- 1Université de Paris, IAME, INSERM, UMR1137, UFR de Médecine, Paris, France
- 2CNR-MyRMA, Centre National de Reference pour les Mycobactéries et les Antituberculeux, APHP, Paris, France
- 3APHP, Hôpital Lariboisière, Service de Microbiologie, Paris, France
- 4Service de Medecine Interne, Hôpital d'Instruction des Armées Sainte Anne, Toulon, France
- 5Service de microbiologie, Hôpital d'Instruction des Armées Sainte Anne, Toulon, France
- 6Ecole du Val-de-Grâce, Paris, France
Invasive cardiovascular infections by Mycobacterium chimaera associated with open-heart surgery have been reported worldwide since 2013. Here, we report a case of a 61 year old man, without any other particular medical background, who underwent cardiac surgery for replacing part of the ascending aorta by a bio-prosthetic graft. Eighteen months later, the patient was painful at the lower back with fever. A pyogenic vertebral osteomyelitis due to M. chimaera associated to graft infection was diagnosed after 6 months of sub-acute infection. The patient presented a disseminated disease with cerebral lesions, chorioretinitis, and chronic renal failure. Despite adequate antimicrobial treatment and graft explantation, the patient died after 6 years. We reviewed the literature on M. chimaera infections associated with open-heart surgery. The worldwide outbreak has been explained by airborne bioaerosol generated by the 3T heater–cooler unit (HCU) used during cardiac by-pass surgical procedures. These infections are difficult to diagnose because of a long latency period (up to several years), with no specific symptoms and a highly specialized microbiological diagnosis. The treatment is based on antibiotics and surgery. These infections are also difficult to treat, since the mortality rate is high around 50%. Prevention is necessary by modifying the use of HCUs in operating rooms.
Background
Invasive cardiovascular infections due to M. chimaera secondary to open-heart surgery were first described in 2015 in Switzerland (1). These infections are difficult to diagnose because of non-specific symptoms, a difficult microbiological diagnostic, and a poor prognostic. They were attributed to contamination from the heater-cooler units (HCU) present in the operating rooms since similar strains of M. chimaera were found in their water tanks. In addition, since the strains from several patients, who underwent surgery at different periods, were also similar, a common reservoir was sought. Since 2015, cases were reported worldwide not only in Europe (Switzerland, Germany, Netherlands, England, France, Italia, Spain, and Ireland) (1–6), but also in North America (United-States, Canada) (7, 8), Hong-Kong (9), New-Zeeland, and Australia (10). Most of the cases were due to the same epidemic strain similar to those found also in the HCUs (11). The epidemic strain has also been found in HCUs in China (12). In 2015, the European Center for Disease Prevention and Control issued a Rapid Risk Assessment (13) and the Food and Drug Administration published as well a safety communication about infections associated with heater-cooler devices and recommendations to deal with the risk (14).
Here, we report the case of a patient diagnosed in France for a M. chimaera infection following cardiac surgery, and subsequently reviewed the literature about this outbreak and discussed the patient case.
Case Presentation
A 61 year old man, without any particular medical background, underwent cardiac surgery in 2012 for replacing part of the ascending aorta by a bio-prosthetic graft and repairing the aortic arch due to a type I aortic dissection. During immediate follow up, a local infection was diagnosed at the coronary angiography insertion site. Since it was show to be caused by Proteus mirabilis and Pseudomonas aeruginosa, the patient was treated with 10 days of ceftazidime.
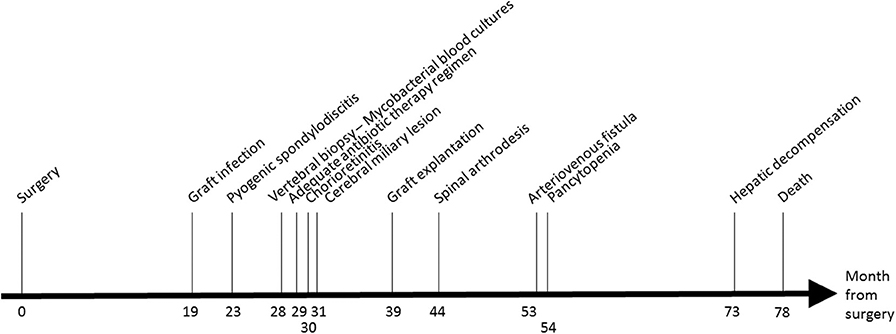
Eighteen months after the surgery (M18, see Figure 1), the patient presented fewer with a lower back pain that was intensified for 1 month. A positron emission tomography/computed tomography (PET-CT) suspected a graft infection with a pseudoaneurysm para-aortic. A transthoracic echocardiography did not show any signs of endocarditis and blood cultures remained negative. An empirical treatment was initiated with piperacillin-tazobactam, teicoplanin, and rifampicin.
At M24, vertebral magnetic resonance imaging (MRI) revealed lesions of the vertebral bodies at T8-T9-L4-L5-S1 and intervertebral disks between T8-T9 and L4-L5-S1, with an epidural abscess of 5 cm at the L3 and L4 levels, consistent with a pyogenic vertebral osteomyelitis (Figure 2). That was consistent with the PET-CT results showing metabolic activity around the peri-aortic graft in favor of infection. Transcutaneous vertebral biopsies, made at M27, were culture-positive for acid fast bacilli (AFB) after 21 days incubation on 7H9 liquid medium (BACT/ALERT® MP, Biomerieux) and subsequently on Lowenstein Jensen solid medium (Bio-Rad). The AFB isolate was identified first as M. intracellulare by GenoType® Mycobacterium CM (Hain Lifescience), and subsequently confirmed as M. chimaera by GenoType® NTM-DR (Hain Lifescience), ITS and hsp65 sequencing. Susceptibility testing of the isolate was performed using a commercial microdilution method, SLOMYCO Myco Sensititre™ (Thermo Scientific™) and showed a wild type susceptibility pattern with a minimal inhibitory concentration (MIC) of clarithromycin at 2 mg/L, MIC of amikacin at 8 mg/L, MIC of linezolid at 32 mg/L and MIC of moxifloxacin at 4 mg/L. Three mycobacterial blood cultures performed at the same period were also positive for M. chimaera. The patient was treated with a 4-antibiotic regimen combining azithromycin, ethambutol, rifampicin, and moxifloxacin. The risk associated with graft explantation was felt to be prohibitively high, and the decision was therefore made to proceed with conservative management.
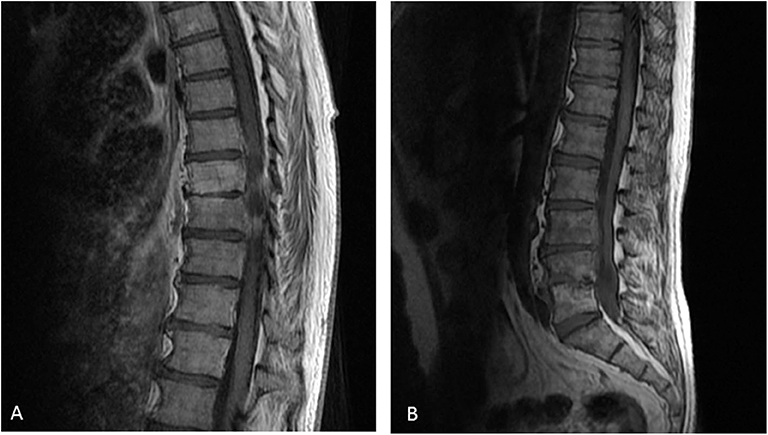
Figure 2. Vertebral magnetic resonance imaging of the vertebral lesions. Vertebral magnetic resonance imaging revealed lesions of the vertebral bodies at T8-T9-L4-L5-S1 and invertebral disks between T8-T9 (A) and L4-L5-S1 (B), with an epidural abscess of 5 cm at the L3 and L4 levels, consistent with a pyogenic vertebral osteomyelitis.
At M 29, 2 months after antimicrobial therapy has started, an ocular examination showed a bilateral chorioretinitis associated with uveitis of the left eye. At M30, a MRI of the brain, performed because of confusion, revealed diffuse hypersignals of both hemispheres consistent with cerebral miliary lesions. The patient showed then a worsening of the vertebral lesions and of the renal function. Because of the disseminated infection, it was decided to replace the aortic graft at M39. M. chimaera was isolated from explanted prosthetic tissues.
At M44, the patient underwent arthrodesis of thoracic spine. Due to the chronic renal failure, an arteriovenous fistula was created at M53. The patient showed pancytopenia at M72. At M73, the patient suffered from hepatic and neurologic decompensation. Unfortunately, the patient died at M78.
The Worldwide Outbreak of Disseminated M. Chimaera Infection Associated to Open-Heart Surgery
The Causative Agent
M. chimaera is a slow growing non-tuberculous mycobacterium (NTM) belonging to the Mycobacterium avium complex (MAC) (15) and was first described in 2004 (16). Like M. avium and M. intracellulare, M. chimaera is predominantly seen in immunocompromised patients and in pulmonary infections in patients with chronic lung diseases. Among patients with sputum culture-positive with MAC, the patients with M. chimaera were less likely to meet criteria for infection than M. avium and M. intracellulare, suggesting a lower virulence or a different reservoir (17, 18). The natural reservoir of M. chimaera is not well-known and is supposed to be similar to other species of MAC. MAC can be found in distribution water systems (19) and M. chimaera was found frequently in household water (20). Drug susceptibility patterns of M. chimaera are comparable to those of the MAC with modal MIC of 2 mg/L for clarithromycin, 0.5 mg/L for rifabutin, 4–8 mg/L for rifampicin and ethambutol, 8 mg/L for amikacin, 4 mg/L for moxifloxacine, and 32 mg/L for linezolid (21).
Burden and Impact of the Disease
Over 120 cases of post-cardiac surgery M. chimaera infections have been reported worldwide. More cases are to be expected since many countries did not register any cases. Using data from Switzerland, the incidence of M. chimaera disseminated disease associated with open heart surgery was estimated to 156–282 cases per year in the 10 major cardiac valve replacement market countries (22). Using data from the national British investigation, the risk of M. chimaera infection for person who underwent cardiothoracic surgery significantly increased since 2012. Out of 10,000 patients undergoing open heart surgery, 300–400 were estimated to experience endocarditis by 5 years post-surgery and one to develop M. chimaera infection (2). A long latency period (median of 21 months) was observed between cardiac surgery and symptoms (23). The reported mortality rate for these infections was remarkably high, around 50% (24, 25). No cases were reported in healthcare workers related to the HCU epidemic. However, one case of M. chimaera pulmonary infection has been described in a healthcare worker previously exposed to HCU (26). Other devices such as thermoregulatory devices used for extracorporeal membrane oxygenation (ECMO) may be also at risk of transmission, but no cases were reported (27).
Transmission Routes
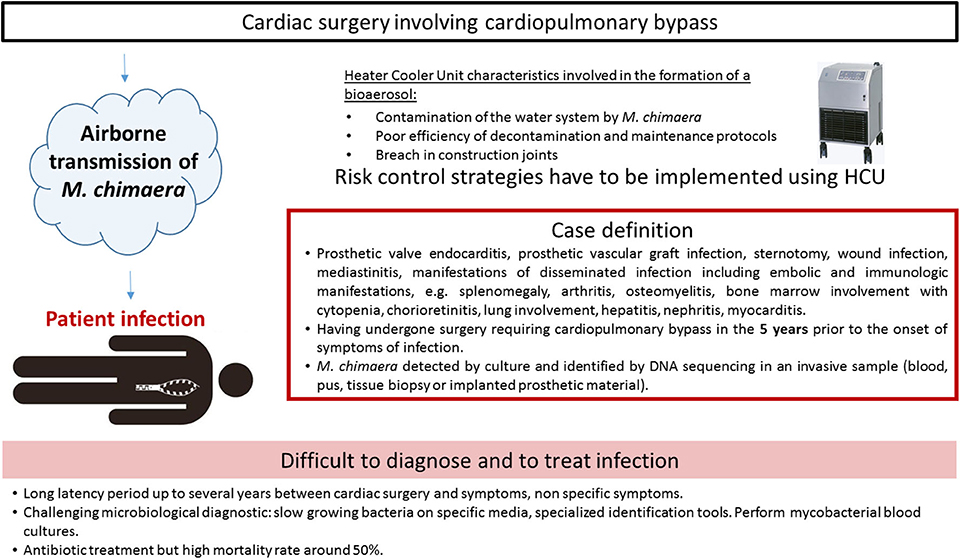
The first report described two cases, caused by closely related M. chimaera strains, as assessed by randomly amplified polymorphic DNA (RAPD)-PCR. The two patients had heart surgery 2 years apart from each other (28). Due to identical RAPD-PCR patterns, a deeper investigation was retrospectively conducted and six cases were finally detected in the institution (1). The prospective on-site observations and microbiological sampling in the hospital environment showed that M. chimaera was present in water circuits of the LivaNova HCU (25). HCU are essential components of cardiopulmonary bypass operation used during open-chest heart surgery. They are connected to the extracorporeal circuit enabling the warming of the patient's blood and the cooling of the cardioplegia solution, since tanks are filled with water. Water in the circuits does not come into direct contact with the patient. Interestingly, air sampling cultures in the operating room were also positive with M. chimaera when a HCU was running, but not when it was turned off. Laser particle measurement and microbial air cultures confirmed that during operation, mycobacterial particles were dispersed from the contaminated HCU into the air of the operating room via aerosolization, despite ultraclean air ventilation (27). The aerosol was generated through a breach in construction joints on the tank cover and released into the operating environment via the rear cooling fan, thereby causing infection (10). Phylogenetic analysis by whole genome sequencing (WGS) showed a strong clustering of all M. chimaera epidemic isolates. They were indeed clonal isolates around the world in Europe, North America, or Australia (2, 29). Moreover, this cluster comprised isolates from patients, HCUs and water at the HCU industry production site (30). The most plausible hypothesis of such genetic similarity is that the devices were contaminated by a point source when manufactured before being sent to the cardiac surgery wards. Contamination and infections characteristics are presented in Figure 3.
Risk Factors
In the majority of cases, patients had undergone cardiac valve or aortic vascular graft surgery prior to diagnosis. However, patients who have undergone other operations that involve cardiopulmonary bypass, including heart or lung transplantation and introduction of ventricular assist devices are also at risk (23). To our knowledge, no cases have been described with other devices than LivaNova, formerly Sorin. It has been shown that the odds of NTM infection increase with the duration time a patient is exposed to a running HCU. The risk reached statistical significance for surgery time longer than 5 h (29). Survival analysis measured for a cohort of 30 cases identified several factors associated with better survival: younger age, mitral valve surgery, mechanical valve replacement, higher serum sodium concentration (30).
Diagnosis and Treatment
Clinical Manifestations
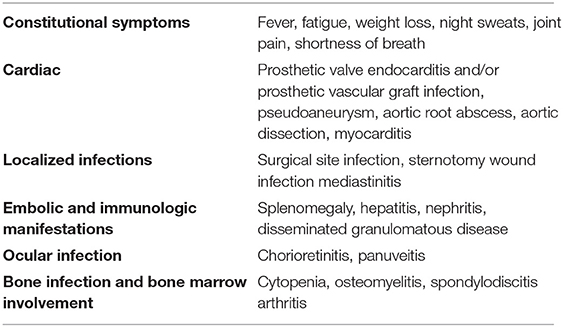
Patients exhibited a wide spectrum of disease including surgical site infection, e.g., prosthetic valve endocarditis (PVE), aortic graft infection or localized thoracic infection, as well as disseminated infection with diverse presentations, such as bacteraemia, osteomyelitis or other bone lesions, cholestatic hepatitis, granulomatous nephritis. Disseminated disease with encephalitis have also been described (31). Patients most commonly complained of non-specific symptoms such as fever, malaise, weight loss, cough, or dyspnea. Laboratory findings included cytopenia and elevated inflammatory markers, transaminase and creatinine blood levels (24). Eye involvement was correlated with the course of the systemic disease. Patients with few choroidal lesions had a favorable outcome, whereas all patients with widespread chorioretinitis died of systemic complications (32). Complications of M. chimaera infection are listed in Table 1.
Diagnosis
The European Center for Disease Prevention (33) and the American Center for Disease Control and Prevention (34) have formulated a case definition for M. chimaera infections associated with open heart surgery based on three criteria: (i) any of the clinical criteria, including prosthetic valve or vascular infection, localized infection, and disseminated infection, (ii) an exposure criteria, e.g., having undergone surgery requiring cardiopulmonary bypass in the 5 years prior to the onset of symptoms of infection, (iii) microbiological criteria, e.g., M. chimaera detected by culture or identified by DNA sequencing in an invasive sample. A 5 years period from surgery to presentation of symptoms has been mentioned, but the delay before diagnosis can be longer, the longest reported time being more than 6 years (35).
Mycobacterial cultures remain the essential investigation for all sample types: blood, tissue and bone biopsy, pus, and urine. Culture of M. chimaera from peripheral blood is the most common method of microbiological diagnosis (30). Its sensitivity increases by performing multiple samples: 3 sets of mycobacterial blood cultures on different days are recommended by the English guidance (36). It is essential to inform the laboratory of the possibility of M. chimaera infection, in order to ensure samples are taken into the correct containers, such as blood culture bottle specific for mycobacterial growth (e.g., BACTEC™ Myco/F Lytic). Molecular technologies based on acid nucleic amplification can be used, especially on sterile samples (tissue biopsies, vascular graft) positive for acid fast bacilli at microscopic examination.
M. chimaera identification is challenging. It is slow growing in liquid and solid media, so growth detection may take between 2 and 8 weeks. Most laboratories can identify a M. chimaera isolate as a species of the M. avium complex (MAC), but precise speciation needs specialized tests (15), such as PCR sequencing of the internal transcribed spacer (ITS) sequence between 16S and 23S ribosomal DNA. M. chimaera is closely related to M. intracellulare, for instance they show only a single nucleotide difference in 16S ribosomal DNA sequences. M. chimaera can be misidentified as M. intracellulare by mass spectrometry (MALDI-TOF MS) or some commercial DNA hybridization probe assays (37). In fact, M. chimaera has been extensively classified as M. intracellulare before 2004 (18). Analysis of WGS is necessary to assess whether a clinical strain is related to the HCU outbreak strain (11). Microbiologic results should be considered alongside histopathology findings, e.g., detection of non-caseating granuloma and foamy macrophages with acid fast bacilli in cardiac or vascular tissues, prosthetic material, or in specimen from the sternotomy wound.
Transesophageal echocardiography (TOE) was shown to be more sensitive than transthoracic echocardiography in diagnosing M. chimaera PVE (3). TOE can be normal at presentation, while the patient later went on be diagnosed with PVE (30). Normal echocardiogram alone cannot be used to exclude infection and serial assessment should be considered. Computed tomography (CT) can assess aortic graft infection. Moreover PET-CT provides additional evidence in which standard CT has been equivocal and has also proved useful in the diagnosis of M. chimaera spondylodiscitis and PVE.
Differential Diagnosis
The presentation and laboratory features of the disease can be very similar to sarcoidosis (29). M. chimaera investigations should be undertaken in all patients for whom a diagnosis of sarcoidosis is being considered and who has an appropriate history of cardiothoracic surgery.
Treatment
The optimal treatment for M. chimaera HCU-related infections is not known. The combination of clarithromycin or azithromycin, rifampin, or rifabutin and ethambutol is the treatment regimen designed on the basis of that recommended for MAC lung disease (38). Antimicrobial susceptibility of M. chimaera strains isolated during the outbreak were fortunately all susceptible to clarithromycin and amikacin (3, 39). Given the disseminated nature of HCU-related infections and the poor outcome, parenteral amikacin, and fluoroquinolones were added (24, 40). According to guidelines, a minimum of 12 months therapy is indicated for non-HIV patients with disseminated MAC disease (38). The optimal duration of therapy in HCU-related infections is unknown and some patients required treatment for more than 24 months. Blood drug concentrations have to be monitored, a study reporting that half of the patients did not reached optimal drug levels (3). The removal of prosthetic materials was associated with a lower risk of mortality for classical PVE (41). Given technical difficulties and the risk of surgery, such decision has to be discussed with the cardiovascular surgeon in case of invasive infection with M. chimaera (30).
Preventive Measures
Several measures were proposed to minimize the risks of M. chimaera infections. The manufacturer updated its disinfection recommendations in 2015 with more frequent cleaning and disinfections of the water system (42). In addition, the manufacturer proposed complete refurbishment and replacement of the internal tubing of devices. Despite intensified cleaning and disinfection, surveillance samples from factory-new units still grew M. chimaera (43). Environmental testing and microbiological screening of HCUs could be performed. However, there is no standardization with regard to the collection of samples and the laboratory methods used (44).
Due to the difficulties to maintain water with good microbiological quality, different strategies were implemented in hospitals (45). The most definitive option was to remove HCUs from the operating room, though this may not be feasible for all facilities. A custom-made airtight housing for the HCU was also used in order to contain the bioaerosol. If a definitive mitigation strategy cannot be implemented, HCU should be oriented so that the aerosol from the exhaust is directed in the opposite way from the patient. However, the utility of this strategy is unproven and may continue to place the patient at risk (12).
Discussion
M. chimaera was initially described in respiratory samples (16). Only one case of vertebral osteomyelitis was reported in a patient with prednisolone treatment, without history of cardiac surgery (46). Cases of disseminated M. chimaera infections were rare until 2015 (25). The involvement of this species in disseminated disease was quite unusual and led to the description of this outbreak associated with cardiac surgery. In the case of our patient, the surgery was performed in 2012 when the risk due to contaminated HCU was not known yet. The microbial diagnosis was rapidly done when the spondylodiscitis diagnosis was made, and isolation and identification of M. chimaera was done in a laboratory with expertise. The strain was genomically sequenced and was shown to cluster with the epidemic isolates. No sample was obtained from the HCU in this hospital at the time of the contamination and later. Although an adequate antimicrobial treatment was immediately given, the patient died 78 months later. We may think that an earlier replacement of aortic graft could have helped in the cure of the infection, but surgery could have been also lethal (40).
This case was the only one registered in this area of France, a second case being registered in the Paris area in 2010 were detailed elsewhere (47).
Data Availability Statement
The data sequencing are now available on the NCBI platform (PRJNA576780, SAMN13008644, SRR10256732).
Ethics Statement
Written informed consent was obtained from the individual's next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
EL, GP, and EC contributed to the manuscript. GP review the clinical case and EL reviewed the literature. EP took care of the patient. FM, HB, and FJ provide clinical and microbiological data. EC supervised this work.
Funding
CNR-MyRMA receives an annual grant from Santé Publique France and DGOS.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the technicians of Lariboisière Bacteriology laboratory for their expert technical assistance: Christine Bisilliat-Gardet, Véronique Charlier, Marie-Emmanuelle Hemet, Marilyne Lemaire, Isabelle Lacrampe, Patricia Lawson-Body, Marie Monjean, Fabienne Meunier, Sylvie Tenza, Odile Vissouarn, and all the others members of the laboratory. Collaborators: Alexandra Aubry, Isabelle Bonnet, Jeremy Jaffre, Vincent Jarlier, Hervé Jacquier, Florence Morel, Jerome Robert, Sabrina Temim, and Wladimir Sougakoff.
References
1. Sax H, Bloemberg G, Hasse B, Sommerstein R, Kohler P, Achermann Y, et al. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis. (2015) 61:67–75. doi: 10.1093/cid/civ198
2. Chand M, Lamagni T, Kranzer K, Hedge J, Moore G, Parks S, et al. Insidious risk of severe Mycobacterium chimaera infection in cardiac surgery patients. Clin Infect Dis. (2017) 64:335–42. doi: 10.1093/cid/ciw754
3. Kohler P, Kuster SP, Bloemberg G, Schulthess B, Frank M, Tanner FC, et al. Healthcare-associated prosthetic heart valve, aortic vascular graft, and disseminated Mycobacterium chimaera infections subsequent to open heart surgery. Eur Heart J. (2015) 36:2745–53. doi: 10.1093/eurheartj/ehv342
4. Cappabianca G, Paparella D, D'Onofrio A, Caprili L, Minniti G, Lanzafame M, et al. Mycobacterium chimaera infections following cardiac surgery in Italy: results from a National Survey Endorsed by the Italian Society of Cardiac Surgery. J Cardiovasc Med. (2018) 19:748–55. doi: 10.2459/JCM.0000000000000717
5. Zegri-Reiriz I, Cobo-Marcos M, Rodriguez-Alfonso B, Millán R, Dominguez F, Forteza A, et al. Successful treatment of healthcare-associated Mycobacterium chimaera prosthetic infective endocarditis: the first Spanish case report. Eur Heart J Case Rep. (2018) 2:yty142. doi: 10.1093/ehjcr/yty142
6. Health Protection Surveillance Centre (HPSC). Report of Case Finding Investigation to Identify Mycobacterium chimaera Infections Potentially Associated with Heater-Cooler Units Used During cardiothoracic Surgery in Ireland (2016).
7. Tan N, Sampath R, Abu Saleh OM, Tweet MS, Jevremovic D, Alniemi S, et al. Disseminated Mycobacterium chimaera infection after cardiothoracic surgery. Open Forum Infect Dis. (2016) 3:ofw131. doi: 10.1093/ofid/ofw131
8. Hamad R, Noly P-E, Perrault LP, Pellerin M, Demers P. Mycobacterium chimaera infection after cardiac surgery: first Canadian outbreak. Ann Thorac Surg. (2017) 104:e43–e5. doi: 10.1016/j.athoracsur.2017.01.115
9. Cheng VCC, Wong SC, Chen JHK, Wong SCY, Yuen KY. Mycobacterium chimaera-contaminated heater-cooler devices: the inner surface as the missing link? J Hosp Infect. (2018) 100:e157–e8. doi: 10.1016/j.jhin.2018.07.010
10. Robertson J, McLellan S, Donnan E, Sketcher-Baker K, Wakefield J, Coulter C. Responding to Mycobacterium chimaera heater-cooler unit contamination: international and national intersectoral collaboration coordinated in the state of Queensland, Australia. J Hosp Infect. (2018) 100:e77–e84. doi: 10.1016/j.jhin.2018.07.024
11. van Ingen J, Kohl TA, Kranzer K, Hasse B, Keller PM, Katarzyna Szafranska A, et al. Global outbreak of severe Mycobacterium chimaera disease after cardiac surgery: a molecular epidemiological study. Lancet Infect Dis. (2017) 17:1033–41. doi: 10.1016/S1473-3099(17)30324-9
12. Zhang X, Lin J, Feng Y, Wang X, McNally A, Zong Z. Identification of Mycobacterium chimaera in heater-cooler units in China. Sci Rep. (2018) 8:7843. doi: 10.1038/s41598-018-26289-5
13. European Centre for Disease Prevention and Control. Invasive Cardiovascular Infection by Mycobacterium chimaera - 30 April 2015. European Centre for Disease Prevention and Control, Stockholm (2015).
14. Safety Communications. Nontuberculous Mycobacterium Infections Associated with Heater-Cooler Devices. FDA Safety Communication. Available online at: http://wayback.archive-it.org/7993/20170722215713/https:/www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm466963.htm (Accessed April 12, 2019).
15. van Ingen J, Turenne CY, Tortoli E, Wallace RJ, Brown-Elliott BA. A definition of the Mycobacterium avium complex for taxonomical and clinical purposes, a review. Int J Syst Evol Microbiol. (2018) 68:3666–77. doi: 10.1099/ijsem.0.003026
16. Tortoli E, Rindi L, Garcia MJ, Chiaradonna P, Dei R, Garzelli C, et al. Proposal to elevate the genetic variant MAC-A, included in the Mycobacterium avium complex, to species rank as Mycobacterium chimaera sp. nov. Int J Syst Evol Microbiol. (2004) 54:1277–85. doi: 10.1099/ijs.0.02777-0
17. Boyle DP, Zembower TR, Reddy S, Qi C. Comparison of clinical features, virulence, and relapse among Mycobacterium avium complex species. Am J Respir Crit Care Med. (2015) 191:1310–7. doi: 10.1164/rccm.201501-0067OC
18. Schweickert B, Goldenberg O, Richter E, Göbel UB, Petrich A, Buchholz P, et al. Occurrence and clinical relevance of Mycobacterium chimaera sp. nov., Germany. Emerging Infect Dis. (2008) 14:1443–6. doi: 10.3201/eid1409.071032
19. Falkinham JO, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl Environ Microbiol. (2001) 67:1225–31. doi: 10.1128/AEM.67.3.1225-1231.2001
20. Wallace RJ, Iakhiaeva E, Williams MD, Brown-Elliott BA, Vasireddy S, Vasireddy R, et al. Absence of Mycobacterium intracellulare and presence of Mycobacterium chimaera in household water and biofilm samples of patients in the United States with Mycobacterium avium complex respiratory disease. J Clin Microbiol. (2013) 51:1747–52. doi: 10.1128/JCM.00186-13
21. Maurer FP, Pohle P, Kernbach M, Sievert D, Hillemann D, Rupp J, et al. Differential drug susceptibility patterns of Mycobacterium chimaera and other members of the Mycobacterium avium-intracellulare complex. Clin Microbiol Infect. (2019) 25:379.e1–e7. doi: 10.1016/j.cmi.2018.06.010
22. Sommerstein R, Hasse B, Marschall J, Sax H, Genoni M, Schlegel M, Widmer AF. Swiss Chimaera taskforce. Global health estimate of invasive Mycobacterium chimaera infections associated with heater-cooler devices in cardiac surgery. Emerging Infect Dis. (2018) 24:576–8. doi: 10.3201/eid2403.171554
23. Sommerstein R, Schreiber PW, Diekema DJ, Edmond MB, Hasse B, Marschall J, et al. Mycobacterium chimaera outbreak associated with heater-cooler devices: piecing the puzzle together. Infect Control Hosp Epidemiol. (2017) 38:103–8. doi: 10.1017/ice.2016.283
24. Kasperbauer SH, Daley CL. Mycobacterium chimaera infections related to the heater-cooler unit outbreak: a guide to diagnosis and management. Clin Infect Dis. (2019) 68:1244–50. doi: 10.1093/cid/ciy789
25. Schreiber PW, Sax H. Mycobacterium chimaera infections associated with heater-cooler units in cardiac surgery. Curr Opin Infect Dis. (2017) 30:388–94. doi: 10.1097/QCO.0000000000000385
26. Rosero CI, Shams WE. Mycobacterium chimaera infection masquerading as a lung mass in a healthcare worker. IDCases. (2019) 15:e00526. doi: 10.1016/j.idcr.2019.e00526
27. Trudzinski FC, Schlotthauer U, Kamp A, Hennemann K, Muellenbach RM, Reischl U, et al. Clinical implications of Mycobacterium chimaera detection in thermoregulatory devices used for extracorporeal membrane oxygenation (ECMO), Germany, 2015 to 2016. Euro Surveill. (2016) 21:30398. doi: 10.2807/1560-7917.ES.2016.21.46.30398
28. Achermann Y, Rössle M, Hoffmann M, Deggim V, Kuster S, Zimmermann DR, et al. Prosthetic valve endocarditis and bloodstream infection due to Mycobacterium chimaera. J Clin Microbiol. (2013) 51:1769–73. doi: 10.1128/JCM.00435-13
29. Lyman MM, Grigg C, Kinsey CB, Keckler MS, Moulton-Meissner H, Cooper E, et al. Invasive nontuberculous mycobacterial infections among cardiothoracic surgical patients exposed to heater-cooler devices1. Emerg Infect Dis. (2017) 23:796–805. doi: 10.3201/eid2305.161899
30. Scriven JE, Scobie A, Verlander NQ, Houston A, Collyns T, Cajic V, et al. Mycobacterium chimaera infection following cardiac surgery in the United Kingdom: clinical features and outcome of the first 30 cases. Clin Microbiol Infect. (2018) 24:1164–70. doi: 10.1016/j.cmi.2018.04.027
31. Lau D, Cooper R, Chen J, Sim VL, McCombe JA, Tyrrell GJ, et al. Mycobacterium chimaera encephalitis post-cardiac surgery: a new syndrome. Clin Infect Dis. (2020) 70:692–5. doi: 10.1093/cid/ciz497
32. Zweifel SA, Mihic-Probst D, Curcio CA, Barthelmes D, Thielken A, Keller PM, et al. Clinical and histopathologic ocular findings in disseminated Mycobacterium chimaera infection after cardiothoracic surgery. Ophthalmology. (2017) 124:178–88. doi: 10.1016/j.ophtha.2016.09.032
33. EU Protocol for Case Detection, Laboratory Diagnosis and Environmental Testing of Mycobacterium chimaera Infections Potentially Associated with Heater-Cooler Units: Case Definition and Environmental Testing Methodology. European Centre for Disease Prevention and Control (2015) Available online at: http://ecdc.europa.eu/en/publications-data/eu-protocol-case-detection-laboratory-diagnosis-and-environmental-testing (Accessed May 25, 2019).
34. Interim Guide for the Identification of Possible Cases of Nontuberculous Mycobacterium Infections Associated with Exposure to Heater-Cooler Units. Centre for Disease Prevention and Control (2016) Available online at: https://www.cdc.gov/hai/outbreaks/heater-cooler.html (Accessed May 25, 2019).
35. Ben Appenheimer A, Diekema DJ, Berriel-Cass D, Crook T, Daley CL, Dobbie D, et al. Mycobacterium chimaera Outbreak Response: experience from four United States healthcare systems. Open Forum Infect Dis. (2016) 3:2392. doi: 10.1093/ofid/ofw195.10
36. Mycobacterium chimaera Infections: Guidance for Secondary Care. GOVUK. Available online at: https://www.gov.uk/government/publications/mycobacterium-chimaera-infections-guidance-for-secondary-care (Accessed May 26, 2019).
37. Lecorche E, Haenn S, Mougari F, Kumanski S, Veziris N, Benmansour H, et al. Comparison of methods available for identification of Mycobacterium chimaera. Clin Microbiol Infect. (2018) 24:409–13. doi: 10.1016/j.cmi.2017.07.031
38. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. (2007) 175:367–416. doi: 10.1164/rccm.200604-571ST
39. Overton K, Mennon V, Mothobi N, Neild B, Martinez E, Masters J, et al. Cluster of invasive Mycobacteria chimaera infections following cardiac surgery demonstrating novel clinical features and risks of aortic valve replacement. Intern Med J. (2018) 48:1514–20. doi: 10.1111/imj.14093
40. Schreiber PW, Hasse B, Sax H. Mycobacterium chimaera infections after cardiac surgery-lessons learned. Clin Microbiol Infect. (2018) 24:1117–8. doi: 10.1016/j.cmi.2018.06.031
41. Mihos CG, Capoulade R, Yucel E, Picard MH, Santana O. Surgical versus medical therapy for prosthetic valve endocarditis: a meta-analysis of 32 studies. Ann Thorac Surg. (2017) 103:991–1004. doi: 10.1016/j.athoracsur.2016.09.083
42. Walker J, Moore G, Collins S, Parks S, Garvey MI, Lamagni T, et al. Microbiological problems and biofilms associated with Mycobacterium chimaera in heater-cooler units used for cardiopulmonary bypass. J Hosp Infect. (2017) 96:209–20. doi: 10.1016/j.jhin.2017.04.014
43. Schreiber PW, Kuster SP, Hasse B, Bayard C, Rüegg C, Kohler P, et al. Reemergence of Mycobacterium chimaera in heater-cooler units despite intensified cleaning and disinfection protocol. Emerging Infect Dis. (2016) 22:1830–3. doi: 10.3201/eid2210.160925
44. Hasse B, Hannan MM, Keller PM, Maurer FP, Sommerstein R, Mertz D, et al. International society of cardiovascular infectious diseases guidelines for the diagnosis, treatment and prevention of disseminated Mycobacterium chimaera infection following cardiac surgery with cardiopulmonary bypass. J Hosp Infect. (2020) 104:214–35. doi: 10.1016/j.jhin.2019.10.009
45. Marra AR, Diekema DJ, Edmond MB. Mycobacterium chimaera infections associated with contaminated heater-cooler devices for cardiac surgery: outbreak management. Clin Infect Dis. (2017) 65:669–74. doi: 10.1093/cid/cix368
46. Moutsoglou DM, Merritt F, Cumbler E. Disseminated Mycobacterium chimaera presenting as vertebral osteomyelitis. Case Rep Infect Dis. (2017) 2017:9893743. doi: 10.1155/2017/9893743
Keywords: HCU, cardiac surgery, non-tuberculous mycobacteria, NTM, spondylodiscitis
Citation: Lecorche E, Pean de Ponfilly G, Mougari F, Benmansour H, Poisnel E, Janvier F and Cambau E (2020) Disseminated Mycobacterium chimaera Following Open-Heart Surgery, the Heater–Cooler Unit Worldwide Outbreak: Case Report and Minireview. Front. Med. 7:243. doi: 10.3389/fmed.2020.00243
Received: 07 October 2019; Accepted: 07 May 2020;
Published: 16 June 2020.
Edited by:
Nicola Petrosillo, Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani (IRCCS), ItalyReviewed by:
Thomas Rogers, Trinity College Dublin, IrelandEmanuele Durante Mangoni, University of Campania Luigi Vanvitelli, Italy
Copyright © 2020 Lecorche, Pean de Ponfilly, Mougari, Benmansour, Poisnel, Janvier and Cambau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emmanuel Lecorche, ZW1tYW51ZWwubGVjb3JjaGVAYXBocC5mcg==
 Emmanuel Lecorche
Emmanuel Lecorche Gauthier Pean de Ponfilly3
Gauthier Pean de Ponfilly3 Frederic Janvier
Frederic Janvier Emmanuelle Cambau
Emmanuelle Cambau