
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 19 June 2020
Sec. Rheumatology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00288
 Kalliopi Klavdianou1†
Kalliopi Klavdianou1† Argyro Lazarini1†
Argyro Lazarini1† Alexandros Grivas2
Alexandros Grivas2 Dimitrios Tseronis2
Dimitrios Tseronis2 Christina Tsalapaki1
Christina Tsalapaki1 Panagiota Rapsomaniki2
Panagiota Rapsomaniki2 Katerina Antonatou1
Katerina Antonatou1 Konstantinos Thomas1
Konstantinos Thomas1 Dimitrios Boumpas2
Dimitrios Boumpas2 Pelagia Katsimbri2†
Pelagia Katsimbri2† Dimitrios Vassilopoulos1*†
Dimitrios Vassilopoulos1*†Background: Real world evidence data regarding secukinumab (SEC) use in biologic-experienced patients with psoriatic arthritis (PsA) are scarce.
Objectives: To assess the real life survival, safety and efficacy of SEC in biologic-experienced patients with PsA.
Methods: All biologic-experienced PsA patients treated with SEC in 2 University Rheumatology Units were included (3/2016-12/2018). Patients' and disease characteristics were recorded at baseline and during SEC therapy.
Results: 75 patients were included; 76% were females with a mean age of 53.9 years, median disease duration of 6.7 years and median SEC treatment duration of 11.1 months. At baseline, 97% had peripheral arthritis, 42% axial involvement, 22% enthesitis, and 12% dactylitis. Regarding previous biologic exposure, 48 (64%) had been exposed to anti-tumor necrosis factor (TNF) agents only, 5 (7%) to the interleukin (IL)-12/23 inhibitor (Ustekinumab-UST) only while 22 (29%) both to anti-TNFs and UST. Fifty-three percent received SEC in combination with non-biologics and 35% with glucocorticoids, respectively. During follow-up, statistically significant improvement in different disease activity indices were noted (DAS28-CRP, DAPSA, BASDAI). SEC survival rate at the end of follow-up was 64% (48/75), without difference between patients exposed to anti-TNFs only (67%) vs. anti-TNFs and UST (68%) as well as to 1 vs. ≥2 anti-TNFs. The rate of serious adverse events and serious infections during follow-up was 4.8 and 1.2/100 patient-years, respectively.
Discussion: In real life, in biologic-experienced patients with PsA, SEC displayed a high retention rate, regardless of the type, and number of previous biologics (anti-TNFs ± anti-IL12/23), without significant side effects.
Secukinumab (SEC) is a recombinant human monoclonal antibody against interleukin-17A (IL17A) that has been approved since 2015 by the European Medicines Agency (EMA) and 2016 by the US Food and Drug Administration (FDA), for the treatment of psoriatic arthritis (PsA) (1, 2). Its efficacy and safety have been demonstrated in the randomized controlled trials (RCTs) that led to its approval (3–6) as well as in their extension studies (7–9).
Until recently, three different classes of biologics had been approved for PsA: the anti-tumor necrosis factor (TNF), the anti-IL12/23 (Ustekinumab—UST) and the anti-IL17 (SEC, ixekizumab) agents (10, 11). In RCTs, SEC has shown efficacy both in anti-TNF naïve as well as anti-TNF-experienced patients (3, 5, 6). So far, real world evidence (RWE) data regarding survival as well as efficacy and safety of SEC in patients with PsA are scarce (12–14), especially for those who had been exposed to both classes of biologics (anti-TNF, anti-IL12/23).
Thus, the aim of our study was to evaluate the use of SEC in biologic-experienced patients with PsA in daily clinical practice.
This was a retrospective, observational, study of patients with PsA attending the Outpatient Rheumatology Clinics of two referral University Hospital Rheumatology Units. All included patients fulfilled the Caspar Classification Criteria (15) for PsA and had initiated treatment with SEC during the study period (March 2016—December 2018), according to their caring physicians' decision.
At baseline and during follow-up (every 3–6 months), data regarding patient (sex, age, gender, body mass index-BMI, smoking status, presence of co-morbidities according to the Rheumatic Diseases Comorbidity Index-RDCI) (16) and disease (disease duration, presence of enthesitis, dactylitis, or axial involvement, disease activity as measured by the Disease Activity in Psoriatic Arthritis Score—DAPSA (17), the Disease Activity Score—DAS28-CRP and Bath Ankylosing Spondylitis Disease Activity Index—BASDAI scores and function as measured by the health Assessment Questionnaire—HAQ and Bath Ankylosing Spondylitis Functional Index—BASFI scores, the percentage of skin disease assessed by the involved Body Surface Area—BSA) characteristics as well as treatment patterns (previous and current treatments including glucocorticoids, non-biologic, and biologics) were recorded for each patient. Furthermore, the number of patients who developed adverse events (AEs) or discontinued treatment were estimated. Reasons for discontinuation were classified as due to primary or secondary inefficacy, adverse events, or other.
All patients received the approved SEC dose of 300 mg subcutaneously (initially every week for the first 4 weeks and then every month). SEC was administered at the scheduled time of their previous bDMARD administration.
Study approval was provided by the local institutional boards of the two participating centers.
Descriptive analysis was initially performed. Categorical data were presented as counts and percentage of patients in each category. Continuous data were presented as mean ± standard deviation (S.D.) if normally distributed and median and interquartile range otherwise. The significance of difference in values obtained at baseline and last follow-up visit was assessed by Wilcoxon test for the continuous variables which were not normally distributed and chi-squared or Fisher's test for categorical variables.
The long-term survival of SEC was calculated until drug discontinuation or patient censoring and presented using Kaplan-Meier survival curves. Differences in drug survival between subgroups of patients were assessed using Log-rank test. Cox regression was used to evaluate predictive factors for drug discontinuation and hazard ratios (HRs) for discontinuation were calculated with univariate and multivariate models. The rates of adverse events and infections were calculated as events per 100 patient-years of follow-up. All statistical tests were two-sided and a p < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics, version 20.
The baseline characteristics of the included patients are summarized in Table 1. All patients had been previously exposed to biologics; 64% (48/75) to anti-TNFs only, 7% (5/75) to UST only while 29% (22/75) had experienced both classes of biologics. Among anti-TNF ever exposed patients (n = 70), 49 (70%) had received ≥ 2 anti-TNF agents. During SEC therapy (Table 1), approximately half of the patients were co-treated with non-biologics (40/75, 53%) and ~ one third with glucocorticoids (35%).
At the end of the follow–up, improvement was noted in several disease indices. Peripheral arthritis as assessed by the DAS28-CRP and the DAPSA scores improved significantly (from a mean of 5.05 to 3.99, p = 0.001, Figure 1A and from 35.06 to 23.02, p = 0.002, Figure 1B, respectively). Similarly, there was improvement in patients' function as assessed by the HAQ score (from a mean of 0.79 to 0.56, p = 0.071). Dactylitis resolved in all patients who had it at baseline (n = 9, p = 0.003, Table 1) while enthesitis resolved in 56% of patients during follow-up (from 16/73, 22% at baseline to 7/73, 10% at last visit, p = 0.043).
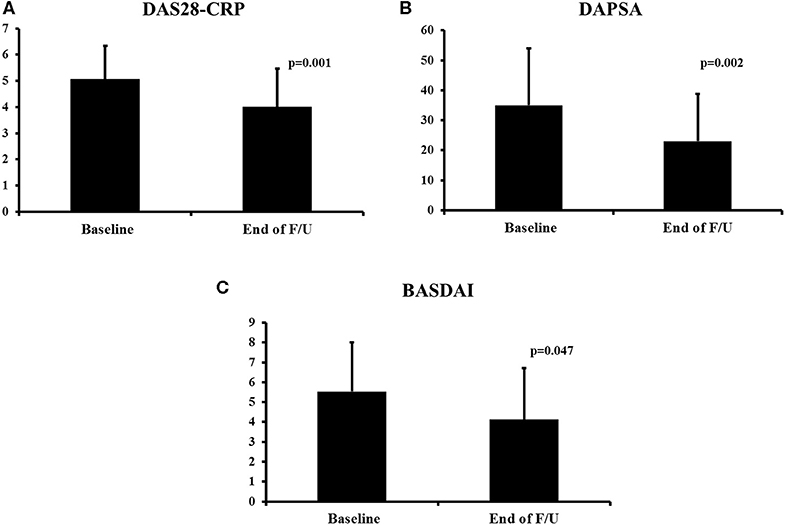
Figure 1. Change in disease activity indices during secukinumab therapy. DAS28-CRP (A), DAPSA (B), and BASDAI (C). The mean ± SD of the respective values at baseline and last visit are shown. DAS28-CRP, Disease Activity Score28- C-Reactive Protein; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; F/U, follow-up.
For patients with axial involvement, there was a statistically significant improvement in the BASDAI score (from a mean of 5.53 to 4.12, p = 0.047, Figure 1C) and an improvement in the BASFI score (from a mean of 6.17 to 4.39, p = 0.141). In terms of the skin disease, the BSA score also decreased significantly (from a mean of 3.0 to 1.3, p = 0.001).
Secukinumab was generally well-tolerated. Overall, nine patients (12%) experienced 11 AEs (13.3/100 patient-years); among those, 4 (5%) were considered serious (SAEs) leading to drug discontinuation at a rate of 4.8/100 patient-years during the follow-up period. These included two neoplasms at a rate of 2.4/100 patient-years (one colon cancer diagnosed in an 81 years old male 3 months and one case of endometrial cancer in a 45 years old female diagnosed 6 months, after SEC initiation, respectively), one case of serious soft tissue infection (requiring IV antibiotics) and one case of proteinuria (due to C3 glomerulopathy). There was one death in the 81 years old male patient who was diagnosed post-mortem with colon cancer (due to peritonitis).
Infections were uncommon (6/75, 8%) and in most cases (5/6) not serious. There were three opportunistic infections; two fungal (one oral-genital, one genital) treated only with local anti-fungals and one case of herpes zoster. The other three, included one case of serious periodontitis and two non-serious soft tissue infections. Only one infection was considered serious (soft tissue infection, requiring intravenous antibiotics) that led to drug discontinuation (overall rate of SIEs: 1.2/100 patient-years).
None of our patients had IBD at baseline and we did not observe any de novo cases of IBD during SEC treatment in our patient population.
The overall drug survival at the last follow-up visit was 64% (48/75) with an estimated median drug survival of 26.8 months (Figure 2A). The estimated 1 and 2 years drug survival was 66% and 56%, respectively.
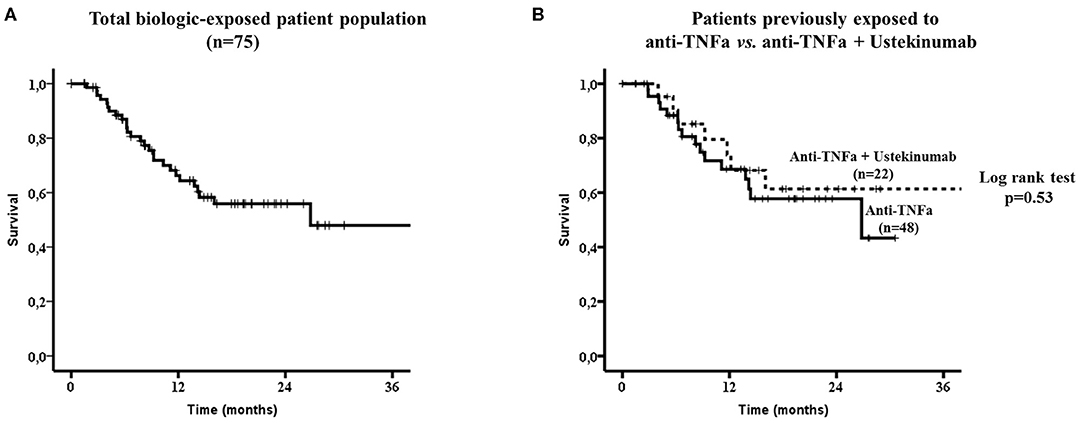
Figure 2. Secukinumab survival in the whole biologic-exposed patient population (n = 75, A) and to those exposed only to anti-TNFa (n = 48) vs. those exposed both to anti-TNFa and Ustekinumab (n = 22) (B). No statistically significant difference in drug survival was noted between the two subgroups by the Log-rank test (p = 0.53). TNFa, tumor necrosis factor; IL12/23, interleukin12/23.
There were no difference in drug survival between patients who had been exposed to anti-TNFs only (32/48, 67%) compared to those who had failed both anti-TNFs and anti-IL12/23 (15/22, 68%, Figure 2B). Although, patients who had been previously exposed to one anti-TNF had overall a better SEC survival compared to those exposed to ≥2 anti-TNFs, this did not reach statistical significance (Figures 3A,B, respectively).
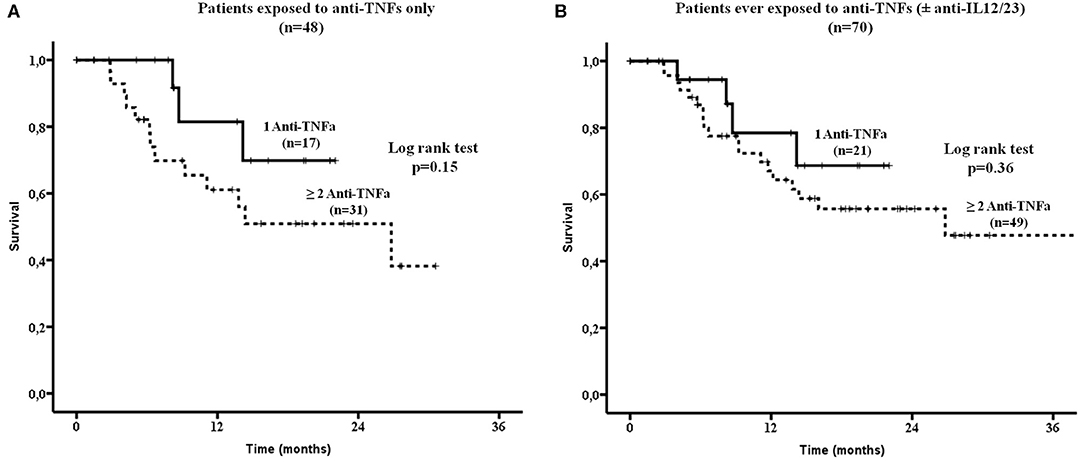
Figure 3. Secukinumab survival was estimated in patients exposed: (A) Only to anti-TNFa (n = 48). There was no statistically significant difference in drug survival between patients exposed to 1 vs. ≥ 2 anti-TNFa (by log rank test, p = 0.15) and (B) To anti-TNFa and/or Ustekinumab (anti-IL12/23, n = 70). Again no statistically significant difference in drug survival was noted between those ever exposed to 1 vs. ≥ 2 anti-TNFa (by log rank test, p = 0.36). TNFa, tumor necrosis factor; IL12/23, interleukin12/23.
The most common reasons for drug discontinuation (n = 27, 36%) was inadequate response (70%, 19/27, 10 due to primary and nine due to secondary lack of response), followed by adverse events (15%, 4/27) or other reasons (15%, 4/27).
No specific baseline or on-treatment factors were associated with SEC discontinuation by uni- and multivariate Cox regression analysis (Table S1).
This is one of the few real world studies assessing the usefulness of SEC in patients with PsA who had previously exposed to two different classes of biologics (anti-TNFs, anti-IL12/23). In this treatment-resistant group, the drug survival of SEC at the end of the follow-up was 64% with an acceptable safety profile similar to what has been previously reported in RCTs.
RCTs and their long term extension studies remain the gold-standard assessing the efficacy and safety of new therapies. On the other hand, real life cohorts provide valuable data regarding the overall safety, efficacy and drug survival of an approved medication in heterogeneous patient populations with various co-morbidities who usually are not registered in RCTs. Our real life patient population had some unique characteristics compared to the RCTs. This was a biologic-experienced, treatment-resistant population since almost all patients had been previously exposed to anti-TNFs (93%) and ~29% both to anti-TNF and anti-IL12/23 agents. Furthermore, these patients had long-standing, established disease (median: 6.7 years) and were recruited from University referral hospitals.
In the 3 RCTs, where a similar to our study SEC dose was used (300 mg), approximately one third of patients (31%) were anti-TNF-experienced (3, 5, 6). In this patient subgroup, the overall response rate at 24 weeks as assessed by the ACR-50 response criteria ranged from 23 to 41% (3, 5, 6). In our cohort, the peripheral joint disease activity (measured by the DAPSA and DAS28-CRP composite indices) as well as the skin disease (assessed by the BSA), improved significantly during SEC therapy. We also noted a statistically significant improvement in dactylitis and enthesitis in the studied population while a similar trend was seen in patients with axial involvement.
Regarding SEC survival, the estimated 1 and 2 years drug survival was 66% and 56%, respectively. So far, long term data regarding SEC survival in daily clinical practice are limited (12, 14, 18, 19). In a recent US study, Oelke et al. analyzed a claims database of 255 PsA patients treated with SEC (12). Among them, 163 patients had been previously exposed to biologics (anti-TNFs: 72%, anti-IL12/23: 45%). The overall 1 year survival rate of SEC in biologic-experienced patients was similar to our study (61% vs. 66% respectively), although no detailed data were given regarding the specific characteristics of these patients (12).
Compared to SEC, multiple patient registries and observational cohort studies have examined the survival of different anti-TNFs in biologic-experienced (mainly anti-TNFs) patients. In these studies, the 1 year drug survival ranged widely from 51 to 77% (12, 20–27). Nevertheless, real life data comparing directly anti-TNF and anti-IL17 (SEC) drug survival are missing. As mentioned above, Oelke et al. showed that the 1 year survival rate in biologic-experienced PsA patients treated with SEC was 61% compared with 53% in anti-TNF treated patients (12).
A novel finding of our study, was that for the first time we presented detailed data for patients with PsA treated with SEC who had been exposed both to anti-TNF and anti-IL12/23 agents. In our study, the overall SEC survival of patients exposed to both classes was not different from those exposed to anti-TNFs alone (68% vs. 67%). In contrast, to studies with anti-TNFs (22, 23, 25), we did not identify any baseline or on-treatment factors that correlated with SEC survival.
The safety profile of SEC was not much different to what has been previously reported in RCTs and their long-term extension studies (3, 5, 6, 9). The overall rate of SAEs was 4.8/100 patient-years which is lower to the rate (7.9/100 patient-years) reported in the long-term extension studies (9). Regarding serious infections, the overall incidence rate was 1.2/100 patient-years which was slightly lower to the 1.9/100 patient-years rate reported in the long term extension studies (9) and in a recent US claims database study (1.9/100 patient-years) (28). In this database study, the comparative incidence of serious infections in biologic-experienced patients treated with anti-TNFs or anti-IL12/23 were 2.7 and 1.7/100 patient-years, respectively (28).
We observed two fungal infections at a rate of 2.4/100 patient-years which is slightly higher to that of the long term extension studies (1.5/100 patient-years) (9). Both patients were treated with topical anti-fungals without interrupting SEC therapy. In our cohort, two malignancies were diagnosed at a rate of 2.4/100 patient-years which was higher to that reported by Deodhar et al. (1.1/100 patient-years) (9). It should be noted though that both neoplasms were diagnosed shortly after SEC initiation (first 6 months) and were considered unrelated to drug administration.
There are certain limitations to our study including its retrospective design, the potential missing data regarding AEs, the short period of follow-up and the absence of a control group of patients treated at the same period with different classes of biologics (anti-TNFs, anti-IL12/23).
In conclusion, we report one of the few real life studies in the literature assessing the survival as well as the safety and efficacy of SEC in patients with PsA who had been previously exposed to one or two different classes of biologics (anti-TNFs, anti-IL12/23). Our findings suggest that SEC is an efficacious and well-tolerated biologic agent in this multi-resistant, difficult to treat patient group.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Hippokration General Hospital, Athens, Greece Attikon University Hospital, Athens, Greece. The patients/participants provided their written informed consent to participate in this study.
KK, AL, PK, and DV: design of the study, data analysis, and manuscript preparation. KK, AL, AG, DT, CT, PR, KA, KT, DB, PK, and DV: acquisition of data. All authors reviewed the manuscript and gave final approval for the work.
This work was supported by research grants from the Special Account for Research Grants (S.A.R.G.), National and Kapodistrian University of Athens, Athens, Greece (DV, Grant # 12085).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.00288/full#supplementary-material
1. Shirley M, Scott LJ. Secukinumab: a review in psoriatic arthritis. Drugs. (2016) 76:1135–45. doi: 10.1007/s40265-016-0602-3
2. Van den Bosch F, Coates L. Clinical management of psoriatic arthritis. Lancet. (2018) 391:2285–94. doi: 10.1016/S0140-6736(18)30949-8
3. McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2015) 386:1137–46. doi: 10.1016/S0140-6736(15)61134-5
4. Strand V, Mease P, Gossec L, Elkayam O, van den Bosch F, Zuazo J. Secukinumab improves patient-reported outcomes in subjects with active psoriatic arthritis: results from a randomised phase III trial (FUTURE 1). Ann Rheum Dis. (2017) 76:203–7. doi: 10.1136/annrheumdis-2015-209055
5. Mease P, van der Heijde D, Landewe R, Mpofu S, Rahman P, Tahir H. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. (2018) 77:890–7. doi: 10.1136/annrheumdis-2017-212687
6. Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. (2018) 20:47. doi: 10.1186/s13075-018-1551-x
7. McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology. (2017) 56:1993–2003. doi: 10.1093/rheumatology/kex301
8. Kavanaugh A, Mease PJ, Reimold AM, Tahir H, Rech J, Hall S. Secukinumab for long-term treatment of psoriatic arthritis: a two-year followup from a phase III, randomized, double-blind placebo-controlled study. Arthritis Care Res. (2017) 69:347–55. doi: 10.1002/acr.23111
9. Deodhar A, Mease PJ, McInnes IB, Baraliakos X, Reich K, Blauvelt A. Long-term safety of secukinumab in patients with moderate-to-severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post-marketing surveillance data. Arthritis Res Ther. (2019) 21:111. doi: 10.1186/s13075-019-1882-2
10. Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. (2016) 68:1060–71. doi: 10.1002/art.39573
11. Gossec L, Smolen JS, Ramiro S, de WM, Cutolo M, Dougados M. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. (2016) 75:499–510. doi: 10.1136/annrheumdis-2015-208337
12. Oelke KR, Chambenoit O, Majjhoo AQ, Gray S, Higgins K, Hur P. Persistence and adherence of biologics in US patients with psoriatic arthritis: analyses from a claims database. J Comp Eff Res. (2019) 8:607–21. doi: 10.2217/cer-2019-0023
13. Sunkureddi P, Latremouille-Viau D, Meiselbach MK, Xie J, Hur P, Joshi R. Characteristics of patients with psoriatic arthritis receiving secukinumab and reasons for initiation: a US retrospective medical chart review. Rheumatol Ther. (2019) 6:89–100. doi: 10.1007/s40744-018-0137-z
14. Mann HF, Zavada J, Senolt L, Bubova K, Nekvindova L, Kristkova Z. Real world use of secukinumab for treatment of axial spondyloarthritis and psoriatic arthritis: nationwide results from the ATTRA registry. Clin Exp Rheumatol. (2019) 37:342–3.
15. Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. (2006) 54:2665–73. doi: 10.1002/art.21972
16. Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2007) 21:885–906. doi: 10.1016/j.berh.2007.06.002
17. Schoels M, Aletaha D, Funovits J, Kavanaugh A, Baker D, Smolen JS. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis. (2010) 69:1441–7. doi: 10.1136/ard.2009.122259
18. Pinto Tasende JA, Maceiras Pan FJ, Mosquera Martinez JA, Fernandez DL, Correa RB, Garcia PC. Secukinumab as biological treatment for psoriatic arthritis in real clinical practice. Reumatol Clin. (in press). doi: 10.1016/j.reuma.2019.07.002
19. Elliott A, Wright G. Real-world data on secukinumab use for psoriatic arthritis and ankylosing spondylitis. Ther Adv Musculoskelet Dis. (2019) 11:1759720X19858510. doi: 10.1177/1759720X19858510
20. Saad AA, Ashcroft DM, Watson KD, Hyrich KL, Noyce PR, Symmons DP. Persistence with anti-tumour necrosis factor therapies in patients with psoriatic arthritis: observational study from the British society of rheumatology biologics register. Arthritis Res Ther. (2009) 11:R52. doi: 10.1186/ar2670
21. Fagerli KM, Lie E, van der Heijde D, Heiberg MS, Kalstad S, Rodevand E. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD study. Ann Rheum Dis. (2013) 72:1840–4. doi: 10.1136/annrheumdis-2012-203018
22. Glintborg B, Ostergaard M, Krogh NS, Andersen MD, Tarp U, Loft AG. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor alpha inhibitor therapy: results from the Danish nationwide DANBIO registry. Arthritis Rheum. (2013) 65:1213–23. doi: 10.1002/art.37876
23. Kristensen LE, Lie E, Jacobsson LT, Christensen R, Mease PJ, Bliddal H. Effectiveness and feasibility associated with switching to a second or third TNF inhibitor in patients with psoriatic arthritis: a cohort study from southern Sweden. J Rheumatol. (2016) 43:81–7. doi: 10.3899/jrheum.150744
24. Soubrier AS, Bele-Philippe P, Cortet B, Ramdane-Sebbane N, Bacle-Boutry MA, Lemeunier L. Treatment response, drug survival and safety of anti-tumour necrosis factor alpha therapy in 193 patients with psoriatic arthritis: a twelve-year “real life” experience. Joint Bone Spine. (2015) 82:31–7. doi: 10.1016/j.jbspin.2014.08.001
25. Aaltonen K, Heinonen A, Joensuu J, Parmanne P, Karjalainen A, Varjolahti-Lehtinen T. Effectiveness and drug survival of TNF-inhibitors in the treatment of psoriatic arthritis: a prospective cohort study. Semin Arthritis Rheum. (2017) 46:732–9. doi: 10.1016/j.semarthrit.2016.09.005
26. Deza G, Notario J, Ferran M, Beltran E, Almirall M, Alcala R. Long-term etanercept survival in patients with psoriatic arthritis: a multicenter retrospective analysis in daily clinical practice in Spain. Rheumatol Int. (2018) 38:2037–43. doi: 10.1007/s00296-018-4144-8
27. Luttropp K, Dozier M, Justo N, Cornillie F, Kachroo S, Govoni M. Real-world treatment persistence of golimumab in the management of immune-mediated rheumatic diseases in Europe: a systematic literature review. BMJ Open. (2019) 9:e027456. doi: 10.1136/bmjopen-2018-027456
Keywords: psoriatic arthritis, secukinumab, tumor necrosis factor inhibitors, biologic agents, real world evidence, ustekinumab
Citation: Klavdianou K, Lazarini A, Grivas A, Tseronis D, Tsalapaki C, Rapsomaniki P, Antonatou K, Thomas K, Boumpas D, Katsimbri P and Vassilopoulos D (2020) Real Life Efficacy and Safety of Secukinumab in Biologic-Experienced Patients With Psoriatic Arthritis. Front. Med. 7:288. doi: 10.3389/fmed.2020.00288
Received: 02 April 2020; Accepted: 22 May 2020;
Published: 19 June 2020.
Edited by:
Tadej Avcin, University Medical Centre Ljubljana, SloveniaReviewed by:
Sule Yavuz, Istanbul Bilim University, TurkeyCopyright © 2020 Klavdianou, Lazarini, Grivas, Tseronis, Tsalapaki, Rapsomaniki, Antonatou, Thomas, Boumpas, Katsimbri and Vassilopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimitrios Vassilopoulos, ZHZhc3NpbG9wQG1lZC51b2EuZ3I=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.