
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Med. , 15 May 2020
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00188
This article is part of the Research Topic Viral Infections In The Intensive Care Unit View all 6 articles
 Jolien Schildermans1†
Jolien Schildermans1† Greet De Vlieger2*†
Greet De Vlieger2*†Cytomegalovirus (CMV) is one of the most pathogenic viruses in human. After a primary infection, CMV resides in the host for life as a latent infection. When immunity is reduced, CMV can escape the suppressive effects of the immune system and lead to viremia and antigenemia. This reactivation, first seen in transplant patients, has also been documented in non-immunocompromised CMV-seropositive critically ill patients and is associated with higher morbidity and mortality. In the latter, it is not clear whether CMV reactivation is an innocent bystander or the cause of this observed worse outcome. Two studies showed no difference in the outcome of CMV-seropositive and seronegative patients. In addition, proof-of-concept studies investigating prophylactic antiviral treatment to prevent CMV reactivation during critical illness, failed to show a beneficial effect on interleukin levels or clinical outcome. Further research is necessary to resolve the question whether CMV replication impairs the prognosis in non-immunocompromised critically ill patients. We here give a concise overview on the available data and propose strategies to further unravel this question. First, post-mortem investigation may be useful to evaluate the effect of viral replication on organ inflammation and function. Second, further research should focus on the question whether the level of viremia needs to exceed a threshold to be associated with worse outcome. Third, clinical and biochemical assessments may help to identify patients at high risk for reactivation. Fourth, preemptive treatment based upon early detection of the virus is currently under investigation. Finally, immune-stimulating biologicals may be beneficial in high-risk groups.
The human cytomegalovirus (CMV) is a beta herpes virus that only infects human after transmission by body fluids such as saliva, blood and urine (1). In immunocompetent hosts, a primary infection frequently passes asymptomatically, although a mononucleosis-like syndrome can develop with fever, myalgia and adenopathy (1). It can be complicated by severe organ disease as colitis, pneumonitis, hepatitis, meningitis and myocarditis and it can trigger a Guillain-Barré syndrome (1). Pathologic examination of infected tissue typically shows “owl's eye inclusions”, a pathognomonic sign of CMV infection (2).
After a primary infection, CMV establishes latency in the body, with the myeloid lineage as main reservoir (1). In developed countries, ~60–80% of the adults have been infected with CMV, and in developing countries incidences rise even to 100%. Risk factors for a latent CMV infection include age, poor socioeconomic status and female gender (3). This latent viral infection is hallmarked by presence of CMV in white blood cells, but with undetectable viral loads because the host defense system suppresses viral replication. Approximately 10% of all circulating CD4+ and CD8+ T cells are involved in this process (4). When immunity is weakened, reactivation can occur and viral antigens spread into the blood (1, 2). This reactivation has first been identified as an important cause of morbidity and mortality in transplant recipients (5) and HIV-infected patients (6); and later it has been increasingly encountered in non-immunocompromised critically ill patients. Here, we will briefly overview the current guidelines to prevent CMV reactivation in transplant recipients, and we will focus in-depth on the available data in critically ill patients.
CMV is the leading viral opportunistic pathogen in immunocompromised patients. In these patients, reactivation can be asymptomatic, when viral replication is detectable without associated symptoms, and is named “CMV infection” (7). When viral replication causes symptoms, it is called “CMV disease.” This disease is subdivided in a “CMV syndrome” when the patient has fever, malaise, leukopenia and/or thrombopenia without organ disease; or in “tissue invasive disease” when organs are involved (7). CMV infection is associated with increased risk of graft failure, high morbidity and mortality, and has been named the “troll” of transplantation as “the virus lurks quietly in the shadows, posed to emerge at any moment and complicate recovery” (8). International guidelines advise to administer antiviral therapy to prevent this reactivation in solid organ and hematopoietic cell transplanted patients (7, 9). Currently, there are two preventive strategies: 1. “prophylaxis” with administration of antiviral medication to patients “at risk”; and 2. “preemptive therapy” with regular monitoring for plasma CMV viral load and administration of antiviral treatment only when a threshold is exceeded (7). This strategy offers the benefit that not all patients are exposed to toxic antiviral treatment. It is recommended to measure the viral load with polymerase chain reaction (PCR) (7). Unfortunately, a lack in standardization of various assays makes it difficult to compare results from different institutes. As from 2010, the World Health Organization published a standard for nucleic acid amplification techniques with the aim to uniform the results and treatment among different care centers (10). However, the threshold above which viral replication should be treated preemptively is still unknown and remains a matter of debate (7).
Assessing the risk for reactivation is necessary to decide which preventive strategy should be applied. This assessment includes the serostatus of donor and receptor, the transplanted organ and the immunosuppressive strategy. Lung transplant recipients and CMV-naive patients who received a CMV infected organ are at highest risk of reactivation and in those, guidelines advise to use the prophylactic strategy. Consequently, no recent data are available on the reactivation rate when no prophylaxis is administered in high-risk patients. In a mixed group of kidney, lung, liver and heart transplant recipients who received preemptive treatment, the incidence of CMV viremia and CMV disease was 48.9 and 6.9%, respectively (11).
For many years, sepsis-induced hyperinflammation has been blamed for the high early mortality in septic patients. Nevertheless, strategies that block this response have been unsuccessful and have even caused additional harm (12). Consequently, these treatments have been largely abandoned. Meanwhile, new insights uncovered that also anti-inflammatory adaptations are engendered and this even at the very early start of critical illness. Indeed, there is also an increase in circulating anti-inflammatory cytokines and the adaptive immune system is affected, as reflected by a reduced human leucocyte antigen DR expression, increased lymphocyte apoptosis and a reduced capacity of lymphocytes to produce cytokines (13). These alterations persist after the acute phase, and are more apparent in this prolonged phase of critical illness when this “immunoparalysis” translates into an increased susceptibility to opportunistic infections such as opportunistic bacterial infections, invasive fungal infections and reactivation of viruses. Many herpes viruses reactivate during critical illness, but CMV is associated with the worst outcome (14). Consequently, CMV reactivation during critical illness has been increasingly studied during last decades.
More than two decades ago, Papazian reported biopsy-proven CMV pneumonitis in 29% to 50% of the intensive care unit (ICU) patients with acute respiratory failure (15–17). Since then, many observational studies have documented CMV reactivation (14, 18–42) (Table 1).
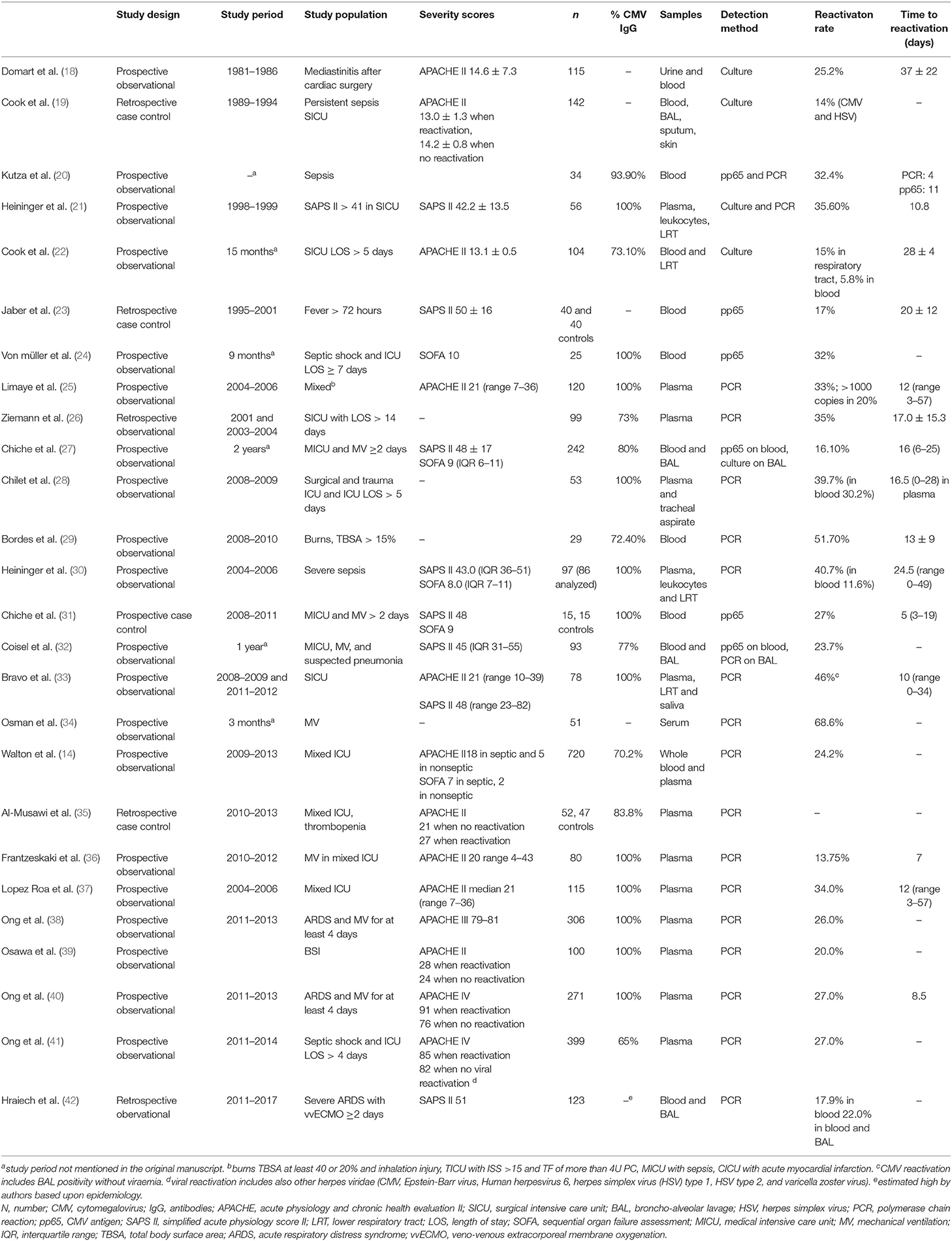
Table 1. Overview of the rate and timing of CMV reactivation in observational studies in critically ill patients.
The reactivation rate depends on the studied population and on the detection method. Early reports used viral cultures, but these are less sensitive and slower than antigen and PCR testing. Antigen detection has a good sensitivity in non-leukopenic patients, but as the test is performed on white blood cells, it is not accurate when patients are leukopenic (7). Nowadays, PCR is the preferred detection method. The highest reactivation rates have been observed in patients with sepsis (21, 43), and there are several biological explanations: CMV reactivation is triggered by cytokines as tumor necrosis factor α and interleukin-1β (44), by endotoxins (45); and by endogenous and exogenous catecholamines (46). Administration of steroids is also associated with higher reactivation rates (22, 23, 40). Finally, the risk increases with higher severity of illness (40, 43).
In critically ill patients, CMV reactivation is associated with prolonged stay in the ICU and in hospital (18, 21–28, 30–33, 47), increased risk for infections (14, 22, 23, 27, 32), prolonged need for mechanical ventilation (MV) (22–24, 26, 27, 30–33), and doubled mortality (19, 22, 23, 26, 32, 34, 35, 40, 41, 43, 47) (Table 2).
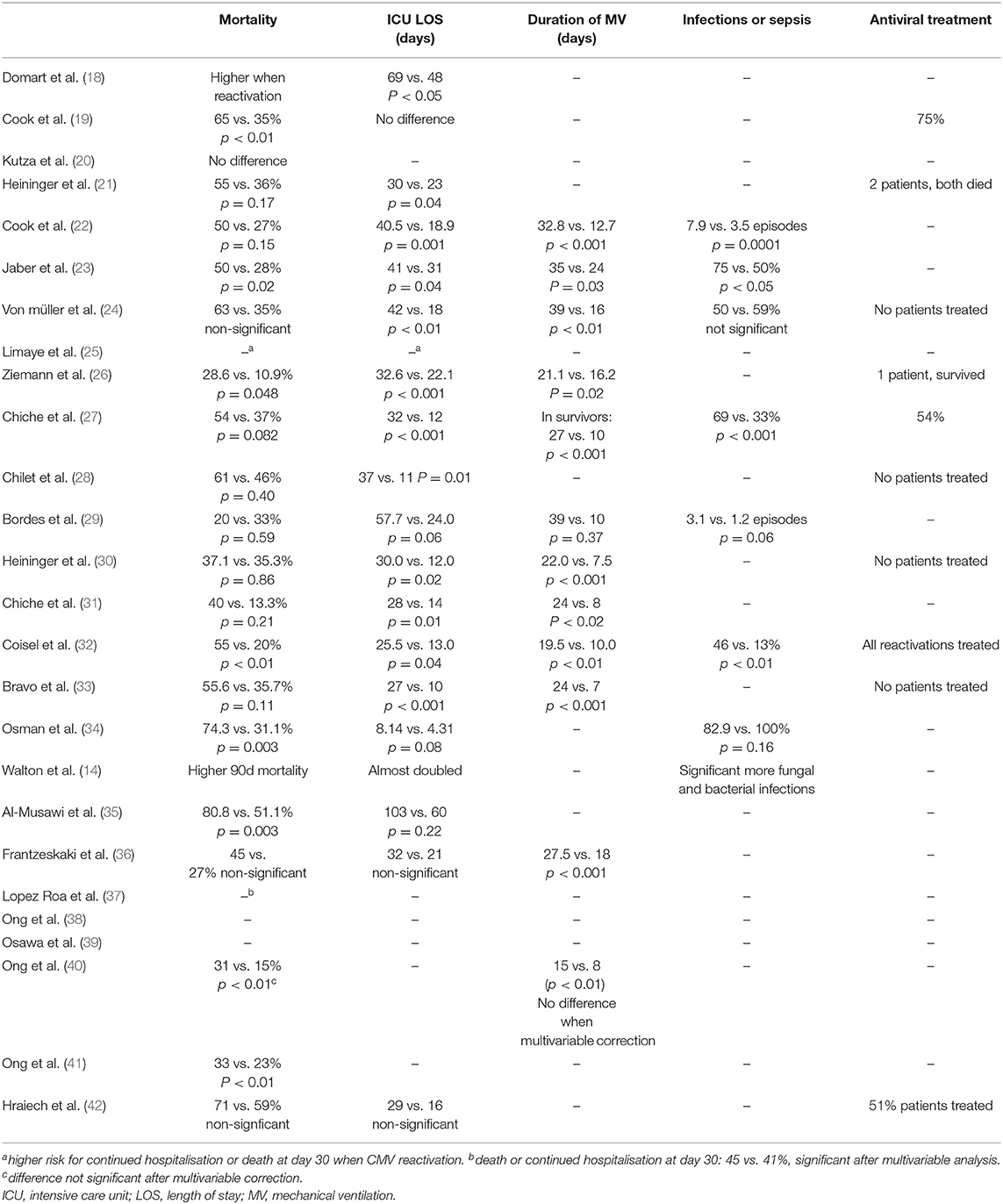
Table 2. Overview of the outcome in CMV reactivating and non-reactivating critically ill patients and the rate of antiviral treatment in observational studies.
Three observational studies reported antiviral treatment in patients with CMV reactivation (19, 27, 32), and in two of them mortality was higher in CMV reactivating than in non-reactivating patients (19, 32). In the study that treated 54% of the patients, treated patients had a non-significant higher mortality than non-treated patients (62 vs. 44%, respectively) (27). As the decision to start antiviral treatment was left to the physician's decision, it is possible that the sickest patients received antivirals more often, which may explain the higher mortality. Indeed, data from observational trials cannot resolve the question whether CMV is a pathogen or bystander of the observed worse outcome. Some experts suggest interventional trials with antiviral treatment to address this question (48–50). It has been shown that CMV replication occurs only in CMV seropositive patients (43), and as such, the presence of CMV antibodies represents a good entry criterion for interventional studies. This theory has been challenged by De Vlieger et al. (51) who investigated the association between CMV seropositivity at ICU admission and outcome. In this study, involving over 1,500 patients with an ICU stay of 3 days or more, no association was found between CMV serostatus and ICU outcome. In addition, there was no difference in ICU or hospital mortality in subgroups with prolonged ICU stay, sepsis, or higher disease severity (51). Ong et al. (38) extended these results in over 300 mechanically ventilated patients with acute respiratory distress syndrome (ARDS), as they found no association between CMV seropositivity and the number of days alive and free of mechanical ventilation (MV) on day 28. However, in a post-hoc defined subgroup of ARDS induced by septic shock, they found an improved outcome in CMV-seronegative as compared to seropositive patients (38). One possible explanation is a higher reactivation rate in patients with sepsis-induced ARDS as compared to the overall ARDS patients (34 vs. 27%, respectively) (40).
In a model with cecal ligation and puncture (CLP), all CMV seropositive mice showed viral reactivation in the lungs, liver, spleen and salivary glands (52). Administration of ganciclovir to prevent this reactivation did not reduce mortality, but shortened the CLP-induced inflammatory response to a duration seen in CMV seronegative mice (53). In addition, lung biopsies showed that blocking the viral replication with antivirals resulted in less fibrosis as compared to untreated mice (53). The results of these studies gave the starting signal for interventional trials in critically ill human.
Recently, two proof-of-concept studies in ICU patients have been performed (54, 55). The Cytomegalovirus Control in Critical Care (CCCC-trial) was an interventional trial in CMV seropositive patients who were mechanically ventilated for at least 24 h. The patients received valaciclovir, valganciclovir, or no treatment. The study was stopped early because a higher mortality was observed in the intervention arms (54).
A second randomized controlled trial compared the effect of ganciclovir to placebo on the evolution of interleukin-6 levels in CMV seropositive patients with sepsis or trauma and respiratory failure (GRAIL-trial) (55). Reactivation occurred more frequently in the ganciclovir group (11.9%) than in the valganciclovir group of the CCCC trial (2.2%). This may be explained by the higher risk of the studied population, as 88% had sepsis upon admission and reactivation in blood occurred in 38.9% of the placebo-treated patients as compared to 27.3% of the non-treated patients in the CCCC trial. In the GRAIL trial, the rate of CMV replication was significantly reduced in the ganciclovir arm, but the evolution of IL-6 did not differ in the two groups (55). This primary outcome was chosen because IL-6 correlated with outcome in a post-hoc analysis of the ARDS network trial (56). Later, it has been shown that the evolution of IL-6 and other cytokines was similar in CMV reactivating patients and a matched cohort of non-reactivating patients, and thus may be not a good surrogate endpoint (57). Interestingly, the GRAIL trail showed that ganciclovir-treated patients had significantly more ventilator free days at 28 days than placebo-treated patients in the subgroup of sepsis-induced respiratory failure (55).
A third randomized controlled trial is investigating preemptive treatment for CMV reactivation in critically ill patients. This study has also evaluated the effect of treating herpes simplex virus oropharyngeal reactivation in mechanically ventilated patients, and found no increase in the ventilator-free days at day 60 as compared to placebo. However, there was a trend to a reduced mortality and a significant increase in the time-to-event analysis for mortality in the intervention arm (58). The results whether preemptive treatment of CMV reactivation is able to improve outcome are expected soon (NCT02152358).
CMV reactivation was first reported in transplant recipients, and it has been increasingly documented in critically ill patients in whom it is associated with high morbidity and mortality. Several years have passed and experts are still debating whether CMV is the culprit or a simple bystander (50, 59). CMV serology was not associated with worse outcome in a large group of patients without additional risk factors, nor in patients with sepsis, prolonged ICU stay or higher severity scores (51). Moreover, two interventional studies have shown negative results and one was even stopped early because of higher mortality in patients who received antiviral treatment (54, 55). The second study showed an increase in ventilator-free days at day 28 in patients with sepsis (55). Although this was a secondary endpoint in a subgroup, it warrants further research. Now is the time to plan an optimal strategy, as new interventional trials in the overall CMV seropositive patients will probably not solve the question and may even induce unnecessary harm. There are several considerations that we must take into account.
First, identification of subgroups at high risk for reactivation is a crucial step to select a target population for future interventional trials, as unnecessary treatment in the patients in whom reactivation does not occur is likely to cause harm without adding benefit. A metaanalysis published in 2009 showed that the rate of reactivation in CMV seropositive patients was 36% with PCR or antigen detection (43). When studies until 2016 were evaluated, reactivation occurred in 31% of the CMV-seropositive patients when PCR was used (60). Thus, CMV reactivation seems to occur less frequently in the studies that were published after 2009 even though the studied patients had additional risk factors such as sepsis or prolonged mechanical ventilation (36, 38, 40, 41). Steroids are frequently administered in septic shock and ARDS and have been identified as risk factor for reactivation (22, 23, 40). Since 2009, the use of steroids has reduced over the time, as the CORTICUS trial showed no beneficial role of steroids in septic patients (61). In addition, other strategies to reduce the hyperinflammatory response have also been abandoned (12). Consequently, it is very likely that the rate of CMV reactivation has changed over time. New, multicenter, observational trials are needed to reevaluate the current incidence of reactivation. It is unlikely that reactivation rates will reach the 49% that has been seen in preemptively treated solid organ transplant recipients (11). As such, critically ill patients have a lower risk of reactivation and will benefit less from prophylactic antiviral treatment than transplanted patients.
Second, there is no consensus definition of reactivation and while many authors report data on viremia, others include viral detection in the respiratory tract (27, 28, 30, 32, 33, 42). Further evaluation of the impact of CMV detection in respiratory samples after transplantation has also been put on the agenda as an urgent Research Topic (7).
Third, it is unknown whether the level of viremia needs to exceed a threshold to be harmful. This has also been overthought in transplant recipients, but data on the untreated history and outcome of viral replication after transplantation are lacking as prevention is generally accepted in these patients (7). In critically ill patients, Limaye et al. (25) showed that the risk for prolonged hospitalization increased when the viral load increases and Bordes et al. (29) found worse outcome in burn patients when viral load exceeded 1,000 copies per milliliter as compared to those with low viral load reactivation. Post-mortem examination may be especially helpful to evaluate whether organ damage occurs above a threshold. More than two decades ago, Papazian found CMV inclusions, a pathognomonic sign of CMV disease, in patients with acute respiratory failure (16). At that time, packed cells were not leukoreduced (62) and it is possible that those patients had a transfusion-related primary infection rather than reactivation. Indeed, recent autopsy studies in critically ill patients have not reported CMV disease in non-immunocompromised patients (63–65). While this may indicate that CMV does not invade organs, another explanation may be the short length of stay (median ICU day 2 or 3) in these autopsy studies while CMV reactivation generally only occurs later during critical illness. It would be interesting to evaluate whether CMV reactivation leads to organ disease and, if so, whether this is related to the viral load.
Fourth, as CMV reactivation is likely caused by a reduced immune response, a strategy that assesses the immunologic response may be helpful to predict reactivation. One possible marker of upcoming reactivation is the interferon-γ (IFN-γ) production by CMV-specific T lymphocytes upon exposure to CMV-antigens. Higher CMV reactivation rates have been documented in critically ill patients in whom this response was lacking (31, 66). Recently, a commercial test to measure this response has become available (CMV-QuantiFERON, Qiagen). In solid organ transplant patients, a low CMV-QuantiFERON-response was associated with a higher risk to develop CMV infection (67, 68). Few studies have evaluated the test in critically ill patients (69). Nowadays, the test is not used in clinical setting, but randomized controlled trials in solid organ transplant recipients are investigating whether this test is useful to individualize the duration of antiviral treatment.
Last, immune stimulating biologicals have been suggested to treat infectious complications in ICU patients. This strategy may be able to target all opportunistic infections that have been typically observed in these patients. In a normally functioning immune system, endogenous immune components act as control mechanisms and evaluate the immune system at several checkpoints (70). These pathways are altered in cancer and sepsis, and these alterations are associated with a worse prognosis (71). In cancer patients, interventions aiming to restore this response have shown to reduce the tumor load and are licensed for therapeutic use (70). The immunological adaptations that occur in critically ill patients have many similarities with the alterations seen in cancer patients (72). Based upon these findings, successful treatments in oncological patients may also improve outcome in critically ill patients. Products that are under investigation in sepsis and/or ARDS are granulocyte-macrophage colony-stimulating factor (73–75), anti-programmed cell death 1 antibodies, anti-programmed cell death ligand 1 antibodies (anti-PD-L1) (76), recombinant INFγ (77), and recombinant human interleukin-7 (78). Patients with CMV reactivation have been suggested good candidates for immune-enhancing therapy (12), but to the best of our knowledge there are currently no trials focusing on these patients.
In conclusion, CMV is one of the most pathogenic viruses in human, and observational reports have described the rate, risk factors and associated outcome of CMV reactivation in critically ill patients. Despite repetitively documented higher morbidity and mortality associated with viral reactivation, it remains to be elucidated whether this is association is causal. Two recent randomized controlled prophylactic trials with a proof-of-concept design were not conclusive. To our opinion, further research should first focus on identifying patients at high risk for reactivation and on the level or viremia, which may indicate patient in whom antiviral prophylaxis may affect outcome. Until then, prophylactic trials are likely to be inconclusive and may induce unnecessary harm.
JS drafted the manuscript. GD critically revised the manuscript and added important research perspectives.
GD received funding of the FWO (Fond Wetenschappelijk Onderzoek, Flemish Government, Belgium) under grant number 1701719N. The funder was not involved in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We thank Yves Debaveye for editing the manuscript.
1. Gandhi MK, Khanna R. Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis. (2004) 4:725–38. doi: 10.1016/S1473-3099(04)01202-2
2. Griffiths P, Baraniak I, Reeves M. The pathogenesis of human cytomegalovirus. J Pathol. (2015) 235:288–97. doi: 10.1002/path.4437
3. Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. (2010) 20:202–13. doi: 10.1002/rmv.655
4. Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. (2005) 202:673–85. doi: 10.1084/jem.20050882
5. Hill RB Jr, Rowlands DT Jr, Rifkind D. Infectious pulmonary disease in patients receiving immunosuppressive therapy for organ transplantation. N Engl J Med. (1964) 271:1021–7. doi: 10.1056/NEJM196411122712001
6. Deayton JR, Sabin CA, Johnson MA, Emery VC, Wilson P, Griffiths PD. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet. (2004) 363:2116–21. doi: 10.1016/S0140-6736(04)16500-8
7. Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. The transplantation society international, the third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. (2018) 102:900–31. doi: 10.1097/TP.0000000000002191
8. Balfour HH Jr. Cytomegalovirus: the troll of transplantation. Arch Intern Med. (1979) 139:279–80. doi: 10.1001/archinte.139.3.279
9. Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, et al. Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. (2019) 19:e260–72. doi: 10.1016/S1473-3099(19)30107-0
10. Fryer J, Heath A, Anderson R, Minor P, Collaborative Study Group. Collaborative study to evaluate the proposed 1st WHO international standard for human cytomegalovirus (HCMV) for nucleic acid amplification Technology. Biologicals. (2016) 44:242–51. doi: 10.1016/j.biologicals.2016.04.00
11. Natori Y, Alghamdi A, Tazari M, Miller V, Husain S, Komatsu T, et al. Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis. Clin Infect Dis. (2018) 66:617–31. doi: 10.1093/cid/cix793
12. Hotchkiss RS, Opal S. Immunotherapy for sepsis–a new approach against an ancient foe. N Engl J Med. (2010) 363:87–9. doi: 10.1056/NEJMcibr1004371
13. Greathouse KC, Hall MW. Critical illness-induced immune suppression: current state of the science. Am J Crit Care. (2016) 25:85–92. doi: 10.4037/ajcc2016432
14. Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, et al. Reactivation of multiple viruses in patients with sepsis. PLoS ONE. (2014) 9:e98819. doi: 10.1371/journal.pone.0098819
15. Papazian L, Fraisse A, Garbe L, Zandotti C, Thomas P, Saux P, et al. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. (1996) 84:280–7. doi: 10.1097/00000542-199602000-00005
16. Papazian L, Thomas P, Bregeon F, Garbe L, Zandotti C, Saux P, et al. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology. (1998) 88:935–44. doi: 10.1097/00000542-199804000-00013
17. Papazian L, Doddoli C, Chetaille B, Gernez Y, Thirion X, Roch A, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. (2007) 35:755–62. doi: 10.1097/01.CCM.0000257325.88144.30
18. Domart Y, Trouillet JL, Fagon JY, Chastre J, Brun-Vezinet F, Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery. Chest. (1990) 97:18–22. doi: 10.1378/chest.97.1.18
19. Cook CH, Yenchar JK, Kraner TO, Davies EA, Ferguson RM. Occult herpes family viruses may increase mortality in critically ill surgical patients. Am J Surg. (1998) 176:357–60. doi: 10.1016/S0002-9610(98)00205-0
20. Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. (1998) 26:1076–82. doi: 10.1086/520307
21. Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. (2001) 29:541–7. doi: 10.1097/00003246-200103000-00012
22. Cook CH, Martin LC, Yenchar JK, Lahm MC, McGuinness B, Davies EA, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. (2003) 31:1923–9. doi: 10.1097/01.CCM.0000070222.11325.C4
23. Jaber S, Chanques G, Borry J, Souche B, Verdier R, Perrigault PF, et al. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. (2005) 127:233–41. doi: 10.1378/chest.127.1.233
24. von Muller L, Klemm A, Weiss M, Schneider M, Suger-Wiedeck H, Durmus N, et al. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. (2006) 12:1517–22. doi: 10.3201/eid1210.060411
25. Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. (2008) 300:413–22. doi: 10.1001/jama.2008.697
26. Ziemann M, Sedemund-Adib B, Reiland P, Schmucker P, Hennig H. Increased mortality in long-term intensive care patients with active cytomegalovirus infection. Crit Care Med. (2008) 36:3145–50. doi: 10.1097/CCM.0b013e31818f3fc4
27. Chiche L, Forel JM, Roch A, Guervilly C, Pauly V, Allardet-Servent J, et al. Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med. (2009) 37:1850–7. doi: 10.1097/CCM.0b013e31819ffea6
28. Chilet M, Aguilar G, Benet I, Belda J, Tormo N, Carbonell JA, et al. Virological and immunological features of active cytomegalovirus infection in nonimmunosuppressed patients in a surgical and trauma intensive care unit. J Med Virol. (2010) 82:1284–91. doi: 10.1002/jmv.21825
29. Bordes J, Maslin J, Prunet B, d'Aranda E, Lacroix G, Goutorbe P, et al. Cytomegalovirus infection in severe burn patients monitoring by real-time polymerase chain reaction: A prospective study. Burns. (2011) 37:434–9. doi: 10.1016/j.burns.2010.11.006
30. Heininger A, Haeberle H, Fischer I, Beck R, Riessen R, Rohde F, et al. Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care. (2011) 15:R77. doi: 10.1186/cc10069
31. Chiche L, Forel JM, Thomas G, Farnarier C, Cognet C, Guervilly C, et al. Interferon-γ production by natural killer cells and cytomegalovirus in critically ill patients. Crit Care Med. (2012) 40:3162–9. doi: 10.1097/CCM.0b013e318260c90e
32. Coisel Y, Bousbia S, Forel JM, Hraiech S, Lascola B, Roch A, et al. Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS ONE. (2012) 7:e51340. doi: 10.1371/journal.pone.0051340
33. Bravo D, Clari MA, Aguilar G, Belda J, Gimenez E, Carbonell JA, et al. Looking for biological factors to predict the risk of active cytomegalovirus infection in non-immunosuppressed critically ill patients. J Med Virol. (2014) 86:827–33. doi: 10.1002/jmv.23838
34. Osman N, Sayes N, Abdel-Rahman S, Hamza S, Abd Al Aziz A. The impact of cytomegalovirus infection on mechanically ventilated patients in the respiratory and geriatric intensive care unitis. Egypt J Chest Dis Tuberc. (2014) 63:239–45. doi: 10.1016/j.ejcdt.2013.09.022
35. Al-Musawi T, Khawaldeh A, Ghoneim A, Sari M, Aldarweesh M. Cytomegalovirus Infection in non-immunocompromised critically ill patients. Eur Conf Clin Microbiol Infect Dis. (2014) 5:571–9. doi: 10.3855/jidc.1487
36. Frantzeskaki FG, Karampi ES, Kottaridi C, Alepaki M, Routsi C, Tzanela M, et al. Cytomegalovirus reactivation in a general, nonimmunosuppressed intensive care unit population: incidence, risk factors, associations with organ dysfunction, and inflammatory biomarkers. J Crit Care. (2015) 30:276–81. doi: 10.1016/j.jcrc.2014.10.002
37. Lopez Roa P, Hill JA, Kirby KA, Leisenring WM, Huang ML, Santo TK, et al. Coreactivation of human herpesvirus 6 and cytomegalovirus is associated with worse clinical outcome in critically ill adults. Crit Care Med. (2015) 43:1415–22. doi: 10.1097/CCM.0000000000000969
38. Ong DS, Klein Klouwenberg PM, Verduyn Lunel FM, Spitoni C, Frencken JF, Dekker HA, et al. Cytomegalovirus seroprevalence as a risk factor for poor outcome in acute respiratory distress syndrome*. Crit Care Med. (2015) 43:394–400. doi: 10.1097/CCM.0000000000000712
39. Osawa R, Wagener M, Singh N. Cytomegalovirus infection in patients with sepsis due to bloodstream infections: lower risk and better outcomes in new versus already hospitalised intensive care unit admissions. Anaesth Intensive Care. (2016) 44:571–80. doi: 10.1177/0310057X1604400514
40. Ong DSY, Spitoni C, Klein Klouwenberg PMC, Verduyn Lunel FM, Frencken JF, Schultz MJ, et al. Cytomegalovirus reactivation and mortality in patients with acute respiratory distress syndrome. Intensive Care Med. (2016) 42:333–41. doi: 10.1007/s00134-015-4071-z
41. Ong DSY, Bonten MJM, Spitoni C, Verduyn Lunel FM, Frencken JF, Horn J, et al. Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis. (2017) 64:1204–10. doi: 10.1093/cid/cix120
42. Hraiech S, Bonnardel E, Guervilly C, Fabre C, Loundou A, Forel JM, et al. Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann Intensive Care. (2019) 9:142. doi: 10.1186/s13613-019-0616-6
43. Kalil AC, Florescu DF. Prevalence and mortality associated with cytomegalovirus infection in nonimmunosuppressed patients in the intensive care unit. Crit Care Med. (2009) 37:2350–8. doi: 10.1097/CCM.0b013e3181a3aa43
44. Stein J, Volk HD, Liebenthal C, Kruger DH, Prosch S. Tumour necrosis factor alpha stimulates the activity of the human cytomegalovirus major immediate early enhancer/promoter in immature monocytic cells. J Gen Virol. (1993) 74:2333–8. doi: 10.1099/0022-1317-74-11-2333
45. Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol. (2006) 80:9151–8. doi: 10.1128/JVI.00216-06
46. Prosch S, Wendt CE, Reinke P, Priemer C, Oppert M, Kruger DH, et al. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology. (2000) 272:357–65. doi: 10.1006/viro.2000.0367
47. Lachance P, Chen J, Featherstone R, Sligl WI. Association between cytomegalovirus reactivation and clinical outcomes in immunocompetent critically ill patients: a systematic review and meta-analysis. Open Forum Infect Dis. (2017) 4:ofx029. doi: 10.1093/ofid/ofx029
48. Cook CH. Cytomegalovirus reactivation and mortality during critical illness: a $64,000 question. Crit Care Med. (2009) 37:2475–6. doi: 10.1097/CCM.0b013e3181ad932e
49. Limaye AP, Boeckh M. CMV in critically ill patients: pathogen or bystander? Rev Med Virol. (2010) 20:372–9. doi: 10.1002/rmv.664
50. Forel JM, Martin-Loeches I, Luyt CE. Treating HSV and CMV reactivations in critically ill patients who are not immunocompromised: pro. Intensive Care Med. (2014) 40:1945–9. doi: 10.1007/s00134-014-3445-y
51. De Vlieger G, Meersseman W, Lagrou K, Wouters P, Wilmer A, Peetermans WE, et al. Cytomegalovirus serostatus and outcome in nonimmunocompromised critically ill patients. Crit Care Med. (2012) 40:36–42. doi: 10.1097/CCM.0b013e31822b50ae
52. Cook CH, Zhang Y, McGuinness BJ, Lahm MC, Sedmak DD, Ferguson RM. Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice. J Infect Dis. (2002) 185:1395–400. doi: 10.1086/340508
53. Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med. (2006) 34:342–9. doi: 10.1097/01.CCM.0000201876.11059.05
54. Cowley NJ, Owen A, Shiels SC, Millar J, Woolley R, Ives N, et al. Safety and efficacy of antiviral therapy for prevention of cytomegalovirus reactivation in immunocompetent critically ill patients: a randomized clinical trial. JAMA Intern Med. (2017) 177:774–83. doi: 10.1001/jamainternmed.2017.0895
55. Limaye AP, Stapleton RD, Peng L, Gunn SR, Kimball LE, Hyzy R, et al. Effect of ganciclovir on il-6 levels among cytomegalovirus-seropositive adults with critical illness: a randomized clinical trial. JAMA. (2017) 318:731–40. doi: 10.1001/jama.2017.10569
56. Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. (2005) 33:1–6. doi: 10.1097/01.CCM.0000149854.61192.DC
57. van de Groep K, Nierkens S, Cremer OL, Peelen LM, Klein Klouwenberg PMC, Schultz MJ, et al. Effect of cytomegalovirus reactivation on the time course of systemic host response biomarkers in previously immunocompetent critically ill patients with sepsis: a matched cohort study. Crit Care. (2018) 22:348. doi: 10.1186/s13054-018-2261-0
58. Luyt CE, Forel JM, Hajage D, Jaber S, Cayot-Constantin S, Rimmele T, et al. Preemptive treatment for herpesviridae study group, acyclovir for mechanically ventilated patients with herpes simplex virus oropharyngeal reactivation: a randomized clinical trial. JAMA Intern Med. (2020) 180:263–72. doi: 10.1001/jamainternmed.2019.5713
59. Chanques G, Jaber S. Treating HSV and CMV reactivations in critically ill patients who are not immunocompromised: con. Intensive Care Med. (2014) 40:1950–3. doi: 10.1007/s00134-014-3521-3
60. Li X, Huang Y, Xu Z, Zhang R, Liu X, Li Y, et al. Cytomegalovirus infection and outcome in immunocompetent patients in the intensive care unit: a systematic review and meta-analysis. BMC Infect Dis. (2018) 18:289. doi: 10.1186/s12879-018-3195-5
61. Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. (2008) 358:111–24. doi: 10.1056/NEJMoa071366
62. Bassuni WY, Blajchman MA, Al-Moshary MA. Why implement universal leukoreduction? Hematol Oncol Stem Cell Ther. (2008) 1:106–23. doi: 10.1016/S1658-3876(08)50042-2
63. Tejerina EE, Padilla R, Abril E, Frutos-Vivar F, Ballen A, Rodríguez-Barbero JM, et al. Autopsy-detected diagnostic errors over time in the intensive care unit. Hum Pathol. (2018) 76:85–90. doi: 10.1016/j.humpath.2018.02.025
64. Maris C, Martin B, Creteur J, Remmelink M, Piagnerelli M, Salmon I, et al. Comparison of clinical and post-mortem findings in intensive care unit patients. Virchows Arch. (2007) 450:329–33. doi: 10.1007/s00428-006-0364-5
65. Winters B, Custer J, Galvagno SM, Colantuoni E, Kapoor SG, Lee H, et al. Diagnostic errors in the intensive care unit: a systematic review of autopsy studies. BMJ Qual Saf. (2012) 21:894–902. doi: 10.1136/bmjqs-2012-000803
66. Clari MA, Aguilar G, Benet I, Belda J, Gimenez E, Bravo D, et al. Evaluation of cytomegalovirus (CMV)-specific T-cell immunity for the assessment of the risk of active CMV infection in non-immunosuppressed surgical and trauma intensive care unit patients. J Med Virol. (2013) 85:1802–10. doi: 10.1002/jmv.23621
67. Deborska-Materkowska D, Perkowska-Ptasinska A, Sadowska A, Gozdowska J, Ciszek M, Serwanska-Swietek M, et al. Diagnostic utility of monitoring cytomegalovirus-specific immunity by QuantiFERON-cytomegalovirus assay in kidney transplant recipients. BMC Infect Dis. (2018) 18:179. doi: 10.1186/s12879-018-3075-z
68. Cantisan S, Lara R, Montejo M, Redel J, Rodriguez-Benot A, Gutierrez-Aroca J, et al. Pretransplant interferon-γ secretion by CMV-specific CD8+ T cells informs the risk of CMV replication after transplantation. Am J Transplant. (2013) 13:738–45. doi: 10.1111/ajt.12049
69. Caston JJ, Cantisan S, Gonzalez-Gasca F, Paez-Vega A, Abdel-Hadi H, Illescas S, et al. Interferon-γ production by CMV-specific CD8+ T lymphocytes provides protection against cytomegalovirus reactivation in critically ill patients. Intensive Care Med. (2016) 42:46–53. doi: 10.1007/s00134-015-4077-6
70. Boussiotis VA. Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med. (2016) 375:1767–78. doi: 10.1056/NEJMra1514296
71. Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. (2011) 15:R99. doi: 10.1186/cc10112
72. Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. (2018) 14:121–37. doi: 10.1038/nrneph.2017.165
73. Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. (2009) 180:640–8. doi: 10.1164/rccm.200903-0363OC
74. Paine R, Standiford TJ, Dechert RE, Moss M, Martin GS, Rosenberg AL, et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. (2012) 40:90–7. doi: 10.1097/CCM.0b013e31822d7bf0
75. Pinder EM, Rostron AJ, Hellyer TP, Ruchaud-Sparagano MH, Scott J, Macfarlane JG, et al. Randomised controlled trial of GM-CSF in critically ill patients with impaired neutrophil phagocytosis. Thorax. (2018) 73:918–25. doi: 10.1136/thoraxjnl-2017-211323
76. Hotchkiss RS, Colston E, Yende S, Angus DC, Moldawer LL, Crouser ED, et al. Immune checkpoint inhibition in sepsis: a phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (BMS-936559). Crit Care Med. (2019) 47:632–42. doi: 10.1097/CCM.0000000000003685
77. Delsing CE, Gresnigt MS, Leentjens J, Preijers F, Frager FA, Kox M, et al. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: a case series. BMC Infect Dis. (2014) 14:166. doi: 10.1186/1471-2334-14-166
Keywords: herpes virus, cytomegalovirus, reactivation, critical illness, immunoparalysis, sepsis
Citation: Schildermans J and De Vlieger G (2020) Cytomegalovirus: A Troll in the ICU? Overview of the Literature and Perspectives for the Future. Front. Med. 7:188. doi: 10.3389/fmed.2020.00188
Received: 26 November 2019; Accepted: 20 April 2020;
Published: 15 May 2020.
Edited by:
David Ong, Sint Franciscus Gasthuis, NetherlandsReviewed by:
Louis Yi Ann Chai, National University Health System, SingaporeCopyright © 2020 Schildermans and De Vlieger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Greet De Vlieger, Z3JlZXQuZGV2bGllZ2VyQHV6bGV1dmVuLmJl
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.