- 1Department of International Medical Center/Ward of General Practice, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Periodical Press, National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
Anticoagulation therapy is an important method of preventing stroke in individuals with atrial fibrillation (AF). Atrial fibrillation is a quivering or irregular heartbeat that can lead to blood clots, stroke, heart failure, and other heart-related complications. Clinical guidelines on AF consistently recommend long-term oral warfarin to treat valvular atrial fibrillation (VAF). However, due to varying risks of blood clots and stroke associated with different types of non-valvular atrial fibrillation NVAF, it is unclear whether direct oral anticoagulant (DOAC) can replace warfarin. Despite a recent increase in evidence on the effectiveness and the importance of anticoagulant therapy in preventing thromboembolic events associated with NVAF, clinical prevention strategies remain complex. Given the complexities associated with clinical use of anticoagulants for patients with NVAF, this review aims to offer guidance on patient anticoagulant use based on current available evidence.
Introduction
Atrial fibrillation (AF) is a common type of arrhythmia. There are currently 335 million individuals with AF worldwide (1), with an overall prevalence rate of 2.9% (2). With an aging global population and changing lifestyles, the incidence of AF is increasing rapidly. The prevalence of AF is around 0.1% for individuals under 55 years old, more than 5% in people over 65 years old, and more than 9% in people over 80 years old (3).
The main negative effects of AF are thrombosis and embolism. For example, the incidence of embolic events in patients with non-valvular atrial fibrillation (NVAF) is 5% per year, which accounts for 15–20% of all cerebral embolism events (4). These consequences of stroke could increase the risks of death and disability by more than 5-fold (5, 6). In general, the fatality rates for stroke are 15, 25, and 50% in the 1-month, 1-year, and 5-years post-stroke periods, respectively (7). However, patients with stroke caused by AF experience persistent recurrences for 5 years as well as higher early mortality rates (7). Therefore, clinical guidelines have identified anticoagulation for individuals with NVAF, as the cornerstone approach to controlling ischemic stroke. However, since clinical risks of atrial fibrillation increase with age, more proactive prevention methods are needed for older individuals.
Over the past 50 years, clinical guidelines have recommended the use of oral anticoagulant (OAC) in NVAF, from the most widely used warfarin to the more effective direct acting oral anticoagulants (DOAC) (8). Most data have shown that the use of OACs in NVAF can reduce the risk of stroke. Studies have shown that anticoagulation therapies can decrease the incidence of stroke by 50% and prevent the recurrence of stroke (9–11). According to data extracted from electronic medical records over the last 10 years in the UK, a 1% increase in anticoagulant use can result in 0.8% decrease in the incidence of stroke associated with AF (12).
In 2010, the Food and Drug Administration (FDA) approved the first DOAC for stroke prevention in AF, dabigatran. Since then, the FDA has approved other DOACs including rivaroxaban in July 2011, apixaban in December 2012, and edoxaban in January 2015. Although several DOACs have become available in the last 10 years, a Phase III trial of more than 100,000 subjects found that the various DOACs have similar efficacy in preventing stroke in patients with NVAF (13–16).
By 2016, DOAC prescriptions exceeded warfarin prescriptions for patients with AF (13). As the use of DOACs has increased, more data have become available on their efficacy for NVAF, as well as on their safety for patients. In 2019, AF clinical guidelines from Europe and the United States prioritized the use of DOACs over vitamin K antagonists (VKAs) for NVAF therapy in most situations (17, 18). However, there are risks associated with these drug use, including potential gastrointestinal bleeding and fatal intracranial hemorrhage. Such side effects can lead to insufficient implementations of prevention strategies. Given the challenges facing the selection of anticoagulants in patients with NVAF, we have summarized the differences in mechanism of action between traditional VKAs and DOACs based on a review of recent evidence and clinical use strategies for different individuals.
Mechanism of Action of VKAs and DOACs
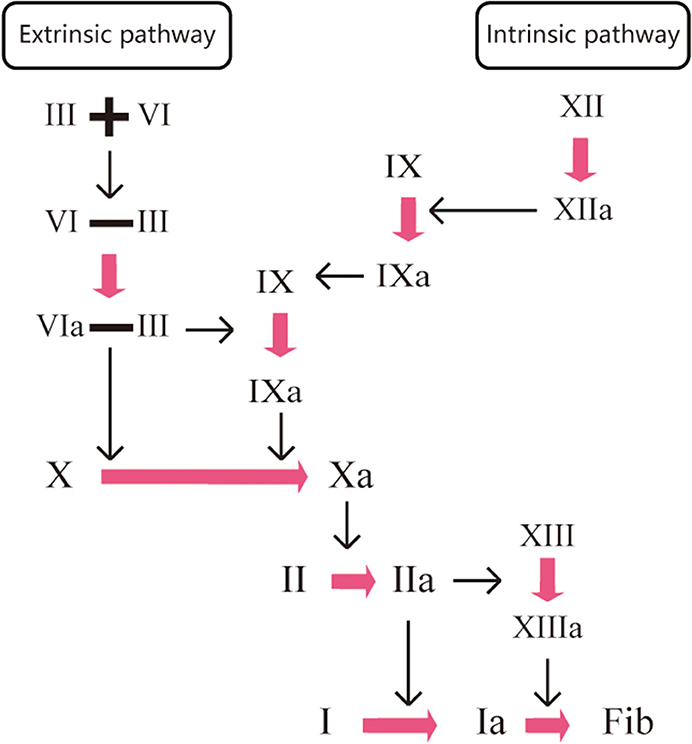
Under normal conditions, the clotting process of the human body is a waterfall-like enzymatic cascade reaction (19). The main principle of anticoagulant drugs is to block the cascade reaction by directly or indirectly inhibiting one or more condensation factors in the coagulation process, thus preventing the development of thrombosis. VKAs induce anticoagulant action by non-specific indirect inhibitions of clotting factors (factors X, IX, IX, IX, VII, and II). Warfarin, a VKA, is a coumarin-derived, multi-target and non-selective oral anticoagulant that relies on vitamin K. It acts on the coagulation factors (VII, IX, and X) at the early stage of the coagulation cascade response to inhibit thrombin production and factor II activation. However, it does not affect the protein synthesis of coagulation factors, instead acting by inhibiting their carboxylation process. Therefore, the process has no effect on coagulation factors that have already been activated in the body. DOACs, due to their high specificity, induce anticoagulants by directly blocking the activities of coagulation factors Xa and IIa cells. An example of a DOAC is the IIa inhibitor dabigatran, which acts on the last step of the coagulation cascade response. Dabigatran directly inactivates the thrombin that has been produced (IIa), exerting anticoagulant effects by blocking fibrinogen cleavage to fibrin. The factor Xa inhibitors, such as rivaroxaban, apixaban, and edoxaban, act on the common pathway of endogenous and exogenous coagulation reactions. The inactivation of one Xa inhibitor can result in the reduction of 1000 IIa cells, which effectively inhibits the production of thrombin (IIa) and achieves anticoagulant effects (Figure 1).
Potential Problems in Anticoagulant Therapy With VKAs in Real-World Observational Studies
In the past half century, warfarin has been used in thrombosis, atrial fibrillation, artificial valve replacement and other indications (20). A meta-analysis of five studies established the effectiveness and safety of warfarin anticoagulant therapy (21–23). Moreover, a meta-analysis found that warfarin reduced the overall stroke risk of patients with AF by 68% and all-cause mortality by 33% (24).
However, systematic reviews of real-world data have shown that there are limitations to the use of warfarin in clinical practice due to its narrow therapeutic window and poor quality of anticoagulation control. Time to treatment (TTR), the proportion of time that the patient's INR between 2 and 3, is the standard method for assessing the quality of anticoagulation control and the risk-benefit profile of warfarin. Potential interactions between warfarin and certain foods or medications can affect INR levels, if the criteria for TTR are not met, there will be high risks of embolism or bleeding (25), thus restricting the use of the medicine in clinical practice. Furthermore, NVAF comorbid conditions, such as other heart disease, liver disease, renal insufficiency, and infections can cause instability of INR results and increase the risk of bleeding. Some foods and drugs interact with warfarin (26, 27), affecting INR levels, so diet and use of other medications are restricted while taking warfarin. Frequent blood draws are needed to detect INR, and this can result in decreased patient compliance. Patient non-compliance, including self-termination of treatment, is a major factor leading to adverse events in individuals treated with warfarin. Due to the risk factors mentioned above, warfarin is not an ideal anticoagulant therapy for stroke prevention and there continues to be a need for improved long-term anticoagulant treatment.
A Comparison of DOACs and VKAs
There is substantial clinical experience with use of DOACs, and patient-reported outcomes have been summarized in a systematic review (28). In addition, clinical trials have found that the newer oral anticoagulants can reduce the stroke rate by 19% compared with warfarin (11, 29). Based on the available evidence, DOACs have been recommended in national guidelines on the prevention of stroke for individuals with NVAF and who have one or more risk factors for stroke. However, in part due to the large sample size required to detect differences between groups in drug trials, there are currently no head-to-head, blinded studies comparing VKAs and DOACs (30).
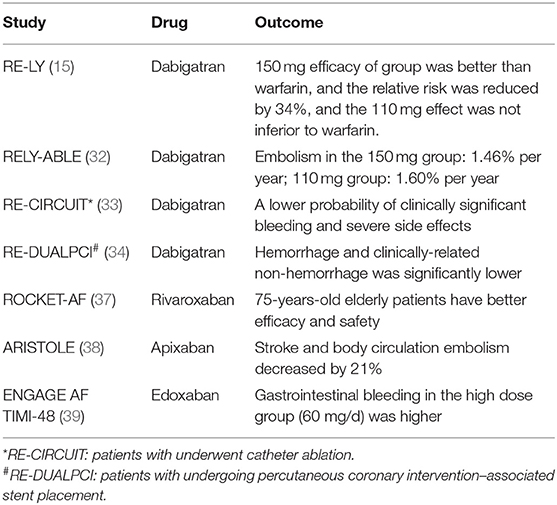
Real-world observational studies of DOACs have been conducted, particularly on dabigatran, which was the first DOAC on the market (31). Observational studies have validated the results of the 2009 RE-LY trial evaluating dabigatran (15). RELY-ABLE (32) was a follow-up to the RE-LY trial and addressed the long-term effectiveness and safety of 150 and 110 mg doses of dabigatran. The RELY-ABLE trial found that annual rate of stroke or systemic embolism was similar in the two groups; 1.46% per year in the 150 mg group and 1.60% per year in the 110 mg group (HR: 0.91, 95% CI: 0.69–1.20). However, individuals in the 150 mg group had significantly more bleeding events than those in the 110 mg group (HR: 1.26, 95% CI: 1.04–1.53), suggesting that bleeding should be carefully observed in patients receiving high-dose dabigatran.
The results of the 2017 RE-CIRCUIT (33) study showed that patients who underwent catheter ablation had a lower probability of clinically significant bleeding and severe side effects with dabigatran than with warfarin (34). The RE-DUALPCI trial (35) found that, in patients with AF undergoing percutaneous coronary intervention–associated stent placement, the incidence of hemorrhage and clinically-related non-hemorrhage in the dabigatran group was significantly lower than in the warfarin triple therapy group at 3 years. The annual incidence of hemorrhage associated with long-term anticoagulant therapy was 1.1–8.1%. The most common sites of bleeding were the skin, mucous membranes, gastrointestinal tract, and genitourinary tract. Intracranial hemorrhage (ICH) is the most serious and can endanger patients' lives. The primary endpoint, risk of bleeding, was reduced by 48 and 28% in the dual therapy dabigatran 110 and 150 mg groups, respectively, compared with the warfarin triple therapy group (36). Clinical trials ROCKET-AF (37), ARISTOLE (38), ENGAGE AF TIMI-48 (39) also showed that DOACs (rivaroxaban, apixaban, edoxaban) is more effective than warfarin, edoxaban gastrointestinal bleeding in the high dose group (60 mg/d) was higher compare with warfarin (see Table 1). In a systematic review of including 170,814 patients treated with apixaban, the majority results showed that warfarin was associated with a lower risk of stroke and systemic embolic events, as well as major bleeding, particularly ICH (46%RRR; p <0.00001) (40). Rutherford et al. (41) recently published a large-scale observational study from Norway, treatment with DOACs for 65,563 AF patients firstly, the results found no statistically significant difference in stroke or SE risk between dabigatran group, rivaroxaban or apixaban. The risk of dabigatran and apixaban was significantly reduced major bleeding compared to rivaroxaban.
According to the latest European guidelines, patients with AF with a CHA2DS2-VASc score >2 are advised to use DOACs or VKAs to reduce the risk of stroke. However, for patients with CHA2DS2-VASc of 1, the risk of thromboembolic is only 0.6–1.3%, but the risk of bleeding will increase, which should be determined according to the individual balance between thromboembolism and bleeding risk. The preference of treatment decision should be to do beneficial, not harmful to patients, not only to avoid stroke. Based on this, it is a key prerequisite to start OAC to evaluate the risk of major bleeding of patients. For patients with CHA2DS2-VASC of 1, if it has been decided to start OAC, DOAC with higher clinical net benefit should be preferred instead of VKA (42, 43).
Because NVAF patients tend to have multiple comorbidities, clinical trials cannot ignore real-world safety studies of warfarin's use in specific high-risk populations.
Numerous studies have shown that increasing age is an independent risk factor for NVAF, leading to increased morbidity and mortality (44). In individuals over 80 years old with NVAF, the mortality rate is 9% (7). In addition, older patients with AF have higher rates of thrombosis and bleeding than younger patients. Several real-world observational studies have included analyses stratified by age, some with a highest age group of 80 years old (45–47). Despite the higher risk of bleeding in older individuals, a Japanese cohort study found that warfarin had a positive net clinical benefit in individuals ≥90 years due a reduced risk of ischemic stroke (48). Research reviews have generally found more favorable outcomes for DOACs than warfarin in the oldest patients, thus DOACs may still be recommended for elderly patients, despite the risks.
About 10–15% of AF patients have chronic kidney disease (CKD), and severe renal insufficiency is an independent risk factor for stroke in AF patients. Given that risks of stroke and hemorrhage are higher in AF patients with renal insufficiency, the selection of oral anticoagulants should be made more carefully for these patients. Warfarin is one of the most commonly used anticoagulants in patients with CKD (49). Clinical practice guidelines consistently recommend warfarin for anticoagulation in AF patients with CKD or end-stage renal disease (ESRD) (50). However, studies on the safety and effectiveness of warfarin in AF patients with CKD have found that, compared with healthy populations, warfarin does not reduce the incidence of ischemic stroke and it increases the risk of intracranial hemorrhage (3 vs. 1% per year). Therefore, it is uncertain whether warfarin can be used as an anticoagulant in patients with severe CKD or ESRD.
In patients with renal insufficiency, clinical guidelines generally recommend initially choosing a lower dose of warfarin and closely monitoring the INR. Due to the difference in renal metabolism of different DOACs, apixaban has been reported to be safer and more effective than dabigatran and rivaroxaban (51). The effectiveness of apixaban is comparable to that of warfarin in patients with AF combined with severe CKD (CrCl <25 mL/min), and apixaban is safer than warfarin. In addition, the safety of apixaban is comparable to that of warfarin in patients with AF with ESRD (GFR <15 with dialysis). With increasing evidence-based support, apixaban has been recommended in updated guidelines for patients with AF who have severe CKD or ESRD, even for those undergoing dialysis.
The Future of AF Anticoagulant Treatment
Factors that limit the long-term use of anticoagulants include the high bleeding risk associated with warfarin and the fact that the INR for effective anticoagulation overlaps with the risk of increased bleeding. Moreover, since AF patients tend to be older, have more comorbidities, and use more medications, it is more difficult to control the bleeding risk in these individuals. Compared with INR 2.0–2.9, the incidence of bleeding is twice as high with INR 3.0–4.4, four times as high with INR 4.5–6.0 and five times as high with INR >7.0 (52). Given this variation and because individual response to warfarin may be genetically determined, some researchers have started to explore the use of genetic testing to determine the therapeutic INR range upon initiation of warfarin therapy (53). In the case of emergent hemorrhage caused by DOACs, idarucizumab can quickly decrease the blood concentration of dabigatran to achieve rapid hemostasis. Alternatively, andexanet alfa, another antagonist of factor Xa inhibitors, is available for clinical use.
Summary and Perspective
This review summarized the anticoagulant effect of warfarin and highlighted the advantages of DOACs for individuals with NVAF. However, the lack of effective monitoring, its price is more expensive than warfarin, and some anticoagulant antagonists are not even on the market in some areas, it is difficult to apply in large-scale clinical applications. Due to the lack of research on DOACs, the limited sample sizes of existing research may not fully reflect its advantages and disadvantages. Another important limitation of this paper is lack of meta-analysis and quantitative results.
The main advantages of DOACs include predictable pharmacokinetics, high efficacy, short half-life and rapid elimination of the effect after discontinuation, lower need for drug and dietary limitations, and lower intracranial hemorrhage risk without the need for frequent monitoring. With DOAC use, it is possible to improve patient compliance with long-term anticoagulant therapy, thereby increasing the treatment effectiveness rate for AF. The safety and effectiveness of DOACs need to be further verified by high-quality research data from more multi-center, double-blind RCTs (Randomized controlled trials).
Author Contributions
XL designed the review. YZ and YL revised the manuscript. JW drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by 1-3-5 Project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (No. 2018HXFH005) and the National Key Research and Development Program of China (No. 2017YFC0907303).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Diane Civic, Ph.D., from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
References
1. Kirchhof P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet. (2017) 390:1873–87. doi: 10.1016/S0140-6736(17)31072-3
2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation. (2019) 139:e56–8. doi: 10.1161/CIR.0000000000000659
3. Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA. (2001) 285:2370–5. doi: 10.1001/jama.285.18.2370
4. Björck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited. Stroke. (2013) 44:3103–8. doi: 10.1161/STROKEAHA.113.002329
5. You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. (2012) 141:e531S−75S. doi: 10.1378/chest.141.4.1129b
6. Lane DA, Lip GY. Lip use of the CHA (2) DS (2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. (2012) 126:860–5. doi: 10.1161/CIRCULATIONAHA.111.060061
8. Kirley K, Qato DM, Kornfield R, Stafford RS, Alexander CG. National trends in oral anticoagulant use in the United States, 2007 to 2011. Circ Cardiovasc Qual Outcomes. (2012) 5:615–21. doi: 10.1161/CIRCOUTCOMES.112.967299
9. Huisman MV, Lip GY, Diener HC, Dubner SJ, Halperin JL, Ma CS, et al. Design and rationale of global registry on long-term oral antithrombotic treatment in patients with atrial fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J. (2014) 167:329–34. doi: 10.1016/j.ahj.2013.12.006
10. Piccini JP, Xu H, Cox M, Matsouaka RA, Fonarow GC, Butler J, et al. Adherence to guideline—directed stroke prevention therapy for atriaI fibrillation iS achievable. Circulation. (2019) 139:1497–506. doi: 10.1161/CIRCULATIONAHA.118.035909
11. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007
12. Cowan JC, Wu J, Hall M, Orlowski A, West RM, Gale PC. A 10 year study of hospitalized atrial fibrillation-related stroke in England and its association with uptake of oral anticoagulation. Eur Heart J. (2018) 39:2975–83. doi: 10.1093/eurheartj/ehy411
13. Barnes GD, Lucas E, Alexander GC, Goldberger DZ. National trends in ambulatory oral anticoagulant use. Am J Med. (2015) 128:1300–5.e1302. doi: 10.1016/j.amjmed.2015.05.044
14. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
15. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2011) 361:1139–51. doi: 10.1056/NEJMoa0905561
16. Connolly SJ, Wallentin L, Yusuf S. Additional events in the RE-LY trial. N Engl J Med. (2014) 371:1464–5. doi: 10.1056/NEJMc1407908
17. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/american heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2019) 74:104-132. doi: 10.1016/j.jacc.2019.01.011
18. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Kardiol Pol. (2016) 74:1359–69. doi: 10.5603/KP.2016.0172
19. Lee CJ, Ansell EJ. Direct thrombin inhibitors. Br J Clin Pharmacol. (2011) 72:581–92. doi: 10.1111/j.1365-2125.2011.03916.x
20. Keeling D, Baglin T, Tait C, Watson H, Perry D, Baglin, et al. Guidelines on oral anticoagulation with warfarin - fourth edition. Br J Haematol. (2011) 154:311–24. doi: 10.1111/j.1365-2141.2011.08753.x
21. Petersen P, Godtfredsen J, Boysen G, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the copenhagen AFASAK study. Lancet. (1989) 1:175–9. doi: 10.1016/S0140-6736(89)91200-2
22. Kistler JP, Singer DE, Millenson MM, Bauer KA, Gress DR, Barzegar S. Effect of low-intensity warfarin anticoagulation on levelofactivityofthehemostaticsystem in patients with atrial fibrillation. Stroke. (1993) 24:1360–5. doi: 10.1161/01.STR.24.9.1360
23. Nademanee K, Kosar EM. Long-term antithrombotic treatment for atrial fibrillation. Am J Cardiol. (1998) 82:37–42. doi: 10.1016/S0002-9149(98)00738-3
24. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/S0140-6736(13)62343-0
25. Moss JD, Cifu AS. Management of anticoagulation in patients with atrial fibrillation. JAMA. (2015) 314:291. doi: 10.1001/jama.2015.3088
26. de Filette J, Michiels V. Bleeding interaction between fluconazole and warfarin. Lancet. (2018) 392:e9. doi: 10.1016/S0140-6736(18)32217-7
27. Ha NB, Yang K, Hanigan S, Kurtz B, Dorsch MP, Mak H, et al. Impact of a guideline for the management of antimicrobial/warfarin interactions in the inpatient setting and across transition of care. Ann Pharmacother. (2016) 50:734–40. doi: 10.1177/1060028016653765
28. Afzal SK, Hasan SS, Babar UZ. A systematic review of patient-reported outcomes associated with the use of direct-acting oral anticoagulants. Br J Clin Pharmacol. (2019) 85:2652–67. doi: 10.1111/bcp.13985
29. PAGB. Summary of Product Characteristics. (2017). Available online at: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf (accessed January 18, 2017).
30. Camm AJ, Fox KAA, Peterson E. Challenges in comparing the non-vitamin K antagonist oral anticoagulants for atrial fibrillation-related stroke prevention. Europace. (2018) 20:1–11. doi: 10.1093/europace/eux086
31. Potpara TS, Lip YG. Postapproval observational studies of non-vitamin k antagonist oral anticoagulants in atrial fibrillation. JAMA. (2017) 317:1115–6. doi: 10.1001/jama.2017.1152
32. Connolly SJ, Wallentin L, Ezekowitz MD, Eikelboom J, Oldgren J, Reilly PA, et al. The long-term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (rely-able) study. Circulation. (2013) 128:237–43. doi: 10.1161/CIRCULATIONAHA.112.001139
33. Hohnloser SH, Calkins H, Willems S, Verma A, Schilling R, Okumura K, et al. Regional differences in patient characteristics and outcomes during uninterrupted anticoagulation with dabigatran versus warfarin in catheter ablation of atrial fibrillation: the RE-CIRCUIT study. J Interv Card Electrophysiol. (2019) 55:145–52. doi: 10.1007/s10840-019-00518-x
34. Calkins H, Willems S, Gerstenfeld EP, Verma A, Schilling R, Hohnloser SH, et al. Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. (2017) 376:1627–36. doi: 10.1056/NEJMoa1701005
35. Ako J, Okumura K, Nakao K, Kozuma K, Morino Y, Okazaki K, et al. Dual anti-thrombotic therapy with dabigatran after percutaneous coronary intervention in atrial fibrillation–Japanese and East-Asian subgroup analysis of the RE-DUAL PCI trial. Circ J. (2019) 83:327–33. doi: 10.1253/circj.CJ-18-0874
36. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. Dual antithrombotic therapy with dabigatran after pci in atrial fibrillation. N Eng J Med. (2017) 377:1513–24. doi: 10.1056/NEJMoa1708454
37. Niessner A. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) V365:2333. doi: 10.1056/NEJMc1112233
38. Granger C, Alexander J, McMurray J, Lopes R, Hylek E, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2011) V365:981–92. doi: 10.1056/NEJMoa1107039
39. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Eng J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
40. Proietti M, Romanazzi I, Romiti GF, Farcomeni A, Lip GYH. Real-world use of apixaban for stroke prevention in atrial fibrillation: a systematic review and meta-analysis. Stroke. (2018) 49:98–106. doi: 10.1161/STROKEAHA.117.018395
41. Rutherford O, Jonasson C, Ghanima W, Söderdahl F, Halvorsen S. Comparison of dabigatran, rivaroxaban and apixaban for effectiveness and safety in atrial fibrillation; a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. (2020) V6N2:75–85. doi: 10.1093/ehjcvp/pvz086
42. Friberg L, Skeppholm M, Terént A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol. (2015) 65:225–32. doi: 10.1016/j.jacc.2014.10.052
43. Sulzgruber P, Wassmann S, Semb AG, Doehner W, Widimsky P, Gremmel T, et al. Oral anticoagulation in patients with non-valvular atrial fibrillation and a CHA2DS2-VASc score of 1. Eur Heart J. (2019) 40:3010–2. doi: 10.1093/eurheartj/ehz650
44. Deitelzweig S, Keshishian A, Li X, Kang A, Dhamane AD, Luo X, et al. Comparisons between oral anticoagulants among older nonvalvular atrial fibrillation patients. J Am Geriatr Soc. (2019) 67:1662–71. doi: 10.1111/jgs.15956
45. Forslund T, Wettermark B, Andersen M, Hjemdahl P. Stroke and bleeding with non-vitamin K antagonist oral anticoagulant or warfarin treatment in patients with non-valvular atrial fibrillation: a population-based cohort study. Europace. (2018) 20:420–8. doi: 10.1093/europace/euw416
46. Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M, et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfa- rin for non-valvular atrial fibrillation. Circulation. (2015) 131:157–64. doi: 10.1161/CIRCULATIONAHA.114.012061
47. Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, et al. Stroke, bleeding, and mor- tality risks in elderly Medicare beneficiaries treated with dabigatran or rivaroxaban for nonvalvular atrial fibrillation. JAMA Intern Med. (2016) 176:1662–71. doi: 10.1001/jamainternmed.2016.5954
48. Chao TF, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF. Oral anticoagulation in very elderly patients with atrial fibrillation - a nationwide cohort study. Circulation. (2018) 138:37–47. doi: 10.1161/CIRCULATIONAHA.117.031658
49. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin k antagonists:american colege of chest physicians evidence-based clinical practice guidelines (8th edition). Chest. (2008) 133:160S−98S. doi: 10.1378/chest.08-0670
50. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ ACC/HRS guideline for the management of patients with atrial fibrilation: a report of the American college of cardiology/american heart asociation task force on practice guidelines and the heart rhythm society. J Am Col Cardiol. (2014) 130:e199–267. doi: 10.1161/CIR.0000000000000041
51. Sarratt SC, Nesbit R, Moye R. Safety outcomes of apixaban compared with warfarin in patients with end-stage renal disease. Ann Pharmacother. (2017) 51:445–50. doi: 10.1177/1060028017694654
52. Patel MR, Hellkamp AS, Fox KA, ROCKET AF, Executive Committee Steering Committee and Investigators. Point-of-care warfarin monitoring in the ROCKET AF trial. N Eng J Med. (2016) 375:390–1. doi: 10.1056/NEJMc1604020
Keywords: atrial fibrillation, anticoagulation, non-valvular heart disease, direct-acting oral anticoagulant, clinical trial
Citation: Wu J, Zhang Y, Liao X and Lei Y (2020) Anticoagulation Therapy for Non-valvular Atrial Fibrillation: A Mini-Review. Front. Med. 7:350. doi: 10.3389/fmed.2020.00350
Received: 17 April 2020; Accepted: 11 June 2020;
Published: 21 July 2020.
Edited by:
Ziyi Li, Guangdong Second Provincial General Hospital, ChinaReviewed by:
Yeen Huang, The Affiliated Hospital of Shenzhen University, ChinaLong-Sheng Lu, Taipei Medical University, Taiwan
Copyright © 2020 Wu, Zhang, Liao and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyang Liao, bGlhb3hpYW95YW5nQHdjaHNjdS5jbg==
 Jia Wu1
Jia Wu1 Yonggang Zhang
Yonggang Zhang Xiaoyang Liao
Xiaoyang Liao