
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Med. , 26 June 2020
Sec. Intensive Care Medicine and Anesthesiology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00372
This article is part of the Research Topic Anesthetic and Critical Care Amid the COVID-19 Pandemic View all 40 articles
 Eric Azabou1*
Eric Azabou1* Guillaume Bao1
Guillaume Bao1 Nicholas Heming2
Nicholas Heming2 Rania Bounab2
Rania Bounab2 Pierre Moine2
Pierre Moine2 Sylvain Chevallier3
Sylvain Chevallier3 Sylvie Chevret4,5,6
Sylvie Chevret4,5,6 Matthieu Resche-Rigon4,5,6
Matthieu Resche-Rigon4,5,6 Shidaps Siami7
Shidaps Siami7 Tarek Sharshar8,9
Tarek Sharshar8,9 Frederic Lofaso1
Frederic Lofaso1 Djillali Annane2
Djillali Annane2The severe respiratory distress syndrome linked to the new coronavirus disease (COVID-19) includes unbearable dyspneic suffering which contributes to the deterioration of the prognosis of patients in intensive care unit (ICU). Patients are put on mechanical ventilation to reduce respiratory suffering and preserve life. Despite this mechanical ventilation, most patients continue to suffer from dyspnea. Dyspnea is a major source of suffering in intensive care and one of the main factors that affect the prognosis of patients. The development of innovative methods for its management, especially non-drug management is more than necessary. In recent years, numerous studies have shown that transcranial direct current stimulation (tDCS) could modulate the perception of acute or chronic pain. In the other hand, it has been shown that the brain zones activated during pain and dyspnea are close and/or superimposed, suggesting that brain structures involved in the integration of aversive emotional component are shared by these two complex sensory experiences. Therefore, it can be hypothesized that stimulation by tDCS with regard to the areas which, in the case of pain have activated one or more of these brain structures, may also have an effect on dyspnea. In addition, our team recently demonstrated that the application of tDCS on the primary cortical motor area can modulate the excitability of the respiratory neurological pathways. Indeed, tDCS in anodal or cathodal modality reduced the excitability of the diaphragmatic cortico-spinal pathways in healthy subjects. We therefore hypothesized that tDCS could relieve dyspnea in COVID-19 patients under mechanical ventilation in ICU. This study was designed to evaluate effects of two modalities of tDCS (anodal and cathodal) vs. placebo, on the relief of dyspnea in COVID-19 patients requiring mechanical ventilation in ICU.
Trial Registration: This protocol is derived from the tDCS-DYSP-REA project registered on ClinicalTrials.gov NCT03640455. It will however be registered under its own NCT number.
Dyspnea is a “symptom” common to various ailments and pathologies such as sepsis, asthma attack, intoxication, severe metabolic disorders known for their association with acute respiratory distress syndrome (ARDS) (1–3). More than half of patients admitted to intensive care for septic shock have an ARDS (4). Coronavirus disease 19 (COVID-19) caused by severe acute respiratory syndrome coronavirus (SARS-CoV-2) presents in its severe forms a severe respiratory distress syndrome requiring patients to be put on mechanical ventilation in intensive care (5–8). This respiratory suffering has a dyspneic component, which often reaches unbearable limits and constitutes a major factor in altering the clinical state and the prognosis of patients (6, 9). Dyspnea usually persists despite adequate treatment of the underlying pathology, or sometimes worsens after it has normalized (10–13). This phenomenon of perceptual dysfunction (exaggerated, persistent perception) is linked to changes in cortical excitability due to neuronal plasticity (14). The pathophysiologic mechanisms of dyspnea are quite complex, but are beginning to be better understood (15). The dysfunctions can occur around the thoracic mechanics, the respiratory muscles and blood gases. They may also occur within the neurological and neurobiological structures ensuring the central integration. In particular there are afferents to the cortex which are compared with the motor pathways via corollary discharge (16, 17). Poor adaptation of the ventilator is also a main cause of dyspnea (3). Dyspnea appears when the respiratory work becomes excessive, in particular when the abnormalities of the respiratory mechanics increases the respiratory work, or when the capacities of ventilation are lower than the needs for the organization (18–21). The length-tension ratio of the respiratory muscle fibers, the numerous neurochemical receptors located in the chest wall, the lungs, the airways, the vascular walls, and also in the cerebral centers of respiration are all actors involved in these mechanisms. The brain correlates of respiratory discomfort have been described by several recent works (2, 22, 23). Analogies are drawn between the pathophysiology of dyspnea sensations and that of pain (14, 24, 25). It is a multidimensional experience resulting from a complex central integration of the interaction between multiple factors, physiological, social, and environmental (26). However, despite the appropriate treatment of the recognized or suspected underlying cause and normalization of the blood gazes, dyspnea is often insufficiently relieved and therefore requires—as with pain—specific treatments for this symptom (1). This applies particularly to the hyperventilation syndrome which often persists after the normalization of the underlying functional impairment, and even more so to “medically unexplained” or “psychogenic” dyspnea (11, 17). Recent years, numerous studies have shown that transcranial direct current stimulation tDCS was able to modulate, the perception of acute (27–29) or chronic pain (30–32) which raised hopes of being able to use this technique in the treatment of refractory pain by conventional therapeutic means. Studies in functional brain imaging have been able to show that the effects of this cortical stimulation—in terms of brain activity—were not limited to the cortical zone next to the stimulation electrode (33) but involved a whole set of brain structures (some of which are quite far from the stimulation site) including the anterior cingulum gyrus, the prefrontal cortex, the thalamus, the brainstem, and even the spinal cord (34, 35). While the role of some of these structures in the central integration of pain is currently well-established, they are likely to be also involved in the central integration of dyspnea. Indeed, functional imaging studies on dyspnea (36), in particular one which jointly assessed pain and dyspnea (37) have highlighted activation zones that are close or even superimposed for pain and dyspnea, probably corresponding to brain structures involved in the integration of the aversive emotional component shared by these two complex sensory experiences. Therefore, it can be hypothesized that stimulation by tDCS with regard to the areas which, in the case of pain activated one or more of these cerebral structures could also have an effect on dyspnea. In addition, our team recently demonstrated that the application of tDCS on the primary cortical motor area can modulate the excitability of the respiratory neurological pathways. Indeed, tDCS in anodal or cathodal modality allowed a reduction in the excitability of the diaphragmatic cortico- spinal pathways in healthy subjects (38).
We hypothesized that tDCS could relieve dyspnea in COVID-19 patient requiring mechanical ventilation in ICU (39, 40). We designed this project to assess the effectiveness of tDCS on the relief of dyspnea in COVID-19 patients requiring mechanical ventilation in ICU.
This study will enroll 63 (3 groups of 21) COVID-19 patients, admitted in ICU with ARDS requiring mechanical ventilator for at least 24 h, and having significant dyspnea (dyspnea level ≥4 on the A1 subscale of the Multidimensional Dyspnea Profile) (1, 41).
- Adult patient, hospitalized in intensive care for COVID-19, having required mechanical ventilation for at least 24 h.
- Not sedated or having a good awakening (Richmond Agitation Score- Sedation Scale (RASS)> −3 at the time of inclusion (42) within 48 h of stopping sedation.
- Able to answer yes or no to simple questions.
- Having significant dyspnea (level≥ 4) on the A1 scale of the Multidimensional Profile of Dyspnea (MPD-A1≥ 4) (1, 41).
- Signature of informed consent by the patient or his family member.
- Patient under guardianship,
- Wake up delay, coma (GCS≤ 8), or severe agitation.
- Chronic respiratory pathology.
- Medical history of respiratory, neuromuscular, or neuro-sensorial handicap (auditory or visual) pathology.
- Language barrier, refusal to participate in the study or to sign the informed consent,
- Pregnant or lactating woman,
- No affiliation to a social security scheme.
The main objective of this study was to determine whether tDCS allowed a significant reduction in dyspnea, measured by the A1 subscale of the multidimensional profile of dyspnea (MPD-A1), in patients admitted to intensive care for COVID-19 placed on mechanical ventilation and suffering dyspnea.
- To evaluate the effect of tDCS on the different components of dyspnea using the other subscales of the multidimensional profile of dyspnea “MPD”: sensory (MPD-QS) and emotional (MPD-A2) subscales.
- To determine if tDCS also allowed a significant reduction in dyspnea measured by the IC-RDOS scale (intensive care respiratory distress observation scale) (43).
- To investigate the presence of pre-inspiratory potentials (PPI) on the EEG in this patient population and determine the effect of tDCS on these PPIs in patients who may have them.
- To evaluate the effect of tDCS on respiratory parameters: mouth pressure (amplitude of variation), PetCO2, tidal volume (VT), and respiratory rate (F) as well as ventilation/minute (calculated from VT and F).
- To evaluate the impact of the possible relief of dyspnea by tDCS on the patient's close outcome during the 28 days following inclusion: mortality in intensive care, in hospital on D28, the cumulative incidence of delirium and its duration until D28, the cumulative incidence of mechanical ventilation, the failure to wean from mechanical ventilation on D28, and the length of stay in intensive care.
- Measurement of the differential of the score on the A1 subscale of the Multidimensional Dyspnea Profile (MPD-A1) (from 0 to 10): between before and after the use of tDCS. This primary judgment criterion will be assessed by an independent, blind observer. The differential will be measured between 30 min before the procedure and 30 min after.
- Differentials of the MDP-QS and MDP-A2 subscales of the Multidimensional Profile of Dyspnea measured between before and after tDCS: in order to assess the effect of tDCS on the different components of dyspnea: sensory (MPD-QS) and emotional qualifiers (MPD-A2 subscales).
- Differential in the IC-RDOS (intensive care respiratory distress observation scale) scale between before and after the use of tDCS. A significant reduction in this score after the use of tDCS will translate into a reduction in respiratory discomfort, especially dyspnea.
- Pre-inspiratory potentials (PPI) assessment: the possible presence of PPI on the EEG in this patient population could be a marker of respiratory suffering, and a possible disappearance of PPI after the use of tDCS could be interpreted as a relieving effect on breathing difficulty.
- The respiratory parameters measurement: mouth pressure (amplitude of variation), PetCO2, tidal volume (VT), and respiratory rate (F) as well as ventilation/minute (calculated from VT and F), between before and after use of tDCS.
- Evaluation of the impact of tDCS on the outcome of patients during the 28 days following inclusion:
(a) Death by D28 in intensive care and in the hospital,
(b) Cumulative incidence of delirium and its duration (CAM-ICU scale) (44).
(c) Proportion of patients with mechanical ventilator dependance beyond D28.
(d) Cumulative incidence of mechanical ventilation on D28,
(e) The duration of the resuscitation stay.
The A1 subscale of the multidimensional profile of dyspnea allows to diagnose dyspnea and to rate its intensity. This score is the equivalent of the visual analog scale. A score of four is considered the lower limit for moderate dyspnea (3). The QS (sensory qualifiers) and A2 (emotional) subscales allow better specifying and defining the type of components that characterize each patient's dyspnea (1, 41). These different subscales of the multidimensional profile of dyspnea will be performed before the start of tDCS, then after the end of tDCS for each patient.
The IC-RDOS scale is derived from the respiratory distress observation scale (RDOS). It is composed of the five items (heart rate, use of the neck muscles during inspiration, paradoxical abdominal movement, facial expression of fear, and additional oxygen) and is validated to serve as tools for objective and reliable evaluation of dyspnea in resuscitation patients (43) and could therefore be used as an alternative to psychometric scales to assess dyspnea in patients who are unable to communicate verbally.
PPIs are slow brain waves generated during the milliseconds preceding the start of inspiration in healthy subjects in a situation of voluntary or forced breathing, and in patients suffering from respiratory discomfort: COPD, asthma, respiratory distress, Ondine (45–50). These potentials are absent in the case of spontaneous breathing in healthy subjects and disappear in patients as soon as the respiratory discomfort is removed. EEG signal was synchronously recorded with the respiratory flow and pressure signals using a Nihon Kohden France manufactured EEG-9100J/K, digital EEG system. Scalp electrodes were placed according to the conventional “10–20” topographic system, via a 19-electrodes cap installed after rubbing and cleaning with alcohol and application of a conductive gel. The ground electrode was positioned at Fpz. The EEG traces are then divided into 3 s sections centered on the start of inspiration (from 2.5 s before the start of inspiration until 0.5 s after the start of inspiration). At least 40–50 EEG samples are required. These 40–50 EEG samples thus cut are then averaged to objectify the PPI. This step of analysis and processing of signals (sampling and averaging) is done automatically using EEG software. The presence or not of the PPI recorded during the 30 min preceding the start of stimulation with tDCS will be recorded, as well as during the 30 min following the cessation of tDCS.
The subject being connected to the ventilator, by measuring devices corresponding to a series connection which comprises—downstream of the subject—a device equipped with a CO2 sensor and a pneumotachograph, the pressure sensor of which also makes it possible to measure the mouth pressure (Pm) (NICO2 sensor combined CO2 adult flow Novametrix Nico).
The oxygen saturation will be determined with your finger using a pulse oximeter (Novametrix Oxymeter). The acquisition of all of these respiratory signals (Pm, instantaneous flow rate, expired CO2 and SatO2) is carried out during a period of 15 min before the introduction of tDCS, then again for 15 min after the end of tDCS.
The following respiratory parameters will be precisely measured and calculated:
- The pressure measured at the mouth (Pm), the amplitude of variation of which (aPm), gives an indirect but fairly practical reflection of the additional respiratory effort, which, in the case of dyspnea linked to laden breathing is one of the parameters that is best correlated with its intensity.
- PETCO2: the partial pressure of CO2 at the end of expiration: by being (in the ideal case), a reflection of capnia, the increase of this being another mechanism inducing dyspnea with a strong unpleasant connotation (air hunger) especially in a context where ventilation is forced to a level lower than that which would have been chosen spontaneously. Thus, the measurement of aPm and PetCO2 will allow us to assess an equivalent of physical stimulus for each of the two types of dyspnea and to assess the relationship of these with the intensity of the dyspnea. In addition, in the case of aPm, it will provide an index of the motor response to the loads.
- Tidal volume and respiratory rate as well as ventilation/minute (calculated from the instantaneous flow signal) will provide us with an interesting insight into the adaptation of breathing to the physiological mechanisms underlying dyspnea.
The confusion assessment method for the intensive care unit (CAM-ICU) will be used for detection and monitoring of delirium during the 28 days of follow-up after inclusion (44).
After verification of the inclusion and exclusion criteria, the patients will be prospectively and randomly included in three groups of 21 patients, depending on the type of tDCS treatment received: anodal tDCS group, cathodal tDCS group, and placebo tDCS group. The tDCS will be applied up on the cortical representation zone of the primary motor and left pre-motor cortex for 30 min; intensity 2 mA (in anodal, cathodal, or placebo modality). The patient will be blinded from the randomization arm. Randomization will be performed on a dedicated and secure specific website (Cleanweb). Randomization will be carried out in a 1: 1: 1 ratio with permutation blocks of size unknown to the investigators.
This is a clinical, interventional, bi-centric, randomized, single-blind, 3-arm trial, including a placebo-controlled arm, and 2 experimental arms, evaluating the effectiveness of a medical device for therapeutic purposes (tDCS) with 63 (3 groups of 21) patients on mechanical ventilation in intensive care with dyspnea. The primary endpoint will be assessed by an independent, blind observer. Transcranial stimulation will be delivered using a medical certified “Starstim 8” brain stimulator controlled via Bluetooth using a laptop computer (Neuroelectrics, Barcelona, Spain), Stimulation will delivered through traditional 5 × 7 cm rectangular sponge electrodes, with a contact area of 35 cm2 (Sponstim, Neuroelectrics, Barcelona, Spain). The tDCS will be applied upon the left hemisphere because of the functional dominance of this hemisphere in humans and in accordance with previous studies having evaluated the effect of tDCS on pain and the respiratory tract (27, 28, 32, 38, 51). As described in our previous work (38), two identical, rectangular, saline-soaked electrodes, each 7 cm long and 5 cm wide (35 cm2), were secured to the scalp. For anodal tDCS, the anode will be placed over the left diaphragmatic primary motor cortex (4 cm lateral to the midline and 1 cm anterior to the binaural line) and the cathode will be placed above the right orbit. These positions will be switched to obtain cathodal tDCS. For both anodal and cathodal tDCS, intensity will be 2 mA and the duration 30 min. The current density used will be 0.057 mA/cm2, which has been proven to be safe (52–55). For the sham condition (placebo tDCS), intensity was also 2 mA but duration was only 2 min. Nitsche and Paulus reported in 2000 that at least 3 min of tDCS was necessary to induce after-effects (31).
The parameters studied (in particular those used to calculate the main judgment criterion) will be measured during the 30 min preceding (pre) and the 30 min following (post) the use of tDCS in each of the three different experimental conditions (anodal tDCS, cathodal tDCS, and placebo tDCS). The placebo tDCS condition constitutes the control condition (fictitious stimulation: absence of delivered current (sham), therefore acting as placebo. The Figure 1 represents a diagram of the course of the experimental procedure.
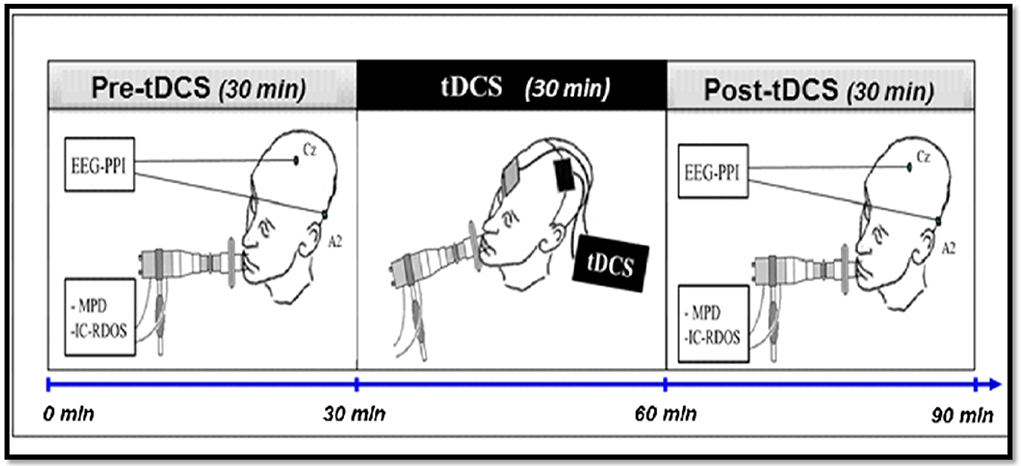
Figure 1. Diagram of the experimental procedure. The practical implementation of the protocol consists of a single session of ~1 h 30 min and will include the three stages. After inclusion and randomization, the set of parameters studied will be measured for each patient, for 30 min before using tDCS, then for 30 min after stopping tDCS. The tDCS will be applied for 30 min to the cortical representation area of the primary motor area and the supplementary left motor area. tDCS, Transcranial stimulation with 2 mA intensity current in anodal, cathodal or placebo polarity, applied to the cortical representation area of the left primary and pre-motor areas; EEG-PPI, EEG to measure the Pre-Inspiratory Potentials; MPD, Scales of the Multidimensional Profile of Dyspnea; IC-RDOS, Scales of the Intensive Care Respiratory Distress Observation Scale.
Side-effects and adverse effects associated with the tDCS during the course of the trial will be assessed using the adverse effects questionnaire proposed by Brunoni et al. for tDCS studies in order to improve systematic reporting of tDCS-related adverse effects (56).
The total duration planned for the study is 12 months. The total duration of participation for each patient is 28 days; because each patient will be followed up for a period of 28 days after inclusion in order to collect the evolution data. The Table 1 summarizes the research chronology.
Inclusion will be made when all the inclusion and non-inclusion criteria are verified and the patient has given informed consent to participate in the study. During inclusion and before the start of the single session of the protocol, the following clinical, drug, and other co- variable data will be collected. These data are in principle systematically measured in intensive care patients.
Demographic data (age and sex), the reason for admission; medical history (neurological, respiratory, cardiological) initial severity by the SAPS-II score (57), the number of organ failures by the SOFA score (58), neurological assessment scores (Glasgow, FOUR score) (59); CAM-ICU (44) and the RASS score (to assess depth of sedation (42). The determinants of secondary cerebral aggression of systemic origin: body temperature, blood pressure, PaO2, PaCO2, natremia, glycemia. The neurological examination which includes: examination of the cranial pairs (spontaneous eye movements, pupil size, photo-motor reflex, oculo-cephalogyr reflex, corneal reflex, reaction to Pierre Marie-Foix's maneuver, cough reflex), the search for archaic reflexes (corneo- mandibular, palmo- mental, yawning, chewing, grasping), osteo-tendinous, and plantar skin reflexes.
The practical implementation of the protocol will consist of a single session of ~1 h 30 m and will include the three stages described in Figure 1. After inclusion and randomization, the set of parameters studied will be measured for each patient, for 30 min before using tDCS, then for 30 min after stopping tDCS. The tDCS will be applied for 30 min to the cortical representation area of the primary motor area and the supplementary left motor area.
The patients will then be followed for a period of 28 days after inclusion in order to collect the evolution data including: Death, measurement of delirium (CAM-ICU scale) until D28; ventilation status (spontaneous or mechanical) and withdrawal (in progress, successful, failure) until D28, the cumulative incidence of mechanical ventilation, and the length of stay in intensive care.
The last visit made on D28 for patients for patients who survived to this date, will be identical to previous visits.
A descriptive analysis of inclusions and monitoring of the protocol will be carried out. The main analysis will be carried out according to the intention to treat principle. Only patients who have withdrawn their consent can be excluded from the analysis. Patients who have decided to discontinue the management planned for the trial, lost to follow-up, or discontinued the trial will be included in the analysis. In general, the quantitative variables will be described by their median and their first and their third quartiles and the qualitative variables will be described by the frequencies of the modalities and the associated percentages. The epidemiological and clinical characteristics of the patients at inclusion will be described by group, without statistical tests being carried out. The protocol violations, the causes of abandonment and loss of sight and the characteristics of these patients will be detailed.
The studied parameters will be measured during the 30 min before (pre) and 30 min following (post) the use of tDCS in all three conditions (tDCS anodal, tDCS cathodal, and tDCS placebo). The placebo tDCS condition is the control condition (fictitious stimulation). The different measures of the judgment criteria will be carried out by one of the investigating doctors blinded in the randomization arm. In order to test the effect of tDCS, the judgment criteria will be compared according to the different experimental conditions (anodal tDCS, vs. cathodal, vs. placebo tDCS). Each experimental arm will be compared to the placebo group at risk 0.025 using Students t-test and applying Bonferroni correction if needed.
- Differentials of the MDP-QS and MDP- A2 subscales of the Multidimensional Profile of Dyspnea measured between before and after tDCS, in order to assess the effect of tDCS on the different components of dyspnea: sensory (MPD-QS) and emotional qualifiers (MPD-A2 subscales). Each experimental arm will be compared to the placebo group at risk 0.05 using a Student test. If the two experimental arms are greater than the placebo arm, they will be compared to each other at risk 0.05.
- Differential in the IC-RDOS (intensive care respiratory distress observation scale) scale: between before and after the use of tDCS. A significant reduction in this score after the use of tDCS will translate into a reduction in respiratory discomfort, especially dyspnea. Each experimental arm will be compared to the placebo group at risk 0.05 using a Student test. If the two experimental arms are greater than the placebo arm, they will be compared to each other at risk 0.05.
- Pre-inspiratory potentials (PPI): the possible presence of PPI on the EEG in this patients population could be a marker of respiratory suffering, and a possible disappearance of PPI after the use of tDCS could be interpreted as a relieving effect on breathing difficulty. Each experimental arm will be compared to the placebo group at 0.05 risk using a Fisher test. If the two experimental arms are greater than the placebo arm, they will be compared to each other at risk 0.05.
- The respiratory parameters: mouth pressure (amplitude of variation), PetCO2, tidal volume (VT), and respiratory rate (F) as well as ventilation/minute (calculated from VT and F). The comparisons of each of these parameters will be carried out. Each experimental arm will be compared to the placebo group at risk 0.05 using a Student test. If the two experimental arms are greater than the placebo arm, they will be compared to each other at risk 0.05.
- Evaluation of the impact of tDCS on the patient's future outcome during the 28 days following inclusion:
(a) Death on D28 in intensive care and in the hospital: Each experimental arm will be compared to the placebo group at 0.05 risk using a Fisher test. If the two experimental arms are greater than the placebo arm, they will be compared to each other at risk 0.05. Kaplan-Meir curves for death.
(b) Cumulative incidence of delirium and its duration (CAM-ICU scale): The cumulative incidence estimates will be made using the gray method and compared according to the previous procedure using a gray test.
(c) Proportion of patients who failed to withdraw from mechanical ventilation on D28: Each experimental arm will be compared to the placebo group at 0.05 risk using a Fisher test. If the two experimental arms are greater than the placebo arm, they will be compared to each other at risk 0.05.
(d) Cumulative incidence of mechanical ventilation on D28: The cumulative incidence estimates will be made using the gray method and compared according to the previous procedure using a gray test.
(e) The duration of the resuscitation stay: Estimates of the median length of stay in intensive care will be made from an inverted Kaplan Meier estimator and compared according to the previous procedure using a log rank test.
The MDP-A1 subscale of the Multidimensional Dyspnea Profile is the main evaluation criterion of this study. This subscale is similar to the visual analog scale. Assuming a difference of 1 (on the primary efficacy endpoint, superiority study) between one of the 2 experimental groups and the placebo group and a standard deviation of 1, with a first species risk (alpha risk) of 2.5% (to take into account the 2 comparisons of each experimental group with placebo) and a power of 80% (beta risk at 20%), it will be necessary to include 21 patients per group or 63 patients in total. This number is consistent with that of other studies in tDCS (29, 60, 61).
The main analysis will be carried out according to the intention to treat principle. Only patients who have withdrawn their consent can be excluded from the analysis. If the period of inclusion in the research is still active, patients who have withdrawn their consent will be replaced. Patients who have decided to discontinue treatment planned in the trial, lost to follow-up or discontinued from the trial will be replaced, as will patients for whom there have been technical problems. Analyzes will be carried out with the intention of treating. Regarding missing data issue, in case of patient drop-out, in order to clearly understanding of the effectiveness of the therapy, we will first report results based on the completed cases, then secondarily with mixed-model or similar approaches which take into account partially available data, and finally with multiple imputation techniques.
This study was approved by our legal ethical committee: Comité de Protection des Personnes Ouest III, Université de Poitiers; CPP number 170946 and renewed on april 28th 2020. Informed consent should be obtained from each patient or family member before inclusion in the study.
Dyspnea is a painful suffering that often reaches unbearable limits. Unfortunately, it is very frequent in intensive care and constitutes a major factor affecting the prognosis of intensive care patients, and more particularly patients under mechanical ventilation. Many COVID-19 patients continue to suffer from it, despite being put on mechanical ventilation and the use of relaxing and analgesic drugs (40). The effectiveness of the treatments currently available therefore remains very limited and there is a pressing need to develop other innovative treatments, including non- medicinal ones, in order to combat this scourge even more effectively and reduce the suffering of patients (39). The tDCS has demonstrated efficacy in pain relief, which shares the same neural substrates as dyspnea. It is a painless, easy to use and non-invasive technique. The originality and the innovative character of this study reside in the development of an effective method of treatment by neuro-modulation non-invasive and easy to use to combat this respiratory suffering in COVID-19 patient. Effective relief of dyspnea with tDCS would also have a significant impact on the prognosis of these patients. Finally, one may argue that it could have been better to conduct a multisession tDCS study, however this study is a pilot, designed to assess whether a single 30 min tDCS session could be beneficial for dyspnea relief in this specific patients' population. According to the findings of the present study we will conceive and assess outcome of other tDCS treatment strategies and designs including multisession ones.
EA, FL, DA, and TS developed the study concept. All authors wrote and drafted the manuscript, read, and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank our institutional sponsors Clinical Research and Innovation Department of Assistance Publique – Hôpitaux de Paris (APHP, DRCI); the University of Versailles Saint Quentin en Yvelines (UVSQ) and the Paris Saclay University. We also thank the Methodology and Clinical Research Unit of the Saint Louis Hospital, Paris (URC Paris Saint Louis) for the methodological supports.
1. Banzett RB, O'Donnell CR, Guilfoyle TE, Parshall MB, Schwartzstein RM, Meek PM, et al. Multidimensional dyspnea profile: an instrument for clinical and laboratory research. Eur Respir J. (2015) 45:1681–91. doi: 10.1183/09031936.00038914
2. Schmidt M, Banzett RB, Raux M, Morelot-Panzini C, Dangers L, Similowski T, et al. Unrecognized suffering in the ICU: addressing dyspnea in mechanically ventilated patients. Intensive Care Med. (2014) 40:1–10. doi: 10.1007/s00134-013-3117-3
3. Schmidt M, Demoule A, Polito A, Porchet R, Aboab J, Siami S, et al. Dyspnea in mechanically ventilated critically ill patients. Crit Care Med. (2011) 39:2059–65. doi: 10.1097/CCM.0b013e31821e8779
4. Annane D, Sebille V, Bellissant E. Effect of low doses of corticosteroids in septic shock patients with or without early acute respiratory distress syndrome. Crit Care Med. (2006) 34:22–30. doi: 10.1097/01.CCM.0000194723.78632.62
5. Gandhi S, Srivastava AK, Ray U, Tripathi PP. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem Neurosci. (2020) 11:1379–81. doi: 10.1021/acschemneuro.0c00217
6. Geier MR, Geier DA. Respiratory conditions in coronavirus disease 2019. (COVID-19): important considerations regarding novel treatment strategies to reduce mortality. Med Hypotheses. (2020) 140:109760. doi: 10.1016/j.mehy.2020.109760
7. Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. (2020). doi: 10.1111/joim.13091
8. Zangrillo A, Beretta L, Scandroglio AM, Monti G, Fominskiy E, Colombo S, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. (2020).
9. Horowitz RI, Freeman PR, Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Respir Med Case Rep. (2020) 30:101063. doi: 10.1016/j.rmcr.2020.101063
10. Peiffer C. Dyspnea relief: more than just the perception of a decrease in dyspnea. Respir Physiol Neurobiol. (2009) 167:61–71. doi: 10.1016/j.resp.2009.04.001
11. Peiffer C. Morphine-induced relief of dyspnea: what are the mechanisms? Am J Respir Crit Care Med. (2011) 184:867–9. doi: 10.1164/rccm.201108-1463ED
12. Peiffer C, Costes N, Herve P, Garcia-Larrea L. Relief of dyspnea involves a characteristic brain activation and a specific quality of sensation. Am J Respir Crit Care Med. (2008) 177:440–9. doi: 10.1164/rccm.200612-1774OC
13. Peiffer C, Poline JB, Thivard L, Aubier M, Samson Y. Neural substrates for the perception of acutely induced dyspnea. Am J Respir Crit Care Med. (2001) 163:951–7. doi: 10.1164/ajrccm.163.4.2005057
14. Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. (2006) 129:1659–73. doi: 10.1093/brain/awl082
15. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. (2012) 185:435–52. doi: 10.1164/rccm.201111-2042ST
16. Mahler DA, O'Donnell DE. Recent advances in dyspnea. Chest. (2015) 147:232–41. doi: 10.1378/chest.14-0800
17. Peiffer C. Dyspnea and emotion: what can we learn from functional brain imaging? Am J Respir Crit Care Med. (2008) 177:937–9. doi: 10.1164/rccm.200802-298ED
18. Banzett RB, Lansing RW, Evans KC, Shea SA. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respir Physiol. (1996) 103:19–31. doi: 10.1016/0034-5687(95)00050-X
19. Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med. (2008) 177:1384–90. doi: 10.1164/rccm.200711-1675OC
20. Lansing RW, Im BS, Thwing JI, Legedza AT, Banzett RB. The perception of respiratory work and effort can be independent of the perception of air hunger. Am J Respir Crit Care Med. (2000) 162:1690–6. doi: 10.1164/ajrccm.162.5.9907096
21. Lansing RW, Moosavi SH, Banzett RB. Measurement of dyspnea: word labeled visual analog scale vs. verbal ordinal scale. Respir Physiol Neurobiol. (2003) 134:77–83. doi: 10.1016/S1569-9048(02)00211-2
22. Banzett RB, O'Donnell CR. Should we measure dyspnoea in everyone? Eur Respir J. (2014) 43:1547–50. doi: 10.1183/09031936.00031114
23. Binks AP, Evans KC, Reed JD, Moosavi SH, Banzett RB. The time-course of cortico-limbic neural responses to air hunger. Respir Physiol Neurobiol. (2014) 204:78–85. doi: 10.1016/j.resp.2014.09.005
24. Barnes PJ. Blunted perception and death from asthma. N Engl J Med. (1994) 330:1383–4. doi: 10.1056/NEJM199405123301910
25. Gracely RH, Undem BJ, Banzett RB. Cough, pain and dyspnoea: similarities and differences. Pulm Pharmacol Ther. (2007) 20:433–7. doi: 10.1016/j.pupt.2006.12.005
26. Banzett RB, Gracely RH, Lansing RW. When it's hard to breathe, maybe pain doesn't matter. focus on “dyspnea as a noxious sensation: inspiratory threshold loading may trigger diffuse noxious inhibitory controls in humans”. J Neurophysiol. (2007) 97:959–60. doi: 10.1152/jn.00970.2006
27. Antal A, Brepohl N, Poreisz C, Boros K, Csifcsak G, Paulus W. Transcranial direct current stimulation over somatosensory cortex decreases experimentally induced acute pain perception. Clin J Pain. (2008) 24:56–63. doi: 10.1097/AJP.0b013e318157233b
28. Boggio PS, Zaghi S, Lopes M, Fregni F. Modulatory effects of anodal transcranial direct current stimulation on perception and pain thresholds in healthy volunteers. Eur J Neurol. (2008) 15:1124–30. doi: 10.1111/j.1468-1331.2008.02270.x
29. Csifcsak G, Antal A, Hillers F, Levold M, Bachmann CG, Happe S, et al. Modulatory effects of transcranial direct current stimulation on laser-evoked potentials. Pain Med. (2009) 10:122–32. doi: 10.1111/j.1526-4637.2008.00508.x
30. Lefaucheur JP, Antal A, Ahdab R, Ciampi de Andrade D, Fregni F, Khedr EM, et al. The use of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) to relieve pain. Brain Stimul. (2008) 1:337–44. doi: 10.1016/j.brs.2008.07.003
31. Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. (2000) (527):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
32. O'Connell NE, Wand BM, Marston L, Spencer S, Desouza LH. Non-invasive brain stimulation techniques for chronic pain. a report of a Cochrane systematic review and meta-analysis. Eur J Phys Rehabil Med. (2011) 47:309–26. doi: 10.1002/14651858.CD008208.pub2
33. Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. (2011) 58:26–33. doi: 10.1016/j.neuroimage.2011.06.018
34. Hayward G, Mehta MA, Harmer C, Spinks TJ, Grasby PM, Goodwin GM. Exploring the physiological effects of double-cone coil TMS over the medial frontal cortex on the anterior cingulate cortex: an H2(15)O PET study. Eur J Neurosci. (2007) 25:2224–33. doi: 10.1111/j.1460-9568.2007.05430.x
35. Peyron R, Faillenot I, Mertens P, Laurent B, Garcia-Larrea L. Motor cortex stimulation in neuropathic pain. correlations between analgesic effect and hemodynamic changes in the brain A PET study. Neuroimage. (2007) 34:310–21. doi: 10.1016/j.neuroimage.2006.08.037
36. Herigstad M, Hayen A, Wiech K, Pattinson KT. Dyspnoea and the brain. Respir Med. (2011) 105:809–17. doi: 10.1016/j.rmed.2010.12.022
37. von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, et al. Dyspnea and pain share emotion-related brain network. Neuroimage. (2009) 48:200–6. doi: 10.1016/j.neuroimage.2009.06.015
38. Azabou E, Roche N, Sharshar T, Bussel B, Lofaso F, Petitjean M. Transcranial direct-current stimulation reduced the excitability of diaphragmatic corticospinal pathways whatever the polarity used. Respir Physiol Neurobiol. (2013) 189:183–7. doi: 10.1016/j.resp.2013.07.024
39. Ahmed T, Shah RJ, Rahim SEG, Flores M, O'Linn A. Coronavirus disease (2019). (COVID-19) complicated by acute respiratory distress syndrome: an internist's perspective. Cureus. (2020) 12:e7482. doi: 10.7759/cureus.7482
40. Altschuler EL, Kast RE. Dapsone, colchicine and olanzapine as treatment adjuncts to prevent COVID-19 associated adult respiratory distress syndrome (ARDS). Med Hypotheses. (2020) 141:109774. doi: 10.1016/j.mehy.2020.109774
41. Meek PM, Banzett R, Parshall MB, Gracely RH, Schwartzstein RM, Lansing R. Reliability and validity of the multidimensional dyspnea profile. Chest. (2012) 141:1546–53. doi: 10.1378/chest.11-1087
42. Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. (2002) 166:1338–44. doi: 10.1164/rccm.2107138
43. Persichini R, Gay F, Schmidt M, Mayaux J, Demoule A, Morélot-Panzini C, et al. Diagnostic accuracy of respiratory distress observation scales as surrogates of dyspnea self-report in intensive care unit patients. Anesthesiology. (2015) 123:830–7. doi: 10.1097/ALN.0000000000000805
44. Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. (2001) 286:2703–10. doi: 10.1001/jama.286.21.2703
45. Raux M, Ray P, Prella M, Duguet A, Demoule A, Similowski T. Cerebral cortex activation during experimentally induced ventilator fighting in normal humans receiving noninvasive mechanical ventilation. Anesthesiology. (2007) 107:746–55. doi: 10.1097/01.anes.0000287005.58761.e8
46. Raux M, Straus C, Redolfi S, Morelot-Panzini C, Couturier A, Hug F, et al. Electroencephalographic evidence for pre-motor cortex activation during inspiratory loading in humans. J Physiol. (2007) 578:569–78. doi: 10.1113/jphysiol.2006.120246
47. Raux M, Tremoureux L, Couturier A, Hug F, Similowski T. Simplified recording technique for the identification of inspiratory premotor potentials in humans. Respir Physiol Neurobiol. (2010) 171:67–70. doi: 10.1016/j.resp.2010.01.002
48. Raux M, Xie H, Similowski T, Koski L. Facilitatory conditioning of the supplementary motor area in humans enhances the corticophrenic responsiveness to transcranial magnetic stimulation. J Appl Physiol. (2010) 108:39–46. doi: 10.1152/japplphysiol.91454.2008
49. Tremoureux L, Raux M, Hudson AL, Ranohavimparany A, Straus C, Similowski T. Does the supplementary motor area keep patients with Ondine's curse syndrome breathing while awake? PLoS ONE. (2014) 9:e84534. doi: 10.1371/journal.pone.0084534
50. Tremoureux L, Raux M, Jutand L, Similowski T. Sustained preinspiratory cortical potentials during prolonged inspiratory threshold loading in humans. J Appl Physiol. (2010) 108:1127–33. doi: 10.1152/japplphysiol.91449.2008
51. Nitsche MA, Cohen LG, Wassermann EM, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. (2008) 1:206–23. doi: 10.1016/j.brs.2008.06.004
52. Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. (2001) 57:1899–901. doi: 10.1212/WNL.57.10.1899
53. Nitsche MA, Paulus W. Noninvasive brain stimulation protocols in the treatment of epilepsy: current state and perspectives. Neurotherapeutics. (2009) 6:244–50. doi: 10.1016/j.nurt.2009.01.003
54. Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. (2005) 568:291–303. doi: 10.1113/jphysiol.2005.092429
55. Roche N, Lackmy A, Achache V, Bussel B, Katz R. Impact of transcranial direct current stimulation on spinal network excitability in humans. J Physiol. (2009) 587:5653–64. doi: 10.1113/jphysiol.2009.177550
56. Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. (2011) 14:1133–45. doi: 10.1017/S1461145710001690
57. Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. (1993) 270:2957–63. doi: 10.1001/jama.1993.03510240069035
58. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
59. Iyer VN, Mandrekar JN, Danielson RD, Zubkov AY, Elmer JL, Wijdicks EF. Validity of the FOUR score coma scale in the medical intensive care unit. Mayo Clin Proc. (2009) 84:694–701. doi: 10.4065/84.8.694
60. Antal A, Paulus W. Transcranial direct current stimulation and visual perception. Perception. (2008) 37:367–74. doi: 10.1068/p5872
Keywords: COVID-19, acute respiratory distress syndrome (ARDS), tDCS, dyspnea relief, brain, neuromodulation, mechanical ventilation, ICU
Citation: Azabou E, Bao G, Heming N, Bounab R, Moine P, Chevallier S, Chevret S, Resche-Rigon M, Siami S, Sharshar T, Lofaso F and Annane D (2020) Randomized Controlled Study Evaluating Efficiency of Low Intensity Transcranial Direct Current Stimulation (tDCS) for Dyspnea Relief in Mechanically Ventilated COVID-19 Patients in ICU: The tDCS-DYSP-COVID Protocol. Front. Med. 7:372. doi: 10.3389/fmed.2020.00372
Received: 01 May 2020; Accepted: 17 June 2020;
Published: 26 June 2020.
Edited by:
Ru-Ping Dai, Central South University, ChinaReviewed by:
Asif Jamil, Leibniz Research Centre for Working Environment and Human Factors (IfADo), GermanyCopyright © 2020 Azabou, Bao, Heming, Bounab, Moine, Chevallier, Chevret, Resche-Rigon, Siami, Sharshar, Lofaso and Annane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eric Azabou, ZXJpYy5hemFib3VAYXBocC5mcg==; ZXJpYy5hemFib3VAdXZzcS5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.