
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 23 June 2020
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00348
This article is part of the Research Topic Coronavirus Disease (COVID-19): Pathophysiology, Epidemiology, Clinical Management and Public Health Response View all 400 articles
 Thushara Galbadage1
Thushara Galbadage1 Brent M. Peterson1
Brent M. Peterson1 Joseph Awada2
Joseph Awada2 Alison S. Buck2
Alison S. Buck2 Danny A. Ramirez2
Danny A. Ramirez2 Jason Wilson3
Jason Wilson3 Richard S. Gunasekera2*
Richard S. Gunasekera2*To successfully mitigate the extraordinary devastation caused by the Coronavirus disease 2019 (COVID-19) pandemic, it is crucial to identify important risk factors for this disease. One such neglected health determinant is the sex of the patient. This is an essential clinical characteristic, as it can factor into a patient's clinical management and preventative measures. Some clinical studies have shown disparities in the proportion between males and females that have more severe clinical outcomes or, subsequently, die from this disease. However, this association has not been unequivocally established. Thus, the purpose of this investigation was to examine the association between male sex and COVID-19 severity. We systematically reviewed the literature, identified studies that matched predetermined selection criteria, and performed a meta-analysis to evaluate the proportion of males among four disease severity categories. Appropriate assessment strategies were implemented to assess and minimize potential biases. The results of this meta-analysis indicated that males constituted a significantly higher proportion of those who had adverse clinical outcomes and died from COVID-19. As the coronavirus spread from the East to the West, male sex remained a consistent risk factor. Our results support the establishment of the male sex as an important risk factor for this disease. Early identification and appropriate medical care for males with lab-confirmed COVID-19 may substantially change the course of clinical prognosis, resulting in greater numbers of lives saved.
Males and females have distinct biological, immunological, and endocrine differences that result in different disease processes and outcomes. Sex-specific differential gene expression and molecular-level variation have been reported to influence blood pressure, cardiovascular health, and kidney function (1–6). Females, in general, have a heightened capability to activate a more robust immune response, offering protection against many infectious disease processes, but may predispose them to an array of autoimmune diseases (7–12). Males and females also express immunological dimorphisms. Females have two X chromosomes in comparison to the XY in males. The random transcriptional inactivation of X chromosomes in females may also help offset certain mutation-related dysregulation of the immune system (13). Differences in endocrine system regulation in females compared to males significantly affect disease processes including respiratory, cardiovascular, and renal disease (6, 14–18). As nations across the world navigate their way through the Coronavirus disease 2019 (COVID-19) pandemic, clinical, research, and public health experts have observed that this disease does not affect all individuals alike.
Since the beginning of 2020, the world's healthcare professionals have tirelessly attempted to mitigate the impact of the COVID-19 pandemic. With over 6.5 million confirmed cases and 387,000 deaths worldwide as of June 5th, 2020, a post-COVID-19 pandemic era is not within the near foreseeable future (19). The United States, one of the epicenters for the disease, has documented over 1.8 million confirmed cases and 108,000 deaths related to COVID-19 (19, 20). Many recent studies have highlighted certain risk factors that cause specific populations to be disproportionately susceptible to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Currently known risk factors for severe clinical outcomes of COVID-19 include: advanced age (65 years and older), chronic lung diseases, immunocompromised status, and other comorbidities such as hypertension, diabetes, or cardiovascular disease (21–25).
Observations in COVID-19 patient data involving clinical characteristics highlight specific disparities in males and females. A recent case-series study looking at COVID-19 and SARS patients showed that while males and females had the similar disease prevalence, males with COVID-19 were at higher risk for worse clinical outcomes and death (26). In this study, as the patient age and the documented comorbidities (i.e., cardiovascular diseases, diabetes, chronic lung diseases, or hypertension) increased, the risk of severity and mortality in both COVID-19 and SARS patients increased. However, the mortality rate in males was 2.4 times that of their age-matched female counterparts (70.3 and 29.7%, respectively).
Furthermore, a nationwide COVID-19 surveillance study conducted in Italy indicated that male mortality rates related to COVID-19 were disproportionately higher than that of female patients with a ratio as much as 4 to 1 (23). Other systematic reviews performed to characterize clinical features or risk factors for COVID-19, have also identified the sex-specific disparities in disease severity and mortality (25, 27). However, the clinical importance of male sex as a risk factor for COVID-19 has mainly been overlooked or explained as a potential confounder to other environmental factors such as smoking or tobacco product usage (28). While various studies have made observations of the sex-specific disparities of COVID-19, this specific relationship has not been adequately established. The sex-specific disease severity is an important clinical consideration as it affects all patient populations. Recognition of male sex as a risk factor for COVID-19 will impact both preventative measures and clinical patient management protocols.
The goal of this systematic review and meta-analysis is to identify whether males are more susceptible to COVID-19, severe forms of the disease, or mortality related to COVID-19. To address this question, we systematically reviewed the literature using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We performed a meta-analysis of the selected study populations comparing male and female COVID-19 patients. This review incorporated three online databases and research studies published between December 15th, 2019, and April 16th, 2020. We characterized the influence of sex as a risk factor for COVID-19 measuring the following clinical outcomes: all lab-confirmed cases, severe cases, critically ill cases, and mortality.
We performed a comprehensive systematic literature search of three online databases, PubMed (LitCOVID), Embase (OVID), and Web of Science (WoS), from December 15th, 2019, to April 16th, 2020. We identified all research articles related to COVID-19 that contained any sex-specific patient or clinical characterizations. The search terms and keywords used to identify research studies for the meta-analysis were: COVID-19, male, female, men, women, sex, and gender (Supplemental Table 1). We reviewed references of review, perspectives, systematic reviews, and meta-analysis articles of the include articles to ensure comprehensiveness of our search. All our search results were evaluated using the PRISMA statement. We reviewed the abstracts and tables of each of the articles to identify the presence of sex-specific (male and female) COVID-19 case numbers. Studies that did not contain an abstract in English were excluded from our study during the screening stage.
The inclusion criteria for research article selection was as stated below. Study population: patients with lab-confirmed COVID-19 diagnosis. Study design: case series or cross-sectional study that did not exclude any lab-confirmed COVID-19 patients. Outcomes measure: at least one outcome reported with male to female ratio among lab-confirmed clinical cases, severe cases, critical cases, and mortality. Research study: only peer-reviewed research publications were included. Commentary articles, perspectives, review articles, and surveillance reports were excluded. The following case definitions were used in this study. All cases were lab-confirmed COVID-19 patients. Severe cases were defined as having at least one of the following clinical findings: (a) breathing rate ≥30/min, (b) oxygen saturation (SpO2) ≤ 93% at rest, or (c) ratio of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) ≤300 mmHg. The severe case definition followed the American Thoracic Society guidelines for community-acquired pneumonia (29). Critical cases were defined as: (a) received mechanical ventilation; (b) clinically diagnosed with shock symptoms, (c) received care in the intensive care unit (ICU) or (d) transfer to a tertiary care hospital.
All articles identified through the keyword search from the online databases were organized into an Excel® spreadsheet. Following the removal of duplicates, articles were subjected to evaluation, and five investigators did data extraction. Research studies were screened using the abstract and any tabulated clinical characteristics of COVID-19 patients. Directly after that, research articles were again screened to identify any discrepancies by an independent investigator. The screened articles were assessed against the study selection criteria by two independent investigators, and any differences in selected articles were revisited, and a definitive determination was made. We organized studies according to the study period, study location, and patient population included in the analysis to ensure we were not using the same COVID-19 cases more than once in our analysis.
A bias risk assessment was conducted on studies included in the meta-analysis utilizing the methodological index for non-randomized studies (Minors) criteria at the study level (30). Each of the selected articles was scored with 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The highest score possible was 16 for non-comparative studies according to Minors guidelines.
Statistical analysis was conducted using R (31) with the meta-analysis packages meta (32) and dmetar (33) (Supplemental Data 1). The principal summary measures of the meta-analysis were proportions of males in four different COVID-19 categories. The four groups were: (a) all confirmed COVID-19 cases, (b) severe cases of COVID-19 as defined in section Data Extraction and Quality Assessment, (c) critically ill cases of COVID-19 as defined in section Data Extraction and Quality Assessment, and (d) deaths associated with COVID-19. Agresti-Coull confidence intervals were used for individual studies. Studies were combined using the inverse variance method on the raw proportions with the DerSimonian-Laird estimator for the between-groups variance estimator (τ2) and the Jackson method for combined confidence intervals. Transformations of raw proportions were calculated for the combined estimates (log, logit, arcsin, and Freeman-Tukey double arcsin), but the results were so similar they are not shown. The proportion of variation in treatment effects was estimated with I2. To assess bias across studies, funnel plots were constructed for each of the four different categories, and Egger's bias test conducted. To determine if there were region-specific differences among populations in Asian and Western countries, we sub-divided the COVID-19 critically ill patient populations into these two regions and analyzed them.
To combine the ages, in 20 of the articles, the median age of patients was given, along with sometimes interquartile range, sometimes min and max. In 10 of the articles, mean and standard deviation (SD) were presented. In one article (34), the mean age was given without SD. We used linear regression on the other 10 (mean, SD) pairs to estimate the SD to be 14.5 years. To combine the ages, we chose to convert means to medians because there would be fewer unknown statistics to estimate, and typical disease distributions are skewed. To convert, we fit a negative binomial distribution to the mean and SD using the method of moments. With the complete list of medians, we used R's metamedian (35) package to obtain summarized confidence intervals for each of the four categories.
We identified 786 research articles that matched our search terms. After the duplicated were removed, 414 unique research articles were screened. Following the screening process, 353 articles with incomplete data were excluded. We then identified 61 research articles with sex-specific case numbers and reviewed full-length articles to assess their eligibility for our study according to the selection criteria. Thirty articles did not fit the selection criteria and were excluded from the meta-analysis. Reasons for exclusion were: not a primary research study (a surveillance report or perspective), did not include consecutive patients or did not meet with the case or severity definitions. The 31 research articles eligible for this meta-analysis were used for qualitative synthesis and quantitative analysis (Figure 1). The 31 eligible articles were subjected to a bias assessment using the Minors criteria at the study level (30). All 31 selected articles scored between 12 and 14 points, with 16 being the highest for non-randomized controlled studies (Table 1). The relatively high scores indicated that we were likely not introducing any significant systematic biases.
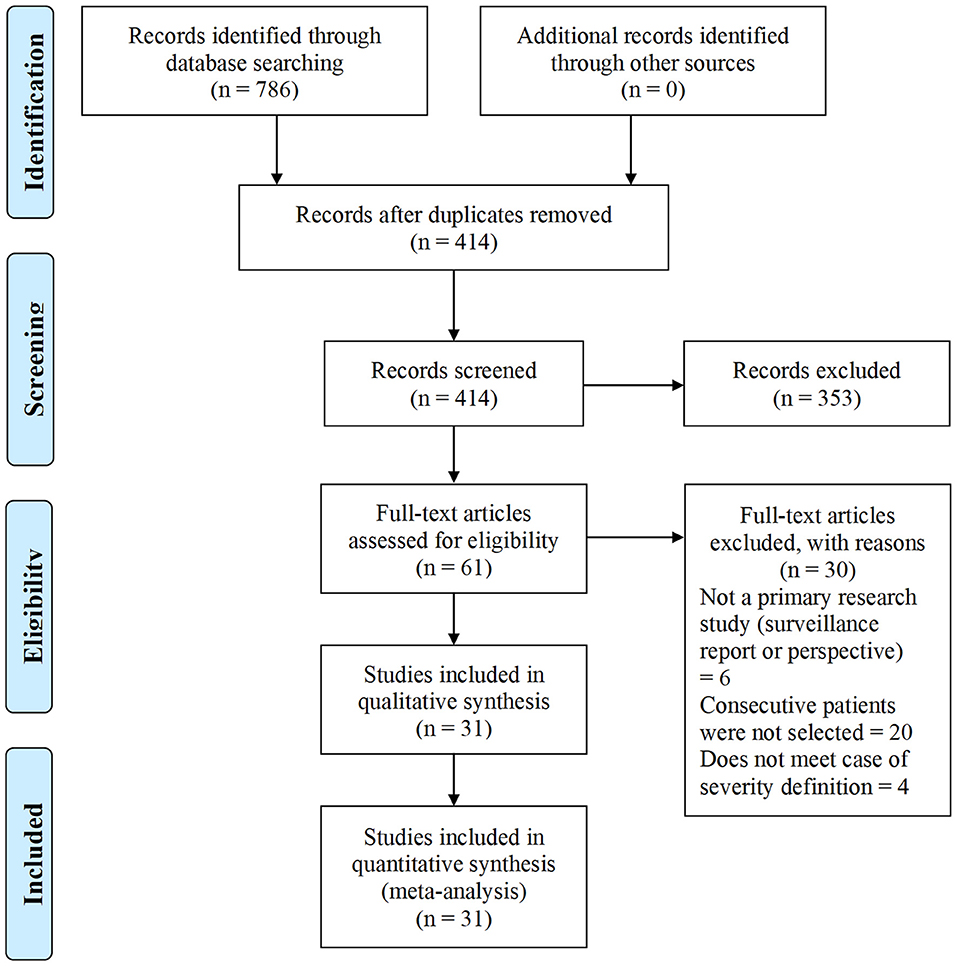
Figure 1. COVID-19 sex-specific clinical outcomes flow diagram of the inclusion criteria of studies eligible for meta-analysis. Flow diagram template adopted from the PRISMA approach to meta-analysis (36).
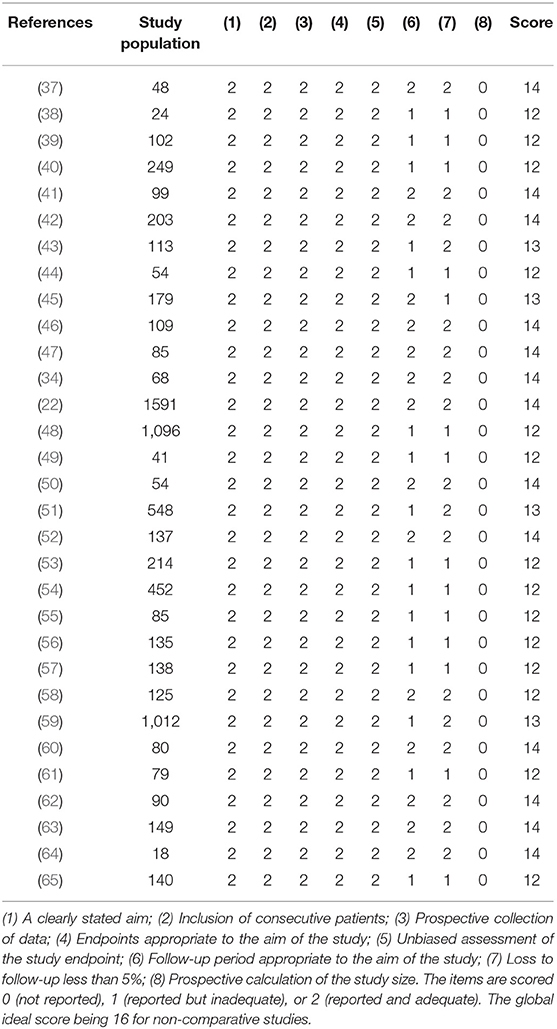
Table 1. Bias risk assessment on the studies included in the meta-analysis using the methodological index for non-randomized studies (Minors) criteria (30).
Within our selected studies, 7,556 lab-confirmed COVID-19 cases were identified. Of these 31 studies, 24 were from various cities in China and included a sample of 5,629 lab-confirmed cases. Two studies were from South Korea and Singapore, which included a sample of 72 lab-confirmed cases. The other five studies were from Europe and North America, having a sample of 1,855 lab-confirmed cases (Figure 2 and Table 2). Most of the early studies came from China with study periods from December 11th, 2019, to February 24th, 2020. Most of the later studies came from other countries with study periods from January 23rd to April 5th, 2020 (Figure 3). These patterns reflect the movement of epicenters for COVID-19 from the East to the West.
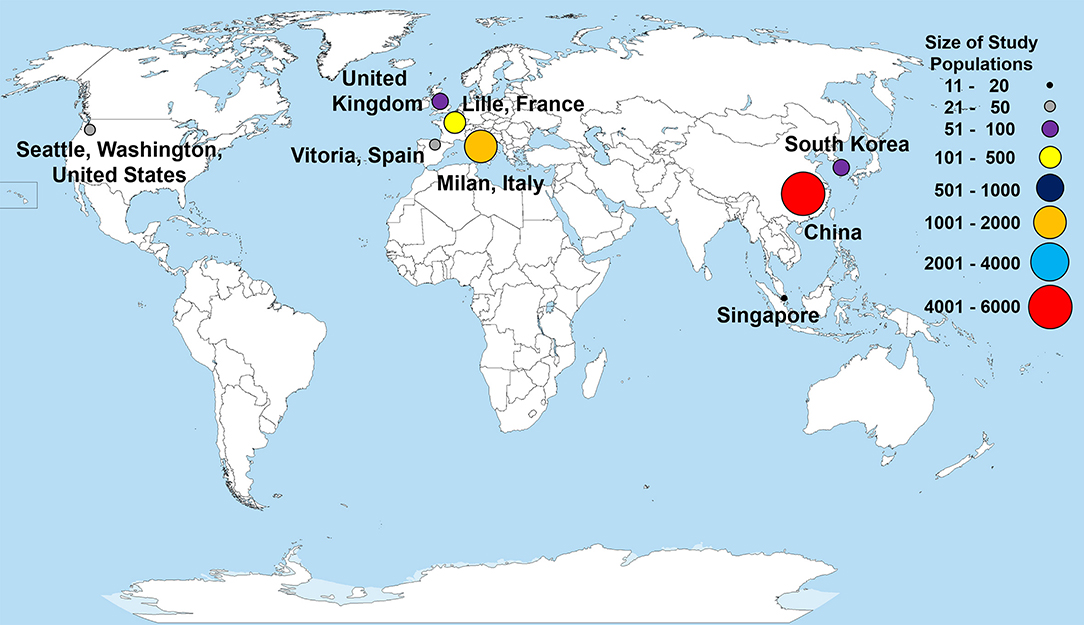
Figure 2. Countries and locations for the selected studies used in the meta-analysis. Total patient populations in each of the study locations are illustrated with a colored circle and correspond to the size of study populations. Each point represents a research study, except for China, which represents the total patient population from 24 different studies. The world map was obtained from Wikimedia Commons, the free media repository licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
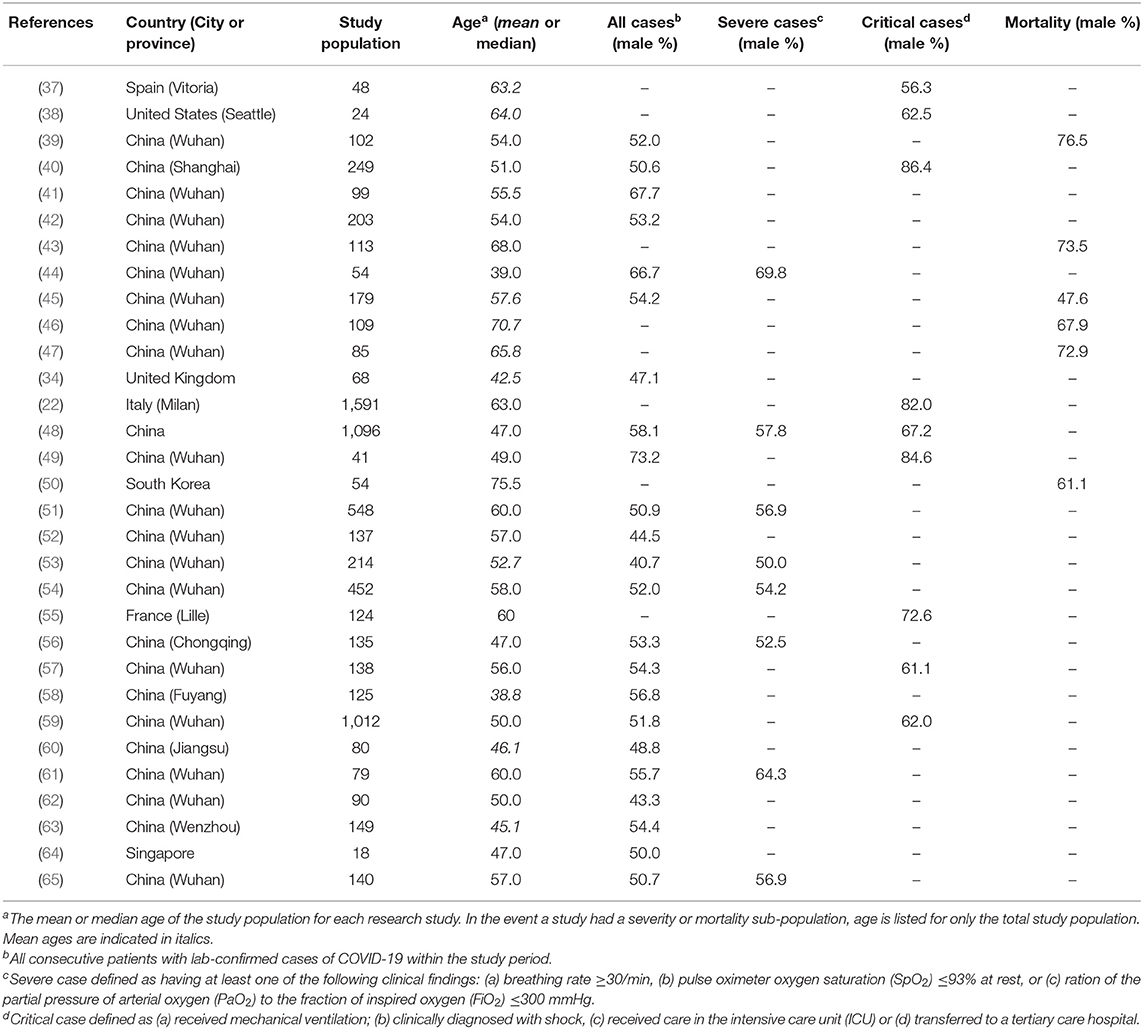
Table 2. Demographics of all studies included in the meta-analysis with sex-specific disease severity.
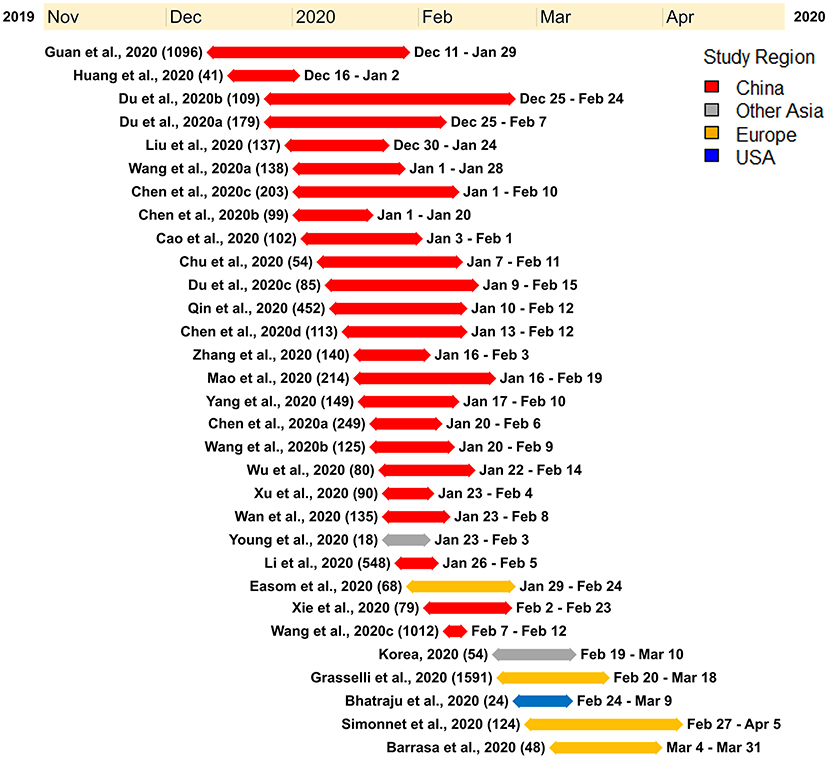
Figure 3. Timeline illustrating the study period of each of the research studies used for the meta-analysis. Each research study used for the meta-analysis is represented by the study name (study sample), duration of the study with a line corresponding to the length of the study, and the start and end date of the study. The studies were ordered according to the start date of each study.
The principal quantitative results are contained in the forest plots shown on the left side of Figures 4, 5. The individual confidence intervals are shown, by study, with the combined proportion for each group and confidence interval at the bottom. A random-effects model was used for the combined proportion to check for heterogeneity (τ2= between-group variation and I2= proportion of total variation in the estimates of treatment effects due to heterogeneity). We used the following guidelines for interpreting I2: I2 = 25% is small heterogeneity; I2 = 50% is medium heterogeneity; and I2 = 75% is large heterogeneity (66). The heterogeneity statistics (τ2 and I2) are shown at the bottom left of the forest plots.
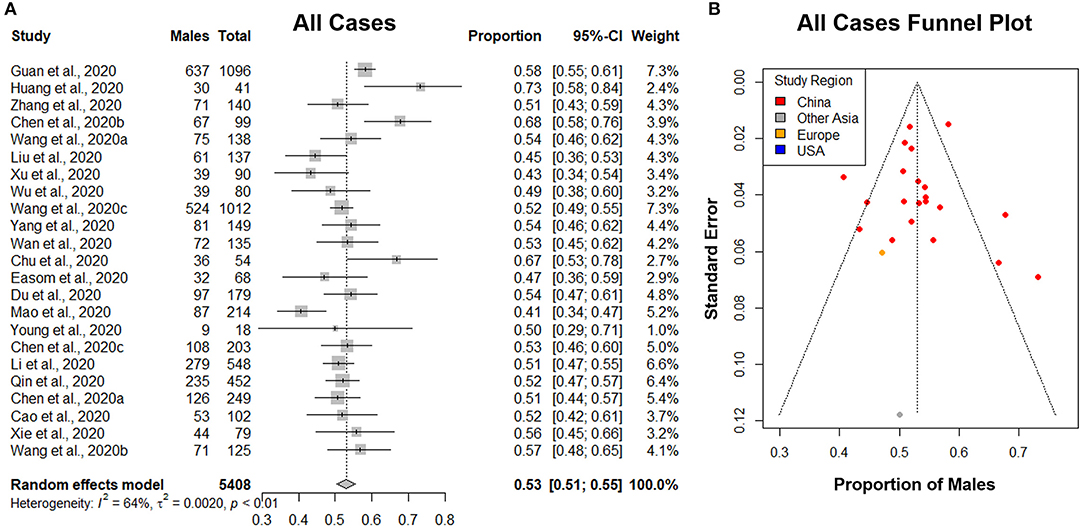
Figure 4. The proportion of males in all lab-confirmed COVID-19 cases. (A) Forest plot of sex-distribution in all lab-confirmed COVID-19 cases in each of the studies. Proportions of males and the 95% confidence intervals (CIs) are indicated. The vertical dotted line represents the combined proportion of all studies. The diamond represents the combined 95% CI, the left and right endpoints of which are the lower and upper bounds of the CI, respectively. (B) Funnel plot with 95% confidence region of sex-distribution in all lab-confirmed COVID-19 cases in each of the studies.
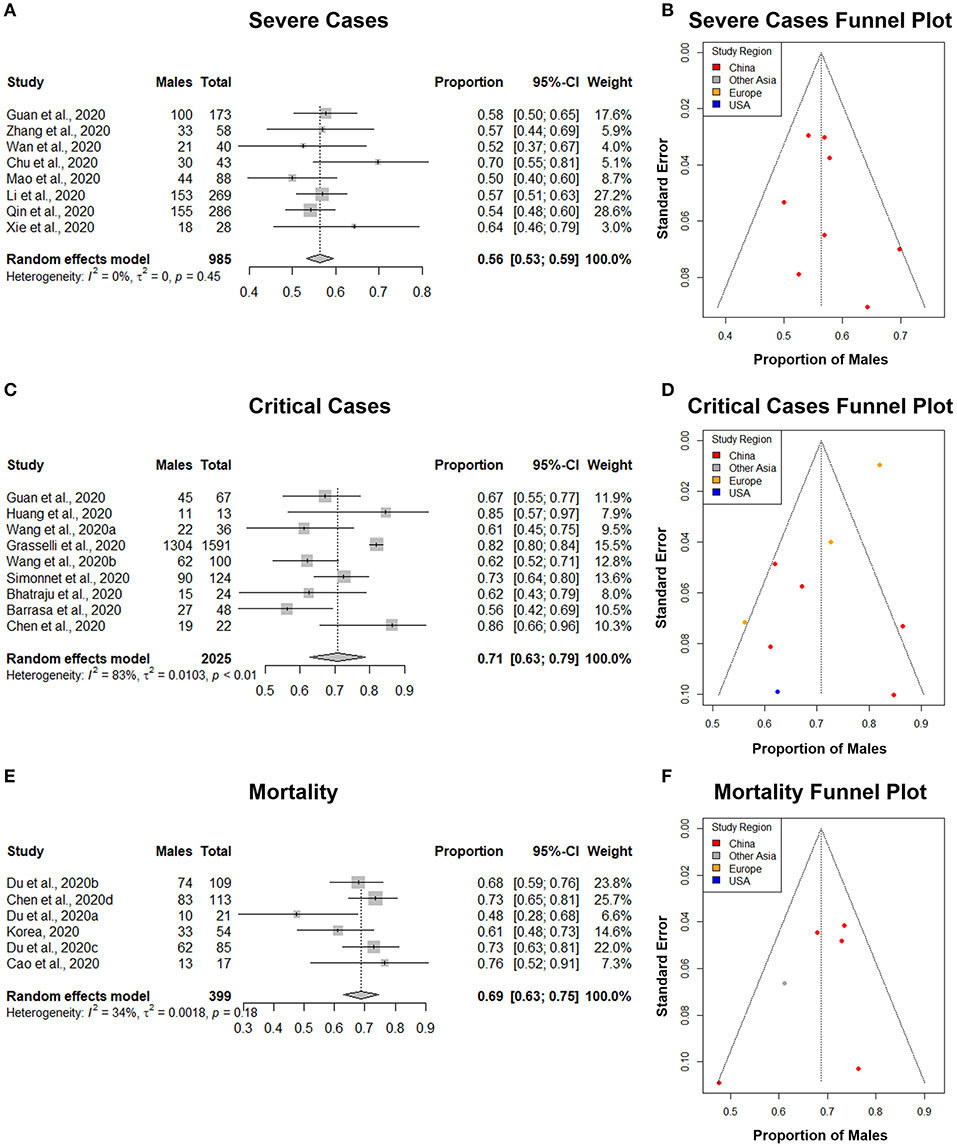
Figure 5. The proportion of males in COVID-19 severe cases, critical cases, and mortalities. (A,C,E) Forest plot of sex-distribution in COVID-19 cases in each of the studies. Proportions of males and the 95% confidence intervals (CIs) are indicated. The vertical dotted lines represent the combined proportion of all studies. The diamond represents the combined 95% CI, the left and right endpoints of which are the lower and upper bounds of the CI, respectively. (A) Severe cases defined as having at least one of the following clinical findings: breathing rate ≥30/min, pulse oximeter oxygen saturation (SpO2) ≤93% at rest, or ration of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) ≤300 mmHg. (C) Critical case defined as: received mechanical ventilation, clinically diagnosed with shock, received care in the intensive care unit (ICU), or transferred to a tertiary care hospital. (E) Mortality defined as all deaths in COVID-19 patients that occurred during the study period. (B,D,F) Funnel plot with 95% confidence region of sex-distribution in COVID-19 severe cases, critical cases, and mortality in each of the studies.
A total of 23 studies with 5,408 lab-confirmed COVID-19 cases were analyzed (Table 3). Our results from the randomized effects model meta-analysis showed that in the sex-distribution of all COVID-19 cases, males accounted for 53% (95% CI [0.51, 0.55]) (Figure 4A). Female patients made up 47% of all COVID-19 cases. There is medium heterogeneity between the set of overall population proportions (I2 = 64%, τ = 0.05). A funnel plot was drawn to assess the publication bias (Figure 4B). The publication bias test results: Egger's test (p = 0.88) indicated that there was no publication bias.
A total of 8 studies with 985 severe COVID-19 cases were analyzed (Table 4). Our results from the randomized effects model meta-analysis showed that in the sex-distribution of all COVID-19 severe cases, males accounted for 56% (95% CI [0.53, 0.59]) (Figure 5A). Female patients made up 44% of all COVID-19 severe cases. There is no heterogeneity for the severe population proportions (I2 = 0%, τ = 0. 0). A funnel plot was drawn to assess the publication bias (Figure 5B). The publication bias test results: Egger's test (p = 0.40) indicated that there was no publication bias.
A total of 9 studies with a total of 2,025 critical COVID-19 cases were analyzed (Table 5). Our results from the randomized effects model meta-analysis showed that in the sex-distribution of all COVID-19 critically ill cases, males accounted for 71% (95% CI [0.63, 0.79]) (Figure 5C). Female patients made up 29% of all COVID-19 critical cases. There was large heterogeneity between the critical population proportions (I2 = 83%, τ = 0.10). A funnel plot was drawn to assess the publication bias (Figure 5D). The publication bias test results: Egger's test (p = 0.02) indicated that there could be some publication bias introduced by the Grasselli et al. (22) study.
A total of 6 studies with a total of 399 mortalities related to COVID-19 cases were analyzed (Table 6). Our results from the randomized effects model meta-analysis showed that in the sex-distribution of all COVID-19 mortalities, males accounted for 69% (95% CI [0.63, 0.75]) (Figure 5E). Female patients made up 31% of all COVID-19 mortalities. The heterogeneity for the mortality population proportions is small (I2 = 34%, τ = 0.04). A funnel plot was drawn to assess the publication bias (Figure 5F). The publication bias test results: Egger's test (p = 0.26) indicated that there was no observable publication bias.
Sex-specific differences in clinical outcomes of COVID-19 cases in China were thought to be related to cultural and social differences in males and females (28). We investigated if our study results hold in different regions of the world. COVID-19 critically ill patient data sets were divided into two groups: Asia and West, and subgroup analyses were performed. We selected the critically ill patient group for Asia and the West as it was the only disease category that included multiple studies from both Asia and West for an appropriate comparison and statistical analysis.
A total of 5 studies from Asia, with a total of 238 critical COVID-19 cases were analyzed. Our results from the randomized effects model meta-analysis showed that in the sex-distribution of COVID-19 critically ill cases from Asia, males accounted for 71% (95% CI [0.61, 0.81]) (Figure 6A). Female patients made up 29% of all COVID-19 critical cases in Asia. There was medium heterogeneity between the critical population proportions (I2 = 64%, τ = 0.0082). A funnel plot was drawn to assess the publication bias in studies from Asia (Figure 6B). The publication bias test results: Egger's test (p = 0.26) indicated that there was no observable publication bias.
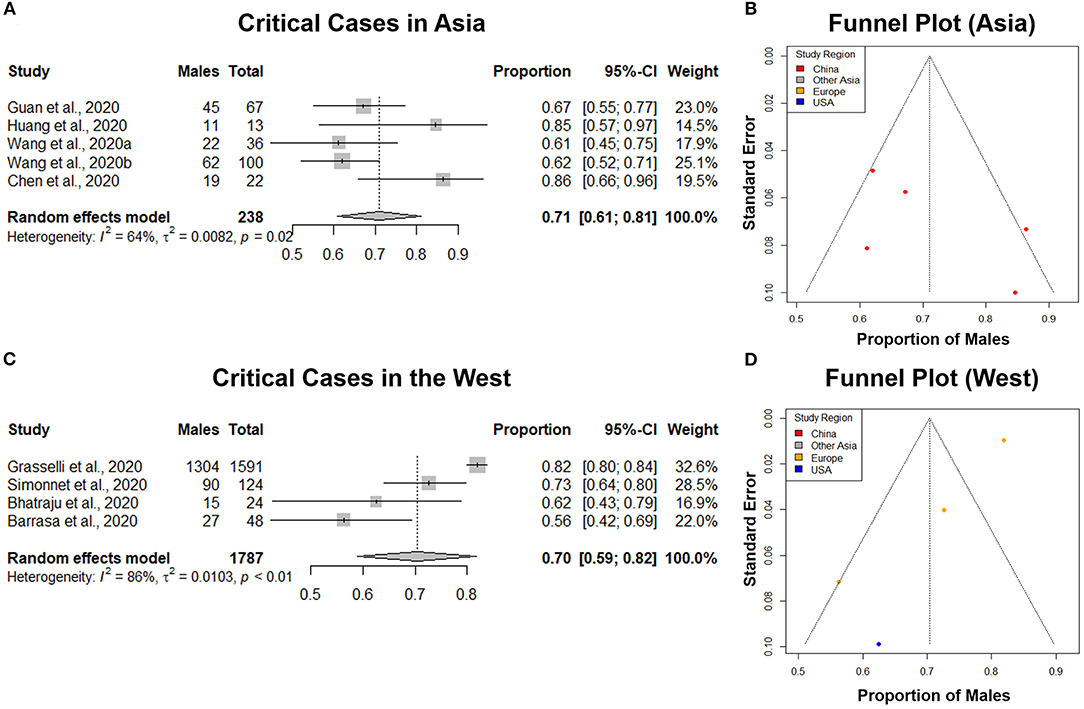
Figure 6. Comparison of the proportion of males in COVID-19 critical cases in Asia and the West. (A,C) Forest plot of sex-distribution in COVID-19 critical cases in each of the studies. Proportions of males and the 95% confidence intervals (CIs) are indicated. The vertical dotted lines represent the combined proportion of all studies. The diamond represents the combined 95% CI, the left and right endpoints of which are the lower and upper bounds of the CI, respectively. Critical case defined as: received mechanical ventilation, clinically diagnosed with shock, received care in the intensive care unit (ICU), or transferred to a tertiary care hospital. (A) Critical cases in Asian countries. (C) Critical Cases in western countries. (B,D) Funnel plot with 95% confidence region of sex-distribution in COVID-19 critical cases in each of the studies.
A total of 4 studies from Western regions with a total of 1,787 critical COVID-19 cases were analyzed. Our results from the randomized effects model meta-analysis showed that in the sex-distribution of COVID-19 critically ill cases from the West, males accounted for 70% (95% CI [0.59, 0.82]) (Figure 6C). Female patients made up 30% of all COVID-19 critical cases in the West. There was large heterogeneity between the critical population proportions (I2 = 86%, τ = 0.0103). A funnel plot was drawn to assess the publication bias in studies from the West (Figure 6D). The publication bias test results: Egger's test (p = 0.04) indicated that there could be some publication bias introduced by the Grasselli et al. (22) study, as indicated previously. We performed a difference of proportions test among critically ill cases in Asia and the West. There was no statistically significant difference between these two groups (p = 0.96). This comparative subgroup analysis of Asia and the West indicated that there was no geography-specific difference in the proportion of critically ill COVID-19 male patients. However, indicated by the moderate to large heterogeneity observed, there are likely variations in male proportion between different studies and regions.
When extracting male and female proportions for each of the four COVID-19 disease severity categories, we obtained the age distributions of the cases stated as a mean ± SD or median and interquartile range (IQR). Using a skewed distribution assumption, the ages were aggregated as medians with 95% confidence intervals. The median age for all COVID-19 cases was 50, severe cases was 61, critically ill cases was 63, and mortality was 70 (Figure 7). A Kruskal-Wallis ranked-sum test conducted on the medians showed that age was significantly different between the COVID-19 disease severity groups (chi-squared = 24.07, df = 3, p = < 0.0001). Our data confirm that advanced age is a risk factor for more severe clinical outcomes and mortality related to COVID-19.
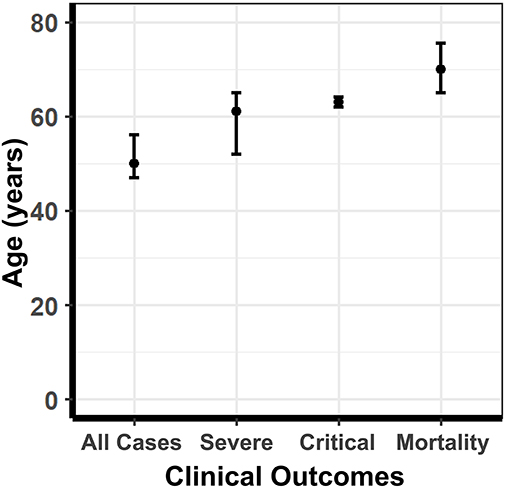
Figure 7. The median age of COVID-19 patients stratified according to disease severity. The median age of COVID-19 patients in all cases, severe cases, critically ill cases, and mortalities. Error bars represent 95% confidence intervals of the median. The median age for all COVID-19 cases was 50, severe cases was 61, critically ill cases was 63, and mortality was 70. A Kruskal-Wallis ranked-sum test conducted on the medians showed that age was significantly different amount the COVID-19 disease severity groups (chi-squared = 24.07, df = 3, p = <0.0001).
In our systematic review and meta-analysis, we set forth to address the question of whether male sex is a risk factor for COVID-19 susceptibility, severe forms of the disease, or mortality related to COVID-19. Systematically reviewing all literature from December 15th, 2019, to April 16th, 2020, we selected 31 research studies that met our selection criteria and performed a meta-analysis on COVID-19 clinical outcomes. Our quality assessment measures indicated small heterogeneity in terms of a single-arm meta-analysis, and the sensitivity analysis showed that there was minimal publication bias. As of the time of completing this manuscript, there were no randomized controlled trials with COVID-19 patients that could address this particular question. The use of non-randomized studies for the meta-analysis is a limitation of this study. However, Abraham et al. (67) suggested that, in the absence of randomized, controlled trials, that a well-designed meta-analysis using non-randomized controlled trials can also present a high level of evidence (67).
The four clinical outcome categories (overall, severe, critical, mortality) exhibited different levels of heterogeneity in our random-effects models. The different heterogeneities observed in some outcome categories is a potential limitation of this study. The explanation for these differences is most likely the region of the studies done within each category. The 23 overall studies exhibited 64% heterogeneity with one from Singapore and one from Great Britain. The eight severe studies exhibited 0% heterogeneity, all being from China. The nine critical studies exhibited 83% heterogeneity, with five from China, three from Europe, and one from the United States. The six mortality studies exhibited 35% heterogeneity, with five from China and one from South Korea. The use of a randomized effects model for our meta-analysis takes into account these heterogeneities observed between different studies and regions. Based on the random-effects models shown, there appears to be a difference in the proportions of males with COVID-19 between at least some of the studies or regions. Due to the study designs, their sampling methods, and limited regions included in this study, it is neither possible nor wise to be more specific. This is a potential avenue for further research.
A few systematic review studies looking at COVID-19 risk factors, clinical characteristics and predictive models identified male sex as a risk factor for either disease incidence or mortality (25, 51, 68–70). Our study findings further confirm these observations. In contrast to previous studies, this study is the first systematic analysis that specifically looks at sex-specific clinical outcomes detailing COVID-19 severity (severe, critically ill, and mortality). Our study selection criteria also allowed the inclusion of a wider representation of sex-specific clinical studies and sample populations, as our study focus was only on patient clinical outcomes.
Our meta-analysis showed that while males accounted for 53% of all COVID-19 cases, males accounted for an increasing proportion of severe cases (56%), critically ill cases (71%), and mortalities (69%) compared to their counterpart. While similar male to female disproportions was observed among a few other studies looking at clinical characteristics of COVID-19, our study provides a comprehensive synthesis of data available across different world regions. This study helps establish male sex as a risk factor for COVID-19 clinical outcomes and shows that it is consistent in Asia and Western regions.
This study results do not come as a surprise. Several studies conducted on the two previous coronavirus epidemics, SARS CoV-1in 2002–2003 and MERS in 2012–2013, showed similar patterns with a male predominance toward greater severity and mortality risks. Studies on mortality rates during the MERS-CoV epidemic showed the male sex to be a risk factor (71–73). Epidemiological studies with SARS-CoV-1 showed similar patterns (74). To further support previous epidemiological observations, in controlled mouse model experiments, SARS-CoV-1 has displayed infectious dose-dependent higher mortality rates in male mice compared to female mice (15). The mounting amount of evidence showing differences among males and female clinical outcomes to coronavirus infections highlights the importance of patient sex in determining the COVID-19 prognosis.
From a clinical standpoint, this information is very pertinent to the practice of patient care. As COVID-19 clinical outcomes are strongly associated with male sex, this can help guide preventative and treatment strategies. Male patients will likely warrant more aggressive inpatient care measures, and especially those that have other COVID-19 risk factors such as advanced age or underlying comorbidities. Susceptible males with other known risk factors may need to take extra precautions to help prevent SARS-CoV-2 infection. Infected males can be encouraged to obtain medical care at an earlier stage of the disease. In cases that require hospitalization, physicians should take into account that medical management could be more difficult in male patients as they are at higher risk of severe disease and mortality.
In addition to preventative and COVID-19 treatment measures, this presents a unique clinical opportunity to address male and female differences at the molecular level, immunological response, and endocrine function (5, 11, 13, 75). For example, SARS-CoV-2 binds to the Angiotensin-converting enzyme 2 (ACE2) receptors and use it as a mechanism for host cell entry (76). Males have been shown to express more ACE2 receptors within the renin-angiotensin-aldosterone system (RAAS) (77). This is likely to play an essential role in the severity of this disease observed in males (77). Differences in male and female immunological responses will also be a clinically significant factor that can be appropriately modulated to better serve COVID-19 patients (8, 12). Besides sex-specific differences in immunological responses, hormonal regulation and the role of estrogen and testosterone in priming the ACE2 receptor sensitivity could hold the key to better explain the higher COVID-19 severity and mortality rates observed in males (78–80). In an age of personalized medicine, if the molecular level of differences in the disease processes of SARS-CoV-2 infection can be characterized with appropriate research, clinicians will be able to use targeted therapy using to promote health equality and help save more lives.
All datasets generated for this study are included in the article/Supplementary Material.
TG led the systematic review, helped prepare the tables and figures, and aided in writing and editing. BP aided in the analytical evaluation of curated articles, writing, and editing. JA, AB, and DR research students analyzed data and joined in discussions. JW performed statistical analyses and drafted statistical sections. RG conceptualized the problem and aided in writing and editing. All authors contributed to the article and approved the submitted version.
This work of RG and TG was supported in part by the Discovery Institute and the Peter & Carla Roth Family.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We acknowledge Dr. Siva Somasundaram, Ms. Avishka Jayasekera, and Dr. John P. Walsh of the University of Southern California for their collaborative support. We also thank Dr. Jeffrey S. Wang, Infectious Disease Specialist at Kaiser Permanente, Anaheim, California, for his clinical insights.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00348/full#supplementary-material
1. Convertino VA. Gender differences in autonomic functions associated with blood pressure regulation. Am J Physiol. (1998) 275:R1909–20. doi: 10.1152/ajpregu.1998.275.6.R1909
2. Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. (2001) 37:1199–208. doi: 10.1161/01.HYP.37.5.1199
3. Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. (2002) 53:672–7. doi: 10.1016/S0008-6363(01)00479-5
4. Kang AK, Miller JA. Effects of gender on the renin-angiotensin system, blood pressure, and renal function. Curr Hypertens Rep. (2002) 4:143–51. doi: 10.1007/s11906-002-0039-9
5. Sandberg K, Ji H. Sex and the renin angiotensin system: implications for gender differences in the progression of kidney disease. Adv Ren Replace Ther. (2003) 10:15–23. doi: 10.1053/jarr.2003.50006
6. Hilliard LM, Sampson AK, Brown RD, Denton KM. The “his and hers” of the renin-angiotensin system. Curr Hypertens Rep. (2013) 15:71–9. doi: 10.1007/s11906-012-0319-y
7. Fairweather D, Rose NR. Women and autoimmune diseases. Emerg Infect Dis. (2004) 10:2005–11. doi: 10.3201/eid1011.040367
8. Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. (2012) 38:J282–91. doi: 10.1016/j.jaut.2011.11.013
9. Rubtsova K, Marrack P, Rubtsov AV. Sexual dimorphism in autoimmunity. J Clin Invest. (2015) 125:2187–93. doi: 10.1172/JCI78082
10. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
11. vom Steeg LG, Klein SL. SeXX matters in infectious disease pathogenesis. PLoS Pathog. (2016) 12:e1005374. doi: 10.1371/journal.ppat.1005374
12. Jaillon S, Berthenet K, Garlanda C. Sexual dimorphism in innate immunity. Clin Rev Allergy Immunol. (2019) 56:308–21. doi: 10.1007/s12016-017-8648-x
13. Taneja V. Sex hormones determine immune response. Front Immunol. (2018) 9:1931. doi: 10.3389/fimmu.2018.01931
14. Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res. (2016) 118:1294–312. doi: 10.1161/CIRCRESAHA.116.307509
15. Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. (2017) 198:4046–53. doi: 10.4049/jimmunol.1601896
16. Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab. (2018) 15:45–55. doi: 10.1016/j.molmet.2018.05.008
17. Vermillion MS, Ursin RL, Attreed SE, Klein SL. Estriol reduces pulmonary immune cell recruitment and inflammation to protect female mice from severe influenza. Endocrinology. (2018) 159:3306–20. doi: 10.1210/en.2018-00486
18. Wensveen FM, Šestan M, Turk Wensveen T, Polić B. ‘Beauty and the beast’ in infection: how immune-endocrine interactions regulate systemic metabolism in the context of infection. Eur J Immunol. (2019) 49:982–95. doi: 10.1002/eji.201847895
19. WHO. Coronavirus Disease (COVID-19) Situation Report 137. (2020). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed June 6, 2020).
20. CDC. Coronavirus Disease 2019 (COVID-19) Cases in the U.S. (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html (accessed June 6, 2020).
21. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID-19: a systematic review and meta-analysis. Arch Acad Emerg Med. (2020) 8:e35.
22. Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. (2020) 323:1574–81. doi: 10.1001/jama.2020.5394
23. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. (2020) 395:1225–8. doi: 10.1016/S0140-6736(20)30627-9
24. Shahid Z, Kalayanamitra R, McClafferty B, Kepko D, Ramgobin D, Patel R, et al. COVID-19 and older adults: what we know. J Am Geriatr Soc. (2020) 68:926–9. doi: 10.1111/jgs.16472
25. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. (2020) doi: 10.1016/j.jinf.2020.04.021. [Epub ahead of print].
26. Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. (2020) 8:152. doi: 10.3389/fpubh.2020.00152
27. Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. (2020) 92:577–83. doi: 10.1002/jmv.25757
28. Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. (2020) 8:e20. doi: 10.1016/S2213-2600(20)30117-X
29. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. (2019) 200:e45–67. doi: 10.1164/rccm.201908-1581ST
30. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
31. R Core Team. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna (2019). Available online at: https://www.R-project.org/ (accessed May 8, 2020).
32. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22:153–60. doi: 10.1136/ebmental-2019-300117
33. Harrer M, Cuijpers P, Furukawa T, Ebert DD. dmetar: Companion R Package For The Guide 'Doing Meta-Analysis in R'. R package version 0.0.9000 (2019). Available online at: http://dmetar.protectlab.org (accessed May 8, 2020).
34. Easom N, Moss P, Barlow G, Samson A, Taynton T, Adams K, et al. Sixty-eight consecutive patients assessed for COVID-19 infection: experience from a UK Regional infectious diseases Unit. Influenza Other Respir Viruses. (2020) 14:374–9. doi: 10.1111/irv.12739
35. McGrath S, Zhao X, Steele R, Thombs BD, Benedetti A. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020). doi: 10.1177/0962280219889080
36. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
37. Barrasa H, Rello J, Tejada S, Martín A, Balziskueta G, Vinuesa C, et al. SARS-Cov-2 in Spanish intensive care: early experience with 15-day survival in vitoria. Anaesth Crit Care Pain Med. (2020). doi: 10.1016/j.accpm.2020.04.001. [Epub ahead of print].
38. Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, et al. Covid-19 in critically ill patients in the seattle region - case series. N Engl J Med. (2020) 382:2012–22. doi: 10.1056/NEJMoa2004500
39. Cao J, Tu WJ, Cheng W, Yu L, Liu YK, Hu X, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis. (2020) ciaa243. doi: 10.1093/cid/ciaa243
40. Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. (2020) 80:e1–6. doi: 10.1016/j.jinf.2020.03.004
41. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
42. Chen T, Dai Z, Mo P, Li X, Ma Z, Song S, et al. Clinical characteristics and outcomes of older patients with Coronavirus Disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. (2020). doi: 10.1093/gerona/glaa089. [Epub ahead of print].
43. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. (2020) 368:m1091. doi: 10.1136/bmj.m1091
44. Chu J, Yang N, Wei Y, Yue H, Zhang F, Zhao J, et al. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J Med Virol. (2020) 2:807–13. doi: 10.1002/jmv.25793
45. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. (2020) 55:2000524. doi: 10.1183/13993003.00524-2020
46. Du RH, Liu LM, Yin W, Wang W, Guan LL, Yuan ML, et al. Hospitalization and critical care of 109 decedents with COVID-19 pneumonia in Wuhan, China. Ann Am Thorac Soc. (2020) doi: 10.1513/AnnalsATS.202003-225OC. [Epub ahead of print].
47. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. (2020) 201:1372–9. doi: 10.1164/rccm.202003-0543OC
48. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
49. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
50. Korea C. Analysis on 54 mortality cases of Coronavirus Disease 2019 in the republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. (2020) 35:e132. doi: 10.3346/jkms.2020.35.e132
51. Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. (2020) doi: 10.1016/j.jaci.2020.04.006. [Epub ahead of print].
52. Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. (2020) 133:1025–31. doi: 10.1097/CM9.0000000000000744
53. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. (2020). doi: 10.1001/jamaneurol.2020.1127
54. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. (2020). doi: 10.2139/ssrn.3541136. [Epub ahead of print].
55. Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. (2020). doi: 10.1002/oby.22831. [Epub ahead of print].
56. Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. (2020) 92:797–806. doi: 10.1002/jmv.25783
57. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
58. Wang R, Pan M, Zhang X, Fan X, Han M, Zhao F, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis. (2020) 95:421–8. doi: 10.1016/j.ijid.2020.03.070
59. Wang X, Fang J, Zhu Y, Chen L, Ding F, Zhou R, et al. Clinical characteristics of non-critically ill patients with novel Coronavirus infection (COVID-19) in a Fangcang hospital. Clin Microbiol Infect. (2020) doi: 10.1016/j.cmi.2020.03.032. [Epub ahead of print].
60. Wu J, Liu J, Zhao X, Liu C, Wang W, Wang D, et al. Clinical characteristics of imported cases of COVID-19 in Jiangsu province: a multicenter descriptive study. Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa199. [Epub ahead of print].
61. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. (2020) 40:1321–6. doi: 10.1111/liv.14449
62. Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. (2020) 47:1275–80. doi: 10.1007/s00259-020-04735-9
63. Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. (2020) 80:388–93. doi: 10.1016/j.jinf.2020.02.016
64. Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. (2020) 323:1488–94. doi: 10.1001/jama.2020.3204
65. Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. (2020). doi: 10.1111/all.14238. [Epub ahead of print].
66. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
67. Abraham NS, Byrne CJ, Young JM, Solomon MJ. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. (2010) 63:238–45. doi: 10.1016/j.jclinepi.2009.04.005
68. Ahmed A, Ali A, Hasan S. Comparison of epidemiological variations in COVID-19 patients inside and outside of China—a meta-analysis. Front Public Health. (2020) 8:193. doi: 10.3389/fpubh.2020.00193
69. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. (2020) 34:101623. doi: 10.1016/j.tmaid.2020.101623
70. Wynants L, Van Calster B, Bonten MMJ, Collins GS, Debray TPA, De Vos M, et al. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. (2020) 369:m1328. doi: 10.1136/bmj.m1328
71. Matsuyama R, Nishiura H, Kutsuna S, Hayakawa K, Ohmagari N. Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health. (2016) 16:1203. doi: 10.1186/s12889-016-3881-4
72. Nam HS, Park JW, Ki M, Yeon MY, Kim J, Kim SW. High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int J Infect Dis. (2017) 58:37–42. doi: 10.1016/j.ijid.2017.02.008
73. Park JE, Jung S, Kim A, Park JE. MERS transmission and risk factors: a systematic review. BMC Public Health. (2018) 18:574. doi: 10.1186/s12889-018-5484-8
74. Karlberg J, Chong DS, Lai WY. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol. (2004) 159:229–31. doi: 10.1093/aje/kwh056
75. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. (2020) 11:29. doi: 10.1186/s13293-020-00304-9
76. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e278. doi: 10.1016/j.cell.2020.02.052
77. Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol. (2010) 24:687–98. doi: 10.1111/j.1472-8206.2010.00854.x
78. Mishra JS, Hankins GD, Kumar S. Testosterone downregulates angiotensin II type-2 receptor via androgen receptor-mediated ERK1/2 MAP kinase pathway in rat aorta. J. Renin Angiotensin Aldosterone Syst. (2016) 17:1470320316674875. doi: 10.1177/1470320316674875
79. Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med. (2017) 242:1412–23. doi: 10.1177/1535370217718808
Keywords: COVID-19, SARS-CoV-2, coronavirus, male, disparity, clinical outcomes, mortality, pandemic
Citation: Galbadage T, Peterson BM, Awada J, Buck AS, Ramirez DA, Wilson J and Gunasekera RS (2020) Systematic Review and Meta-Analysis of Sex-Specific COVID-19 Clinical Outcomes. Front. Med. 7:348. doi: 10.3389/fmed.2020.00348
Received: 11 May 2020; Accepted: 10 June 2020;
Published: 23 June 2020.
Edited by:
Zisis Kozlakidis, International Agency for Research on Cancer (IARC), FranceReviewed by:
Marco Iosa, Santa Lucia Foundation (IRCCS), ItalyCopyright © 2020 Galbadage, Peterson, Awada, Buck, Ramirez, Wilson and Gunasekera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard S. Gunasekera, cmljaGFyZC5ndW5hc2VrZXJhQGJpb2xhLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.