
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 09 July 2020
Sec. Rheumatology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00316
This article is part of the Research Topic Advance In B-cell therapies For The Treatment of Rheumatic and Musculoskeletal Diseases View all 10 articles
B cell hyperactivity and breach of tolerance constitute hallmarks of systemic lupus erythematosus (SLE). The heterogeneity of disease manifestations and relatively rare prevalence of SLE have posed difficulties in trial design and contributed to a slow pace for drug development. The anti-BAFF monoclonal antibody belimumab is still the sole targeted therapy licensed for SLE, lending credence to the widely accepted notion that B cells play central roles in lupus pathogenesis. However, more therapeutic agents directed toward B cells or B cell-related pathways are used off-label or have been trialed in SLE. The anti-CD20 monoclonal antibody rituximab has been used to treat refractory SLE during the last two decades, and the anti-type I IFN receptor anifrolumab is currently awaiting approval after one phase III clinical trial which met its primary endpoint and one phase III trial which met key secondary endpoints. While the latter does not directly affect the maturation and antibody production activity of B cells, it is expected to affect the contribution of B cells in proinflammatory cytokine excretion. The proteasome inhibitor bortezomib, primarily directed toward the plasma cells, has been used in few severe cases as an escape regimen. Collectively, current clinical experience and primary results of ongoing clinical trials prophesy that B cell therapies of selective targets will have an established place in the future personalized therapeutic management of lupus patients.
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can affect multiple organ systems (1). The treatment of SLE has traditionally been non-specific, with antimalarial agents as the therapeutic cornerstone due to the wide variety of beneficial effects associated with their use (2, 3), and broad immunosuppression being used to hamper the inflammatory state and protect against end-organ damage accrual (4–6). Several of the medications used to treat patients with SLE still have not received approval by regulatory drug agencies. Following the timeline of drug development in the field of rheumatology at large, the development of new therapies for SLE has been hampered due to several reasons.
First, the pronounced heterogeneity of clinical phenotypes poses challenges in developing outcome measures which unanimously and reliably capture response to treatment regarding activity in the afflicted organs, and which also reflect the global SLE disease activity. As a result, the lack of reliable measures for treatment evaluation makes it challenging to design clinical trials to assess drug efficacy. Recruitment of participants has been slow and inadequate in organ-specific trials, whereas the applicability of currently available outcome measures has been questioned in clinically heterogeneous study populations. Borrowed from e.g., rheumatoid arthritis (RA), the treat-to-target concept has also gained attention in SLE (7), and composite measures have been developed to serve as tools for assessing clinical improvement. The SLE Responder Index (SRI) (8) was initially designed to serve as an outcome measure in clinical trials of belimumab (9–11), and the British Isles Lupus Assessment Group (BILAG)-based combined lupus assessment (BICLA) was first used in a phase IIb clinical trial of epratuzumab (12). They were both developed to reflect improvement in SLE disease activity. Other composite tools have been developed to reflect low disease activity, e.g., the Lupus Low Disease Activity State (LLDAS) (13), or remission, e.g., the Definitions of Remission in SLE (DORIS) (14). Both LLDAS and DORIS were designed to be applicable on specific evaluation occasions, and are independent of preceding degree of activity.
Using such tools, the first successful trials (10, 11) resulted in the approval of the first biological agent for the treatment of SLE about one decade ago (15). This agent was belimumab, a monoclonal antibody against the B cell activating cytokine BAFF, further discussed later, and the target was no other than B cells of early maturation stages, lending credence to the historical notion that they have a central role in lupus pathogenesis (16). Indeed, even before the official approval of belimumab as a treatment option, several therapies targeting B cells at different developmental stages have been used off-label (17). This review summarizes the rationale and clinical application of the B cell therapy panorama in SLE.
The complex SLE disease is characterized by loss of self-tolerance, which leads to immune responses toward endogenous nuclear and cytoplasmic material. In response to these autoantigens, clones of plasma cells produce autoantibodies, which are considered a hallmark of the disease. Autoantibodies may induce inflammation through the formation of immune complexes and through activation of Fc-γ receptors. Arguing for a pathogenic role, autoantibodies such as anti-Smith (Sm) and anti-double stranded DNA (anti-dsDNA) are associated with the clinical presentation of the disease (18), and the level of anti-dsDNA frequently correlates with SLE disease activity (19).
Apart from the production of autoantibodies, B cells play additional roles in the pathogenesis of SLE. In lupus prone mice, B cells that do not secrete autoantibodies are still important to disease progression (20). This indicates that other B cell functions, such as antigen presentation to T cells may be of importance. Furthermore, B cells display hyperactivity in SLE (21), as well as increased expression of several toll-like receptors (TLRs) compared with healthy individuals (22), which may contribute to the inflammatory state. Thus, B cells are important players in several aspects of the SLE pathogenesis, and reducing the stimulation and numbers of B cells has been an important part of drug research.
B cells initially develop in the fetal liver and adult bone marrow and can be characterized by the use of surface markers such as CD19, CD20 and CD22, expressed at different stages of maturation. The development and survival of B cells depend upon stimulation by the B cell activating factor belonging to the tumor necrosis factor (TNF) family (BAFF), also known as B lymphocyte stimulator (BLyS). BAFF is a member of the TNF ligand superfamily of proteins, and is mainly produced by myeloid and stromal cells (23). Stimulation with BAFF improves B cell survival, proliferation, and antibody production through binding to three known receptors expressed in B cells at different stages of maturation, i.e., the BAFF-Receptor (BAFF-R; also known as BLyS receptor 3, BR3), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and B cell maturation antigen (BCMA). BAFF transgenic mice develop symptoms characteristic of SLE (24), and BAFF levels are increased in patients with SLE compared with healthy controls and correlate with disease activity (25–28). In addition to BAFF, B cells are stimulated by cytokines such as a proliferation-inducing ligand (APRIL), which mainly serves as a plasma cell survival factor, interleukin (IL)-6, IL-21 and type I interferons (IFNs).
To inhibit B cell responses in SLE, two main pathways are currently used, i.e., (i) BAFF inhibition, and (ii) B cell depletion targeting the cell surface receptor CD20. The BAFF inhibitor belimumab was the first biological medication approved in 2011 by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for use in SLE. Belimumab is a recombinant human IgG1-λ monoclonal antibody that inhibits the soluble form of BAFF, preventing its interaction with BAFF receptors, thus inhibiting B cell survival and maturation. In contrast, rituximab is a chimeric anti-CD20 IgG1 monoclonal antibody that targets the CD20 molecule on the surface of B cells. This leads to B cell depletion through apoptosis, antibody dependent cell mediated cytotoxicity (ADCC), or antibody-dependent phagocytosis (ADP). Pharmaceuticals directly and indirectly targeting B cells that are used or have been trialed in SLE are illustrated in Figure 1, and summarized in Table 1.
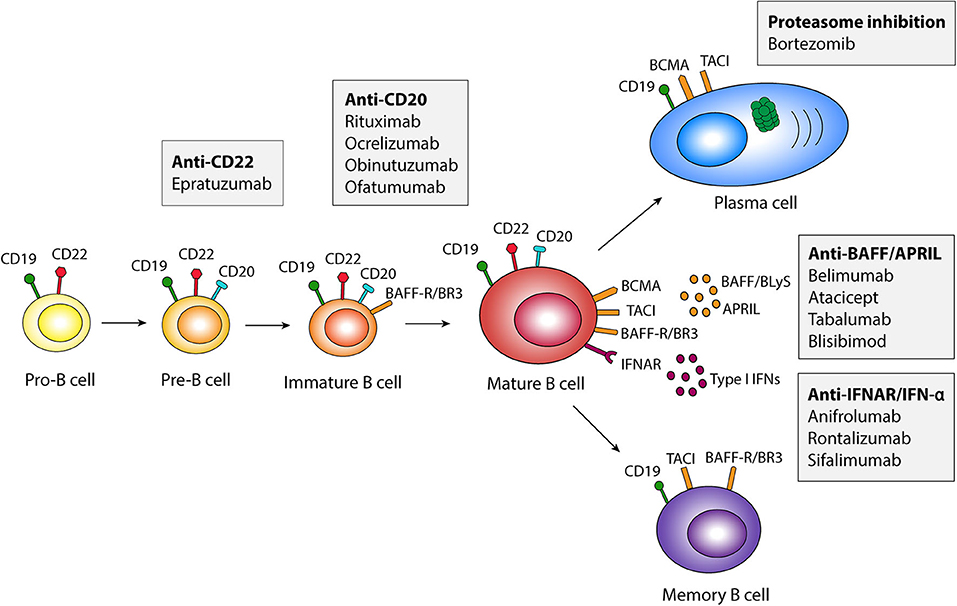
Figure 1. Schematic illustration of pharmaceuticals targeting B cells in different developmental stages. APRIL, a proliferation-inducing ligand; BAFF, B cell activating factor belonging to the tumor necrosis factor family; BAFF-R, BAFF Receptor; BCMA, B cell maturation antigen; BLyS, B lymphocyte activator; BR3, BLyS receptor 3; IFN, interferon; IFNAR, type I IFN receptor; TACI, transmembrane activator and calcium modulator and cyclophilin ligand interactor.
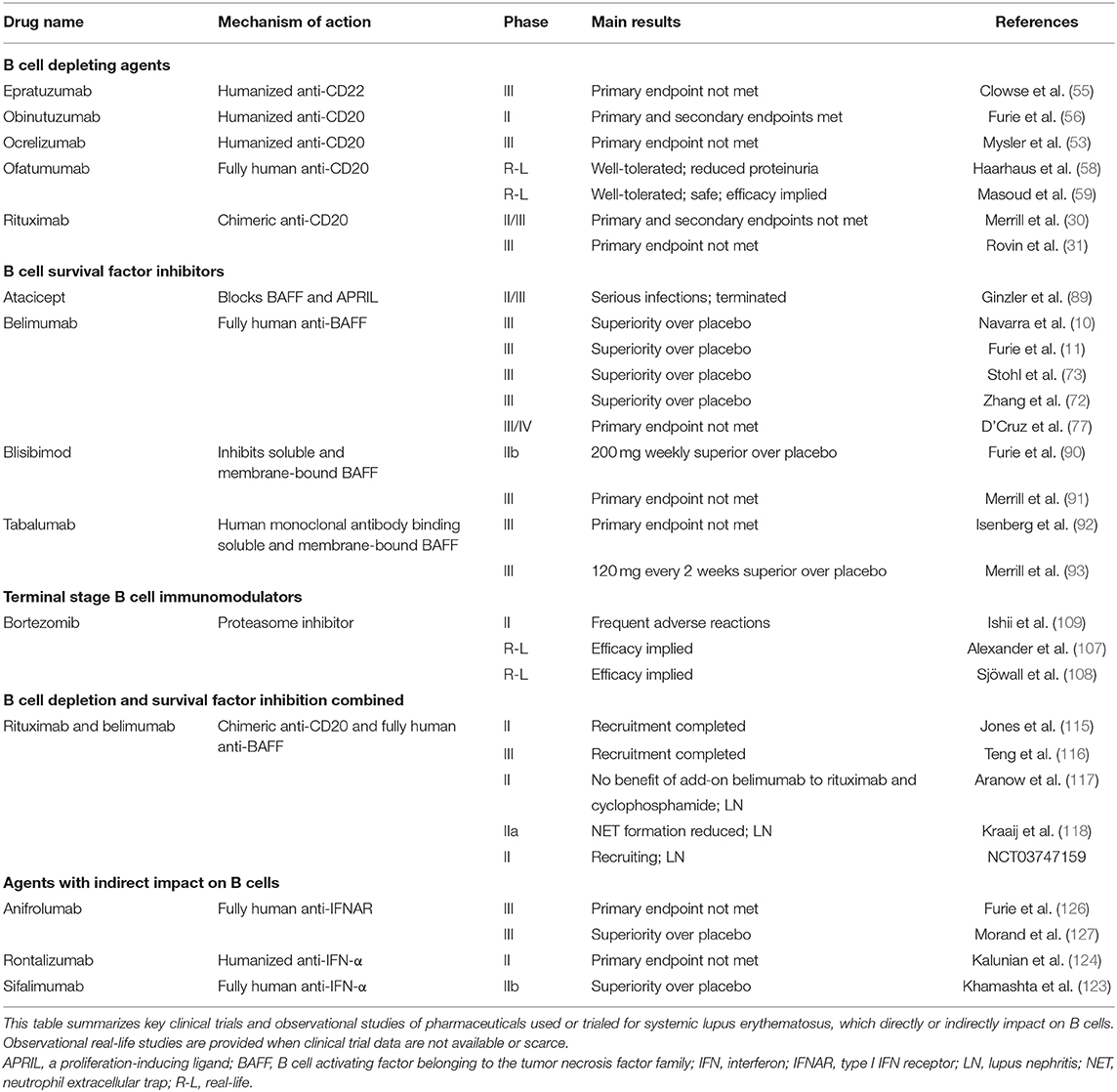
Table 1. Pharmaceuticals with direct or indirect impact on B cells currently used or trialed for systemic lupus erythematosus.
The chimeric anti-CD20 monoclonal antibody rituximab was approved by the FDA in 2006 for use in RA, and has been used off-label in the treatment of refractory SLE (29). The initial uncontrolled studies of rituximab in SLE showed encouraging results with improvements in both the clinical and laboratory compartment of the disease. However, two phase III randomized controlled trials have been performed, the EXPLORER trial in non-renal SLE (30) and the LUNAR trial in renal disease (31), none of which met their primary endpoints of significant reduction of disease activity compared with placebo (32).
Based on experience from rheumatoid arthritis (RA), the most commonly used regimen for rituximab in clinical practice consists of two intravenous infusions of 1,000 mg each, given 14–21 days apart. In the EXPLORER study, 257 patients with moderate to severe non-renal SLE were randomized to receive rituximab or placebo. Rituximab in EXPLORER was administered at a dose of 1,000 mg at week 0, 2, 24, and 26 on a background of azathioprine, methotrexate, or mycophenolic acid therapy. At week 52, there was no difference between the active treatment and placebo groups in the primary endpoints (30), which comprised achievement and maintenance of a major, partial or no clinical response assessed using the eight British Isles Lupus Assessment Group (BILAG) index organ system scores (33). Nonetheless, in a subgroup analysis, rituximab showed benefit over placebo regarding major clinical response in African-American and Hispanic patients (30). In the LUNAR trial, 144 patients with class III or IV lupus nephritis on mycophenolic acid were randomized to receive placebo or rituximab, again at a dose of 1,000 mg at weeks 0, 2, 24 and 26. Also in this study, rituximab failed to achieve the primary endpoint, and there was no significant difference between the placebo and treatment arms regarding the proportion of patients who achieved complete or partial renal response (31). Afterwards, concerns have been raised regarding the concomitant use of high doses of glucocorticoids and immunosuppressive therapy in the EXPLORER and LUNAR trials, potentially clouding the effect exerted by rituximab. Several other factors may have played roles in the disappointing results of these trials, including inappropriate endpoints, the size of study populations and patient heterogeneity (32).
Despite the negative clinical trials, the European League Against Rheumatism (EULAR) recommendations for the management of SLE prompt consideration of rituximab for organ-threatening SLE that has been refractory or shown intolerance to standard of care immunosuppressants (4). Moreover, the joint EULAR/European Renal Association—European Dialysis and Transplant Association (ERA-EDTA) recommendations for the management of lupus nephritis (34) and the American College of Rheumatology (ACR) guidelines for the management of renal SLE (35) recommend the use of rituximab as a rescue treatment in active renal SLE that has been non-responsive to standard therapy.
Indeed, targeting CD20 with rituximab has been endorsed in several centers where it is used as an off-label therapeutic option in SLE, mostly for refractory renal disease, either alone or as an add-on treatment to cyclophosphamide or mycophenolic acid (36–43), but also for other organ manifestations when conventional treatment has failed, e.g., severe lupus polyarthritis, hematological aberrancies and neuropsychiatric lupus (43–48). However, the use of rituximab has also raised some concerns regarding untoward effects, such as infusion-related reactions (49–51) and an increased frequency of post-rituximab late-onset neutropenia in SLE compared with other diseases, which calls for an attentive surveillance of rituximab-treated patients (52).
Besides rituximab, some additional biological therapies targeting B cells have been trialed in SLE. The anti-CD20 humanized monoclonal antibody ocrelizumab was evaluated in a phase III trial which included 381 cases with severe lupus nephritis. However, the trial was terminated early due to an imbalance in serious infections in the treatment arm, and ocrelizumab has not been studied further (53). Epratuzumab is a humanized monoclonal antibody directed against CD22, which was well-tolerated and yielded encouraging results in a phase IIb study, with an evident superiority of epratuzumab 2,400 mg monthly in inducing BICLA response compared with placebo (12, 54). Unfortunately, none of the two subsequent phase III trials of epratuzumab in lupus were able to show improvements in response frequencies when compared with placebo (55).
Obinutuzumab is another humanized anti-CD20 monoclonal antibody with superior B cell cytotoxic effects over rituximab implicated for patients with RA and SLE. This drug has been studied in a phase II clinical trial of lupus nephritis (NOBILITY; NCT02550652), designed to evaluate the safety and efficacy of the type II anti-CD20 monoclonal antibody obinutuzumab in patients with proliferative kidney disease. The first results were reported in the form of a conference abstract, where greater frequencies of complete and partial renal response were observed among patients who received obinutuzumab vs. placebo, both as an add-on to mycophenolate mofetil and glucorticoids (56). Finally, the fully human monoclonal antibody ofatumumab, approved for the treatment of chronic lymphocytic leukemia, has shown encouraging results in smaller groups of patients with lupus manifestations such as autoimmune hemolytic anemia, immune-mediated thrombocytopenia and lupus nephritis (57, 58). These last two agents could be of particular interest for patients in whom rituximab has shown efficacy but infusion reactions have prompted discontinuation (59), or patients who did not achieve complete B cell depletion following treatment with rituximab (50).
Due to its important role in B cell homeostasis, BAFF has been of central interest as a target molecule in B cell pharmacotherapy in SLE. Belimumab, formerly known as Lympho-Stat B, was the first drug to be licensed for SLE in more than 60 years, and is still the sole biological agent approved for use in adult SLE since 2011 and pediatric and adolescent SLE since 2019. The efficacy of belimumab in reducing lupus activity was first shown in two phase III randomized, placebo-controlled clinical trials (10, 11), and patients with serological activity, high BAFF levels, low baseline B cell counts, limited or no organ damage and no exposure to tobacco were later demonstrated to be more benefited (60–67). Belimumab is a recombinant human IgG1-λ monoclonal antibody that specifically binds to the soluble form of BAFF. Normally, the binding of BAFF to B cells prolongs their survival and promotes their maturation and differentiation toward immunoglobulin and autoantibody production (68). BAFF signaling also leads to increases in anti-apoptotic proteins (69). As defective clearance of apoptotic cells is implicated in the pathogenesis of SLE and stimulation of autoantibody production, reductions in anti-apoptotic proteins upon BAFF inhibition may be expected to hamper this B cell-driven component of lupus pathogenesis.
Early trials of belimumab in SLE were inconclusive. A phase II trial that comprised 449 patients failed to meet its primary endpoints (9). However, a significant proportion of study participants (30%) had no elevated titres of antinuclear antibodies (ANA) at baseline, and the validity of their diagnosis was later questioned. To this point, it is important to mention that ANA have been shown to be less common than generally assumed in established cases of SLE (70, 71), which still is a matter of debate.
The first successful randomized controlled trial of belimumab in SLE was the BLISS-52 trial. BLISS-52 comprised 865 patients with a moderate to severe SLE and positivity for immunological markers. Modest but consistent improvements through week 52 were displayed in patients who received belimumab across various clinical outcomes, and the trial met its primary endpoint, i.e., a significantly greater proportion of patients who received belimumab 10 mg/kg at week 0, 2, 4 and thereafter every fourth week met the SRI-4 criteria for response compared with placebo (10). A second phase III clinical trial of similar design, the BLISS-76 trial, comprised 819 patients. The main difference compared with BLISS-52 was that the observation period in BLISS-76 was prolonged to a total of 76 weeks. The primary efficacy endpoint was the same as that in BLISS-52, and was set to the evaluation visit of week 52. Although this endpoint was reached at week 52 with belimumab 10 mg/kg resulting in a greater proportion of SRI-4 responders than placebo, the results of the subsequent study period until week 76 were rather inconclusive (11). Since then, three more phase III trials have been performed. One assessed belimumab efficacy in a North East Asian SLE population (72), and another one assessed the efficacy of subcutaneous administration (73, 74); both reached their primary endpoint, i.e., SRI-4 response frequency at week 52. Another phase III/IV trial assessed the efficacy of belimumab in SLE patients of black race (EMBRACE) using the same primary endpoint, however with a modification in the SLE Disease Activity Index (SLEDAI) assessment for the proteinuria item to meet the SLEDAI-2K standard (75), as compared with scoring according to Safety of Estrogens in Lupus Erythematosus National Assessment (SELENA)-SLEDAI (76) in the original SRI (8). While the primary endpoint of EMBRACE was not achieved, patients with high disease activity were benefited (77). Finally, reports from several real-life clinical settings have confirmed clinical efficacy and steroid-sparing effects (61, 78–84).
The BLISS trials of belimumab excluded patients with severe active lupus nephritis, but a large proportion of study participants had a history of renal involvement and low to moderate proteinuria at the time of inclusion (10, 11). A post-hoc analysis demonstrated that these patients benefited from belimumab with regard to several organ-specific aspects, including rates of renal flares (85). A phase III randomized controlled trial has been designed to specifically assess the effect of belimumab as an add-on to standard of care therapy in patients with active renal SLE, i.e., the BLISS-LN trial (NCT01639339), and publication of the first results is awaited. In a recent press release, the pharmaceutical company announced that BLISS-LN met its primary and key secondary endpoints (86), which paves the way for increasing use of B cell-targeted immunomodulation in this severe lupus manifestation (87).
Atacicept is another BAFF-blocking biological agent that has been studied as a candidate pharmaceutical for SLE. Being a receptor construct that combines TACI with the Fc portion of human IgG, atacicept blocks the effects of both BAFF and its homologous B cell cytokine APRIL (88). Unfortunately, a clinical trial of atacicept in lupus nephritis was prematurely terminated due to adverse events in the form of hypogammaglobulinemia and infections (89), but attempts with adjusted dosing have not been totally abandoned.
Blisibimod is a fusion protein consisting of four high-affinity BAFF-binding domains and the Fc domain of human IgG1, and targets both soluble and membrane-bound BAFF. A dose-ranging phase IIb clinical trial (90) determined a safe and effective dose of blisibimob to be further studied in a subsequent phase III clinical trial, which however failed to meet its primary endpoint (91).
Only one of the two phase III clinical trials of tabalumab, a fully human monoclonal antibody that targets soluble and membrane-bound BAFF, met its primary endpoint, i.e., proportion of patients achieving SRI-5 at week 52 (92, 93), and no further development of this drug was therefore planned for SLE. However, it is worth noting that no dose-ranging phase II studies had preceded the phase III trials. Several key outcomes in both trials still justify the rationale of targeting both the cleaved and membrane-bound BAFF counterparts (94, 95).
The majority of the immunosuppressants used in SLE exert their therapeutic effects on B cells, plasmablasts and short-lived plasma cells (96). However, to achieve effects beyond this, i.e., on the long-lived plasma cells, the only available alternatives are autologous stem cell transplantation, atacicept (blocking both BAFF and APRIL) and proteasome inhibition (97–99). This was the rationale for using bortezomib in SLE cases resistant to conventional therapy.
Bortezomib is a specific, reversible, and cell permeable dipeptide boronic acid inhibitor of the chymotryptic activity of the 20S subunit of the proteasome, approved for the treatment of multiple myeloma and mantle cell lymphoma (100). Proteasome inhibition causes accumulation of defective immunoglobulin chains, resulting in endoplasmic reticulum stress, misfolded protein response, and subsequent apoptosis of plasma cells (101, 102). In addition, the long-lived plasma cells are vigorous antibody producers, and are thus highly sensitive to proteasome inhibition (99). On the other hand, proteasome inhibitors also effectively function as inhibitors of the production of pro-inflammatory cytokines through the regulation of NF-κB activation (103). Promising results in experimental lupus models and reports on use of bortezomib for allograft rejection in kidney transplantation (104, 105) have given rise to the concept of using bortezomib for patients with refractory lupus (106).
Several cases with refractory and life-threating manifestations of SLE in Germany and Sweden were treated with bortezomib and encouraging results were reported (107, 108). In a recent Japanese multicentre double-blind randomized controlled phase II trial, which enrolled 14 patients with persistently raised disease activity, patients were randomized to receive either bortezomib as an add-on therapy to their concomitant immunosuppressants or placebo (109). Unfortunately, albeit obvious clinical efficacy was seen in several patients, some of the patients who received bortezomib experienced adverse reactions, i.e., fever, severe hypersensitivity, or other infusion reactions. The authors recommended to carefully select patients for bortezomib therapy, and use protocols to prevent side-effects.
Since rituximab induces B cell depletion, but also results in elevation of BAFF levels, studies have examined whether the increased BAFF levels may promote re-expansion of autoreactive B cells and by extension an earlier relapse. The effects of rituximab are dependent on the degree of B cell depletion, and incomplete depletion has been shown to be associated with lower frequencies of clinical response (27). In patients with refractory SLE with high levels of anti-dsDNA antibodies, relapse occurred at lower B cell numbers, and plasmablasts represented a larger percentage of the B cell population (110). Following rituximab administration, levels of BAFF rise (111), and BAFF levels are higher at relapse after rituximab treatment compared with disease flare before rituximab treatment (112). Further, quantifiable BAFF in serum has been associated with shorter clinical response to rituximab in patients with refractory SLE (113). Thus, a contributing factor to the lack of efficacy of rituximab in randomized clinical trials may be the increased BAFF levels following rituximab administration. Theoretically, combining rituximab with belimumab could give a more thorough and sustained inhibition of B cell responses, as speculated in early investigations (28, 111, 112, 114). This is currently evaluated in several clinical trials, e.g., BEAT Lupus (115) and BLISS-BELIEVE (116).
It is of particular importance that the merit of combining B cell therapies has also been conceptualized in the context of lupus nephritis. The Rituximab and Belimumab for Lupus Nephritis (CALIBRATE; NCT02260934) (117) and the investigator-initiated Synergetic B cell Immunomodulation in SLE (SynBioSe) trials (SynBioSe 1: NCT02284984; SynBioSe 2: NCT03747159) were designed to assess the efficacy of rituximab and belimumab combined in active lupus nephritis. The proof-of-concept open label SynBioSe 1 is completed, and a first report demonstrated reductions in antinuclear antibodies and neutrophil extracellular trap (NET) formation (118). SynBioSe 2 is currently recruiting, and results may be anticipated by the end of 2023.
Autoreactive B cells are indubitably key cells in the pathogenesis of SLE, but the theoretical merit has hitherto seldom culminated in the anticipated outcomes in drug development. The lack of success in clinical trials has not been for lack of trying. Apart from pharmaceuticals which predominantly exert effects on B cells, numerous other therapeutic modalities have been trialed for SLE, several of them expected to indirectly impact on B cells and B cell functions. For example, in lupus prone mice, targeting other B cell stimulating cytokines, such as IL-6, decreased disease progression, but this strategy did not succeed in subsequent clinical trials (119). Targeting the co-stimulatory molecule CD40 led to modest clinical improvement, but also unacceptable side-effects in the form of thromboembolic events (120).
Activation of the type I IFN pathway is prominent in the pathogenesis of SLE, and type I IFNs stimulate BAFF production. In patients with SLE, the type I IFN pathway is overexpressed, and the IFN-α protein in particular has shown associations with both disease activity (121) and risk of relapse (122). IFNs are pleiotropic cytokines with numerous functions in the immune response equilibrium, including an impact on B cells. Thus, albeit not exclusive, the effects of IFN inhibition are attractive also in the B cell context.
The first reports to support the efficacy of direct IFN-α inhibition in SLE originated from a phase IIb clinical trial of sifalimumab (123). The results were modest, but in favor of sifalimumab. Unfortunately, a phase II trial of the anti-INF-α rontalizumab demonstrated that rontalizumab was superior over placebo in SLE patients with low IFN-regulated gene expression, but not in patients with high IFN gene signature (124), contrary to what expected considering its biologic mechanism.
Following promising results in a phase II clinical trial (125), the type I IFN receptor (IFNAR) inhibitor anifrolumab was evaluated in two phase III trials, i.e., TULIP-1 and TULIP-2. In TULIP-1, the primary outcome, i.e., SRI-4 response, was not met (126). By contrast, a greater proportion of patients receiving anifrolumab vs. placebo in TULIP-2 met the primary outcome, i.e., BICLA (127). Possible reasons for the discrepancy between the TULIP trials may include the choice of outcomes and the study populations. The primary endpoint in TULIP-2 was initially planned to be SRI-4. However, this was changed at a later stage, upon a subanalysis of TULIP-1 where proportions of BICLA unlike SRI-4 responders favored anifrolumab. Notably, in TULIP-2 both SRI-4 and BICLA showed ability to separate treatment arms.
An interesting trend is targeting B cell intracellular signaling, such as through inhibition of Bruton's Tyrosine Kinase (BTK), which is a strategy approved for the treatment of B cell malignancies. Inhibition of BTK has shown efficacy in lupus prone mice, which resulted in reduced kidney damage and increased survival (128). Another development originating in the area of cancer therapy was the chimeric auto-antigen receptor (CAAR) T cells. CAAR-T cells have been genetically engineered to kill human autoreactive B cells specific toward desmoglein-3 in pemphigus vulgaris (129), and in two lupus mice models, use of CAAR-T cells targeting the CD19 surface molecule resulted in reduced kidney damage and increased survival (130). Although long-term data are not available, evidence suggests that the CAAR-T cells acquire a long-term memory phenotype and persist in peripheral tissue of patients.
To summarize, B cell hyperactivity and breach of tolerance constitute hallmarks of SLE, and it is widely accepted that B cells play central roles in the pathogenesis. However, the contribution of B cells to disease initiation and perpetuation is less well understood. B cells in SLE constitute the main autoantibody producers and probably facilitate the priming of autoreactive T cells and function as antigen-presenting cells, as well as constitute a source of the cytokines involved in immune dysregulation (131). As a result, many of the therapeutic agents that have been trialed in SLE target B cell-related pathways.
Even though drug development in the field of SLE has been slow, B cell-targeting therapies have been increasingly used during the last two decades and contributed to improved management and improved prognosis. The amount and primary results of ongoing clinical trials prophesy that B cell therapies of selective targets will have an established place in the future personalized therapeutic management of lupus patients.
All authors contributed to the manuscript draft, critically reviewed all parts of the manuscript, accepted its final version prior to submission, and account for its content.
IP was funded by the Swedish Rheumatism Association, King Gustaf V's 80-year Anniversary foundation Professor Nanna Svartz Foundation, Ulla and Roland Gustafsson Foundation, and Region Stockholm and Karolinska Institutet. MS was funded by the Gothenburg Society of Medicine. CS was funded by the Swedish Rheumatism Association, Region Östergötland (ALF grants), King Gustaf V's 80-year Anniversary foundation, and King Gustaf V and Queen Victoria's Freemasons foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Lina Wirestam for the schematic illustration.
1. Kaul A, Gordon C, Crow MK, Touma Z, Urowitz MB, van Vollenhoven R, et al. Systemic lupus erythematosus. Nat Rev Dis Primers. (2016) 2:16039. doi: 10.1038/nrdp.2016.39
2. Ruiz-Irastorza G, Khamashta MA. Hydroxychloroquine: the cornerstone of lupus therapy. Lupus. (2008) 17:271–3. doi: 10.1177/0961203307086643
3. Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. (2010) 69:20–8. doi: 10.1136/ard.2008.101766
4. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. (2019) 78:736–45. doi: 10.1136/annrheumdis-2019-215089
5. Barile-Fabris L, Ariza-Andraca R, Olguin-Ortega L, Jara LJ, Fraga-Mouret A, Miranda-Limon JM, et al. Controlled clinical trial of IV cyclophosphamide versus IV methylprednisolone in severe neurological manifestations in systemic lupus erythematosus. Ann Rheum Dis. (2005) 64:620–5. doi: 10.1136/ard.2004.025528
6. Contreras G, Tozman E, Nahar N, Metz D. Maintenance therapies for proliferative lupus nephritis: mycophenolate mofetil, azathioprine and intravenous cyclophosphamide. Lupus. (2005) (14 Suppl 1):s33–8. doi: 10.1177/096120330501400107
7. van Vollenhoven RF, Mosca M, Bertsias G, Isenberg D, Kuhn A, Lerstrom K, et al. Treat-to-target in systemic lupus erythematosus: recommendations from an international task force. Ann Rheum Dis. (2014) 73:958–67. doi: 10.1136/annrheumdis-2013-205139
8. Furie RA, Petri MA, Wallace DJ, Ginzler EM, Merrill JT, Stohl W, et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. (2009) 61:1143–51. doi: 10.1002/art.24698
9. Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. (2009) 61:1168–78. doi: 10.1002/art.24699
10. Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. (2011) 377:721–31. doi: 10.1016/S0140-6736(10)61354-2
11. Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3918–30. doi: 10.1002/art.30613
12. Wallace DJ, Kalunian K, Petri MA, Strand V, Houssiau FA, Pike M, et al. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Ann Rheum Dis. (2014) 73:183–90. doi: 10.1136/annrheumdis-2012-202760
13. Franklyn K, Lau CS, Navarra SV, Louthrenoo W, Lateef A, Hamijoyo L, et al. Asia-pacific lupus, definition and initial validation of a lupus low disease activity state (LLDAS). Ann Rheum Dis. (2016) 75:1615–21. doi: 10.1136/annrheumdis-2015-207726
14. van Vollenhoven R, Voskuyl A, Bertsias G, Aranow C, Aringer M, Arnaud L, et al. A framework for remission in SLE: consensus findings from a large international task force on definitions of remission in SLE (DORIS). Ann Rheum Dis. (2017) 76:554–61. doi: 10.1136/annrheumdis-2016-209519
15. Parodis I, Axelsson M, Gunnarsson I. Belimumab for systemic lupus erythematosus: a practice-based view. Lupus. (2013) 22:372–80. doi: 10.1177/0961203313476154
16. Harvey PR, Gordon C. B-cell targeted therapies in systemic lupus erythematosus: successes and challenges. BioDrugs. (2013) 27:85–95. doi: 10.1007/s40259-013-0015-8
17. Gunnarsson I, Sundelin B, Jonsdottir T, Jacobson SH, Henriksson EW, van Vollenhoven RF. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum. (2007) 56:1263–72. doi: 10.1002/art.22505
18. Swaak AJ, Huysen V, Nossent JC, Smeenk RJ. Antinuclear antibody profiles in relation to specific disease manifestations of systemic lupus erythematosus. Clin Rheumatol. (1990) 9:82–94. doi: 10.1007/BF02205555
19. Frodlund M, Dahlstrom O, Kastbom A, Skogh T, Sjowall C. Associations between antinuclear antibody staining patterns and clinical features of systemic lupus erythematosus: analysis of a regional Swedish register. BMJ Open. (2013) 3:e003608. doi: 10.1136/bmjopen-2013-003608
20. Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. (1999) 189:1639–48. doi: 10.1084/jem.189.10.1639
21. Suzuki N, Sakane T. Induction of excessive B cell proliferation and differentiation by an in vitro stimulus in culture in human systemic lupus erythematosus. J Clin Invest. (1989) 83:937–44. doi: 10.1172/JCI113979
22. Klonowska-Szymczyk A, Wolska A, Robak T, Cebula-Obrzut B, Smolewski P, Robak E. Expression of toll-like receptors 3, 7, and 9 in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Mediators Inflamm. (2014) 2014:381418. doi: 10.1155/2014/381418
23. Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. (2009) 9:491–502. doi: 10.1038/nri2572
24. Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. (2000) 404:995–9. doi: 10.1038/35010115
25. Petri M, Stohl W, Chatham W, McCune WJ, Chevrier M, Ryel J, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. (2008) 58:2453–9. doi: 10.1002/art.23678
26. Salazar-Camarena DC, Ortiz-Lazareno PC, Cruz A, Oregon-Romero E, Machado-Contreras JR, Munoz-Valle JF, et al. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus. (2016) 25:582–92. doi: 10.1177/0961203315608254
27. Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum. (2011) 63:3038–47. doi: 10.1002/art.30466
28. Parodis I, Zickert A, Sundelin B, Axelsson M, Gerhardsson J, Svenungsson E, et al. Evaluation of B lymphocyte stimulator and a proliferation inducing ligand as candidate biomarkers in lupus nephritis based on clinical and histopathological outcome following induction therapy. Lupus Sci Med. (2015) 2:e000061. doi: 10.1136/lupus-2014-000061
29. Eko SL, van Vollenhoven RF. Rituximab and lupus–a promising pair? Curr Rheumatol Rep. (2014) 16:444. doi: 10.1007/s11926-014-0444-5
30. Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. (2010) 62:222–33. doi: 10.1002/art.27233
31. Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum. (2012) 64:1215–26. doi: 10.1002/art.34359
32. Touma Z, Urowitz MB, Gladman DD. Systemic lupus erythematosus: an update on current pharmacotherapy and future directions. Expert Opin Biol Ther. (2013) 13:723–37. doi: 10.1517/14712598.2013.764411
33. Isenberg DA, Rahman A, Allen E, Farewell V, Akil M, Bruce IN, et al. BILAG 2004. Development and initial validation of an updated version of the british isles lupus assessment group's disease activity index for patients with systemic lupus erythematosus. Rheumatology. (2005) 44:902–6. doi: 10.1093/rheumatology/keh624
34. Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. Update of the joint European league against rheumatism and european renal association-european dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. (2020) 79:713–23. doi: 10.1136/annrheumdis-2020-216924
35. Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, et al. American College of, American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res. (2012) 64:797–808. doi: 10.1002/acr.21664
36. Jonsdottir T, Gunnarsson I, Mourao AF, Lu TY, van Vollenhoven RF, Isenberg D. Clinical improvements in proliferative vs membranous lupus nephritis following B-cell depletion: pooled data from two cohorts. Rheumatology. (2010) 49:1502–4. doi: 10.1093/rheumatology/keq055
37. Jonsdottir T, Sundelin B, Welin Henriksson E, van Vollenhoven RF, Gunnarsson I. Rituximab-treated membranous lupus nephritis: clinical outcome and effects on electron dense deposits. Ann Rheum Dis. (2011) 70:1172–3. doi: 10.1136/ard.2010.129288
38. Jonsdottir T, Zickert A, Sundelin B, Henriksson EW, van Vollenhoven RF, Gunnarsson I. Long-term follow-up in lupus nephritis patients treated with rituximab–clinical and histopathological response. Rheumatology. (2013) 52:847–55. doi: 10.1093/rheumatology/kes348
39. Weidenbusch M, Rommele C, Schrottle A, Anders HJ. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. (2013) 28:106–11. doi: 10.1093/ndt/gfs285
40. Davies RJ, Sangle SR, Jordan NP, Aslam L, Lewis MJ, Wedgwood R, et al. Rituximab in the treatment of resistant lupus nephritis: therapy failure in rapidly progressive crescentic lupus nephritis. Lupus. (2013) 22:574–82. doi: 10.1177/0961203313483376
41. Zhang J, Zhao Z, Hu X. Effect of rituximab on serum levels of anti-c1q and antineutrophil cytoplasmic autoantibodies in refractory severe lupus nephritis. Cell Biochem Biophys. (2015) 72:197–201. doi: 10.1007/s12013-014-0437-z
42. Gomez Mendez LM, Cascino MD, Garg J, Katsumoto TR, Brakeman P, Dall'Era M, et al. Peripheral blood B cell depletion after rituximab and complete response in lupus nephritis. Clin J Am Soc Nephrol. (2018) 13:1502–9. doi: 10.2215/CJN.01070118
43. Alshaiki F, Obaid E, Almuallim A, Taha R, El-Haddad H, Almoallim H. Outcomes of rituximab therapy in refractory lupus: a meta-analysis. Eur J Rheumatol. (2018) 5:118–26. doi: 10.5152/eurjrheum.2018.17096
44. Jonsdottir T, Gunnarsson I, Risselada A, Henriksson EW, Klareskog L, van Vollenhoven RF. Treatment of refractory SLE with rituximab plus cyclophosphamide: clinical effects, serological changes, and predictors of response. Ann Rheum Dis. (2008) 67:330–4. doi: 10.1136/ard.2007.079095
45. Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, et al. Club rhumatismes et, safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French autoimmunity and rituximab registry. Arthritis Rheum. (2010) 62:2458–66. doi: 10.1002/art.27541
46. Ramos-Casals M, Garcia-Hernandez FJ, de Ramon E, Callejas JL, Martinez-Berriotxoa A, Pallares L, et al. Off-label use of rituximab in 196 patients with severe, refractory systemic autoimmune diseases. Clin Exp Rheumatol. (2010) 28:468–76. Available online at: https://www.clinexprheumatol.org/abstract.asp?a=747
47. Witt M, Grunke M, Proft F, Baeuerle M, Aringer M, Burmester G, et al. German registry of autoimmune diseases, clinical outcomes and safety of rituximab treatment for patients with systemic lupus erythematosus (SLE) - results from a nationwide cohort in Germany (GRAID). Lupus. (2013) 22:1142–9. doi: 10.1177/0961203313503912
48. Ryden-Aulin M, Boumpas D, Bultink I, Callejas Rubio JL, Caminal-Montero L, Castro A, et al. Off-label use of rituximab for systemic lupus erythematosus in Europe. Lupus Sci Med. (2016) 3:e000163. doi: 10.1136/lupus-2016-000163
49. Karmacharya P, Poudel DR, Pathak R, Donato AA, Ghimire S, Giri S. Rituximab-induced serum sickness: a systematic review. Semin Arthritis Rheum. (2015) 45:334–40. doi: 10.1016/j.semarthrit.2015.06.014
50. Md Yusof MY, Shaw D, El-Sherbiny YM, Dunn E, Rawstron AC, Emery P, et al. Predicting and managing primary and secondary non-response to rituximab using B-cell biomarkers in systemic lupus erythematosus. Ann Rheum Dis. (2017) 76:1829–36. doi: 10.1136/annrheumdis-2017-211191
51. Hennessey A, Lukawska J, Cambridge G, Isenberg D, Leandro M. Adverse infusion reactions to rituximab in systemic lupus erythematosus: a retrospective analysis. BMC Rheumatol. (2019) 3:32. doi: 10.1186/s41927-019-0082-7
52. Parodis I, Soder F, Faustini F, Kasza Z, Samuelsson I, Zickert A, et al. Rituximab-mediated late-onset neutropenia in systemic lupus erythematosus - distinct roles of BAFF and APRIL. Lupus. (2018) 27:1470–8. doi: 10.1177/0961203318777116
53. Mysler EF, Spindler AJ, Guzman R, Bijl M, Jayne D, Furie RA, et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum. (2013) 65:2368–79. doi: 10.1002/art.38037
54. Wallace DJ, Goldenberg DM. Epratuzumab for systemic lupus erythematosus. Lupus. (2013) 22:400–5. doi: 10.1177/0961203312469692
55. Clowse ME, Wallace DJ, Furie RA, Petri MA, Pike MC, Leszczynski P, et al. Efficacy and safety of epratuzumab in moderately to severely active systemic lupus erythematosus: results from two phase III randomized, double-blind, placebo-controlled trials. Arthritis Rheumatol. (2017) 69:362–75. doi: 10.1002/art.39856
56. Furie R, Aroca G, Alvarez A, Fragoso-Loyo H, Santillán E, Rovin B, et al. A phase II randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of obinutuzumab or placebo in combination with mycophenolate mofetil in patients with active class III or IV lupus nephritis. Arthritis Rheumatol. (2019) 71:0939.
57. Montillo M, O'Brien S, Tedeschi A, Byrd JC, Dearden C, Gill D, et al. Ibrutinib in previously treated chronic lymphocytic leukemia patients with autoimmune cytopenias in the RESONATE study. Blood Cancer J. (2017) 7:e524. doi: 10.1038/bcj.2017.5
58. Haarhaus ML, Svenungsson E, Gunnarsson I. Ofatumumab treatment in lupus nephritis patients. Clin Kidney J. (2016) 9:552–5. doi: 10.1093/ckj/sfw022
59. Masoud S, McAdoo SP, Bedi R, Cairns TD, Lightstone L. Ofatumumab for B cell depletion in patients with systemic lupus erythematosus who are allergic to rituximab. Rheumatology. (2018) 57:1156–61. doi: 10.1093/rheumatology/key042
60. van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, et al. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Ann Rheum Dis. (2012) 71:1343–9. doi: 10.1136/annrheumdis-2011-200937
61. Parodis I, Sjowall C, Jonsen A, Ramskold D, Zickert A, Frodlund M, et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev. (2017) 16:343–51. doi: 10.1016/j.autrev.2017.02.005
62. Parodis I, Gomez A, Frodlund M, Jonsen A, Zickert A, Sjowall C, et al. Smoking reduces the efficacy of belimumab in mucocutaneous lupus. Expert Opin Biol Ther. (2018) 18:911–20. doi: 10.1080/14712598.2018.1494719
63. Ramskold D, Parodis I, Lakshmikanth T, Sippl N, Khademi M, Chen Y, et al. B cell alterations during BAFF inhibition with belimumab in SLE. EBioMedicine. (2019) 40:517–27. doi: 10.1016/j.ebiom.2018.12.035
64. Parodis I, Johansson P, Gomez A, Soukka S, Emamikia S, Chatzidionysiou K. Predictors of low disease activity and clinical remission following belimumab treatment in systemic lupus erythematosus. Rheumatology. (2019) 58:2170–76. doi: 10.1093/rheumatology/kez191
65. Sohrabian A, Parodis I, Carlstromer-Berthen N, Frodlund M, Jonsen A, Zickert A, et al. Increased levels of anti-dsDNA antibodies in immune complexes before treatment with belimumab associate with clinical response in patients with systemic lupus erythematosus. Arthritis Res Ther. (2019) 21:259. doi: 10.1186/s13075-019-2056-y
66. Parodis I, Gomez A, Emamikia S, Chatzidionysiou K. Established organ damage reduces belimumab efficacy in systemic lupus erythematosus. Ann Rheum Dis. (2019) 78:1006–7. doi: 10.1136/annrheumdis-2018-214880
67. Parodis I, Åkerström E, Sjöwall C, Sohrabian A, Jönsen A, Gomez A, Frodlund M, et al. Autoantibody and cytokine profiles during treatment with belimumab in patients with systemic lupus erythematosus. Int J Mol Sci. (2020) 21:3463. doi: 10.3390/ijms21103463
68. Baker KP, Edwards BM, Main SH, Choi GH, Wager RE, Halpern WG, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. (2003) 48:3253–65. doi: 10.1002/art.11299
69. Schweighoffer E, Tybulewicz VL. Signalling for B cell survival. Curr Opin Cell Biol. (2018) 51:8–14. doi: 10.1016/j.ceb.2017.10.002
70. Sjowall C, Sturm M, Dahle C, Bengtsson AA, Jonsen A, Sturfelt G, et al. Abnormal antinuclear antibody titers are less common than generally assumed in established cases of systemic lupus erythematosus. J Rheumatol. (2008) 35:1994–2000. Available online at: https://pubmed.ncbi.nlm.nih.gov/18785312/
71. Frodlund M, Wettero J, Dahle C, Dahlstrom O, Skogh T, Ronnelid J, et al. Longitudinal anti-nuclear antibody (ANA) seroconversion in systemic lupus erythematosus: a prospective study of Swedish cases with recent-onset disease. Clin Exp Immunol. (2020) 199:245–54. doi: 10.1111/cei.13402
72. Zhang F, Bae SC, Bass D, Chu M, Egginton S, Gordon D, et al. A pivotal phase III, randomised, placebo-controlled study of belimumab in patients with systemic lupus erythematosus located in China, Japan and South Korea. Ann Rheum Dis. (2018) 77:355–63. doi: 10.1136/annrheumdis-2017-211631
73. Stohl W, Schwarting A, Okada M, Scheinberg M, Doria A, Hammer AE, et al. Efficacy and safety of subcutaneous belimumab in systemic lupus erythematosus: a fifty-two-week randomized, double-blind, placebo-controlled study. Arthritis Rheumatol. (2017) 69:1016–27. doi: 10.1002/art.40049
74. Doria A, Stohl W, Schwarting A, Okada M, Scheinberg M, van Vollenhoven R, et al. Efficacy and safety of subcutaneous belimumab in anti-double-stranded DNA-positive, hypocomplementemic patients with systemic lupus erythematosus. Arthritis Rheumatol. (2018) 70:1256–64. doi: 10.1002/art.40511
75. Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. (2002) 29:288–91. Available online at: https://pubmed.ncbi.nlm.nih.gov/11838846/
76. Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. (2005) 353:2550–8. doi: 10.1056/NEJMoa051135
77. D'Cruz D, Maksimowicz-McKinnon K, Oates J, Santiago MB, Bass D, Burriss S, et al. 200 Efficacy and safety of belimumab in patients of black race with systemic lupus erythematosus: results from the EMBRACE study. Lupus Sci Med. (2019) 6:A149–50. doi: 10.1136/lupus-2019-lsm.200
78. Iaccarino L, Bettio S, Reggia R, Zen M, Frassi M, Andreoli L, et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res. (2017) 69:115–23. doi: 10.1002/acr.22971
79. Fanouriakis A, Adamichou C, Koutsoviti S, Panopoulos S, Staveri C, Klagou A, et al. Low disease activity-irrespective of serologic status at baseline-associated with reduction of corticosteroid dose and number of flares in patients with systemic lupus erythematosus treated with belimumab: a real-life observational study. Semin Arthritis Rheum. (2018) 48:467–74. doi: 10.1016/j.semarthrit.2018.02.014
80. Collins CE, Dall'Era M, Kan H, Macahilig C, Molta C, Koscielny V, et al. Response to belimumab among patients with systemic lupus erythematosus in clinical practice settings: 24-month results from the OBSErve study in the USA. Lupus Sci Med. (2016) 3:e000118. doi: 10.1136/lupus-2015-000118
81. Cortes J, Andreu JL, Calvo J, Garcia-Aparicio AM, Coronell CG, Diaz-Cerezo S. Evaluation of use of belimumab in clinical practice settings (Observe Study) in spain: health resource utilization and labour absenteeism. Value Health. (2014) 17:A534. doi: 10.1016/j.jval.2014.08.1703
82. Schwarting A, Schroeder JO, Alexander T, Schmalzing M, Fiehn C, Specker C, et al. First real-world insights into belimumab use and outcomes in routine clinical care of systemic lupus erythematosus in Germany: results from the OBSErve Germany study. Rheumatol Ther. (2016) 3:271–90. doi: 10.1007/s40744-016-0047-x
83. Touma Z, Sayani A, Pineau CA, Fortin I, Matsos M, Ecker GA, et al. Belimumab use, clinical outcomes and glucocorticoid reduction in patients with systemic lupus erythematosus receiving belimumab in clinical practice settings: results from the OBSErve Canada study. Rheumatol Int. (2017) 37:865–73. doi: 10.1007/s00296-017-3682-9
84. von Kempis J, Duetsch S, Reuschling N, Villiger R, Villiger PM, Vallelian F, et al. Clinical outcomes in patients with systemic lupus erythematosus treated with belimumab in clinical practice settings: a retrospective analysis of results from the OBSErve study in Switzerland. Swiss Med Wkly. (2019) 149:w20022. doi: 10.4414/smw.2019.20022
85. Dooley MA, Houssiau F, Aranow C, D'Cruz DP, Askanase A, Roth DA, et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus. (2013) 22:63–72. doi: 10.1177/0961203312465781
86. GlaxoSmithKline. GSK Announces Positive Headline Results in Phase 3 Study of Benlysta in Patients with Lupus Nephritis. (2019) Available online at: https://www.gsk.com/en-gb/media/press-releases/gsk-announces-positive-headline-results-in-phase-3-study-of-benlysta-in-patients-with-lupus-nephritis/ (June 22, 2020).
87. Anders HJ, Saxena R, Zhao MH, Parodis I, Salmon JE, Mohan C. Lupus nephritis. Nat Rev Dis Primers. (2020) 6:7. doi: 10.1038/s41572-019-0141-9
88. van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis Rheum. (2011) 63:1782–92. doi: 10.1002/art.30372
89. Ginzler EM, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther. (2012) 14:R33. doi: 10.1186/ar3738
90. Furie RA, Leon G, Thomas M, Petri MA, Chu AD, Hislop C, et al. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann Rheum Dis. (2015) 74:1667–75. doi: 10.1136/annrheumdis-2013-205144
91. Merrill JT, Shanahan WR, Scheinberg M, Kalunian KC, Wofsy D, Martin RS. Phase III trial results with blisibimod, a selective inhibitor of B-cell activating factor, in subjects with systemic lupus erythematosus (SLE): results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. (2018) 77:883–9. doi: 10.1136/annrheumdis-2018-213032
92. Isenberg DA, Petri M, Kalunian K, Tanaka Y, Urowitz MB, Hoffman RW, et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. (2016) 75:323–31. doi: 10.1136/annrheumdis-2015-207653
93. Merrill JT, van Vollenhoven RF, Buyon JP, Furie RA, Stohl W, Morgan-Cox M, et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. (2016) 75:332–40. doi: 10.1136/annrheumdis-2015-207654
94. Gatto M, Saccon F, Zen M, Bettio S, Iaccarino L, Punzi L, et al. Success and failure of biological treatment in systemic lupus erythematosus: a critical analysis. J Autoimmun. (2016) 74:94–105. doi: 10.1016/j.jaut.2016.06.014
95. Houssiau FA, Doria A. Targeting BAFF/BLyS in lupus: is the glass half-full or half-empty? Ann Rheum Dis. (2016) 75:321–2. doi: 10.1136/annrheumdis-2015-208312
96. Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. (2009) 113:214–23. doi: 10.1182/blood-2008-07-168286
97. Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, et al. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J Exp Med. (2004) 199:1577–84. doi: 10.1084/jem.20040168
98. Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. (2008) 180:3655–9. doi: 10.4049/jimmunol.180.6.3655
99. Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K, et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med. (2008) 14:748–55. doi: 10.1038/nm1763
100. Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, et al. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. (2006) 107:4907–16. doi: 10.1182/blood-2005-08-3531
101. Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Assessment of proteasome inhibition for extending remissions, bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. (2005) 352:2487–98. doi: 10.1056/NEJMoa043445
102. Alexander T, Cheng Q, Klotsche J, Khodadadi L, Waka A, Biesen R, et al. Proteasome inhibition with bortezomib induces a therapeutically relevant depletion of plasma cells in SLE but does not target their precursors. Eur J Immunol. (2018) 48:1573–9. doi: 10.1002/eji.201847492
103. van der Vlag J, Berden JH. Proteasome inhibition: a new therapeutic option in lupus nephritis? Nephrol Dial Transplant. (2008) 23:3771–2. doi: 10.1093/ndt/gfn536
104. Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. (2008) 86:1754–61. doi: 10.1097/TP.0b013e318190af83
105. Walsh RC, Alloway RR, Girnita AL, Woodle ES. Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int. (2012) 81:1067–74. doi: 10.1038/ki.2011.502
106. Nencioni A, Grunebach F, Patrone F, Ballestrero A, Brossart P. Proteasome inhibitors: antitumor effects and beyond. Leukemia. (2007) 21:30–6. doi: 10.1038/sj.leu.2404444
107. Alexander T, Sarfert R, Klotsche J, Kuhl AA, Rubbert-Roth A, Lorenz HM, et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis. (2015) 74:1474–8. doi: 10.1136/annrheumdis-2014-206016
108. Sjowall C, Hjorth M, Eriksson P. Successful treatment of refractory systemic lupus erythematosus using proteasome inhibitor bortezomib followed by belimumab: description of two cases. Lupus. (2017) 26:1333–38. doi: 10.1177/0961203317691371
109. Ishii T, Tanaka Y, Kawakami A, Saito K, Ichinose K, Fujii H, et al. Multicenter double-blind randomized controlled trial to evaluate the effectiveness and safety of bortezomib as a treatment for refractory systemic lupus erythematosus. Mod Rheumatol. (2018) 28:986–92. doi: 10.1080/14397595.2018.1432331
110. Lazarus MN, Turner-Stokes T, Chavele KM, Isenberg DA, Ehrenstein MR. B-cell numbers and phenotype at clinical relapse following rituximab therapy differ in SLE patients according to anti-dsDNA antibody levels. Rheumatology. (2012) 51:1208–15. doi: 10.1093/rheumatology/ker526
111. Vallerskog T, Heimburger M, Gunnarsson I, Zhou W, Wahren-Herlenius M, Trollmo C, et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. (2006) 8:R167. doi: 10.1186/ar2076
112. Carter LM, Isenberg DA, Ehrenstein MR. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum. (2013) 65:2672–9. doi: 10.1002/art.38074
113. Cambridge G, Isenberg DA, Edwards JC, Leandro MJ, Migone TS, Teodorescu M, et al. B cell depletion therapy in systemic lupus erythematosus: relationships among serum B lymphocyte stimulator levels, autoantibody profile and clinical response. Ann Rheum Dis. (2008) 67:1011–6. doi: 10.1136/ard.2007.079418
114. Cambridge G, Stohl W, Leandro MJ, Migone TS, Hilbert DM, Edwards JC. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: relationships with B cell depletion, circulating antibodies, clinical relapse. Arthritis Rheum. (2006) 54:723–32. doi: 10.1002/art.21650
115. Jones A, Muller P, Dore CJ, Ikeji F, Caverly E, Chowdhury K, et al. Belimumab after B cell depletion therapy in patients with systemic lupus erythematosus (BEAT Lupus) protocol: a prospective multicentre, double-blind, randomised, placebo-controlled, 52-week phase II clinical trial. BMJ Open. (2019) 9:e032569. doi: 10.1136/bmjopen-2019-032569
116. Teng YKO, Bruce IN, Diamond B, Furie RA, van Vollenhoven RF, Gordon D, et al. Phase III, multicentre, randomised, double-blind, placebo-controlled, 104-week study of subcutaneous belimumab administered in combination with rituximab in adults with systemic lupus erythematosus (SLE): BLISS-BELIEVE study protocol. BMJ Open. (2019) 9:e025687. doi: 10.1136/bmjopen-2018-025687
117. Aranow C, Dall'Era M, Byron M, Ding L, Smilek D, Diamond B, et al. FRI0305 Phase 2 trial of induction therapy with anti-cd20 (RITUXIMAB) followed by maintenance therapy with anti-baff (BELIMUMAB) in patients with active lupus nephritis. Ann Rheum Dis. (2018) 77:690. doi: 10.1136/annrheumdis-2018-eular.5711
118. Kraaij T, Kamerling SWA, de Rooij ENM, van Daele PLA, Bredewold OW, Bakker JA, et al. The NET-effect of combining rituximab with belimumab in severe systemic lupus erythematosus. J Autoimmun. (2018) 91:45–54. doi: 10.1016/j.jaut.2018.03.003
119. Wallace DJ, Strand V, Merrill JT, Popa S, Spindler AJ, Eimon A, et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: a phase II dose-ranging randomised controlled trial. Ann Rheum Dis. (2017) 76:534–42. doi: 10.1136/annrheumdis-2016-209668
120. Sidiropoulos PI, Boumpas DT. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus. (2004) 13:391–7. doi: 10.1191/0961203304lu1032oa
121. Mathian A, Mouries-Martin S, Dorgham K, Devilliers H, Barnabei L, Ben Salah E, et al. Monitoring disease activity in systemic lupus erythematosus with single-molecule array digital enzyme-linked immunosorbent assay quantification of serum interferon-alpha. Arthritis Rheumatol. (2019) 71:756–65. doi: 10.1002/art.40792
122. Mathian A, Mouries-Martin S, Dorgham K, Devilliers H, Yssel H, Garrido Castillo L, et al. Ultrasensitive serum interferon-alpha quantification during SLE remission identifies patients at risk for relapse. Ann Rheum Dis. (2019) 78:1669–76. doi: 10.1136/annrheumdis-2019-215571
123. Khamashta M, Merrill JT, Werth VP, Furie R, Kalunian K, Illei GG, et al. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: a randomised, double-blind, placebo-controlled study. Ann Rheum Dis. (2016) 75:1909–16. doi: 10.1136/annrheumdis-2015-208562
124. Kalunian KC, Merrill JT, Maciuca R, McBride JM, Townsend MJ, Wei X, et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-alpha) in patients with systemic lupus erythematosus (ROSE). Ann Rheum Dis. (2016) 75:196–202. doi: 10.1136/annrheumdis-2014-206090
125. Furie R, Merrill J, Werth V, Khamashta M, Kalunian K, Brohawn P, et al. Anifrolumab, an anti-interferon alpha receptor monoclonal antibody, in moderate to severe systemic lupus erythematosus. Arthritis Rheumatol. (2015) 67:3223.
126. Furie RA, Morand EF, Bruce IN, Manzi S, Kalunian KC, Vital EM, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomised, controlled, phase 3 trial. Lancet Rheumatol. (2019) 1:e208–19. doi: 10.1016/S2665-9913(19)30076-1
127. Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active systemic lupus erythematosus. N Engl J Med. (2020) 382:211–21. doi: 10.1056/NEJMoa1912196
128. Satterthwaite AB. Bruton's tyrosine kinase, a component of B cell signaling pathways, has multiple roles in the pathogenesis of lupus. Front Immunol. (2017) 8:1986. doi: 10.3389/fimmu.2017.01986
129. Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. (2016) 353:179–84. doi: 10.1126/science.aaf6756
130. Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. (2019) 11:eaav1648. doi: 10.1126/scitranslmed.aav1648
Keywords: B cells, systemic lupus erythematosus, therapy, biologics, plasma cells, plasmablasts, lupus nephritis
Citation: Parodis I, Stockfelt M and Sjöwall C (2020) B Cell Therapy in Systemic Lupus Erythematosus: From Rationale to Clinical Practice. Front. Med. 7:316. doi: 10.3389/fmed.2020.00316
Received: 21 April 2020; Accepted: 01 June 2020;
Published: 09 July 2020.
Edited by:
Md Yuzaiful Md Yusof, University of Leeds, United KingdomReviewed by:
Chris Wincup, University College London, United KingdomCopyright © 2020 Parodis, Stockfelt and Sjöwall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ioannis Parodis, aW9hbm5pcy5wYXJvZGlzQGtpLnNl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.