- 1Department of Experimental Diagnostic and Specialty Medicine (DIMES), Nephrology, Dialysis and Renal Transplant Unit, St. Orsola Hospital, University of Bologna, Bologna, Italy
- 2Unit of General and Transplant Surgery, Department of Medical and Surgical Sciences, University of Bologna, S. Orsola Malpighi Hospital Bologna, Bologna, Italy
Cholesterol embolization (CE) is a rare and alarming post-transplant complication, responsible for primary non-function (PNF) or delayed graft function (DGF). Its incidence is expected to rise due to increasingly old donors and recipients and the extended criteria for donation. Therapy with statins and steroids has not been shown to be effective, while agonism of prostaglandin I2 has been reported to be useful in systemic CE. We report two cases of acute post-transplant CE in which intravenous iloprost (0.05 mg/kg/day) was added to standard statin and steroid therapy. In the first instance, CE was due to embolization from the kidney artery resulting in embolization of the small vessels; after a long DGF and 15 days of iloprost therapy, renal function recovered. The second instance is a case of embolization from the iliac artery of the recipient, where CE manifested as a partial renal infarction. After 5 days of iloprost administration, creatinine levels improved. Iloprost acts on vasodilation and on different inflammatory pathways, improving the anti-inflammatory profile. Post-transplant CE is difficult to diagnose and, if not treated, can lead to loss of function. Iloprost added to standard therapy could be beneficial in accelerating renal function recovery immediately after transplant.
Introduction
Cholesterol embolization (CE) is a rare but alarming complication in renal allograft. Its reported frequency is roughly 0.4% (1–3) and, when it presents acutely after transplant, is recognized as one of the causes of primary non-function (PNF) and delayed graft function (DGF) (2, 4, 5).
Considering the increase in transplants from extended criteria donors (ECDs), from donation after circulatory death (DCD), and the tendency for recipients to be older, the possibility of embolization arising from either donor or recipient vessels is expected to increase (6–10). Moreover, since embolization leads to focal and patchy damage, diagnosis is difficult, and injury severity may be underestimated (2, 11–13).
In the absence of a standard and effective therapy, strategies usually aim at stabilizing the plaque by using statins associated with steroids if the disease is recurring and systemic. Reports describe the effectiveness of iloprost, a synthetic analog of prostaglandin I2, as a rescue therapy in systemic CE (2, 11, 14–16). Moreover, in the coronary angiography setting, where ischemic damage to renal tissue is the leading pathogenic mechanism, a reduction in the incidence of contrast-induced nephropathy has been reported in patients with baseline renal insufficiency undergoing coronary intervention (17).
To the best of our knowledge, there are no recent reports on the use of iloprost in CE after kidney transplantation (2, 16, 18).
Here we report two cases of acute post-transplant CE in which the addition of iloprost to the standard care helped accelerate the recovery of kidney function.
Written informed consent was obtained from the participants for the publication of these case reports.
Case Report
Case 1
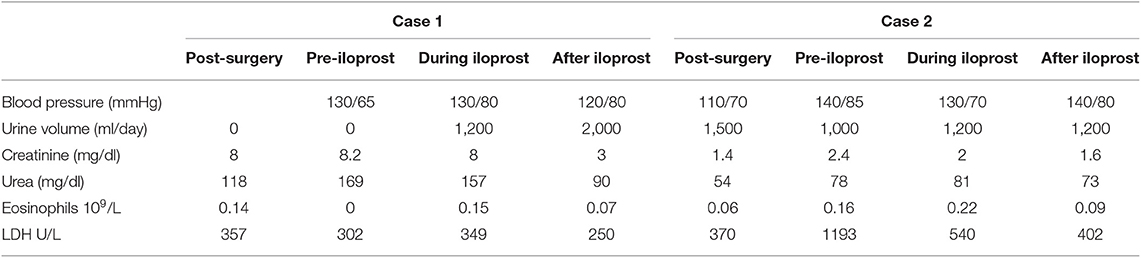
A 44-year-old man received a kidney transplant from a brain-dead donor (DBD). The donor was 59 years old, had died from cerebral hemorrhage, his Kidney Donor Profile Index (KDPI) was 83%, Karpinsky's score was 3, and he had been a smoker with a past history of prostate cancer for which he was in regular follow-up (19). The surgeon described atheromatous plaques in the renal artery that were particularly evident at the confluence with the aorta and were partially removed before implantation. Immunosuppressive therapy consisted of basiliximab, steroids, and tacrolimus. Owing to persistent oligo-anuria, a kidney biopsy was performed on the eighth post-operative day (POD). Histology showed severe acute tubular necrosis (ATN), diffuse cholesterol embolism in the arterioles, inflammatory mixed infiltrate, and interstitial edema. A borderline cellular rejection was diagnosed, and thymoglobulin (ATG) therapy at a dose of 3 mg/kg was administered. Because of the persistence of DGF, the kidney biopsy was repeated on POD 16. The sample showed regression of the interstitial infiltrate, with persistence of ATN and diffuse CE (Figure 1). Therefore, we started rescue therapy with intravenous iloprost at a dose of 0.05 mg/kg/day for 15 days. We observed a slow but progressive recovery of kidney function. No peripheral signs of embolism were observed on physical examination. After 3 months, creatinine was 3 mg/dl; at the 1 year of follow-up, it had improved to 2 mg/dl (Table 1). In this case, the probable source of embolization was the donor renal artery, which presented as a severe atherosclerotic plaque at retrieval.
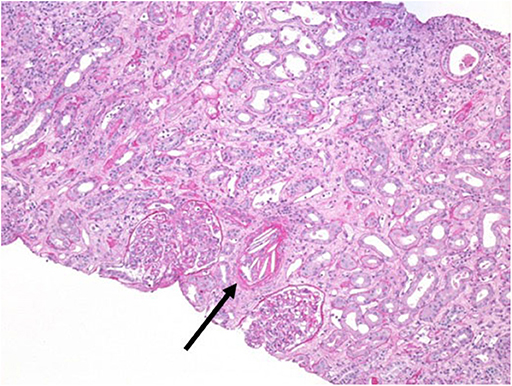
Figure 1. Kidney biopsy at post-operative day 16. Periodic acid–Schiff (PAS) staining, magnification 20×. Arrow indicates a massive cholesterol embolization occluding the arteriolar lumen.
Case 2
A 71-year-old hypertense woman underwent a DBD double kidney transplant. The iliac vessels of the recipient, a smoker, presented with severe atheromatous plaques such that it was difficult to find a suitable vessel to perform the arterial anastomoses; some plaques were fixed to the walls of the vessel with 6-0 prolene. The 81-year-old donor had died from a cerebral hemorrhage, had a KDPI of 99%, and a Karpinsky's score of 4 in both kidneys. Immunosuppressive therapy consisted of ATG, steroids, and tacrolimus. The graft function was prompt, with creatinine levels of 1.7 mg/dl on POD 4, and routine ultrasounds were normal. On POD 13, we observed an abrupt rise in creatinine (2.4 mg/dl), lactate dehydrogenase (LDH) 1,100 U/l, and a slight decrease in diuresis. A contrast-enhanced ultrasonography showed a lack of vascularization in the upper pole of one of the kidneys compatible with a partial infarction. Intravenous iloprost at a dose of 0.05 mg/kg/day was administered as a rescue therapy for 5 days. After 3 days, we started to see progressive recovery of kidney function; after 3 months, the creatinine level was 1.5 mg/dl (Table 1). No peripheral signs of embolism were observed on physical examination. In this case, the most likely source of embolization was the recipient's iliac artery.
Discussion
Atheroembolic renal disease in kidney transplantation is recognized as a possible cause of graft loss. It can occur in the early days post-transplant as well as in the late phases of transplant follow-up (2, 4, 5).
When presenting acutely post-transplant, CE usually occurs due to an acute embolization from either the aorta or the renal artery of the donor during organ harvesting or from the vascular axis of the recipient during surgery.
As a result of the increasing number of ECD and of the aging of both donor and recipient population, atherosclerosis of the vascular axis of the graft and of the recipient is becoming a serious challenge in the field of organ transplantation (20–23).
In our first case, we described the embolization of the donor artery in which plaque disruption probably occurred at harvesting or during the preliminary vascular manipulation made before implantation. In the second instance, the likely cause the acute deterioration of function was crystal embolization from the recipient iliac artery. Our final diagnosis was difficult to prove since no peripheral or systemic signs of CE were present, no other causes of acute kidney injury were identified, and an ischemic area was clearly identified by contrast-enhanced ultrasound. We were aware that the patient was severely atherosclerotic from the results of multiple computed tomography-angiographies performed during the time spent on the waiting list. The surgeon, due to our experience in high atherosclerotic patients, defined her to be suitable for transplant; her condition, however, was found to be worse than predicted.
When the plaque disrupts, microemboli spray downstream, and occlude the vascular lumen of small arteries. The ensuing damage is a combination of tissue ischemia, direct cytotoxic effects of crystals, and necrosis due to the local inflammatory reaction. Soon after embolization, the first damage occurs to endothelium mitochondria (24). Then, because of the large dimensions of cholesterol crystals (1 μm−1 mm), macrophages are not able to digest them completely; this “frustrated phagocytosis” triggers an intracellular danger signal mediated by damage-associated molecular patterns, interleukin (IL)-1α, IL-1β, and nuclear factor (NF)-κB (24–27). The vicious cycle of necroinflammation eventually leads to necrosis (28, 29). Moreover, it has been demonstrated that cholesterol crystals also activate the complement-dependent inflammasome and cytokines (30, 31). Overall, the ischemic and inflammatory pathways activated by this phenomenon increase the already high cardiovascular and inflammatory risk profile of transplant recipients (32).
Since the damage caused by CE is patchy, it is well-known that histologic diagnosis is difficult and often underestimated; this also occurs in native kidneys (12, 13). Moreover, cholesterol crystals are not always present in the sample, and the only lesions seen are ATN and inflammatory infiltrates (2). In light of this, the mild interstitial mixed infiltrate already present in the biopsy of our first case could be explained as related more to an inflammatory reaction to the severe and diffuse embolism rather than to cellular rejection, especially considering the ischemic lesions present in the sample.
Given the key role of inflammation in CE, therapies have always been based on adding steroids to statins, although there is no clear evidence of its effectiveness (2). There are also very few reports showing positive results in the use of the synthetic prostacyclin iloprost as a rescue therapy in systemic CE (2, 11, 14–16, 33, 34). Recently, prophylactic intravenous iloprost therapy has shown some effectiveness in reducing the incidence of contrast-induced nephropathy in the coronary angiography setting in patients with baseline renal insufficiency undergoing coronary intervention, a setting in which toxic ischemic damage is the leading pathogenic event to renal cells (17).
In the 80s and 90s, scientific literature put great emphasis on the prostacyclin system and on the use of prostacyclin analogs in kidney disease (14, 35, 36). The main application field was ischemic injury, but there are also some reported experiences in the field of transplantation. In fact, pretransplant graft perfusion or administration of iloprost in the early days post-transplant led to some benefits in cases of DGF and of cyclosporin-induced toxicity (18, 35, 37–40).
Regarding CE in kidney transplantation, there are no reports exploring the effectiveness of PGI2 agonism.
Iloprost is an analog to PGI2 that exerts different effects both on the vascular wall and blood cells. Acting directly on endothelial cells, smooth muscle, and adventitia, it stimulates angiogenesis, endothelial cell integrity, and relaxation of smooth muscle cells. Moreover, PGI2 has inhibitory effects on the activation of endothelial cells and on the proliferation and migration of smooth muscle cells (41–43). PGI2 acts on leukocytes stimulating the production of anti-inflammatory cytokines and inhibiting the release of IL-1, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ. PGI2 also regulates macrophage functions, promoting their anti-inflammatory profile (44, 45). Effects on the inhibition of platelet aggregation have also been described (20, 43).
The acute continuous iloprost therapy we administered to our patients may have partially counteracted the necroinflammation and vasoconstriction caused by the emboli through vasodilatation, the inhibition of IL-1 and TNF, and the production of other cytokines; in combination with high steroid doses commonly used in the early post-transplant phases, iloprost may have strengthened the positive effects that the reduction of oxidative damage exerts on the outcome of the transplant (46).
Our cases are a good example of increasingly common complications related to detrimental vascular characteristics of grafts and recipients. Moreover, in the case of transplantation, this phenomenon could be restricted to the graft, without the occurrence of peripheral or systemic lesions. Since the embolization could be patchy, the pathognomonic lesion could be invisible in the histologic sample, making the final diagnosis even more difficult. It is important to note that the only lesion seen at biopsy could be ATN associated with an inflammatory infiltrate, easily attributable to cellular rejection (2).
Conclusions
Acute post-transplant CE seems to be increasingly diagnosed in patients with severe atherosclerosis and ECD donors. In the context of transplantation, diagnosis can be difficult since CE can be limited to the graft and the histology can be confused with cellular rejection. As prompt treatment can help in reducing the risk of PNF and in the recovery of function, CE should always be suspected in cases of persistent DGF or acute cellular rejection not responding to therapy. Iloprost, with its vasodilator and anti-inflammatory effects, could potentially act on the molecular pathways activated by cholesterol crystals; it is our opinion that prompt intravenous therapy with iloprost, added to statins and steroids, has accelerated the good outcome of the two patients whose cases we have described in this report.
Of course, the effectiveness of iloprost infusion in hindering the inflammatory and ischemic cascades induced by CE in the immediate post-transplant setting should be investigated in depth, especially considering that prompt intervention is essential. Larger case control studies and clinical trials are needed to prove the causality between iloprost administration and the improvement of kidney function and investigate when prompt intervention is essential.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
VCo and GC contributed to conception, design of the work, analysis, and interpretation of data. MR, VA, FO, AA, IC, and VCu contributed to the acquisition of data for the work. GL revising it critically for important intellectual content. All the authors provide approval for publication of the content.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Lai CK, Randhawa PS. Cholesterol embolization in renal allografts: a clinicopathologic study of 12 cases. Am J Surg Pathol. (2007) 31:536–45. doi: 10.1097/PAS.0b013e31802b30e3
2. Scolari F, Ravani P. Atheroembolic renal disease. Lancet. (2010) 375:1650–60. doi: 10.1016/S0140-6736(09)62073-0
3. Ripple MG, Charney D, Nadasdy T. Cholesterol embolization in renal allografts. Transplantation. (2000) 69:2221–5. doi: 10.1097/00007890-200005270-00050
4. Singh I, Killen PD, Leichtman AB. Cholesterol dysfunction emboli presenting as acute allograft after renal transplantation. J Am Soc Nephrol. (1995) 6:165–70.
5. Chaudhury PR, Alexander JW, First MR, Peddi VR, Munda R, Cavallo T. Immediate allograft dysfunction due to atheroembolic disease. Am J Kidney Dis. (2001) 37:423–6. doi: 10.1053/ajkd.2001.21334
6. Messina M, Diena D, Dellepiane S, Guzzo G, Lo Sardo L, Fop F, et al. Long-term outcomes and discard rate of kidneys by decade of extended criteria donor age. Clin J Am Soc Nephrol. (2017) 12:323–31. doi: 10.2215/CJN.06550616
7. Del Río F, Andrés A, Padilla M, Sánchez-Fructuoso AI, Molina M, Ruiz Á, et al. Kidney transplantation from donors after uncontrolled circulatory death: the Spanish experience. Kidney Int. (2019) 95:420–8. doi: 10.1016/j.kint.2018.09.014
8. Stevenson RP, Shapter O, Aitken E, Stevenson K, Shiels PG, Kingsmore DB. Has the expansion in extended criteria deceased donors led to a different type of delayed graft function and poorer outcomes? Transplant Proc. (2018) 50:3160–4. doi: 10.1016/j.transproceed.2018.07.022
9. Shamali A, Kassimatis T, Phillips BL, Burton H, Kessaris N, Callaghan C. Duration of delayed graft function and outcomes after kidney transplantation from controlled donation after circulatory death donors: a retrospective study. Transpl Int. (2019) 32:635–45. doi: 10.1111/tri.13403
10. Sagban olga A, Baur B, Schelzig H, Grabitz K, Duran M. Vascular challenges in renal transplantation. Ann Transplant. (2014) 19:464–71. doi: 10.12659/AOT.890893
11. Scolari F, Tardanico R, Pola A, Mazzucchelli C, Maffeis R, Bonardelli S, et al. Cholesterol crystal embolic disease in renal allografts. J Nephrol. (2003) 16:139–143.
12. Fries C, Roos M, Gaspert A, Vogt P, Salomon F, Wüthrich RP, et al. Atheroembolic disease-a frequently missed diagnosis results of a 12-year matched-pair autopsy study. Medicine. (2010) 89:126–32. doi: 10.1097/MD.0b013e3181d5eb39
13. Perrone ME, Chang A, Henriksen KJ. Medical renal diseases are frequent but often unrecognized in adult autopsies. Mod Pathol. (2018) 31:365–73. doi: 10.1038/modpathol.2017.122
14. Grenader T, Lifschitz M, Shavit L. Iloprost in embolic renal failure. Mt Sinai J Med. (2005) 72:339–41.
15. Elinav E, Chajek-Shaul T, Stern M. Improvement in cholesterol emboli syndrome after iloprost therapy. BMJ. (2002) 324:268–69. doi: 10.1136/bmj.324.7332.268
16. Sevillano ÁM, Hernández E, Caro J, Molina-Gómez M, Gutiérrez-Martínez E, Morales-Ruiz E, et al. Cholesterol atheroembolism and combined treatment with steroids and iloprost. Nefrologia. (2012) 32:824–828. doi: 10.3265/Nefrologia.pre2012.Aug.11645
17. Kassis HM, Minsinger KD, McCullough PA, Block CA, Sidhu MS, Brown JR. A review of the use of iloprost, a synthetic prostacyclin, in the prevention of radiocontrast nephropathy in patients undergoing coronary angiography and intervention. Clin Cardiol. (2015) 38:492–8. doi: 10.1002/clc.22407
18. Neumayer HH, Schreiber M, Wagner K. Prevention of delayed graft function in cadaveric kidney transplants by the calcium antagonist diltiazem and the prostacyclin-analogue iloprost–outcome of a prospective randomized clinical trial. Prog Clin Biol Res. (1989) 301:289–95.
19. Noale M, Maggi S, Artibani W, Bassi PF, Bertoni F, Bracarda S, et al. Pros-IT CNR: an Italian prostate cancer monitoring project. Aging Clin Exp Res. (2017) 29:165–72. doi: 10.1007/s40520-017-0735-6
20. Miller SB. Prostaglandins in health and disease: an overview. Semin Arthritis Rheum. (2006) 36:37–49. doi: 10.1016/j.semarthrit.2006.03.005
21. Hernández D, Triñanes J, Armas AM, Ruiz-Esteban P, Alonso-Titos J, Duarte A, et al. Vascular damage and kidney transplant outcomes: an unfriendly and harmful link. Am J Med Sci. (2017) 354:7–16. doi: 10.1016/j.amjms.2017.01.004
22. Nanmoku K, Watarai Y, Narumi S, Goto N, Yamamoto T, Tsujita M, et al. Surgical techniques and procedures for kidney transplant recipients with severe atherosclerosis. Exp Clin Transplant. (2017) 15:594–601. doi: 10.6002/ect.2016.0207
23. Khan MA, El-Hennawy H, Jones KC, Harriman D, Farney AC, Rogers J, et al. Eversion endarterectomy of the deceased donor renal artery to prevent kidney discard. Clin Transplant. (2018) 32:e13275. doi: 10.1111/ctr.13275
24. Mulay SR, Anders HJ. Crystallopathies. N Engl J Med. (2016) 374:2465–76. doi: 10.1056/NEJMra1601611
25. Kiyotake R, Oh-hora M, Ishikawa E, Miyamoto T, Ishibashi T, Yamasaki S. Human mincle binds to cholesterol crystals and triggers innate immune responses. J Biol Chem. (2015) 290:25322–32. doi: 10.1074/jbc.M115.645234
26. Corr EM, Cunningham CC, Dunne A. Cholesterol crystals activate Syk and PI3 kinase in human macrophages and dendritic cells. Atherosclerosis. (2016) 251:197–205. doi: 10.1016/j.atherosclerosis.2016.06.035
27. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. (2010) 464:1357–61. doi: 10.1038/nature08938
28. Franklin BS, Mangan MS, Latz E. Crystal formation in inflammation. Annu Rev Immunol. (2016) 34:173–202. doi: 10.1146/annurev-immunol-041015-055539
29. Linkermann A, Stockwell BR, Krautwald S, Anders H-J. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. (2014) 14:759–67. doi: 10.1038/nri3743
30. Niyonzima N, Samstad EO, Aune MH, Ryan L, Bakke SS, Rokstad AM, et al. Reconstituted high-density lipoprotein attenuates cholesterol crystal–induced inflammatory responses by reducing complement activation. J Immunol. (2015) 195:257–64. doi: 10.4049/jimmunol.1403044
31. Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol. (2014) 192:2837–45. doi: 10.4049/jimmunol.1302484
32. La Manna G, Cappuccilli ML, Cianciolo G, Conte D, Comai G, Carretta E, et al. Cardiovascular disease in kidney transplant recipients: the prognostic value of inflammatory cytokine genotypes. Transplantation. (2010) 89:1001–8. doi: 10.1097/TP.0b013e3181ce243f
33. Scolari F, Tardanico R, Zani R, Pola A, Viola BF, Movilli E, et al. Cholesterol crystal embolism: a recognizable cause of renal disease. Am J Kidney Dis. (2000) 36:1089–109. doi: 10.1053/ajkd.2000.19809
34. Meyrier A. Cholesterol crystal embolism: diagnosis and treatment. Kidney Int. (2006) 69:1308–12. doi: 10.1038/sj.ki.5000263
35. Hansen JM, Christensen NJ, Fogh-Andersen N, Strandgaard S. Effects of the prostacyclin analogue iloprost on cyclosporin-induced renal hypoperfusion in stable renal transplant recipients. Nephrol Dial Transplant. (1996) 11:340–6.
36. Nakamoto H, Fujita T, Origasa H, Isono M, Kurumatani H, Okada K, et al. A multinational phase IIb/III trial of beraprost sodium, an orally active prostacyclin analogue, in patients with primary glomerular disease or nephrosclerosis (CASSIOPEIR trial), rationale and study design. BMC Nephrol. (2014) 15:153. doi: 10.1186/1471-2369-15-153
38. Alcaraz A, Luque P, Mendes DR, Calatrava P, Luque P, Rodriguez A, et al. Experimental kidney transplantation in pigs from non-heart-beating donors: evaluation of vasoactive substances and renal artery flow. Transplant Proc. (2001) 33:2971–2. doi: 10.1016/s0041-1345(99)00373-5
39. Fukushima N, Shirakura R, Chang J, Izutani H, Inoue M, Yamaguchi T, et al. Successful multiorgan transplants from non-heart-beating donors using percutaneous cardiopulmonary support. Transplant Proc. (1998) 30:3783–3784. doi: 10.1016/S0041-1345(98)01235-4
40. Pliquett RU, Asbe-Vollkopf A, Scheuermann EH, Gröne E, Probst M, Geiger H, et al. Cholesterol-crystal embolism presenting with delayed graft function and impaired long-term function in renal transplant recipients: two case reports. J Med Case Rep. (2009) 3:6839. doi: 10.1186/1752-1947-3-6839
41. Kawabe J, Ushikubi F, Hasebe N. Prostacyclin in vascular diseases. Recent insights and future perspectives. Circ J. (2010). 74:836–43. doi: 10.1253/circj.cj-10-0195
42. Zardi EM, Zardi DM, Cacciapaglia F, Dobrina A, Amoroso A, Picardi A, et al. Endothelial dysfunction and activation as an expression of disease: role of prostacyclin analogs. Int Immunopharmacol. (2005) 5:437–59. doi: 10.1016/j.intimp.2004.10.016
43. Majed BH, Khalil RA. Molecular mechanisms regulating the vascular prostacyclin pathways and their adaptation during pregnancy and in the newborn. Pharmacol Rev. (2012) 64:540–82. doi: 10.1124/pr.111.004770
44. Aronoff DM, Peres CM, Serezani CH, Ballinger MN, Carstens JK, Coleman N, et al. Synthetic prostacyclin analogs differentially regulate macrophage function via distinct analog-receptor binding specificities. J Immunol. (2007) 178:1628–34. doi: 10.4049/jimmunol.178.3.1628
45. Tsai M-K, Hsieh C-C, Kuo HF, Yang SN, Kuo CH, Huang MY, et al. Effect of prostaglandin I2 analogs on macrophage inflammatory protein 1α in human monocytes via I prostanoid receptor and cyclic adenosine monophosphate. J Investig Med. (2014) 62:332–9. doi: 10.2310/JIM.0000000000000042
Keywords: cholesterol embolism, kidney transplant, prostaglandin agonism, delayed graft function, extended criteria donors
Citation: Corradetti V, Comai G, Ravaioli M, Cuna V, Aiello V, Odaldi F, Angeletti A, Capelli I and La Manna G (2020) Iloprost in Acute Post-kidney Transplant Atheroembolism: A Case Report of Two Successful Treatments. Front. Med. 7:41. doi: 10.3389/fmed.2020.00041
Received: 15 August 2019; Accepted: 28 January 2020;
Published: 28 February 2020.
Edited by:
Thomas Friedrich Mueller, University Hospital Zürich, SwitzerlandReviewed by:
Gautam Bhave, Vanderbilt University, United StatesZaid A. Abassi, Technion Israel Institute of Technology, Israel
Copyright © 2020 Corradetti, Comai, Ravaioli, Cuna, Aiello, Odaldi, Angeletti, Capelli and La Manna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaetano La Manna, Z2FldGFuby5sYW1hbm5hQHVuaWJvLml0
 Valeria Corradetti
Valeria Corradetti Giorgia Comai1
Giorgia Comai1 Andrea Angeletti
Andrea Angeletti Irene Capelli
Irene Capelli Gaetano La Manna
Gaetano La Manna