- 1Department of Medicine, King Edward Medical University, Lahore, Pakistan
- 2Department of Medicine, Khyber Medical College, Peshawar, Pakistan
- 3Department of Medicine, Allama Iqbal Medical College, Lahore, Pakistan
- 4Department of Medicine, Ziauddin University, Karachi, Pakistan
- 5Department of Medicine, Dow University of Health Sciences, Karachi, Pakistan
- 6Department of Gastroenterology, Pakistan Kidney and Liver Institute, Lahore, Pakistan
- 7Department of Cardiology, Baylor College of Medicine, Houston, TX, United States
Background: The recent COVID-19 pandemic sweeping the globe has caused great concern worldwide. Due to the limited evidence available on the dynamics of the virus and effective treatment options available, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has had a huge impact in terms of morbidity and mortality. The economic impact is still to be assessed.
Aims: The purpose of this article is to review the evidence for the multiple treatment options available, to consider the future of this global pandemic, and to identify some potential options that could revolutionize the treatment of COVID-19. Moreover, this article underscores the sheer importance of repurposing some of the available antiviral and antimicrobial agents that have long been in use so as to have an effective and expeditious response to this widespread pandemic and the need to conduct a multicenter global randomized controlled trial to find an effective single antiviral agent or a cocktail of available antimicrobial agents.
Method: We thoroughly searched and reviewed various case reports, retrospective analyses, and in vitro studies published in PubMed, EMBASE, and Google Scholar regarding the treatment options used for SARS-CoV, MERS-CoV, and SARS-CoV-2 since its outbreak in an attempt to highlight treatments with the most promising results.
Conclusion: We are currently facing one of the worst pandemics in history. Although SARS-CoV-2 is associated with a lower mortality rate than are SARS-CoV and MERS-CoV, its higher infectivity is making it a far more serious threat. Unfortunately, no vaccine against SARS-CoV-2 or effective drug regimen for COVID-19 currently exists. Drug repurposing of available antiviral agents may provide a respite; moreover, a cocktail of antiviral agents may be helpful in treating this disease. Here, we have highlighted a few available antimicrobial agents that could be very effective in treating COVID-19; indeed, a number of trials are underway to detect and confirm the efficacy of these agents.
Introduction
In December 2019, the city of Wuhan, China, saw the outbreak of an unusual disease manifesting as severe pneumonia and respiratory distress. This disease epidemic was later shown to be caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has now engulfed the world. The disease has spread across borders, leading to a global pandemic, and is currently showing no significant plateauing. SARS-CoV-2, formerly known as novel coronavirus (2019-nCOV), is a positive single-stranded RNA virus belonging to the family coronaviridae (1). Currently, there is not much strong evidence from randomized clinical trials to show improved outcomes or a decrease in terms of mortality with regards to the various treatment options available or prophylactic treatment. With little known about the virus and treatments available, we here highlight some of the leading therapeutic options and compare and contrast these in an attempt to determine which may be the most promising. We try to highlight up-to-date published clinical data and the treatment strategies for this novel pandemic so far.
Given the rapid spread so far and the new treatment modalities under consideration, the main focus lay on the repurposing of existing drugs, with several trials underway in attempts to find the most revolutionizing one.
Treatment Modalities Compared and Contrasted
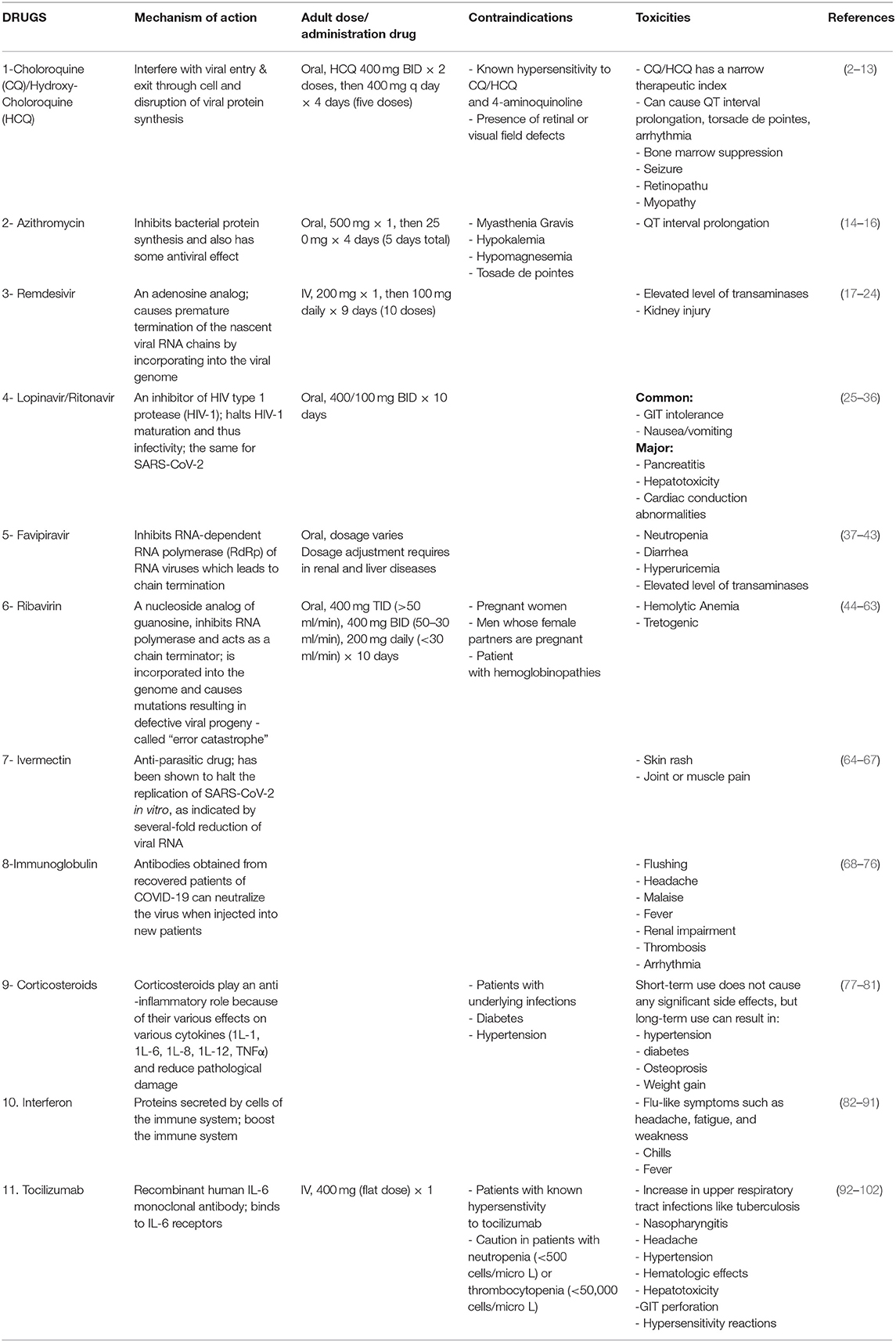
The treatment options available for COVID-19 and their mechanisms of action are briefly outlined in Table 1.
Chloroquine (CQ)/Hydroxychloroquine (HCQ)
CQ and HCQ have been used for the treatment of malaria for 70 years. CQ and HCQ act on multiple pathways of virus entry into and exit from cells and cause disruption of the essential viral protein synthesis (2). The in-vitro activities of CQ and HCQ have been shown to have an inhibitory effect on SARS-CoV-2 mRNA production, with HCQ showing greater efficacy than CQ (3, 4). However, in vitro activity cannot be interpreted as clinical activity against COVID-19; in vitro activity of CQ/HCQ against many other viruses, such as Ebola virus (5), Chikungunya virus (6), influenza virus (7), HIV (8), and dengue virus (9), has been reported previously, but their clinical efficacy did not reach that seen in vitro.
In a non-randomized trial in France on 36 patients with COVID-19, HCQ was administered alone or in combination with azithromycin. After 6 days of treatment, 100% of patients treated with the hydroxychloroquine and azithromycin combination had no detectable viral load in nasopharyngeal swabs compared to 57.1% of patients treated with hydroxychloroquine only and 12.5% of the control group (p < 0.001) (10). In another report from China in 100 patients with COVID-19, those treated with HCQ showed better clinical outcomes than control patients (11).
Triggered somewhat by the media and an intense pressure to prescribe a medication to COVID-19 patients and also due to the general perception of CQ/HCQ efficacy, clinicians may turn to off-label use of CQ/HCQ. Off-label use of CQ/HCQ is occurring globally, including in some hospitals in the USA but should be approached cautiously, as CQ and HCQ have a narrow therapeutic index and can cause QT interval prolongation, torsade de pointes, arrhythmia (12), bone marrow suppression, seizure, retinopathy, and myopathy.
Given the lack of evidence, we strongly call on public health organizations to collaborate effectively with local governments to support unified randomized controlled trials (RCTs) to test the potential therapeutic effects of CQ/HCQ against COVID-19. If the ethical use, safety, and advanced clinical efficacy of CQ/HCQ can be established by RCTs, as proposed by the WHO, it would be a significant advancement in the treatment of COVID-19 patients. Global multicenter RCTs would be the most effective approach for collecting accurate data about the safety and clinical efficacy of CQ/HCQ for the treatment of COVID-19, and this strategy would allow robust data to be available in the near future (13).
Azithromycin
Azithromycin is a bacteriostatic belonging to the macrolides class that inhibits bacterial protein synthesis and thus interferes with bacterial growth. It is also known to have antiviral effects in addition to its antibacterial properties. It has been used to treat respiratory viral infection due to this former property (14).
In a small non-randomized study conducted by Gautret et al., azithromycin in combination with HCQ has demonstrated substantial antiviral activity against SARS-CoV-2 (10). Literature on azithromycin alone as a treatment option for COVID-19 is scarce, and it is not clear whether macrolides can be used alone or should be in combination with HCQ. Masashi et al. believe that macrolides alone, or in combination with other drugs, are effective against SARS-CoV-2 (15).
Several clinical trials are being conducted to check the efficacy of HCQ-azithromycin for SARS-CoV-2. An interventional clinical trial is underway to determine the efficacy and safety of HCQ-azithromycin (16).
Remdesivir
Remdesivir is an adenosine analog that interferes with the synthesis of new viral RNA by chain termination. Although it was developed to be used against Ebola virus and Marburg virus infections (17), it showed antiviral activity against many other RNA viruses such as Lassa virus, respiratory syncytial virus, and coronaviruses such as SARS-CoV and MERS-CoV. Due to its antiviral activity against SARS-CoV and MERS-CoV, it has also been tested against SARS-CoV-2.
Remdesivir achieved satisfactory results in the Ces1c (–/–) mouse SARS model. It significantly reduced lung virus titers and improved pulmonary function when administered one day after disease onset (p < 0.0001). Virus titers were discernibly reduced, but with high mortality of mice, when administered 2 days after disease onset. This study concluded that when lung injury reaches a peak, simply reducing the virus titers can no longer suppress the strong immune responses in mice, but remdesivir can significantly improve the symptoms and mortality (p = 0.0037) in mice when administered at early stages (18).
A case has been reported in which a patient with a SARS-CoV-2 infection confirmed by RT-PCR (performed on a nasopharyngeal swab) showed drastic improvement in one day with remdesivir (19). On account of the broad-spectrum anti-CoV activity of remdesivir, a randomized, double-blinded clinical trial was planned and is still ongoing (20). This study includes 308 participants, randomized to either remdesivir or placebo. Another phase 3 randomized, double-blinded, placebo-controlled study is underway focusing on the safety and efficacy of remdesivir in 452 hospitalized adults with severe respiratory symptoms from SARS-CoV-2 (21).
In an in vitro study, remdesivir inhibited the growth of bat-CoVs and human CoV (22). Another study revealed that remdesivir and chloroquine are very effective against SARS-CoV-2 in vitro (23).
Preliminary results from a recent randomized, placebo-controlled, double-blind phase 3 clinical trial in hospitalized patients with COVID-19 revealed that compared to placebo, remdesivir was associated with shorter time to recovery (11 vs. 15 days) (24)
Lopinavir/Ritonavir
Lopinavir is an inhibitor of HIV type 1 protease (HIV-1), halting the maturation of HIV-1 and thus its infectivity (25). Ritonavir, which is also a protease inhibitor, is administered in combination with lopinavir to enhance its bioavailability by inhibiting its metabolic inactivation (25). This combination is considered to be a highly effective antiretroviral agent, and some studies even advocate the use of monotherapy as a therapeutic option in certain HIV-infected patients (26). Along with other drugs (chloroquine, chlorpromazine, and loperamide), lopinavir was found to inhibit the in vitro replication of MERS-CoV and SARS-CoV (27). In patients with SARS associated with SARS-CoV infection, the combination of lopinavir/ritonavir and ribavirin resulted in a lower rate of acute respiratory distress syndrome (ARDS) or death at day 21 when compared to the historical control group treated with ribavirin only (2.4 vs. 28.8%, p < 0.001) (28). The lopinavir/ritonavir and ribavirin combination also allowed a reduction in steroid dosages and resulted in a decreased incidence of nosocomial infection (28). Lopinavir/ritonavir also reduced mortality in marmosets with MERS-like disease. The mortality rate at 36 h post-inoculation was 0–33% with lopinavir/ritonavir treatment vs. 67% in untreated or mycophenolate-treated animals (29). A case was reported in which a patient with MERS-CoV pneumonia improved and showed viral clearance after 6 days of triple antiviral therapy with lopinavir/ritonavir, ribavirin, and pegylated interferon (IFN)-alpha 2a (30). In another case, a patient with MERS-CoV pneumonia who later developed renal failure was started on triple antiviral therapy (lopinavir/ritonavir, ribavirin, and pegylated interferon) and showed resolution of viremia 2 days after treatment initiation, though virus shedding continued, highlighting the importance of starting ribavirin treatment early (31).
Given the effectiveness of lopinavir/ritonavir against MERS-CoV and SARS-CoV, it was thus tested for the treatment of SARS-CoV-2. Lopinavir, but not ritonavir, inhibits the in vitro replication of SARS-CoV-2 (32). Lopinavir/ritonavir was recommended for the treatment of SARS-CoV-2 pneumonia in China (33). A small report showed that out of four patients (two with mild SARS-CoV-2 pneumonia and two with severe) treated with lopinavir/ritonavir, umifenovir, and Shufeng Jiedu Capsule (a traditional Chinese medicine), three patients showed significant improvement and were discharged, while the other patient (with severe pneumonia) showed signs of improvement (34). In a patient with SARS-CoV-2 mild pneumonia, administration of lopinavir/ritonavir resulted in a decrease in the viral load from the very next day, and viral titers were later undetectable (35). The author highlighted that the decrease in viral titers could be due to the natural course of the disease; therefore, further studies are needed to determine the direct antiviral effect of lopinavir/ritonavir. Another young woman treated with lopinavir/ritonavir for SARS-CoV-2 pneumonia showed improvement after 7 days of therapy (36).
Favipiravir
Favipiravir inhibits RNA-dependent RNA polymerase (RdRp) of RNA viruses (but not cellular RNA and DNA synthesis) (37) and shows broad-spectrum antiviral activity against RNA viruses (38). Favipiravir (T-705) can induce mutations in the genome of the influenza virus, which reduces the infectivity of the virus in vitro. This mechanism of lethal mutagenesis is proposed to be the key antiviral mechanism of favipiravir (39). It was originally developed against the influenza virus (38) and was the first effective drug against Ebola virus infection in an animal model (40). Favipiravir has shown in vitro effectiveness against the rabies virus (RABV) but is ineffective in vivo, especially after neuroinvasion. Although favipiravir blocked RABV replication at the site of inoculation in mice, it was not effective in the CNS, which means a method for its adequate penetration into the CNS needs to be devised (41). A randomized clinical trial in China comparing the efficacy of favipiravir and umifenovir for moderate symptoms showed that favipiravir is superior to umifenovir, having a higher recovery rate (71.4 vs. 55.9% for favipiravir and umifenovir, respectively; p = 0.0199). The time to cough relief and fever reduction by favipiravir was also shorter than that by umifenovir (p < 0.0001) (42). Further clinical trials of favipiravir in adult patients with SARS-CoV-2 pneumonia have been approved in China (43), and similar trials are being conducted at Harvard University and also in Japan.
Ribavirin
Ribavirin is a guanosine analog that acts as a chain terminator by inhibiting RNA polymerase (44). Alternative potential mechanisms could include its incorporation into the HCV genome, causing mutations and resulting in the production of defective viral progeny in a process called “error catastrophe” (45), or the inhibition of inosine monophosphate dehydrogenase (46). It is being used in combination with interferon in patients with chronic hepatitis C (47) and showed good results in patients with respiratory syncytial virus, especially immunocompromised patients (48).
The use of ribavirin in addition to corticosteroids in patients with SARS-CoV pneumonia resulted in resolution of fever and lung opacities within 2 weeks (49). In another study in Canada, ribavirin was administered to patients with clinical improvement, but no clear benefit was found. However, this study did highlight the side effects of ribavirin, as 49% of the patients showed a decrease in hemoglobin levels of 2 g/dL, and 76% showed signs of hemolysis, diagnosed by a 1.5 times increase in bilirubin or decreased haptoglobin level (50). In a series of 31 patients with SARS, 1 patient recovered with antibiotics only, 17 showed a rapid response to combination therapy (ribavirin and methylprednisolone), while the remaining required step-up or pulsed methylprednisolone therapy. This highlights the importance of ribavirin therapy in SARS (51). Ribavirin, when used in combination with interferon β, inhibits SARS-associated coronavirus replication in vitro; in another study, it showed antiviral activity against SARS coronavirus when used synergistically with interferon 1α and interferon β (52, 53). In a further study, it was able to lower the viral load in five out of eight patients (54). Ribavirin and interferon α2a given to MERS-CoV patients resulted in 14/20 (70%) survival as compared to 7/24 (29%) survival with no treatment at day 14 (p = 0.04) but 6/20 (30%) survival in the treatment group vs. 4/24 (17%) survival with no treatment at day 28 (p = 0.54). There was no significant difference between the later groups (55). It did not show any advantages in SARS-CoV patients (56, 57). Ribavirin used with interferon α in MERS-CoV resulted in improvement in 4 days in one patient and 6 days in another (58). No treatment advantage was seen with ribavirin in MERS-CoV after meta-analysis (59, 60). Ribavirin showed in vitro antiviral effects against SARS-CoV-2 (24). Ribavirin can be used for COVID-19 (61), and it has also been recommended to use for COVID-19 via intravenous infusion (62), as it binds tightly with SARS-CoV-2 RdRp and stops polymerase function (63). However, we need a randomized control trial to elucidate the antiviral potential of ribavirin. Moreover, it should be used in combination with either interferon or lopinavir/ritonavir to enhance its antiviral activity against SARS-CoV-2.
Ivermectin
This FDA-approved anti-parasitic drug has been shown to halt the replication of SARS-CoV-2 in vitro, as indicated by a several-fold reduction in viral RNA ivermectin-treated samples (64). However, further evaluation is needed to determine its efficacy in combating COVID-19 in humans. Ivermectin also shows broad-spectrum antiviral activity. It inhibits yellow fever virus replication, specifically targeting NS3 helicase activity (65). Ivermectin also inhibits HIV-1 (66) and Dengue virus (66, 67) replication by inhibiting importin alpha/beta, which facilitates the transport of proteins between the cytoplasm and nucleus, as these viruses use these proteins for their replication.
Immunoglobulin
IgG antibodies have two functional parts: Fab fragments, which help in antigen recognition, and the Fc fragment, which helps in the activation of the immune system (68). Intravenous immunoglobulin (IVIG) is effectively used for autoimmune diseases and chronic inflammatory diseases such as lupus, multiple sclerosis, Kawasaki disease, and dermatomyositis (69, 70). It has been used for the treatment of various bacterial, viral, and fungal infections in humans and in many experimental models (71, 72).
Likewise, SARS-Cov-2 infections could be treated using polyclonal antibodies from recovered COVID-19 patients (73). It would be preferable to extract the immunoglobulin from patients in the same city or the same area, as lifestyle, diet, and the environment are implicated in the development of specific antibodies against the virus. Immune IgG collected in China may be different from that collected in Europe or the USA (74).
In an uncontrolled case series, five critically ill COVID-19 patients on ventilators and receiving methylprednisolone and antiviral agents were transfused with convalescent plasma containing SARS-CoV-2 specific antibody (IgG) at a binding titer >1:1,000 that had been obtained from five recovered COVID-19 patients. Convalescent plasma was transfused between days 10–22 after admission. Out of five patients, three were weaned from the ventilator after 2 weeks, and four out of five recovered from ARDS after 12 days of transfusion of convalescent plasma. Three patients were discharged after 51-, 53-, and 55-days stays at hospital, and the remaining two were in stable condition after 37 days of convalescent plasma transfusion (75). There were a few limitations to the above study. Firstly, this was a small case series with no control patients, and secondly, these patients had already been given antiviral agents and steroids.
This method of passive antibody therapy can provide an effective treatment against the rapidly rising pandemic of COVID-19 (76). Though serum antibodies have been in use as a treatment for a relatively long time, further clinical trials with control groups are needed to support the idea of using serum antibodies as a treatment option for COVID-19.
Corticosteroids
Corticosteroids are a class of steroid hormones that play a key physiological role in inflammation and the immune system. The use of corticosteroids for COVID-19 has been controversial since the outbreak of this disease (77). In the past, corticosteroids have been widely used for treatment during SARS-CoV outbreaks because of their effects on various cytokines [IL-1, IL-6, IL-8, IL-12, and tumor necrosis factor-α (TNF-α)] (78, 79). Studies in humans have shown that corticosteroids are effective in reducing pathological damage, but the main concern is their adverse effects, such as acute respiratory syndrome (79).
A study was conducted on the treatment of porcine respiratory coronavirus with dexamethasone and showed that one or two doses at earlier stages are effective in reducing pro-inflammatory responses but prolonged use may play a role in enhancing viral replication (80). Another Chinese study was conducted in which SARS-CoV patients were divided into four groups; this showed that early and high doses of steroids with quinolone had an effective response (56).
A randomized clinical trial will be conducted to determine the effectiveness of systemic glucocorticoids in patients with severe novel coronavirus pneumonia (6). The use of corticosteroids for COVID-19 is controversial because of the risks of acute respiratory syndrome and further enhancement of viral replication (81).
Interferon
Interferons are naturally occurring proteins produced and secreted by cells of the immune system, e.g., white blood cells, epithelial cells, and fibroblasts. There are three major classes of interferon (alpha, beta, and gamma). Each class has a different and diverse action. Interferons boost the immune system against invading antigens, such as viruses and bacteria, and affect not only the stimulated cell but also neighboring cells (82).
Literature reviews highlight that interferons have been in use for many years against emerging viruses when no other treatment options have been available (83). Interferons have also been used for SARS-CoV and MERS-CoV in the past and have shown promising results both in vitro and in vivo in decreasing viral replication (83–86). Most of the time, interferons were used in combination with ribavirin or lopinavir/ritonavir, but the potential benefits did not meet expectations, most probably because of their administration at later, post-infectious, stages (59).
From previous studies, we can assume that interferons may be an effective treatment option against SARS-CoV-2 (87). SARS-CoV and MERS-CoV are able to disrupt interferon signaling pathways by interfering with proteins involved in interferon expression, such as Orf6 and Orf3b (88). The excessive in vitro sensitivity of SARS-CoV-2 to interferons is potentially because SARS-CoV-2 might have lost these anti-interferon actions due to their truncated Orf6 and Orf3b proteins (89). This suggests that interferons may be a better potential treatment option for SARS-CoV-2 than for SARS-CoV. As interferon treatment is more effective at earlier stages, they can be used prophylactically against SARS-CoV-2, and this is further supported by the in vitro efficacy of interferon pretreatment against the virus (89). Shen et al. stated that interferon-2α can effectively reduce the infection rate of SARS-CoV-2, which further supports the above hypothesis (90).
The recommended guidelines for the treatment of SARS-CoV-2 in China include administering 5M units of interferon α via an inhaler in combination with oral ribavirin twice a day (62). The advantage of inhalation therapy is that it acts directly on the respiratory tract; however, the pharmacokinetics and pharmacodynamics of this route are not precisely known (87). A clinical trial is underway to determine the effectiveness of interferon α with ribavirin and lopinavir/ritonavir in COVID-19 patients (91).
Due to the greater sensitivity of SARS-CoV-2 to interferon α in comparison to its family members (SARS-CoV and MERS-CoV), it can be used as an effective treatment option for COVID-19 patients. However, it will be necessary to wait for the results from current clinical trials to understand the exact efficacy of interferon (87).
Tocilizumab
Tocilizumab is a recombinant anti-human interleukin (IL)-6 receptor monoclonal antibody. It binds to both membrane-bound and soluble IL-6 receptors (IL-6R) and prevents further inflammatory cascades (92). It has been seen that critical SARS-CoV-2 patients have a surge of inflammatory cytokines, called a cytokine storm, as was previously seen with SARS and MERS. These inflammatory markers (IL-1B, IL-1RA, IL-7, IL-8, IL-9, IL-10, fibroblast growth factor, granulocyte-macrophage colony stimulating factor, IFNγ, granulocyte-colony stimulating factor, interferon-γ-inducible protein, monocyte-derived growth factor, TNFα, and vascular endothelial growth factor) were high in COVID-19 patients, leading to systemic inflammation and multi-organ failure (93, 94). IL-6 and IL-2 receptor (IL-2R) can be used to predict the severity of COVID-19-related pneumonia, as significant differences between the levels of IL-6 and IL-2R were seen between the three clinically differentiated groups (p < 0.05). The study showed that the more severe the disease, the higher the levels of IL-6 and IL-2R (95). Several other reports have shown elevated levels of IL-6 in COVID-19 critical patients (96). IL-6 is a key substance in cytokine release syndrome, so blocking IL-6R with tocilizumab can save patients with severe COVID-19 (97). In a small clinical trial in China, 21 patients with severe or critical COVID-19 were treated with tocilizumab. Within a few days of treatment, fever resolved in all patients, 15/20 (75%) needed less oxygen (one needed no oxygen), and CT scans showed resolution of pulmonary lesions in 19/21 (90.5%) patients. Lymphocytes in 10/19 (52.6%) patients and C-reactive protein (CRP) in 16/19 (84.2%) patients also returned to normal (98). In another retrospective study, tocilizumab was administered to 15 patients, and a significant improvement was seen in CRP levels, which dropped from 126.9 (10.7–257.9) to 11.2 (0.02–113.7) mg/L (p < 0.01) (99). Many individual cases have been reported in which the use of tocilizumab resulted in a significant improvement in patients. A 60-year-old patient with a previous history of multiple myeloma presented with chest tightness, and his chest CT showed multiple ground-glass opacities. He was admitted and given moxifloxacin for 3 days. Later, he was given umifenovir (arbidol), as the diagnosis of COVID-19 was confirmed by real time RT-PCR performed on a nasopharyngeal swab.
Two weeks later, he was transferred to another hospital as his chest tightness had worsened, and his oxygen saturation had become low. A CT scan showed bilateral, multiple ground-glass opacities. He was given methylprednisolone on days 2–6 of admission to improve his chest tightness and dyspnea. The patient still had bilateral, multiple ground-glass opacities on a chest CT performed on day 8. His laboratory results showed a high level of serum IL-6, and he was administered 8 mg/kg of IV tocilizumab. His IL-6 level started decreasing, chest tightness improved, and his CT on day 19 showed a decrease in ground-glass opacities (100).
Another 42-year-old patient with a history of renal cell carcinoma presented with fever and received ceftriaxone. After 6 days, he developed cough and fever, and his real-time PCR results for SARS-CoV-2 were positive. A further CT scan showed bilateral ground-glass opacities, and he was started on lopinavir/ritonavir (for 5 days) on day 7. On day 8, he developed dyspnea with decreasing oxygen saturation and was put on oxygen supplementation. He was given two doses of tocilizumab, 8 h apart, and his condition started improving. On day 12, partial regression of pulmonary infiltrates and ground-glass opacities was seen on chest CT. His CRP (a marker of cytokine storm) decreased from 225 mg/L to 33 mg/L in 4 days (101).
A 45-year-old male patient with a history of sickle cell disease presented with vaso-occlusive crises, no pulmonary findings, no dyspnea, no cough, no fever, and oxygen saturation of 98%. On day 1, the patient developed fever, and his oxygen saturation dropped to 91% with auscultatory crepitations. He was given amoxicillin-clavulanic acid and hydroxychloroquine, while a specimen was sent for RT-PCR testing for SARS-CoV-2. On day 3, his saturation dropped to 80% and his chest CT showed abnormal findings consistent with SARS-CoV-2-related pneumonia and acute chest syndrome. After RT-PCR results indicated SARS-CoV-2, a single dose of tocilizumab was injected and the patient improved and was discharged after blood transfusion (his hemoglobin was low) (102).
These cases highlight that tocilizumab can be used to successfully treat COVID-19 patients with respiratory failure by limiting cytokine-related pulmonary damage.
Vaccines
Vaccination can be the only definitive and preventive treatment option for COVID-19. A number of vaccine clinical trials are being conducted. A clinical trial by the University of Oxford is currently in phase 2/3 (103), and another phase 2 trial by the Institute of Biotechnology, Academy of Military Medical Sciences, PLA of China, is in progress. (104).
Conclusion
After reviewing a number of studies and case reports, we conclude that remdesivir and hydroxychloroquine/chloroquine with or without azithromycin are promising treatment options for patients with mild and moderate COVID-19. However, tocilizumab and immunoglobulin therapy seem to be effective in treating severe disease. There is a need for randomized control trials involving the entire globe to determine the efficacy and potency of these available potential treatment options.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ahmed SF, Quadeer AA, McKay MR. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. (2020) 12:254. doi: 10.3390/v12030254
2. Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. (2020) 55:105960. doi: 10.1016/j.ijantimicag.2020.105960
3. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. (2020) 9:ciaa237. doi: 10.1093/cid/ciaa237. [Epub ahead of print].
4. Weston S, Haupt R, Logue J, Matthews K, Frieman M. FDA approved drugs with broad anti-coronaviral activity inhibit SARS-CoV-2 in vitro. BioRxiv. (2020). doi: 10.1101/2020.03.25.008482
5. Dowall SD, Bosworth A, Watson R, Bewley K, Taylor I, Rayner E, et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J Gen Virol. (2015) 96:3484–92. doi: 10.1099/jgv.0.000309
6. de Lamballerie X, Boisson V, Reynier JC, Enault S, Charrel RN, Flahault A, et al. On chikungunya acute infection and chloroquine treatment. Vector Borne Zoonotic Dis. (2008) 8:837–9. doi: 10.1089/vbz.2008.0049
7. Paton NI, Lee L, Xu Y, Ooi EE, Cheung YB, Archuleta S, et al. Chloroquine for influenza prevention: a randomised, double-blind, placebo controlled trial. Lancet Infect Dis. (2011) 11:677–83. doi: 10.1016/S1473-3099(11)70065-2
8. Sperber K, Louie M, Kraus T, Proner J, Sapira E, Lin S, et al. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin Ther. (1995) 17:622–36. doi: 10.1016/0149-2918(95)80039-5
9. Tricou V, Minh NN, van TP, Lee SJ, Farrar J, Wills B, et al. A randomized controlled trial of chloroquine for the treatment of dengue in Vietnamese adults. PLoS Negl Trop Dis. (2012) 4:e785. doi: 10.1371/annotation/8683caec-b309-46d7-bc47-dc9cc27108e4
10. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. (2020) 56:105949. doi: 10.1016/j.ijantimicag.2020.105949
11. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. (2020) 14:72–3. doi: 10.5582/bst.2020.01047
12. Nord JE, Shah PK, Rinaldi RZ, Weisman MH. Hydroxychloroquine cardiotoxicity in systemic lupus erythematosus: a report of 2 cases and review of the literature. Semin Arthritis Rheum. (2004) 33:336–51. doi: 10.1016/j.semarthrit.2003.09.012
13. ClinicalTrials.gov Schilling W. National Library of Medicine (U.S). (2020). March, 11-. Identifier: NCT04303507 chloroquine/Hydroxychloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting (COPCOV). Available online at: https://clinicaltrials.gov/ct2/show/NCT04303507 (accessed Jun 24, 2020).
14. Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediators Inflamm. (2012) 2012:649570. doi: 10.1155/2012/649570
15. Ohe M, Shida H, Jodo S, Kusunoki Y, Seki M, Furuya K, et al. Macrolide treatment for COVID-19: Will this be the way forward? Biosci Trends. (2020) 14:159–60. doi: 10.5582/bst.2020.03058
16. ClinicalTrials.gov. National Library of Medicine (U.S). (2020). March, 25-. Identifier: NCT04321278 Safety and Efficacy of hydroxychloroquine associated with azithromycin in SARS-CoV-2 virus (coalition Covid-19 Brasil II). Available online at: https://clinicaltrials.gov/ct2/show/NCT04321278 (accessed Jun 24, 2020).
17. Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. (2016) 531:381–5. doi: 10.1038/nature17180
18. Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. (2020) 11:222. doi: 10.1038/s41467-019-13940-6
19. Holshue ML, deBolt C, Lindquist S, Lofy KH, Wiesman J, Bruc H, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. (2020) 382:929–36. doi: 10.1056/NEJMoa2001191
20. ClinicalTrials.gov. National Library of Medicine (U.S). (2020). Feb, 05-. Identifier: NCT04252664 A Trial of Remdesivir in Adults with Mild and Moderate COVID-19. Available online at: https://clinicaltrials.gov/ct2/show/results/NCT04252664 (accessed June 24, 2020).
21. ClinicalTrials.gov. National Library of Medicine (U.S). (2020). Feb, 06-. Identifier: NCT04257656 A Trial of Remdesivir in Adults with severe COVID-19. Available from: https://clinicaltrials.gov/ct2/show/NCT04257656 (accessed June 24, 2020).
22. Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, et al. Coronavirus susceptibility to the antiviral Remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. (2018) 9:e00221-18. doi: 10.1128/mBio.00221-18
23. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
24. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of covid-19 - preliminary report. N Engl J Med. (2020) NEJMoa2007764. doi: 10.1056/NEJMoa2007764
25. Cvetkovic RS, Goa KL. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. (2003) 63:769–802. doi: 10.2165/00003495-200363080-00004
26. Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. (2008) 4:1023–33. doi: 10.2147/TCRM.S3285
27. de Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, van Nieuwkoop S, Bestebroer TM, et al. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. (2014) 58:4875–84. doi: 10.1128/AAC.03011-14
28. Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, et al. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. (2004) 59:252–6. doi: 10.1136/thorax.2003.012658
29. Chan JF, Yao Y, Yeung ML, Deng W, Bao L, Jia L, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. (2015) 212:1904–13. doi: 10.1093/infdis/jiv392
30. Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for middle East respiratory syndrome. Antivir Ther. (2016) 21:455–9. doi: 10.3851/IMP3002
31. Spanakis N, Tsiodras S, Haagmans BL, Raj VS, Pontikis K, Koutsoukou A, et al. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents. (2014) 44:528–32. doi: 10.1016/j.ijantimicag.2014.07.026
32. Choy KT, Wong AY, Kaewpreedee P, Sia SF, Chen D, Hui KPY, et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. (2020) 178:104786. doi: 10.1016/j.antiviral.2020.104786
33. Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan Y-P, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. (2020) 7:4. doi: 10.1186/s40779-020-0233-6
34. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. (2020) 14:64–8. doi: 10.5582/bst.2020.01030
35. Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. (2020) 35:e79. doi: 10.3346/jkms.2020.35.e79
36. Kim JY, Choe PG, Oh Y, Kim J, Park SJ, Park JH, et al. The first case of 2019. novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. (2020) 35:e61. doi: 10.3346/jkms.2020.35.e61
37. Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, et al. Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother. (2005) 49:981–6. doi: 10.1128/AAC.49.3.981-986.2005
38. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. (2017) 93:449–63. doi: 10.2183/pjab.93.027
39. Baranovich T, Wong SS, Armstrong J, Marjuki H, Webby RJ, Webster RG, et al. T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol. (2013) 87:3741–751. doi: 10.1128/JVI.02346-12
40. Oestereich L, Lüdtke A, Wurr S, Rieger T, Muñoz-Fontela C, Günther S. Successful treatment of advanced Ebola virus infection with T-705 (favipiravir) in a small animal model. Antiviral Res. (2014) 105:17–21. doi: 10.1016/j.antiviral.2014.02.014
41. Yamada K, Noguchi K, Kimitsuki K, Kaimori R, Saito N, Komeno T, et al. Reevaluation of the efficacy of favipiravir against rabies virus using in vivo imaging analysis. Antiviral Res. (2019) 172:104641. doi: 10.1016/j.antiviral.2019.104641
42. Chen C, Huang J, Cheng Z, Wu J, Chen S, Zhang Y, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. MedRxiv [Preprint]. (2020) doi: 10.1101/2020.03.17.20037432
43. Du YX, Chen XP. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clin Pharmacol Ther. (2020) 108:242–7.doi: 10.1002/cpt.1844
44. Maag D, Castro C, Hong Z, Cameron CE. Hepatitis C virus RNA-dependent RNA polymerase (NS5B) as a mediator of the antiviral activity of ribavirin. J Biol Chem. (2001) 276:46094–8. doi: 10.1074/jbc.C100349200
45. Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci USA. (2001) 98:6895–900. doi: 10.1073/pnas.111085598
46. Zhou S, Liu R, Baroudy BM, Malcolm BA, Reyes GR. The effect of ribavirin and IMPDH inhibitors on hepatitis C virus subgenomic replicon RNA. Virology. (2003) 310:333–42. doi: 10.1016/S0042-6822(03)00152-1
47. Chemello L, Cavalletto L, Bernardinello E, Guido M, Pontisso P, Alberti A. The effect of interferon alfa and ribavirin combination therapy in naive patients with chronic hepatitis C. J Hepatol. (1995) 23(Suppl. 2):8–12.
48. Marcelin JR, Wilson JW, Razonable RR, Mayo Clinic Hematology/Oncology and Transplant Infectious Diseases Services. Oral ribavirin therapy for respiratory syncytial virus infections in moderately to severely immunocompromised patients. Transpl Infect Dis. (2014) 16:242–50. doi: 10.1111/tid.12194
49. Lee N, Hui D, Wu A, et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. (2003) 348:1986–94. doi: 10.1056/NEJMoa030685
50. Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. (2003) 289:2801–9. doi: 10.1001/jama.289.21.JOC30885
51. So LK, Lau AC, Yam LY, Cheung TMT, Poon E, Yung RWH, et al. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. (2003). 361:1615–7. doi: 10.1016/S0140-6736(03)13265-5
52. Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. (2005) 326:905–8. doi: 10.1016/j.bbrc.2004.11.128
53. Wang WK, Chen SY, Liu IJ, Kao CL, Chen HL, Chiang BL, et al. Temporal relationship of viral load, ribavirin, interleukin (IL)-6, IL-8, and clinical progression in patients with severe acute respiratory syndrome. Clin Infect Dis. (2004) 39:1071–5. doi: 10.1086/423808
54. Chen F, Chan KH, Jiang Y, Kao RY, Lu HT, Fan KW, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. (2004) 31:69–75. doi: 10.1016/j.jcv.2004.03.003
55. Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, et al. Ribavirin and interferon alfa-2a for severe middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. (2014) 14:1090–5. doi: 10.1016/S1473-3099(14)70920-X
56. Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, et al. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol. (2003) 52(Pt 8):715–20. doi: 10.1099/jmm.0.05320-0
57. Leong HN, Ang B, Earnest A, Teoh C, Xu W, Leo YS. Investigational use of ribavirin in the treatment of severe acute respiratory syndrome, Singapore, 2003. Trop Med Int Health. (2004) 9:923–7. doi: 10.1111/j.1365-3156.2004.01281.x
58. Khalid M, Al Rabiah F, Khan B, Al Mobeireek A, Butt TS, Al Mutairy E. Ribavirin and interferon-α2b as primary and preventive treatment for Middle East respiratory syndrome coronavirus: a preliminary report of two cases. Antivir Ther. (2015) 20:87–91. doi: 10.3851/IMP2792
59. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. (2006) 3:e343. doi: 10.1371/journal.pmed.0030343
60. Morra ME, van Thanh L, Kamel MG, Ghazy AA, Altibi AMA, Dat LM, et al. Clinical outcomes of current medical approaches for middle East respiratory syndrome: a systematic review and meta-analysis. Rev Med Virol. (2018) 28:e1977. doi: 10.1002/rmv.1977
61. Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. (2020) 248:117477. doi: 10.1016/j.lfs.2020.117477
62. Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019. (COVID-19). Drug Discov Ther. (2020). 14:58–60. doi: 10.5582/ddt.2020.01012
63. Elfiky AA. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. (2020) 253:117592. doi: 10.1016/j.lfs.2020.117592
64. Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. (2020) 178:104787. doi: 10.1016/j.antiviral.2020.104787
65. Mastrangelo E, Pezzullo M, de Burghgraeve T, Kaptein S, Pastorino B, Dallmeier K, et al. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother. (2012) 67:1884–94. doi: 10.1093/jac/dks147
66. Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. (2012) 443:851–6. doi: 10.1042/BJ20120150
67. Tay MY, Fraser JE, Chan WK, Moreland NJ, Rathore AP, Wang C, et al. Nuclear localization of dengue virus (DENV) 1-4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. (2013). 99:301–6. doi: 10.1016/j.antiviral.2013.06.002
68. Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. (2017) 29:491–8. doi: 10.1093/intimm/dxx039
69. Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. (2005) 142:1–11. doi: 10.1111/j.1365-2249.2005.02834.x
70. Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J. Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clin Exp Immunol. (2011) 164(Suppl. 2):2–5. doi: 10.1111/j.1365-2249.2011.04387.x
71. Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Intravenous immunoglobulin for infectious diseases: back to the pre-antibiotic and passive prophylaxis era? Trends Pharmacol Sci. (2004) 25:306–10. doi: 10.1016/j.tips.2004.04.002
72. Ben-Nathan D, Lustig S, Tam G, Robinzon S, Segal S, Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. (2003) 188:5–12. doi: 10.1086/376870
73. Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. (2020) 41:355–9. doi: 10.1016/j.it.2020.03.007
74. Jawhara S. Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int J Mol Sci. (2020) 21:2272. doi: 10.3390/ijms21072272
75. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. (2020) 323:1582–9. doi: 10.1001/jama.2020.4783
76. Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. (2020) doi: 10.1001/jama.2020.4940. [Epub ahead of print].
77. Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19-a systematic review of current evidence. Ecancermedicalscience. (2020) 14:1022. doi: 10.3332/ecancer.2020.1022
78. Chihrin S, Loutfy MR. Overview of antiviral and anti-inflammatory treatment for severe acute respiratory syndrome. Expert Rev Anti Infect Ther. (2005) 3:251–62. doi: 10.1586/14787210.3.2.251
79. Lam CW, Chan MH, Wong CK. Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin Biochem Rev. (2004) 25:121–32.
80. Zhang X, Alekseev K, Jung K, Vlasova A, Hadya N, Saif LJ. Cytokine responses in porcine respiratory coronavirus-infected pigs treated with corticosteroids as a model for severe acute respiratory syndrome. J Virol. (2008) 82:4420–8. doi: 10.1128/JVI.02190-07
81. Qin YY, Zhou YH, Lu YQ, Sun F, Yang S, Harypursat V, et al. Effectiveness of glucocorticoid therapy in patients with severe coronavirus disease 2019: protocol of a randomized controlled trial. Chin Med J (Engl). (2020). 133:1080–6. doi: 10.1097/CM9.0000000000000791
82. Totura AL, Bavari S. Broad-spectrum coronavirus antiviral drug discovery. Expert Opin Drug Discov. (2019) 14:397–412. doi: 10.1080/17460441.2019.1581171
83. Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, et al. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon- alpha. J Infect Dis. (2004) 189:1164–7. doi: 10.1086/382597
84. Falzarano D, de Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. (2013) 3:1686. doi: 10.1038/srep01686
85. Falzarano D, de Wit E, Rasmussen AL, Feldmann F, Okumura A, Scott DP, et al. Treatment with interferon-α2b and ribavirin improves outcome in MERS-CoV-infected rhesus macaques. Nat Med. (2013) 19:1313–7. doi: 10.1038/nm.3362
86. Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Treatment of SARS with human interferons. Lancet. (2003). 362:293–4. doi: 10.1016/S0140-6736(03)13973-6
87. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. (2020) 178:104791. doi: 10.1016/j.antiviral.2020.104791
88. Kopecky-Bromberg SA, Martínez-Sobrido L, Frieman M, Baric RA, Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol. (2007) 81:548–57. doi: 10.1128/JVI.01782-06
89. Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R, Menachery VD. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.03.07.982264
90. Shen KL, Yang YH. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J Pediatr. (2020) 16:219–21. doi: 10.1007/s12519-020-00344-6. [Epub ahead of print].
91. Zeng YM, Xu XL, He XQ, Tang SQ, Li Y, Huang YQ, et al. Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol. Chin Med J (Engl). (2020) 133:1132–4. doi: 10.1097/CM9.0000000000000790
92. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. (2020) 111:102452. doi: 10.1016/j.jaut.2020.102452
93. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. (2017) 39:529–39. doi: 10.1007/s00281-017-0629-x
94. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. Natl Sci Rev. (2020) 7:998–1002. doi: 10.1093/nsr/nwaa041
95. Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, et al. [Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi. (2020). 43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005
96. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. (2020) 46:846–8. doi: 10.1007/s00134-020-05991-x
97. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. (2020) 55:105954. doi: 10.1016/j.ijantimicag.2020.105954
98. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. (2020) 117:10970–5. doi: 10.1073/pnas.2005615117
99. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. (2020) 92:814–8. doi: 10.1002/jmv.25801
100. Zhang X, Song K, Tong F, Fei M, Guo H, Lu Z, et al. First case of COVID-19 in a patient with multiple myeloma successfully treated with tocilizumab. Blood Adv. (2020) 4:1307–10. doi: 10.1182/bloodadvances.2020001907
101. Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, et al. Tocilizumab, an anti-IL-6 receptor antibody, to treat COVID-19-related respiratory failure: a case report. Ann Oncol. (2020) 31:961–4. doi: 10.1016/j.annonc.2020.03.300
102. de Luna G, Habibi A, Deux JF, Colard M, Pham Hung d', Alexandry d', et al. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol. (2020) 95:876–8. doi: 10.1002/ajh.25833
103. ClinicalTrials.gov. National Library of Medicine (U.S). (2020). Identifier: NCT04341389 A phase II Trial to evaluate the Recombinant Vaccine for COVID-19 (Adenovirus Vector) (CTII-nCoV). Available online at: https://clinicaltrials.gov/ct2/show/NCT04341389 (accessed June 24, 2020).
104. ClinicalTrials.gov. National Library of Medicine (U.S). (2020). Identifier: NCT04400838 Investigating a Vaccine against COVID-19. Available online at: https://clinicaltrials.gov/ct2/show/NCT04400838?term=vaccine&cond=covid-19&phase=2&draw=2&rank=8 (accessed June 24, 2020).
Keywords: COVID-19, SARS-CoV-2, chloroquine/hydroxychloroquine, ivermectin, remdesivir, immunoglobulin, tocilizumab
Citation: Ali MJ, Hanif M, Haider MA, Ahmed MU, Sundas F, Hirani A, Khan IA, Anis K and Karim AH (2020) Treatment Options for COVID-19: A Review. Front. Med. 7:480. doi: 10.3389/fmed.2020.00480
Received: 02 May 2020; Accepted: 15 July 2020;
Published: 31 July 2020.
Edited by:
Abdallah Samy, University of Kansas, United StatesReviewed by:
Junki Maruyama, University of Texas Medical Branch at Galveston, United StatesIshtiaq Hussain, Cleveland Clinic Florida, United States
Koichi Yuki, Boston Children's Hospital and Harvard Medical School, United States
Copyright © 2020 Ali, Hanif, Haider, Ahmed, Sundas, Hirani, Khan, Anis and Karim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Hanif, aGFuaWZhZnJpZGkyNzNAZ21haWwuY29t
 Mukarram Jamat Ali
Mukarram Jamat Ali Muhammad Hanif
Muhammad Hanif Muhammad Adnan Haider
Muhammad Adnan Haider Muhammad Umer Ahmed
Muhammad Umer Ahmed FNU Sundas2
FNU Sundas2 Izhan Ali Khan
Izhan Ali Khan