- 1Department of Cardio-Pulmonary Circulation, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai, China
- 2Department of Cardiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Background: Superoxide dismutases (SODs) are an important family of antioxidant enzymes that modulate reactive oxygen species levels. It is largely unknown which SOD isoform(s) change in vivo in idiopathic pulmonary arterial hypertension (IPAH) patients.
Methods: A total of 133 consecutive adult IPAH patients who underwent bone morphogenetic protein receptor type 2 (BMPR2) genetic counseling were enrolled in this prospective study. The plasma activities of three subtypes of SOD [copper–zinc (Cu/Zn-SOD), manganese (Mn-SOD), and extracellular SOD (Ec-SOD)] were examined.
Results: The activities of SODs were significantly lower in IPAH patients than in healthy subjects. However, only Ec-SOD activity in BMPR2 mutation patients was significantly decreased compared to those in patients without a mutation. The reduced Ec-SOD activity was markedly associated with mean pulmonary arterial pressure, pulmonary vascular resistance (PVR), and 6-min walking distance (6MWD). The reduction of Mn-SOD activity was only associated with 6MWD. There was no association between Cu/Zn-SOD and hemodynamics. Patients with a lower Ec-SOD level had a worse survival compared to those with a higher baseline. The reduced Ec-SOD activity and the raised PVR increased the mortality risk.
Conclusions: Ec-SOD was correlated with BMPR2 mutation, hemodynamic dysfunction, and poor outcomes. Circulating Ec-SOD could be a potentially vital antioxidant enzyme in the pathogenesis of IPAH.
Introduction
The enhanced production of superoxide anions and other reactive oxygen species (ROS) contributes to the pathogenesis of pulmonary arterial hypertension (PAH) (1). The superoxide or ROS inactivates endothelium-derived nitric oxide (NO) and promotes the progression of endothelial dysfunction. Accordingly, steady-state levels of superoxide are dependent on both its rate of production and the activity of various antioxidant enzymes (2, 3). Superoxide dismutases (SODs) are one family of important antioxidant enzymes that defend against superoxide radicals in vascular protection. The expression and the activity of SODs presumably have a profound effect on the responses of vascular cells to both acute and chronic oxidative stress (4).
Blood vessels express three isoforms of SODs: copper–zinc SOD (Cu/Zn-SOD, SOD 1), which locates in the cytosol, manganese SOD (Mn-SOD, SOD 2), which locates in the mitochondrial matrix, and an extracellular form of Cu/Zn-SOD (Ec-SOD, SOD 3) (4, 5). In mammals, different SOD isoforms are encoded by distinct genes but catalyze the same reaction. A reduction of SOD activity has been implicated in patients with PAH and in animal models of PAH (1, 6–12). For example, Cu/Zn-SOD knockout mice spontaneously displayed signs of elevated right ventricular systolic pressure and pulmonary arterial remodeling under normoxia (6). Mn-SOD deficiency was likewise evident in pulmonary arteries and plexiform lesions. Tissue-specific, epigenetic Mn-SOD deficiency initiated and sustained a heritable form of PAH by impairing redox signaling and resulting in proliferation and apoptosis resistance of pulmonary arterial smooth muscle cells (PASMC) (7). In a recent phase I and open-label clinical study in IPAH patients, the plasma Mn-SOD level change was used as a potential marker of drug effect (8). Remarkably, both Ec-SOD mRNA expression and activity were decreased in the lung tissue of idiopathic PAH (IPAH) (9). The loss of function or selective depletion of Ec-SOD exacerbated PAH (10, 11). The intratracheal delivery of adenoviruses overexpressing Ec-SOD could suppress monocrotaline-induced PAH in rats (12). Taken together, these data suggested that SODs are important antioxidant enzymes in the pathogenesis of PAH.
Identifying the link between defective SOD activity and clinical characteristics in IPAH patients is important, which, in turn, can be used as an epigenetic biomarker for a new and improved therapeutic strategy. Approximately 20% of patients with IPAH carried mutations in bone morphogenetic protein receptor type 2 (BMPR2), where a loss of BMPR2 function may compromise the integrity of the endothelial barrier and contribute to endothelial dysfunction by mediating endothelium-derived nitric oxide bioactivity (2, 13, 14). To date, there is limited information on plasma SOD alteration in IPAH patients with or without BMPR2 mutation. Thus, the objective of the present study was to prospectively determine whether (a) the abnormalities of SOD levels were related to hemodynamic dysfunction and clinical characteristics, (b) patients with BMPR2 mutation had a more severe reduction of SOD levels, and (c) plasma SOD level could be a predictor for prognosis and clinical outcome.
Materials and Methods
Study Subjects
One hundred thirty-three consecutive adult IPAH patients (≥18 years of age at diagnosis) who underwent BMPR2 genetic counseling at the time of their first right heart catheterization were prospectively enrolled in this study between January 2010 and July 2013. One hundred thirty control subjects were selected from a cohort of healthy volunteers. The median age of the control subjects was 40 (20–57) years, and the ratio of women to men was 3:1. IPAH was diagnosed according to standard criteria: mean pulmonary artery pressure (mPAP) ≥25 mmHg and pulmonary vascular resistance (PVR) at rest >3 Wood units, in the presence of a normal pulmonary artery wedge pressure (PAWP ≤15 mmHg) (15). Patients were excluded if they have definite causes for PAH, such as connective tissue disease and congenital heart disease, and also those with portopulmonary hypertension, chronic pulmonary thromboembolism, pulmonary hypertension due to left heart diseases, and lung diseases and/or hypoxemia. Other exclusion criteria for the study included potential confounding factors associated with plasma antioxidant enzyme production: cigarette smoking and excessive alcohol consumption, hypertension, and type 2 diabetes mellitus (16, 17). We also excluded the participants who completed an acute exercise and were with vitamin C and E supplementation (18). We prospectively followed up these patients for a mean of 26 ± 9 months after enrollment, and no patient received lung or heart–lung transplantation. The major endpoint was defined as all-cause mortality.
The study was conducted according to the principles of the Declaration of Helsinki and was approved by the Shanghai Pulmonary Hospital Ethics Committee (number K16-055). Written informed consent was obtained from all the participants.
Blood Sampling and Plasma SOD Assay
Venous blood was collected from all subjects after fasting overnight (>12 h) to minimize the influence of foods and beverages on the plasma SOD concentrations. All the samples were collected directly into specially prepared sodium ethylene diamine tetra-acetic acid tubes containing a preservative to retard auto-oxidation. After centrifuging at 3,000 rpm at 4 for 15 min, the supernatant was separated for SOD activity immediate determinations. The whole procedure was completed within 20 min. For the oxidant test, the presence of 0.005% butylated hydroxytoluene (without glutathione) in plasma was allowed for anti-ex vivo oxidation and improving the stability, routinely. Each sample was measured within a month of collection, and at least two different dilutions of the same sample were tested. To minimize the inter- and the intra-assay coefficients of variation, each analyte was duplicated on three different days within 1 month. The plasma Cu/Zn-SOD and Mn-SOD activities were determined by an SOD assay kit (Cayman Chemical Company, item no. 706002) using a tetrazolium salt reaction (9). The method utilized tetrazolium salt for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. Detection of only Mn-SOD activity needs the addition of potassium cyanide to inhibit both Cu/Zn-SOD and Ec-SOD. The samples can be assayed in the absence of xanthine oxidase to generate a sample background. This sample background absorbance (at 440–460 nm) was subtracted from the sample absorbance generated in the presence of xanthine oxidase, thus correcting for non-SOD-generated absorbance.
The plasma activity of Ec-SOD was performed based on the competitive ELISA assay kit (Lifespan Biosciences, NBP1-90377) according to the manufacturer's instructions (19). Each well of the supplied microtiter plate has been pre-coated with a target-specific capture antibody. Standards or samples are added to the wells as well as a fixed quantity of biotin-conjugated target antigen. The antigens in the standards or samples compete with the biotin-conjugated antigen to bind to the capture antibody. An avidin–horseradish peroxidase (HRP) conjugate is then added, which binds to the biotin. A 3,3′,5,5′-tetramethylbenzidine substrate was used for testing for Ec-SOD, and the optical density of the well is measured at a wavelength of 450 ± 2 nm.
Statistical Analysis
Results were expressed as numbers, percentages, means with corresponding standard deviations, or medians with corresponding 25th and 75th percentiles [interquartile range (IQR)]. Continuous variables were compared with baseline characteristics, hemodynamic parameters, and SOD levels using Student's t-test or Mann–Whitney U-test according to normality. The proportions were compared with Pearson chi-square test or Fisher's exact test, as appropriate. Continuous variables were assessed for linearity of their relationship with the outcome variable. Spearman's ρ was investigated for correlations, with a Bonferroni correction for each variable. If these variables were not found to be linearly related to the outcome, they were grouped into quartiles and modeled to avoid violating model assumptions. A univariate analysis of covariance adjusted for age and sex was carried out to compare the means among the three groups (control subjects, BMPR2 mutation carrier group, and BMPR2 wild-type group). Bonferroni method was applied to correct the p-value for multiple comparisons in post hoc tests.
For comparison of the prognostic values of SODs and selected hemodynamic parameters, receiver operating characteristic curves (ROC) were generated and the areas under the curves were calculated. The optimal thresholds for baseline Ec-SOD for death prediction was determined using Youden index (sensitivity + specificity−1). The value of Ec-SOD corresponding to the maximum value of Youden's index was considered as the optimal cutoff point for Ec-SOD. Survival analyses were performed using Kaplan–Meier method and were compared by means of log-rank test. Two steps were performed to analyze the survival. First, a univariable Cox proportional regression was used for time-to-event analysis to estimate hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality according to the stratified covariates (SODs, hemodynamic parameters, 6MWD, WHO FC, female gender, and age). All variables with a p < 0.05 were then tested in a stepwise forward Cox regression analyses; the variables were entered at a p < 0.05. In the second step of the survival analysis, a multivariable stepwise forward Cox regression model was used to estimate the HR and the 95% CI for association in different SOD groups and outcomes adjusted for age and sex. For all analyses, p < 0.05 were considered as statistically significant. All calculations were performed using the SPSS 14.0 statistical software package (Statistical Package for Social Science, Chicago, IL, USA) or StatView 5.0.1 (SAS Institute, Cary, NC, USA).
Results
SOD Levels and Characteristics of the Study Population
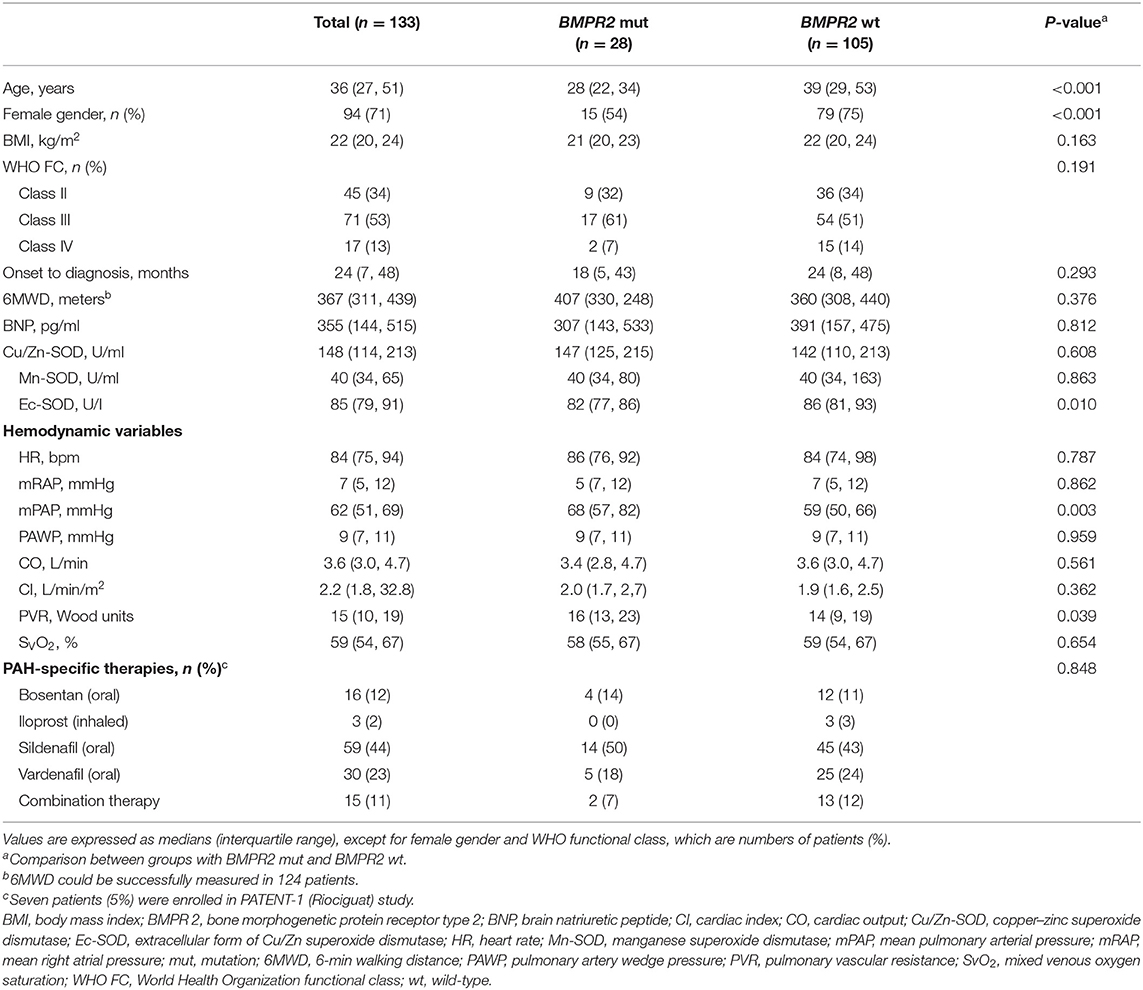
Among the 133 IPAH patients recruited, 28 (21%) were BMPR2 mutation carriers (BMPR2 mut); the other 105 patients were BMPR2 wild-type (BMPR2 wt). The clinical characteristics are summarized in Table 1. The patients with BMPR2 mut had a younger median age at diagnosis (28 years; IQR, 22–34 years) than those of with BMPR2 wt (39 years; IQR, 29–53 years; p < 0.001). The sex ratio of females to males was 2.4:1 (n = 94/39) in the total population. In BMPR2 wt patients, the female/male ratio was 3.0:1 (n = 79/26), whereas in the BMPR2 mut group, the female/male ratio was 1.2:1 (n = 15/13, p < 0.001). In patients with IPAH, plasma Cu/Zn-SOD (148 U/ml; 95% CI, 114–213), Mn-SOD (40 U/ml; 95% CI, 34–115), and Ec-SOD (85 U/L, 95% CI, 79–119) activities were significantly lower compared to those in the 130 control subjects (212 U/ml, 95% CI: 153–266; 144 U/ml, 95% CI: 114–157; and 175 U/L, 95% CI: 143–193, respectively; p < 0.01 or p < 0.001). Among the three comparison groups (control subjects, BMPR2 mut group, and BMPR2 wt group), plasma Ec-SOD activity was still lowest in the BMPR2 mut group, adjusted for female gender (F = 6.679, p = 0.010) and age (F = 1.420, p = 0.002). There was no difference of Cu/Zn-SOD and Mn-SOD in the multiple-comparison tests. Only Ec-SOD activity in patients with BMPR2 mut group was statistically decreased compared to those in the BMPR2 wt group (BMPR2 wt, p = 0.02, Figure 1). However, there was no significant difference regarding WHO functional class severity in both BMPR2 mut and BMPR2 wt groups as well as total patients.
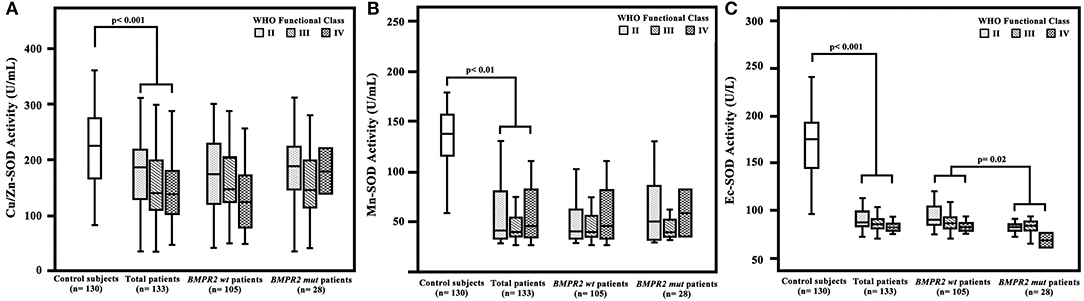
Figure 1. Plasma superoxide dismutase (SOD) activity in patients with idiopathic pulmonary arterial hypertension (IPAH), control subjects, patients with BMPR2 mutation (BMPR2 mut), and those with wild-type BMPR2 (BMPR2 wt), associated with WHO functional class. (A) The plasma Cu/Zn level was significantly decreased in patients with IPAH compared to that in the control subjects. (B) The Mn-SOD activity was significantly lower in patients with IPAH compared to that in the control subjects. (C) The Ec-SOD activities in patients with IPAH were significantly lower compared to those in the control subjects. The Ec-SOD activity in patients of the BMPR2 mut group was statistically decreased compared to that in patients of the BMPR2 wt group. The line through the center of the boxes represents the median. BMPR 2, bone morphogenetic protein receptor type 2; Cu/Zn-SOD, copper–zinc superoxide dismutase; Mn-SOD, manganese superoxide dismutase; Ec-SOD, extracellular form of Cu/Zn superoxide dismutase.
Correlation of SOD Activity With Hemodynamic Variables
The BMPR2 mut patients had a more severe hemodynamic compromise, with a significantly higher mPAP and PVR in comparison with the BMPR2 wt patients (Table 1). The baseline plasma Ec-SOD activities were negatively correlated with mPAP (r = −0.22; p = 0.04) and PVR (r = −0.21; p = 0.02). The Ec-SOD and the Mn-SOD activities were correlated positively with 6MWD (r = 0.26; p = 0.003, r = 0.19; p = 0.04, respectively, Figure 2). The other baseline SOD activities did not correlate with age, mRAP, and mixed venous oxygen saturation (SVO2) (Supplementary Figures S1–S8).
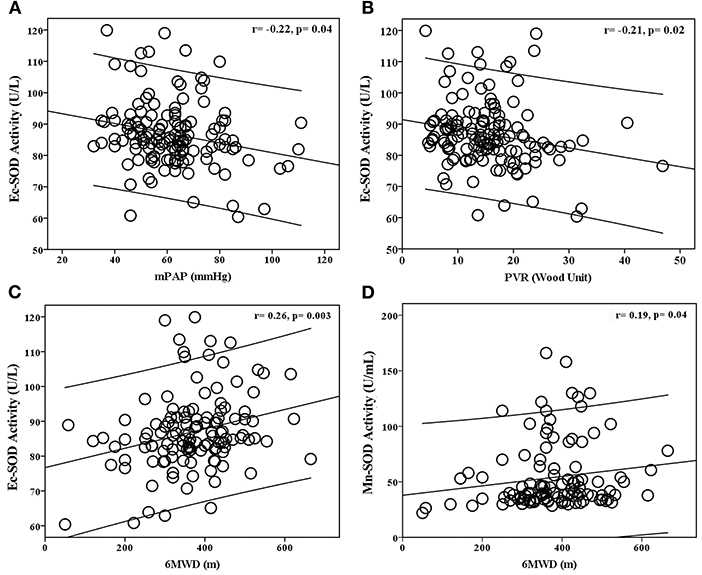
Figure 2. Relationship between baseline plasma Ec-SOD activity with (A) mean pulmonary arterial pressure (mPAP), (B) pulmonary vascular resistance (PVR), (C) 6-min walking distance (6MWD), and (D) plasma Mn-SOD activity with 6MWD in patients with idiopathic pulmonary arterial hypertension (IPAH). The baseline plasma Ec-SOD activities were negatively correlated with mPAP and PVR. The Ec-SOD and Mn-SOD activities were correlated positively with 6MWD.
SOD Activities in Relation to Other Markers of Adverse Outcomes
A univariate analysis identified several factors related to mortality. An elevated mRAP, an increased PVR, and a reduced Ec-SOD were all significantly associated with an increased risk of death. By a stepwise multivariate Cox regression analysis, only the lower Ec-SOD and the higher PVR remained as significant predictors of adverse outcomes (Figure 3).
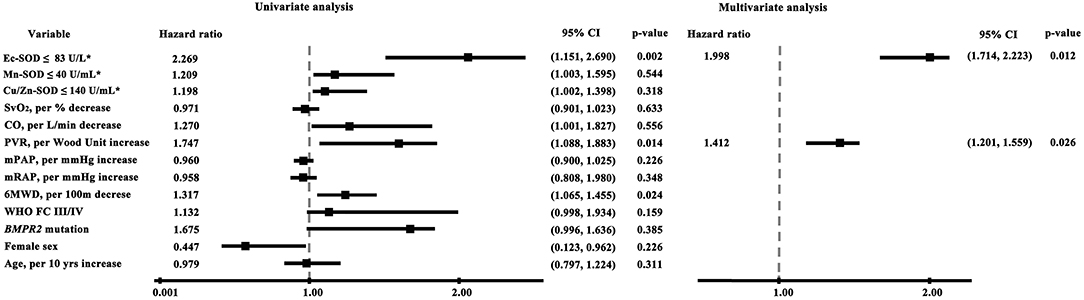
Figure 3. Cox proportional hazards model of factors associated with survival in idiopathic pulmonary arterial hypertension (univariate and multivariate analyses). The lower Ec-SOD and the higher PVR remained as significant predictors of adverse outcomes. The asterisk indicates identification by the receiver operating characteristic analysis curve-derived cutoff value. BMPR 2, bone morphogenetic protein receptor type 2; CO, cardiac output; Cu/Zn-SOD, copper–zinc superoxide dismutase; Ec-SOD, extracellular form of Cu/Zn superoxide dismutase; Mn-SOD, manganese superoxide dismutase; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; 6MWD, 6-min walking distance; PVR, pulmonary vascular resistance; SvO2, mixed venous oxygen saturation.
The ROC curve analyses further illustrated that Ec-SOD was a strong indicator of adverse outcomes in IPAH. The best Ec-SOD cutoff level for predicting outcome was 83 U/L, giving a sensitivity rate of 69% and a specificity rate of 71%. The c-statistic for Ec-SOD level was 0.72 (95% CI, 0.64–0.85), which was similar to PVR (0.73; 0.60–0.86) but numerically superior to 6MWD (0.66; 0.55–0.76), mPAP (0.58; 0.44–0.71), Mn-SOD (0.54; 0.41–0.67), and Cu/Zn-SOD (0.47; 0.32–00.61, Figure 4A).
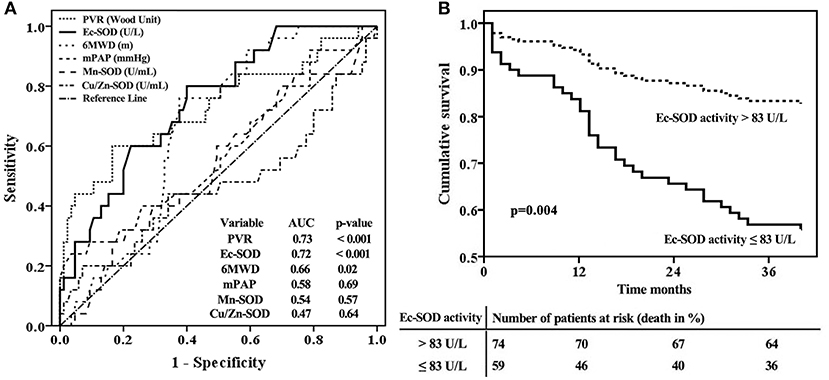
Figure 4. Extracellular form of Cu/Zn superoxide dismutase (Ec-SOD) activity in relation to other markers for an adverse prognosis according to (A) receiver operation characteristic analyses and (B) survival curves for the baseline cutoff plasma Ec-SOD activity in patients with idiopathic pulmonary arterial hypertension, adjusted by age and female gender. The best Ec-SOD cutoff level for predicting outcome was 83 U/L. Survival was significantly better in patients with Ec-SOD activity >83 U/L.
Survival Analysis
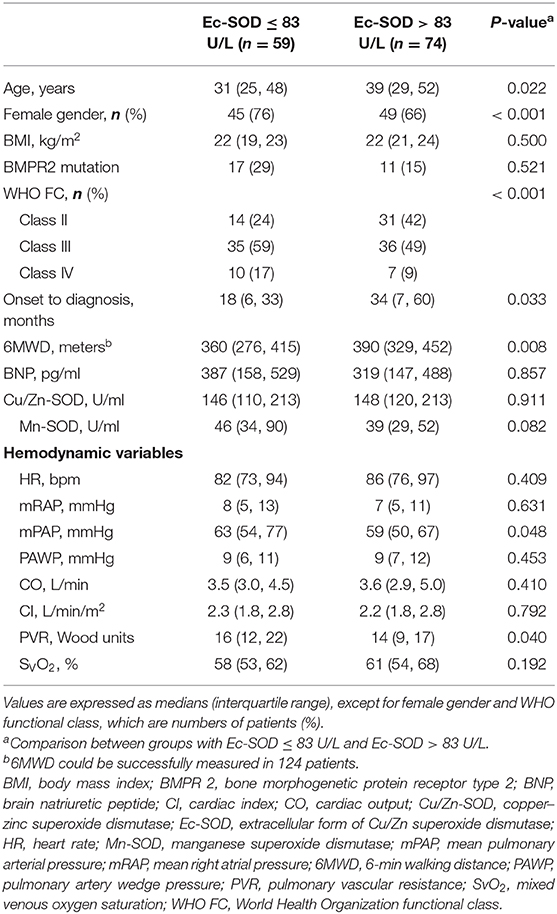
The observed cardiopulmonary mortality was 26% (35 patients) in the current cohort of IPAH patients. Twenty-five of the deaths were directly related to right ventricular failure; six had a sudden death, and the cause of death was not able to be ascertained in four cases. The total patients with IPAH were divided into two groups based on the cutoff levels for Ec-SOD calculated by ROC analysis to detect mortality.
In the group of 59 patients with Ec-SOD activity ≤83 U/L, 24 (41%) died, but only 11 of 74 (15%) patients were in the group with Ec-SOD activity >83 U/L (p < 0.001). The patients with Ec-SOD activity >83 U/L had a significantly lower mPAP and PVR than those with Ec-SOD activity ≤83 U/L. Moreover, the proportion of female gender and the severity of WHO functional class in the group with Ec-SOD ≤83U/L were higher than those with Ec-SOD >83 U/L (Table 2). Survival was significantly better in patients with Ec-SOD activity >83 U/L (Figure 4B). The 1- and 3- year survival estimates were 84 and 63%, respectively, in patients with Ec-SOD activity ≤83 U/L and 95 and 86%, respectively, in patients with Ec-SOD activity >83U/L (p = 0.004), adjusted by age and female gender.
Discussion
Antioxidant enzymes play prominent roles against oxidative damage and provide protective signals in pathological remodeling of the pulmonary vasculature during the development of PAH. Understanding the SOD pathway will greatly help in the clinical development of medications for PAH therapy. However, it is unclear whether an abnormality of SOD activity is associated with hemodynamic dysfunction and poor outcomes in patients with IPAH. Our results demonstrate that (a) the plasma SOD activity was markedly reduced in patients with IPAH compared to that in healthy subjects, (b) patients with BMPR2 mutation had a lower Ec-SOD level than the no-mutation subjects, (c) only Ec-SOD activity was correlated with hemodynamic abnormalities and survival, and (d) a decreased Ec-SOD activity was associated with an increased mortality risk, suggesting that the use of Ec-SOD may be superior to Cu/Zn-SOD or Mn-SOD for supplemental treatment if possible.
Oxidative stress in PAH is rather inescapable with the increased lipid peroxidation and reduced antioxidant defenses (20). The expression and the activity of SODs presumably have profound effects on the responses of vascular cells to oxidative stress (4). It is noteworthy that the plasma activity of all SODs' isoforms is statistically decreased in patients with IPAH compared with that in the healthy subjects in our study, especially for Ec-SOD activity. The decreases in Ec-SOD activities might not be attributed solely to superoxide or ROS level; the mechanisms of post-translation modification, genetic polymorphisms, and epigenetic regulation are included (21–23). For example, exogenous hydrogen peroxide (H2O2) inactivates Ec-SOD in persistent pulmonary hypertension of newborn lambs. Through oxidation of histidine residues in copper-containing catalytic sites, H2O2 has been shown to inhibit Ec-SOD activity (21). Moreover, Nozik-Grayck et al. have reported that histone deacetylation contributed to low Ec-SOD expression in PASMC from IPAH patients, specifically via class I histone deacetylase 3 (9, 23). There is also evidence that miR21 could inhibit Ec-SOD expression in lung epithelial cells in PAH (23). Collectively, Ec-SOD activity may be regulated by comprehensive processes in pulmonary hypertension; substantial work is geared on how to use Ec-SOD to evaluate the oxidative stress system.
Despite important advances in understanding the genetics of PAH (such as mutation in BMPR2 in familial PAH) and the recognition of somatic chromosomal abnormalities in sporadic PAH, the cause of most cases of PAH remains yet unclear (24, 25). It has been reported that patients with BMPR2 mutations exhibited a reduced level of NO production (26). Despite finding 21% of IPAH patients to have BMPR2 mutations, not all patients with a mutation in the present cohort had a corresponding lower activity of SODs. It has been shown that Ec-SOD is the only extracellular isoform and occurs in bodily fluids such as plasma, lymph, and synovial and cerebrospinal fluid in the human organism (27). Ec-SOD can bind to the surface of endothelial cells by a high abundance of heparin sulfate. Consequently, it is highly expressed in lung tissue (28). Notably, Ec-SOD is the predominant isoform responsible for up to 70% of all SOD activity in the cardiovascular system (29, 30). Xu et al. reported that Ec-SOD gene mutation (SOD 3E124D) in rats or SOD 3 knockout in mice aggravated the development of PAH under stress conditions (10). In addition, Ec-SOD preserves NO levels as it diffuses from the endothelium to its major target (soluble guanylate cyclase) in the vascular muscle (4, 31). The activity of Ec-SOD was markedly lower in BMPR2 mutation patients in our present study, possibly due to the decreased bioavailability of NO and/or decreased responsiveness to NO in PAH.
We found that the reduced Ec-SOD activity was closely associated with the severity of hemodynamic impairment in the study population as a whole, implying that antioxidant enzyme deficiency might partly reflect pulmonary vascular resistance under oxidative stress status. ROS could stimulate vasoconstriction or proliferation of PASMC through NO reduction, leading to the pathogenesis of PAH (12). Ec-SOD might halt the ROS cascade by disproportioning superoxide anion and maintaining NO bioavailability in pulmonary arteries (12). Our group has reported that patients with IPAH who carry the BMPR2 mutation had further reduced NO metabolites and worse hemodynamics (27). Sustained Ec-SOD expression in the pulmonary artery might exert a central role in extracellular antioxidative properties (32). Hence, the low NO availability perhaps disturbs the balance and the distribution of SODs.
The role of SODs in pulmonary vasculature has not been fully understood. In a competing reaction, superoxide reacts six times faster with NO than with any isoform of the SODs (33, 34). The endogenous Ec-SOD does not participate in the development of PAH under basal conditions but plays a role of protecting the lung from the development of PAH under stress conditions (9). Reports of the association of genes encoding the SOD enzymes in cardiovascular complications are scarce (35). One study found a rare functional variant rs1799895 (Arg213Gly) in the heparin-binding domain of Ec-SOD. The Gly allele was associated with reduced Ec-SOD affinity for heparin and decreased tissue binding (36). Accordingly, the associations of the Gly allele with cardiovascular risk factors, morbidity, or mortality have been reported (35, 37, 38). Although we did not design and detect the genetic variant of Ec-SOD in patients with IPAH, it is an important finding that Ec-SOD, but not Cu/Zn-SOD or Mn-SOD levels, could predict long-term outcomes in our study as the strength of risk prediction for Ec-SOD activity was robust. The vascular wall contains large amounts of Ec-SOD, implicating that reduced Ec-SOD activity might contribute to endothelial dysfunction and NO degradation. Thus, SODs, which are responsible for preventing oxidative damage, might be beneficial for the supplemental treatment for IPAH.
Several limitations of the present study must be noted while interpreting the results. First, since plasma SOD levels were only measured at baseline, it was difficult for us to evaluate the variability and the prognosis of level changes over time or the impact of different therapies. Second, blood samples for measuring the plasma SOD levels were collected after an overnight (>12 h) fast in all patients and subjects; the best time to collect samples for prediction remains unclear. However, there are studies reporting that SOD activities were stable in samples initially kept frozen, whose activities were not subject to protein alteration as a result of the freezing procedure (39). The difference between inter-batch and inter-operator variability was reasonable. Furthermore, another potential limitation is lack of race; the findings may be applicable to the Chinese Han population. It remains to be explained whether the results of this study are exclusive to the selected population. Finally, we had better to perform the sensitivity analyses in order to assess the validity and the robustness of the analyses based on the cutoff value.
Conclusion
In summary, we demonstrated that the baseline plasma SOD activities were significantly lower in patients with IPAH than in healthy control subjects. Only the Ec-SOD level was associated with the hemodynamic measures of disease severity and BMPR2 mutation. Patients with reduced Ec-SOD activity had increased risks for mortality independent of clinical characteristics and other risk factors. Decreased circulating Ec-SOD could potentially be used as a biomarker in the prognosis of IPAH patients.
What Is Already Known on This Subject?
▶ Antioxidant enzymes play prominent roles against oxidative damage and protective signals in pathological remodeling of the pulmonary vasculature during the development of idiopathic pulmonary arterial hypertension (IPAH). Superoxide dismutases (SODs) are an important family of antioxidant enzymes that modulate reactive oxygen species levels.
WHat Might This Study Add?
▶ This study adds to the present knowledge that plasma SOD activities were significantly lower in patients with IPAH than in healthy control subjects. Only Ec-SOD level was associated with the hemodynamic measures of disease severity and BMPR2 mutation. Patients with reduced Ec-SOD activity had increased risks for mortality, independent of clinical characteristics and other risk factors. Ec-SOD is a vital antioxidant enzyme and superior to the other two isoforms in PAH pathogenesis.
How Might This Impact on Clinical Practice?
▶ Understanding the SOD pathway will greatly help in the clinical development of medications for PAH therapy. This study demonstrated that the use of Ec-SOD may be superior to the other two SOD isoforms for supplemental treatment.
Data Availability Statement
All datasets presented in this study are included in the article/Supplementary Material.
Ethics Statement
The studies involving human participants were reviewed and approved by Shanghai Pulmonary Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
All authors participated in the design of this study and/or patient enrolment and met the criteria for authorship. Z-CJ contributed to the study design, conduct of the study, data analysis, scientific overview, and editing of the manuscript and was directly involved in the recruitment and care of the participants. RZ contributed to the data analysis, scientific interpretation, and drafting and editing of the manuscript. LW, Q-HZ, RJ, S-GG, XJ, X-QX, Y-YH, and YL were directly involved in the recruitment and care of the participants and in data collection. All the authors had full access to all data of this study and had final responsibility for the decision to submit for publication. All the authors have reviewed the manuscript and approved the final version for submission.
Funding
This study was supported in part by Shanghai Pujiang Program 18PJD043 and the International Cooperation Project of Science and Technology Commission Shanghai Municipality 19410741000 (RZ), the Program of Natural Science Foundation of Shanghai 18ZR1431500 (LW), the Cohort Study of Orphan Diseases, Major Program of the National Natural Science Foundation of China, Precision Medicine, the 13th 5-Year Plan 2016YFC0901500, the Key Program of National Natural Science Foundation of China 81630003, and CAMS Innovation Fund for Medical Sciences 2016-I2M-1-002, 2017-I2M-B&R-02 (Z-CJ). The sponsors had no involvement in the study design, data analysis, data interpretation, and writing or revision of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the contribution of all investigators who participated in this study. We also thank the patients who participated in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00509/full#supplementary-material
Abbreviations
BMI, body mass index; BMPR2, bone morphogenetic protein receptor type 2; BNP, brain natriuretic peptide; CI, cardiac index; 95% CI, 95% confidence interval; CO, cardiac output; Cu/Zn-SOD, copper–zinc superoxide dismutase; Ec-SOD, extracellular form of Cu/Zn superoxide dismutase; IPAH, idiopathic pulmonary arterial hypertension; Mn-SOD, manganese superoxide dismutase; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; 6MWD, 6-min walk distance; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; ROC, receiver operation characteristic; SOD, superoxide dismutase; SvO2, mixed venous oxygen saturation.
References
1. Bowers R, Cool C, Murphy RC, Tuder RM, Hopken MW, Flores SC, et al. Oxidative stress in severe pulmonary hypertension. Am J Respir Crit Care Med. (2004) 169:764–9. doi: 10.1164/rccm.200301-147OC
2. Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. (2008) 10:1713–65. doi: 10.1089/ars.2008.2027
3. Wei EP, Kontos HA, Christman CW, DeWitt DS, Povlishock JT. Superoxide generation and reversal of acetylcholine-induced cerebral arteriolar dilation after acute hypertension. Circ Res. (1985) 57:781–7. doi: 10.1161/01.RES.57.5.781
4. Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. (2004) 24:1367–73. doi: 10.1161/01.ATV.0000133604.20182.cf
5. Fukai T, Ushio-Fukai M. Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal. (2011) 15:1583–606. doi: 10.1089/ars.2011.3999
6. Ramiro-Diaz JM, Nitta CH, Maston LD, Codianni S, Giermakowska W, Resta TC, et al. Nfat is required for spontaneous pulmonary hypertension in superoxide dismutase 1 knockout mice. Am J Physiol Lung Cell Mol Physiol. (2013) 304:L613–25. doi: 10.1152/ajplung.00408.2012
7. Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. (2010) 121:2661–71. doi: 10.1161/CIRCULATIONAHA.109.916098
8. Hemnes AR, Rathinasabapathy A, Austin EA, Brittain EL, Carrier EJ, Chen X, et al. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J. (2018) 51:1702638. doi: 10.1183/13993003.02638-2017
9. Nozik-Grayck E, Woods C, Stearman RS, Venkataraman S, Ferguson BS, Swain K, et al. Histone deacetylation contributes to low extracellular superoxide dismutase expression in human idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. (2016) 311:L124–34. doi: 10.1152/ajplung.00263.2015
10. Xu D, Guo H, Xu X, Lu Z, Fassett J, Hu X, et al. Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension. (2011) 58:303–9. doi: 10.1161/HYPERTENSIONAHA.110.166819
11. Nozik-Grayck E, Woods C, Taylor JM, Benninger RK, Johnson RD, Villegas LR, et al. Selective depletion of vascular ec-sod augments chronic hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. (2014) 307:L868–76. doi: 10.1152/ajplung.00096.2014
12. Kamezaki F, Tasaki H, Yamashita K, Tsutsui M, Koide S, Nakata S, et al. Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. Am J Respir Crit Care Med. (2008) 177:219–26. doi: 10.1164/rccm.200702-264OC
13. Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. (2009) 54:S32–42. doi: 10.1016/j.jacc.2009.04.015
14. Gangopahyay A, Oran M, Bauer EM, Wertz JW, Comhair SA, Erzurum SC, et al. Bone morphogenetic protein receptor ii is a novel mediator of endothelial nitric-oxide synthase activation. J Biol Chem. (2011) 286:33134–40. doi: 10.1074/jbc.M111.274100
15. Galiè N, Humbert M, Vachiery JL, Bibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS): endorsed by: association for european paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Repir J. (2015) 46:903–75. doi: 10.1183/13993003.01032-2015
16. Bandeira Sde M, Guedes Gda S, da Fonseca LJ, Pires AS, Gelain DP, Moreira JC, et al. Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and sod activity. Oxid Med Cell Longev. (2012) 2012:819310. doi: 10.1155/2012/819310
17. Dong X, Li D, Liu H, Zhao Y. Sod3 and enos genotypes are associated with sod activity and nox. Exp Ther Med. (2014) 8:328–34. doi: 10.3892/etm.2014.1720
18. Morrison D, Hughes J, Della Gatta PA, Mason S, Lamon S, Russell AP, et al. Vitamin C and E supplementation prevents some of the cellular adaptations to endurance-training in humans. Free Radic Biol Med. (2015) 89:852–62. doi: 10.1016/j.freeradbiomed.2015.10.412
19. Lu Z, Xu X, Hu X, Zhang P, vanDell ED, French JP, et al. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. (2008) 51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186
20. Anwar A, Ruffenach G, Mahajan A, eEghbali M, Umar S. Novel biomarkers for pulmonary arterial hypertension. Respir Res. (2016) 17:88. doi: 10.1186/s12931-016-0396-6
21. Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal. (2011) 15:1497–506. doi: 10.1089/ars.2010.3630
22. Zelko IN, Folz RJ. Regulation of oxidative stress in pulmonary artery endothelium. modulation of extracellular superoxide dismutase and NOX4 expression using histone deacetylase class I inhibitors. Am J Respir Cell Mol Biol. (2015) 53:513–24. doi: 10.1165/rcmb.2014-0260OC
23. Boucherat O, Potus F, Bonnet S. microRNA and pulmonary hypertension. Adv Exp Med Biol. (2015) 888:237–52. doi: 10.1007/978-3-319-22671-2_12
24. Archer SL. Acquired mitochondrial abnormalities, including epigenetic inhibition of superoxide dismutase 2, in pulmonary hypertension and cancer: therapeutic implications. Adv Exp Med Biology. (2016) 903:29–53. doi: 10.1007/978-1-4899-7678-9_3
25. Aldred MA, Comhair SA, Varella-Garcia M, Asosingh K, Xu W, Noon GP, et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. (2010) 182:1153–60. doi: 10.1164/rccm.201003-0491OC
26. Zhang R, Wang XJ, Zhang HD, Sun XQ, Zhao QH, Wang L, et al. Profiling nitric oxide metabolites in patients with idiopathic pulmonary arterial hypertension. Eur Respir J. (2016) 48:1386–95. doi: 10.1183/13993003.00245-2016
27. Pinto A, Immohr MB, Jahn A, Jenke A, Boeken U, Lichtenberg A, et al. The extracellular isoform of superoxide dismutase has a significant impact on cardiovascular ischaemia and reperfusion injury during cardiopulmonary bypass. Eur J Cardiothorac Surg. (2016) 50:1035–44. doi: 10.1093/ejcts/ezw216
28. Nozik-Grayck E, Suliman HB, Majka S, Albietz J, Van-Rheen Z, Roush K, et al. Lung ec-sod overexpression attenuates hypoxic induction of egr-1 and chronic hypoxic pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. (2008) 295:L422–30. doi: 10.1152/ajplung.90293.2008
29. Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med. (2003) 35:236–56. doi: 10.1016/S0891-5849(03)00275-2
30. Stralin P, Karlsson K, Johansson BO, Marklund SL. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler Thromb Vasc Biol. (1995) 15:2032–6. doi: 10.1161/01.ATV.15.11.2032
31. Wolin MS. Interactions of oxidants with vascular signaling systems. Arterioscler Thromb Vascu Biol. (2000) 20:1430–42. doi: 10.1161/01.ATV.20.6.1430
32. Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. (2006) 174:1370–7. doi: 10.1164/rccm.200605-676OC
33. Brown DI, Griendling KK. Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Cir Res. (2015) 116:531–49. doi: 10.1161/CIRCRESAHA.116.303584
34. Gheddouchi S, Mokhtari-Soulimane N, Merzouk H, Bekhti F, Soulimane F, Guermouche B, et al. Low sod activity is associated with overproduction of peroxynitrite and nitric oxide in patients with acute coronary syndrome. Nitric Oxide. (2015) 49:40–6. doi: 10.1016/j.niox.2015.05.007
35. Mohammedi K, Bellili-Munoz N, Marklund SL, Driss F, Le-Nagard H, Patente TA, et al. Plasma extracellular superoxide dismutase concentration, allelic variations in the sod3 gene and risk of myocardial infarction and all-cause mortality in people with type 1 and type 2 diabetes. Cardiovasc Diabetol. (2015) 14:845. doi: 10.1186/s12933-014-0163-2
36. Chu Y, Alwahdani A, Iida S, Lund DD, Faraci FM, Heistad DD. Vascular effects of the human extracellular superoxide dismutase R213g variant. Circulation. (2005) 112:1047–53. doi: 10.1161/CIRCULATIONAHA.104.531251
37. Juul K, Tybjaerg-Hansen A, Marklund S, Heegaard NH, Steffensen R, Sillesen H, et al. Genetically reduced antioxidative protection and increased ischemic heart disease risk: the copenhagen city heart study. Circulation. (2004) 109:59–65. doi: 10.1161/01.CIR.0000105720.28086.6C
38. Marklund SL, Nilsson P, Israelsson K, Schampi I, Peltonen M, Asplund K. Two variants of extracellular-superoxide dismutase: Relationship to cardiovascular risk factors in an unselected middle-aged population. J Intern Med. (1997) 242:5–14. doi: 10.1046/j.1365-2796.1997.00160.x
39. Ito Y, Nakachi K, Imai K, Hashimoto S, Watanabe Y, Inaba Y, et al. Stability of frozen serum levels of insulin-like growth factor-i, insulin-like growth factor-ii, insulin-like growth factor binding protein-3, transforming growth factor beta, soluble fas, and superoxide dismutase activity for the jacc study. J Epidemiol. (2005) 15 (Suppl. 1): S67–73. doi: 10.2188/jea.15.S67
Keywords: idiopathic pulmonary arterial hypertension, superoxide dismutases, extracellular SOD, biomarkers, prognosis
Citation: Zhang R, Wang L, Zhao Q-H, Jiang R, Gong S-G, Jiang X, Xu X-Q, He Y-Y, Li Y and Jing Z-C (2020) Alteration of Extracellular Superoxide Dismutase in Idiopathic Pulmonary Arterial Hypertension. Front. Med. 7:509. doi: 10.3389/fmed.2020.00509
Received: 08 May 2020; Accepted: 23 July 2020;
Published: 17 November 2020.
Edited by:
Mahmood Yaseen Hachim, Mohammed Bin Rashid University of Medicine and Health Sciences, United Arab EmiratesReviewed by:
Mohamed A. Saleh, University of Sharjah, United Arab EmiratesClaudia Mickael, University of Colorado Denver, United States
Viviane De Maertelaer, Université Libre de Bruxelles, Belgium
Copyright © 2020 Zhang, Wang, Zhao, Jiang, Gong, Jiang, Xu, He, Li and Jing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Cheng Jing, amluZ3poaWNoZW5nQHZpcC4xNjMuY29t
†These authors have contributed equally to this work
 Rui Zhang
Rui Zhang Lan Wang1†
Lan Wang1† Rong Jiang
Rong Jiang Yang-Yang He
Yang-Yang He Yuan Li
Yuan Li Zhi-Cheng Jing
Zhi-Cheng Jing