
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 27 March 2020
Sec. Nephrology
Volume 7 - 2020 | https://doi.org/10.3389/fmed.2020.00099
This article is part of the Research Topic Frailty and Sarcopenia in Various Cachectic Kidney Diseases View all 6 articles
 Jia-Feng Chang1,2,3,4,5,6
Jia-Feng Chang1,2,3,4,5,6 Chang-Chin Wu7,8
Chang-Chin Wu7,8 Chih-Yu Hsieh6
Chih-Yu Hsieh6 Yen-Yao Li9,10
Yen-Yao Li9,10 Ting-Ming Wang11
Ting-Ming Wang11 Jian-Chiun Liou12*
Jian-Chiun Liou12*Background: Cardiac sympathetic response (CSR) and malnutrition-inflammation syndrome (MIS) score are validated assessment tools for patients' health condition. We aim to evaluate the joint effect of CSR and MIS on all-cause and cardiovascular (CV) mortality in patients with hemodialysis (HD).
Methods: Changes in normalized low frequency (ΔnLF) during HD were utilized for quantification of CSR. Unadjusted and adjusted hazard ratios (aHRs) of mortality risks were analyzed in different groups of ΔnLF and MIS score.
Results: In multivariate analysis, higher ΔnLF was related to all-cause, CV and sudden cardiac deaths [aHR: 0.78 (95% confidence interval (CI): 0.72–0.85), 0.78 (95% CI: 0.70–0.87), and 0.74 (95% CI: 0.63–0.87), respectively]. Higher MIS score was associated with incremental risks of all-cause, CV and sudden cardiac deaths [aHR: 1.36 (95% CI: 1.13–1.63), 1.33 (95% CI: 1.06 – 1.38), and 1.50 (95% CI: 1.07–2.11), respectively]. Patients with combined lower ΔnLF (≤6.8 nu) and higher MIS score were at the greatest risk of all-cause and CV mortality [aHR: 5.64 (95% CI: 1.14–18.09) and 5.86 (95% CI: 1.64–13.65), respectively].
Conclusion: Our data indicate a joint evaluation of CSR and MIS score to identify patients at high risk of death is more comprehensive and convincing. Considering the extremely high prevalence of cardiac autonomic neuropathy and malnutrition-inflammation cachexia in HD population, a non-invasive monitoring system composed of CSR analyzer and MIS score calculator should be developed in the artificial intelligence-based prediction of clinical events.
Interest is increasing rapidly in the application of non-invasive monitoring systems and artificial intelligence (AI)-based decision support technologies to predict clinical events in patients undergoing maintenance hemodialysis (HD), such as sudden death, emergency visit, muscle spasm, and hospitalization (1, 2). Cardiovascular (CV) diseases still top the list as the leading causes of fatal events in HD patients worldwide (3–5). Over time, the major causes of CV death shift from acute myocardial infarction and heart failure to sudden cardiac death (SCD) (6). The impaired cardiac sympathetic response (CSR) highly prevalent among HD patients is intricately involved in intradialytic hypotension and SCD (7–9). Meanwhile, diabetic autonomic neuropathy (DAN), a serious complication of diabetes mellitus (DM), plays a pivotal role in the pathogenesis of cardiac autonomic neuropathy (CAN) (10). Since end-stage renal disease (ESRD) is most often caused by DM, the heart rate variability (HRV) used for quantification of CAN serves as a valuable non-invasive monitoring tool for patient safety (11, 12).
From another point of view, the severity of malnutrition-inflammation cachexia (MIC) is in parallel with risks of all-cause and CV mortality (13). Malnutrition-inflammation syndrome (MIS) score online calculator is a validated tool in predicting composite outcomes and fatal CV events (14, 15). In light of this, electrocardiogram signals during HD and MIS score should be measured and merged into a continuous surveillance system. Nonetheless, there has been no study to evaluate the joint effect of impaired CSR and MIC on clinical outcomes in HD patients. Thus, we aimed to investigate the interaction between lower CSR and higher MIS score on death risks in this population-based study, providing preliminary data bank for future AI-based prediction models.
The study protocol was approved by the Institutional Review Board of the En Chu Kong Hospital (ECKIRB1041102; ECKIRB1071202). All clinical investigations were conducted according to the principles of the Declaration of Helsinki. The prospective cohort was conducted in HD patients at blood purification centers from March 2016 until February 2020. A written informed consent was obtained from the participants of this study. Patients undergoing HD treatment for at least 3 months were eligible for inclusion. All patients had to be older than 18 years of age and receive thrice-weekly HD. Patients were excluded from the study if they had terminal illness, active infections, active malignancy, or personal reasons. HD vintage was defined as the duration of time between the first day of HD treatment and the first day that the patient entered the study cohort. Each HD session was performed for 3.5–4.5 h with a blood flow rate of 200–300 mL/min and dialysate flow rate of 500 mL/min. Blood pressure was recorded in the horizontal recumbent position before dialysis session. Pre-dialysis blood samples were obtained from the existing vascular access.
The detailed measurements and procedures for HRV analysis were previously reported (12, 16–18). All measurements for the spectral analysis were conducted in a quiet HD room. On the days of HD, all study patients received twice HRV analysis (before and after HD sessions). The protocol for HRV analysis was designed according to the standard method. After patients laid and breathed smoothly, the electrocardial signals were continuously recorded for 5 min via electrodes placed on four limbs. Cardiac autonomic signals were detected using a HRV analysis device (USPTO Application #: #20180092631) with an analog-to-digital converter. Digitized signals were analyzed online and stored in a hard disk for off-line verification. A computer algorithm was used for digital signal processing, identifying each QRS complex and excluding ventricular premature complex or noises. Stationary R-R values were re-sampled and interpolated at a rate of 7.11 Hz to produce continuity in the time domain (19). Fast Fourier transformation (FFT) was applied as a non-parametric method for frequency-domain analysis. Based on FFT, our algorithm evaluated the power spectrum density for each time segment with appropriate corrections. The power spectrum quantified by the standard frequency-domain analysis was divided into very low frequency (VLF) (0.003–0.04 Hz), low frequency (LF) (0.04–0.15 Hz), and high frequency (HF) (0.15–0.40 Hz). All HRV data were logarithmically transformed to adjust for the skewness of the distribution (16). Emergent evidences have shown patients with impaired sympathetic response during HD are at high risk of adverse clinical outcomes (8, 12). Normalized LF (nLF) has been well-described as an index of cardiac sympathetic activity: nLF (%) = LF/(total power-VLF)*100. Thus changes in nLF (ΔnLF) before and after HD represent CSR in HD patients (12).
The following bio-demographic and laboratory parameters of each patient were recorded at baseline: age, gender, hypertension, DM, coronary artery disease (CAD), HD vintage, blood pressure, potassium, calcium, phosphorus, creatinine, pre-dialysis blood urea nitrogen, fasting glucose, alanine aminotransferase, albumin, C-reactive protein (CRP), uric acid, Kt/V (clearance of urea multiplied by dialysis duration and normalized for urea distribution volume), total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, iron, ferritin, total iron-binding capacity (TIBC), hemoglobin, and platelet count. We adjusted serum calcium according to the following equation: adjusted calcium = measured calcium+ [(4.0 – serum albumin in g/dL) * 0.8]. All laboratory tests were performed by standard procedures with certified methods. MIS score parameters were estimated through an online scientific calculator (http://www.touchcalc.com/calculators/mis). MIS score includes seven conventional components of the subjective global assessment (weight loss in the preceding 6 months, gastrointestinal symptoms, dietary food intake, functional capacity, comorbidities, subcutaneous fat loss and signs of muscle wasting) and three new elements (body mass index, serum albumin, and TIBC). Each MIS component has four levels of severity from 0 (normal) to 3 (very severe).
CV mortality in study patients was defined as death attributable to CAD, myocardial ischemia and infarction, heart failure, fatal arrhythmia, cardiac arrest because of other causes, cerebrovascular diseases, pulmonary embolism, peripheral artery diseases, and sudden otherwise unexplained death. SCD was defined by World Health Organization criteria: a sudden, natural, non-violent and unexpected death with all potentially lethal non-cardiac causes ruled out (20). Non-CV mortality was defined as all other causes of death, i.e., infection, malignancies, gastrointestinal hemorrhage, accidents, and miscellaneous. All-cause mortality included CV and non-CV death. Patients were censored if they met one of the criteria listed below during the follow-up: (1) patients were transferred to another dialysis unit; (2) patients abandoned HD treatment; (3) patients switched to peritoneal dialysis; (4) patients had recovery of kidney function and stopped HD treatment; and (5) patients received kidney transplant.
Statistical analysis was performed using SPSS 22.0 for windows (SPSS Inc. Chicago, USA). Continuous variables were presented as mean ± standard deviation (SD). Categorical variables were expressed as patient number (n) and percentage (%). The univariate Cox regression analysis was performed to investigate the independence of risk factors associated all-cause, CV mortality and SCD. The included subjects for final analysis were further stratified into higher and lower concentration groups by median values of ΔnLF and MIS score, respectively. Unadjusted and multivariable adjusted hazard ratios (aHRs) of mortality risks were calculated for different categories of ΔnLF and MIS score in the Cox regression model. The modification effect between ΔnLF and MIS score on mortality risks was determined using an interaction product term. According to methods previously described, an interaction occurs when the impact of a risk factor on outcome is changed by the value of a third variable, sometimes referred to as effect modification.
The multiplicative interaction term derived from the product of ΔnLF and MIS score acts as a third variable. We evaluated if the effect of ΔnLF on mortality risks was modified by MIS score through incorporating an interaction term in the multivariate model. The cumulative survival probability and proportional hazards were presented by graphical methods. A significant product term indicates that there is an interaction between ΔnLF and MIS score on the probability of mortality risks. A P < 0.05 was considered statistically significant.
The final study sample included 160 patients with maintenance HD obtaining complete medical records and follow-up. Baseline bio-clinical data of the whole study population with comparison between DM and non-DM are summarized in Table 1. The mean age was 60.7 ± 10.3 years, approximately 47% were male. Prevalence of DM, hypertension, and previous CAD was 46.3, 41.9, and 21.9%, respectively. The mean duration of follow-up was 31.9 ± 7.4 months. The overall mortality rate was 25.0% during 5097.6 person-months of follow-up, corresponding to an annual mortality rate of 9.4%. Twenty-four patients (60.0%) died from CV causes, and 16 (40.0%) were non-CV deaths. Thirteen patients (32.5%) died from SCD, and 11 (27.5%) were non-sudden CV deaths. With respect to bio-clinical parameters, there were many significant differences between DM and non-DM group. Older age and CAD history were more frequent in DM group. Furthermore, DM patients had lower levels of ΔnLF/post-dialysis nLF and higher levels of MIS score, higher pre-dialysis glucose, and phosphate. The comparisons among survivors, non-CV deaths, non-sudden CV deaths, and SCD are summarized in Table 2. The bio-clinical parameters that differed significantly included age, DM, prior CAD, ΔnLF, post-dialysis nLF, MIS score, albumin, CRP, HDL, and TIBC. It is noteworthy that subjects with SCD and non-sudden CV deaths had a higher prevalence of DM and CAD, lower levels of ΔnLF, and higher levels of MIS score.
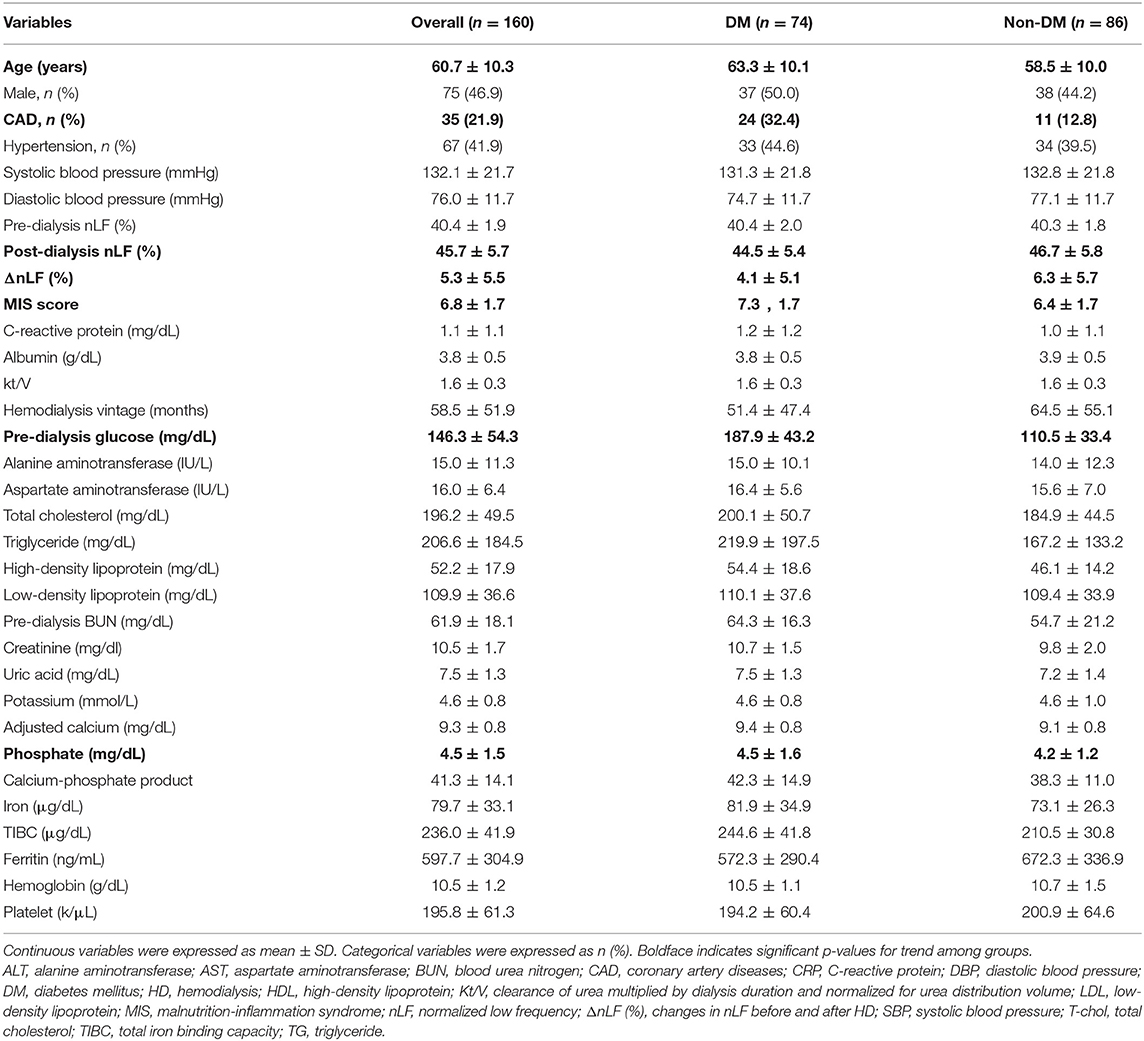
Table 1. Bio-clinical data of the whole study population with comparisons between DM and non-DM groups.
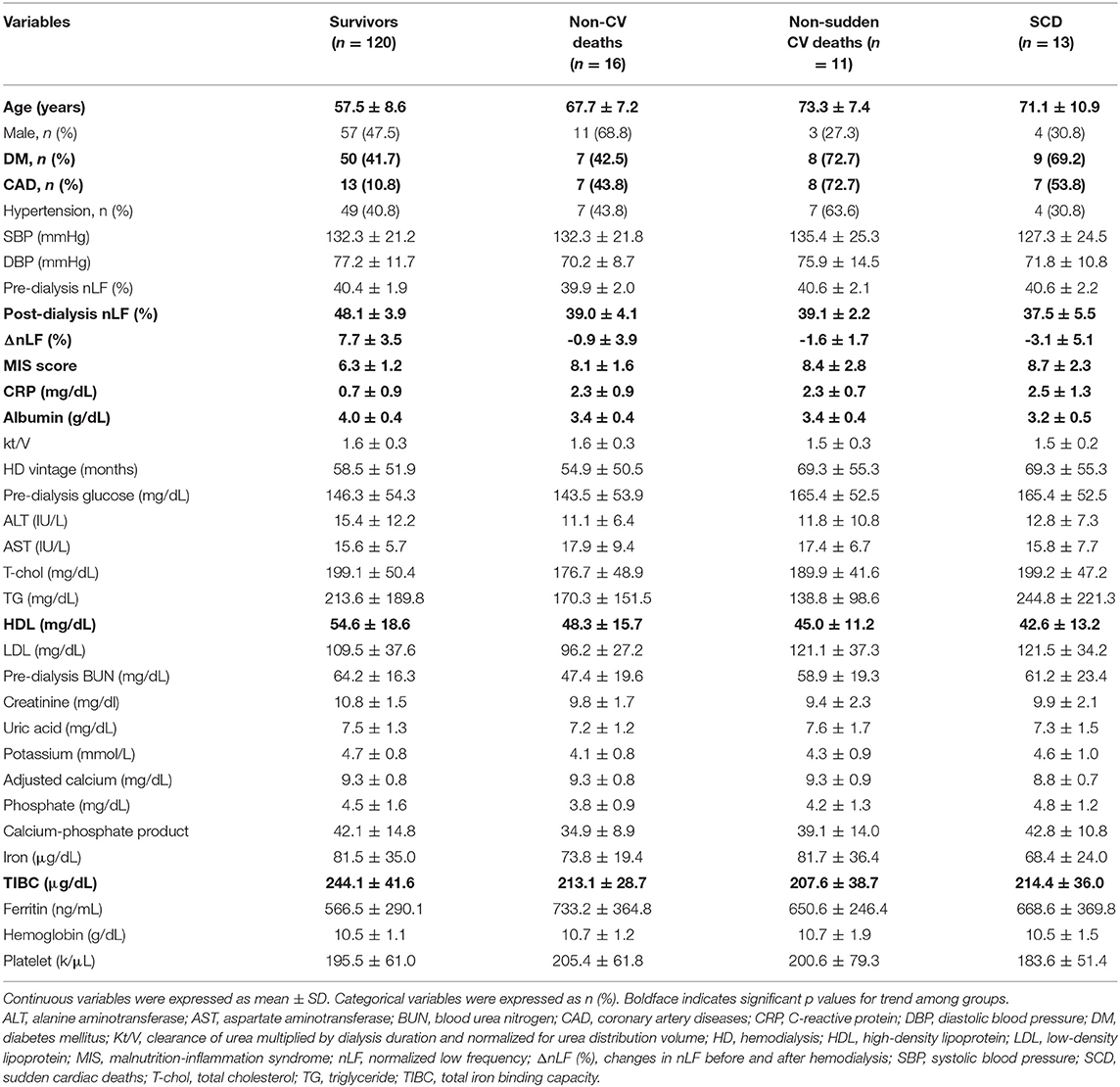
Table 2. Comparison of demographic characteristics and relevant laboratory data among survivors, non-CV deaths, non-sudden CV deaths, and SCD.
In the univariate Cox regression analysis of prognostic factors, ΔnLF, MIS score, age, DM, CAD, albumin, CRP, HDL, ferritin, and TIBC were significantly associated with all-cause mortality (Table 3). In addition, the association between above prognostic factors and CV mortality remained significant. Table 4 unveiled that ΔnLF, MIS score, age and CAD were still strongly associated with SCD. Furthermore, we investigated possibly interesting covariates in the multivariate Cox regression model, including patient age, DM, ΔnLF, MIS score, and prior CAD. Multivariable-adjusted results demonstrated ΔnLF and MIS score were still significantly associated with all-cause and CV mortality [aHR: 0.781 (95% CI: 0.698–0.8740 and 1.333 (95% CI: 1.058–1.678), respectively] (Table 5).
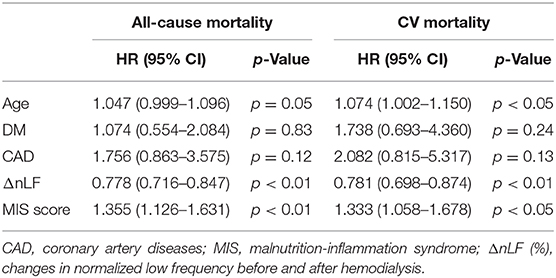
Table 5. Multivariate Cox regression analysis of key prognostic factors for all-cause and CV mortality.
Figure 1 illustrates cumulative survival curves of all-cause mortality with respect to different categories of ΔnLF after adjusting for age, diabetes mellitus, CAD, MIS score, and ΔnLF in the Cox regression model during 5097.6 person-months of follow-up. The multivariable-adjusted result demonstrated the patient group with combined lower levels of ΔnLF (<6.8 nu) and higher levels of MIS score (>6.1 nu) was associated with a highest risk of all-cause mortality [aHR: 6.158 (95% CI: 1.120–13.843), P = 0.037]. Nonetheless, the p-value for the interaction product term was insignificant (P = 0.062), suggesting the association between ΔnLF and all-cause mortality was not limited to high level of MIS score.
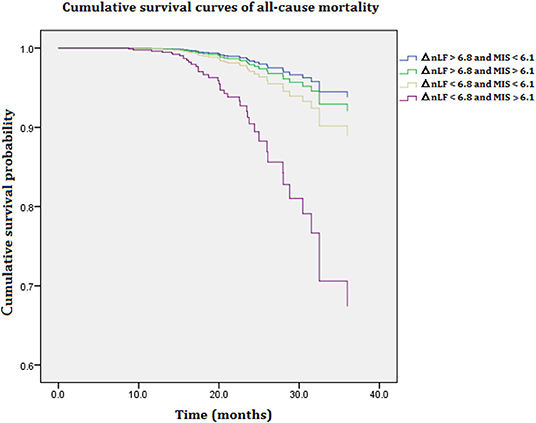
Figure 1. Cumulative survival curves of all-cause mortality with respect to different categories of ΔnLF and MIS score after adjusting for age, diabetes mellitus, CAD, MIS score, and ΔnLF during 5097.6 person-months of follow-up. Combined lower levels of ΔnLF (<6.8 nu) and higher levels of MIS score (>6.1 nu) was associated with a highest risk of all-cause mortality (P = 0.037). CAD, coronary artery diseases; MIS, malnutrition-inflammation syndrome; ΔnLF (%), changes in normalized low frequency before and after hemodialysis.
Figure 2 illustrates cumulative survival curves of CV mortality with respect to different categories of ΔnLF after adjusting for age, diabetes mellitus, CAD, MIS score, and ΔnLF in the Cox regression model during 5097.6 person-months of follow-up. The multivariable-adjusted result demonstrated the patient group with combined lower levels of ΔnLF (<6.8 nu) and higher levels of MIS score (>6.1 nu) was associated with a highest risk of CV mortality [aHR: 5.671 (95% CI: 1.060–14.708), P = 0.046]. Nonetheless, the p-value for the interaction product term was insignificant (P = 0.182), suggesting the association between ΔnLF and CV mortality was not limited to high levels of MIS score.
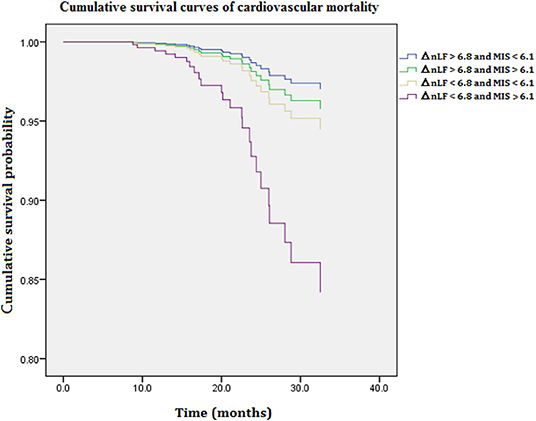
Figure 2. Cumulative survival curves of cardiovascular mortality with respect to different categories of ΔnLF and MIS score after adjusting for age, diabetes mellitus, CAD, MIS score, and ΔnLF during 5097.6 person-months of follow-up. Combined lower levels of ΔnLF (<6.8 nu) and higher levels of MIS score (>6.1 nu) was associated with a highest risk of cardiovascular mortality (P = 0.041). CAD, coronary artery diseases; MIS, malnutrition-inflammation syndrome; ΔnLF (%), changes in normalized low frequency before and after hemodialysis.
The services to improve patient safety are delivery of non-invasive early detection and sustained monitoring of high-risk subjects. In this prospective cohort study we present the brand new idea that the joint evaluation of ΔnLF and MIS score provides a more robust predictive value for not only all-cause but also CV mortality with the application of a non-invasive monitoring system. Our data indicate that higher ΔnLF was related to all-cause, CV and SCD [aHR: 0.78 (95% CI: 0.72–0.85), 0.78 (95% CI: 0.70–0.87), and 0.74 (95% CI: 0.63–0.87), respectively]. Higher MIS score was associated with incremental risks of all-cause, CV and sudden cardiac deaths [aHR: 1.36 (95% CI: 1.13–1.63), 1.33 (95% CI: 1.06–1.38), and 1.50 (95% CI: 1.07–2.11), respectively]. Patients with combined lower ΔnLF (≤6.8 nu) and higher MIS score were at the greatest risk of all-cause and CV mortality [aHR: 5.64 (95% CI: 1.14–18.09) and 5.86 (95% CI: 1.64–13.65), respectively]. Apparently, there is an intricate relationship between impaired CSR, MIC and fatal events. Several important findings in this work deserve further discussion.
It is of prime importance to understand the interplay between the sympathetic and parasympathetic regulatory coordination, which serves to speed up and slow down the heart rate, respectively (21). HRV is a useful non-invasive method to analyze the function of the autonomic nervous system in high-risk population. The first study of HRV in predicting mortality among 900 case of myocardial infarction patients was reported by Kleiger et al. (22). Emerging evidence supported the view that impaired CSR was a predictor of clinical events, especially for SCD (7–9, 11, 12). Thence, HRV is widely used in the medical inspection. Furthermore, HRV abnormalities have been linked to immune dysfunction and inflammation, including CV disease, diabetes, osteoporosis, arthritis, Alzheimer's disease, periodontal disease, and cancers (23, 24). To the best of our knowledge, it is the first study to investigate the interaction between impaired CSR and malnutrition-inflammation cachexia syndrome. The fact that CV disease remains the leading cause of morbidity and mortality in ESRD patients, the non-invasive measurement of ΔnLF and MIS score online scoring system should be applied and monitored in routine HD.
DAN, a serious and common complication of DM, consists of composite nerve damaging disorders (10). As the disease progresses with age, the incidence of DAN and CAN increased in DM patients (10, 25). CAN measured by HRV is strongly associated with an incremental risk of silent myocardial ischemia and death, responsible for the most critical form of DAN. Despite its relationship to death risks and multidisciplinary diseases, the significance of DAN has not been fully appreciated. Hyperglycemia is generally considered to be the major culprit of neuropathy, but the exact pathogenesis of DAN is still not clear. Elevated oxidative stress and free radicals cause vascular endothelium damages and reduce nitric oxide bioavailability (26, 27). Alternately, excess nitric oxide production may result in peroxynitrite production, endothelial damages, and neuron injuries, a process referred to as nitrosative stress (28, 29). Meanwhile, the burden of pro-inflammatory and pro-oxidant state is reminiscent of uremic milieu in chronic kidney disease (30). In light of this, ESRD patients superimposed on DM would be at the greatest risk of impaired CSR related death. Indeed, our data indicated that HD patients with DM had a lower level of CSR and higher level of MIS score (Table 1). Moreover, SCD population had the lowest level of CSR and highest level of MIS score (Table 2). In the univariate and multivariate Cox regression model, impaired CSR remained the strongest predictor of various fatal events (Tables 3–5).
In this study, the modification effect between MIS score and ΔnLF on all-cause and CV mortality was examined using an interaction product term according to previous methods of moderation analysis (31). As expected, HD patients with higher lower ΔnLF (<6.8 nu) and higher MIS score (>6.1 nu) and have the greatest risk of all-cause and CV death (Figures 1, 2). Although the association between ΔnLF and all-cause/ CV mortality was not limited to MIS score (P = 0.062 and 0.182 for the interaction term, respectively), our data provided a preliminary data bank for future AI-based prediction models. The majority of maintenance HD patients have CV diseases, and their CV mortality is 20 times higher than in the general population (32). The reasons why HD patients are at particular risk for CV death are complicated, e.g., ventricular hypertrophy as well as non-traditional risk factors, such as chronic volume overload, anemia, inflammation, oxidative stress, chronic kidney disease–mineral bone disorder and other aspects of the “uremic milieu.” Better understanding the effects of these numerous factors on CV death would be an important step for prevention and treatment. Accordingly, it is of prime importance to collect various aspects of clinical information from HD patients in future AI studies.
AI has influenced all aspects of human life and medical care is no exception to this growing trend. Since MIS score is a validated predictive model for various fatal events in HD patients (33, 34), we merged the online automatic MIS score calculator into the non-invasive HRV monitoring system in our study. The hope is that AI-based decision support technologies will guide multidisciplinary specialists to identify the right level of care in clinical practice, particularly in high-risk HD population. Thakur et al. successfully developed a supervised machine-learning-based prediction model to predict clinical events based on the sensor data and demographic information (1). In this manner, AI-based models help clinicians not only analyze medical data in disease prevention, diagnosis, patient monitoring and development of new protocols, but also early detect early changes to vital parameters more accurately and efficiently.
Our study has several limitations. First, the changes of electrocardiogram signals during HD and laboratory values might not reflect substantial intra-individual variability over time. Second, our sample size was relatively small and our patients were predominantly Asian patients, limiting the statistical power in the multivariate adjustment and generalization to other populations. Third, every-other-day HD preserves circadian rhythm, but a second day without HD is characterized by parasympathetic withdrawal. We did not record the electrocardiogram signals on the second day.
With respect to the extremely high mortality rates in HD population, the improvement of patient safety with sustained monitoring and early detection of high-risk subjects are of prime importance. ΔnLF and MIS score predict all-cause, CV death and SCD in HD patients, yet a combination monitoring system provides more robust predictive powers. While considering the high prevalence of CAN and MIC in HD patients, ΔnLF, and MIS score could serve as a more promising dual predictor for prognostic assessment. A non-invasive monitoring system composed of HRV signal analyzer and MIS score calculator should be developed in the artificial intelligence-based prediction of clinical events.
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to Jia-Feng Chang;Y2pmNjY5OUBnbWFpbC5jb20=.
The studies involving human participants were reviewed and approved by Institutional Review Board of the En Chu Kong Hospital (ECKIRB1041102). The patients/participants provided their written informed consent to participate in this study.
J-FC and J-CL were responsible for study concept and design, interpretation of data, writing of the manuscript, study supervision, and drafting of the manuscript. C-YH assisted with methodology, investigation, biochemical, and digital analysis. T-MW, Y-YL, and C-CW were responsible for the manuscript revision.
This research was funded by the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (DP2-108-21121-01-O-05-04), Ministry of Science and Technology (MOST 108-2320-B-385-001-), and Taipei Medical University Research Foundation (TMU106-AE1-B17). This work was also financially supported by the Academia and Industry Collaboration Project of Taipei Medical University & En Chu Kong Hospital & Pharmofoods Medical Editing Co., Ltd. (A-108-031).
The authors declare that this study received funding from Pharmofoods Medical Editing Co., Ltd. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We gratefully acknowledge the cooperation among Taipei Medical University, En Chu Kong Hospital, Renal Care Joint Foundation, and Pharmofoods Medical Editing Co., Ltd.
1. Thakur SS, Abdul SS, Chiu Roy BR, Huang PY, Malwade S, et al. Artificial-intelligence-based prediction of clinical events among hemodialysis patients using non-contact sensor data. Sensors. (2018) 18:E2833. doi: 10.3390/s18092833
2. Putra FR, Nursetyo AA, Thakur SS, Roy RB, Syed-Abdul S, Malwade S, et al. Prediction of clinical events in hemodialysis patients using an artificial neural network. Stud Health Technol Inform. (2019) 264:1570–1.
3. Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. (1998) 32:S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470
4. Goodkin DA, Young EW, Kurokawa K, Prütz KG, Levin NW. Mortality among hemodialysis patients in Europe, Japan, and the United States: case-mix effects. Am J Kidney Dis. (2004) 44:16–21. doi: 10.1016/S0272-6386(04)01100-X
5. Robinson BM, Zhang J, Morgenstern H, Bradbury BD, Ng LJ, Mccullough KP, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. (2014) 85:158–65. doi: 10.1038/ki.2013.252
6. Collins AJ, Foley RN, Gilbertson DT, Chen SC. United States renal data system public health surveillance of chronic kidney disease and end-stage renal disease. Kidney Int. Suppl. (2015) 5:2–7. doi: 10.1038/kisup.2015.2
7. Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am College Cardiol. (1996) 27:1053–60. doi: 10.1016/0735-1097(95)00615-X
8. Pelosi G, Emdin M, Carpeggiani C, Morales MA, Piacenti M, Dattolo P, et al. Impaired sympathetic response before intradialytic hypotension: a study based on spectral analysis of heart rate and pressure variability. Clin Sci. (1999) 96:23–31. doi: 10.1042/cs0960023
9. Carreira MAM, Nogueira AB, Pena FM, Kiuchi MG, Rodrigues RC, Lugon JR. Detection of autonomic dysfunction in hemodialysis patients using the exercise treadmill test: the role of the chronotropic index, heart rate recovery, and RR variability. PLoS ONE. (2015) 10:e0128123. doi: 10.1371/journal.pone.0128123
10. Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. (2003) 26:1553–79. doi: 10.2337/diacare.26.5.1553
11. Suzuki M, Hiroshi T, Aoyama T, Tanaka M, Ishii H, Kisohara M, et al. Nonlinear measures of heart rate variability and mortality risk in hemodialysis patients. Clin J Am Soc Nephrol. (2012) 7:1454–60. doi: 10.2215/CJN.09430911
12. Chen SC, Huang JC, Tsai YC, Mai RHC, Chen RJH, Kuo PL, et al. Heart rate variability change before and after hemodialysis is associated with overall and cardiovascular mortality in hemodialysis. Sci Reports. (2016) 6:20597. doi: 10.1038/srep20597
13. Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr. (2013) 23:77–90. doi: 10.1053/j.jrn.2013.01.001
14. Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G. Comparing outcome predictability of markers of malnutrition–inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transpl. (2004) 19:1507–19. doi: 10.1093/ndt/gfh143
15. Ho LC, Wang HH, Chiang CK, Hung KY, Wu KD. Malnutrition-inflammation score independently determined cardiovascular and infection risk in peritoneal dialysis patients. Blood Purif. (2010) 29:308–16. doi: 10.1159/000280641
16. Electrophysiology TFOTESOCTNASOP. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. (1996) 93:1043–65. doi: 10.1161/01.CIR.93.5.1043
17. Liu C, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. Am J Physiol Heart Circ Physiol. (2003) 285:H2188–93. doi: 10.1152/ajpheart.00256.2003
18. Chen CJ, Kuo TB, Tseng YJ, Yang CC. Combined cardiac sympathetic excitation and vagal impairment in patients with non-organic erectile dysfunction. Clin Neurophysiol. (2009) 120:348–52. doi: 10.1016/j.clinph.2008.10.155
19. Kuo TB, Lin T, Yang CC, Li C-L, Chen C-F, Chou P. Effect of aging on gender differences in neural control of heart rate. Am J Physiol Heart Circ Physiol. (1999) 277:H2233–9. doi: 10.1152/ajpheart.1999.277.6.H2233
20. Tseng ZH, Olgin JE, Vittinghoff E, Ursell PC, Kim AS, Sporer K, et al. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD study. Circulation. (2018) 137:2689–700. doi: 10.1161/CIRCULATIONAHA.117.033427
21. Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger A, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. (1981) 213:220–2. doi: 10.1126/science.6166045
22. Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. (1987) 59:256–62. doi: 10.1016/0002-9149(87)90795-8
23. Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. (2000) 51:245–70. doi: 10.1146/annurev.med.51.1.245
24. Kiecolt-Glaser JK, Mcguire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol. (2002) 53:83–107. doi: 10.1146/annurev.psych.53.100901.135217
25. Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes. (2014) 5:17. doi: 10.4239/wjd.v5.i1.17
26. Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes. (1997) 46(Suppl. 2):S31–37. doi: 10.2337/diab.46.2.S31
27. Low PA, Nickander KK, Tritschler HJ. The roles of oxidative stress and antioxidant treatment in experimental diabetic neuropathy. Diabetes. (1997) 46(Suppl. 2):S38–42. doi: 10.2337/diab.46.2.S38
28. Vinik AI, Erbas T, Park TS, Stansberry KB, Scanelli JA, Pittenger GL. Dermal neurovascular dysfunction in type 2 diabetes. Diabetes Care. (2001) 24:1468–75. doi: 10.2337/diacare.24.8.1468
29. Hoeldtke RD, Bryner KD, Mcneill DR, Hobbs GR, Riggs JE, Warehime SS, et al. Nitrosative stress, uric Acid, and peripheral nerve function in early type 1 diabetes. Diabetes. (2002) 51:2817–25. doi: 10.2337/diabetes.51.9.2817
30. Chang JF, Liang SS, Thanasekaran P, Chang HW, Wen LL, Chen CH, et al. Translational medicine in pulmonary-renal crosstalk: therapeutic targeting of p-cresyl sulfate triggered nonspecific ROS and chemoattractants in dyspneic patients with uremic lung injury. J Clin Med. (2018) 7. doi: 10.3390/jcm7090266
31. Chang JF, Yeh JC, Chiu YL, Liou JC, Hsiung JR, Tung TH. Periodontal pocket depth, hyperglycemia, and progression of chronic kidney disease: a population-based longitudinal study. Am J Med. (2017) 130:61–69.e61. doi: 10.1016/j.amjmed.2016.08.024
32. Cozzolino M, Mangano M, Stucchi A, Ciceri P, Conte F, Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dialysis Transpl. (2018) 33:iii28–34. doi: 10.1093/ndt/gfy174
33. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. (2001) 38:1251–63. doi: 10.1053/ajkd.2001.29222
Keywords: cardiac sympathetic response, malnutrition-inflammation score, autonomic neuropathy, protein energy wasting, mortality, hemodialysis
Citation: Chang J-F, Wu C-C, Hsieh C-Y, Li Y-Y, Wang T-M and Liou J-C (2020) A Joint Evaluation of Impaired Cardiac Sympathetic Responses and Malnutrition-Inflammation Cachexia for Mortality Risks in Hemodialysis Patients. Front. Med. 7:99. doi: 10.3389/fmed.2020.00099
Received: 27 November 2019; Accepted: 05 March 2020;
Published: 27 March 2020.
Edited by:
Kunihiro Sakuma, Tokyo Institute of Technology, JapanReviewed by:
Maria-Eleni Roumelioti, University of New Mexico, United StatesCopyright © 2020 Chang, Wu, Hsieh, Li, Wang and Liou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Chiun Liou, amNsaW91QHRtdS5lZHUudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.