. Latour
Jos M. Latour Yuxia Zhang
Yuxia Zhang