
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 29 January 2020
Sec. Nuclear Medicine
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00336
This article is part of the Research TopicReviews in: Radiopharmaceuticals in Nuclear MedicineView all 5 articles
 Francesco Martucci1
Francesco Martucci1 Mariarosa Pascale2
Mariarosa Pascale2 Maria Carla Valli1
Maria Carla Valli1 Gianfranco A. Pesce1
Gianfranco A. Pesce1 Patrizia Froesch3
Patrizia Froesch3 Luca Giovanella4
Luca Giovanella4 Antonella Richetti1
Antonella Richetti1 Giorgio Treglia4,5,6*
Giorgio Treglia4,5,6*Background: Molecular imaging methods are currently used in the management of patients with lung cancer. Compared to non-small cell lung cancer, less data are available about the impact of molecular imaging using fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in staging patients with small cell lung cancer (SCLC). Performing a systematic review and meta-analysis, we aimed to provide quantitative data about the impact of 18F-FDG PET/CT in staging SCLC.
Methods: A comprehensive literature search of studies on the use of 18F-FDG PET/CT in patients with SCLC was performed. Three different databases were screened (PubMed/MEDLINE, EMBASE, and Cochrane library databases) until June 2019. Only articles describing the impact of 18F-FDG PET/CT in staging patients with SCLC were selected. A pooled analysis evaluating the change of binary SCLC staging (limited-stage vs. extensive-stage disease) using 18F-FDG PET/CT was carried out.
Results: Nine articles including 721 patients with SCLC were included in the systematic review. Compared to conventional staging, a superior diagnostic accuracy of 18F-FDG PET/CT was found. A change of binary SCLC staging using 18F-FDG PET/CT was demonstrated in 15% (95% confidence interval, 9–21%) of patients with SCLC. Currently, it is not clearly demonstrated that the use of 18F-FDG PET/CT for staging may improve the survival outcome of patients with SCLC.
Conclusions: 18F-FDG PET/CT is a useful molecular imaging method for staging patients with SCLC because it can change the management in a significant number of patients. More large prospective studies and cost-effectiveness analyses on the impact of 18F-FDG PET/CT in staging patients with SCLC are needed.
Neuroendocrine tumors are ~20% of all lung cancers, and small cell lung cancer (SCLC) is the most frequent neuroendocrine tumor of the lung. Most cases of SCLC are related to smoking, and the estimated incidence of this tumor in the United States for the year 2019 is 29,660 new cases/year with a male/female incidence ratio of 1:1 (1, 2).
SCLC is an aggressive high-grade neuroendocrine tumor characterized by rapid growth and early development of metastatic spread. Most SCLC patients have metastatic disease at the initial diagnosis, and about one-third of them has limited disease confined to the chest, whereas two-thirds of them has hematogenous metastases. Even if SCLC is usually highly sensitive to chemotherapy and radiation therapy, however, most patients develop recurrent disease (2, 3).
Most of the literature classifies SCLC patients based on a binary classification scheme to define the extent of the disease. Limited-stage disease (LD-SCLC) is confined to the ipsilateral hemithorax (including contralateral mediastinal and ipsilateral supraclavicular lymph nodal metastases) and can be included within a radiation field. Extensive-stage disease (ED-SCLC) is spread beyond the ipsilateral hemithorax (including hematogenous metastases and malignant pleural or pericardial effusion) (2–4). In patients with LD-SCLC, the standard treatment is usually chemotherapy plus thoracic radiation therapy. In this setting of patients, prophylactic cranial irradiation is also indicated to increase overall survival. In ED-SCLC, long-term survival is rare, and systemic therapy alone is considered a palliative treatment (2–4). Correct staging of SCLC is pivotal to assess the indication of thoracic radiation therapy, which is useful mainly in patients with LD-SCLC. Therefore, using a binary staging system in SCLC patients, only disagreement on the presence or absence of metastatic lesions outside one hemithorax or malignant pleural effusion will have a significant impact on patient management (4).
Computed tomography (CT) of chest and abdomen and brain magnetic resonance imaging (MRI) are the most used imaging methods for staging SCLC (4). However, if LD-SCLC is suspected, molecular imaging using fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET) can be performed to assess for distant metastases. In particular, hybrid imaging using 18F-FDG PET/CT, providing both functional and morphological data, has been demonstrated to be superior to 18F-FDG PET alone, and it is the current state of art for molecular imaging of lung cancer (5). As SCLC is an aggressive neuroendocrine tumor with increased metabolism and 18F-FDG is a radiolabeled glucose analog, an increased uptake of this radiopharmaceutical is expected by SCLC lesions (6–8). Compared to non-small cell lung cancer, less data are available about the impact of molecular imaging using 18F-FDG PET/CT in staging patients with SCLC. Therefore, we aimed to provide timely evidence-based data on the use of this imaging method for staging SCLC.
This evidence-based article was written according to the “Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies” (9–11). This review has been registered on the online database PROSPERO (international prospective register of systematic reviews) after submission, as requested by the reviewers.
The review question was formulated according to the Patient/Intervention/Comparison/Outcome process:
- Patients: individuals with histologically proven SCLC;
- Intervention: 18F-FDG PET/CT performed for disease staging;
- Comparison: staging without 18F-FDG PET/CT (e.g., using conventional imaging methods such as contrast-enhanced CT or bone scintigraphy);
- Outcomes: the main outcome was the change of binary SCLC staging using 18F-FDG PET/CT; secondary outcomes were the diagnostic accuracy of 18F-FDG PET/CT staging compared to conventional staging in SCLC and the impact of 18F-FDG PET/CT staging on survival of SCLC patients.
Two authors (FM and GT) performed a comprehensive computer literature search of three different bibliographic databases (PubMed/MEDLINE, EMBASE, and Cochrane library) to find published articles on the impact of 18F-FDG PET/CT in staging patients with SCLC. A search algorithm combining several keywords related to the index test and the target condition was used: (A) “SCLC” and (B) “PET/CT” or “PET-CT.” No beginning date limit nor language restrictions were used. The literature search was performed until June 30th, 2019. To expand the literature search, we also screened the references of the retrieved articles searching for additional studies, which could be included in the systematic review.
Inclusion criteria were studies or subsets of studies investigating the impact of 18F-FDG PET/CT in staging patients with SCLC histologically proved. The exclusion criteria were (a) articles outside the field of interest of this review (including articles using 18F-FDG PET only without hybrid technology for staging, studies evaluating the prognostic value of 18F-FDG PET/CT or its use for radiotherapy planning, and articles evaluating the role of 18F-FDG PET/CT for treatment response assessment or restaging after treatment); (b) review articles, letters, comments, editorials, and conference proceedings; and (c) case reports or small case series.
Two researchers (FM and GT) independently reviewed the titles and abstracts of the retrieved articles, applying the inclusion and exclusion criteria mentioned above. The same two researchers then independently reviewed the full text of the selected articles to assess their eligibility for inclusion. In cases of studies performing both 18F-FDG PET and PET/CT, whether sufficient information on the findings of the subgroup performing 18F-FDG PET/CT were not provided, we have excluded them from the qualitative analysis. A consensus meeting among the researchers was performed to solve any disagreement.
For each study included in the qualitative analysis, we collected the following information: authors, year of publication, country of origin, study design and level of evidence, patient characteristics (overall number of SCLC patients performing 18F-FDG PET/CT, mean age, sex ratio), technical aspects (hybrid imaging modality, injected radiopharmaceutical activity, time interval between radiopharmaceutical injection and image acquisition, image analysis, comparison with other imaging methods), diagnostic accuracy of 18F-FDG PET/CT for staging compared to conventional imaging (on a patient-based analysis), percentage of change of binary SCLC staging (from LD-SCLC to ED-SCLC and vice versa) using 18F-FDG PET/CT, and impact of 18F-FDG PET/CT on the survival outcome of SCLC patients.
The overall quality of the studies included in the systematic review and meta-analysis was performed using the revised “Quality Assessment of Diagnostic Accuracy Studies” tool (12).
The change of binary SCLC staging by 18F-FDG PET/CT was defined as the ratio between the number of SCLC patients with a change of staging obtained by 18F-FDG PET/CT (upstaging from LD-SCLC to ED-SCLC or downstaging from ED-SCLC to LD-SCLC, respectively) and the overall number of SCLC patients who underwent the 18F-FDG PET/CT scan. Pooled analyses were performed using a random-effects model (taking into account the variability between studies). Pooled data were presented with their respective 95% confidence interval (95% CI) values and displayed using forest plots. Heterogeneity was estimated using the I-square index (I2) (13). Publication bias was assessed through the Egger's test (14). StatsDirect software version 3 (StatsDirect Ltd., Cambridge, UK) was used for the meta-analysis.
The results of the literature search are reported in Figure 1. The comprehensive computer literature search revealed 165 articles. Reviewing titles and abstracts, 152 records were excluded: 136 because they were outside the field of interest of this systematic review; 10 as reviews, comments, editorials, or letters; and 6 as case reports. Thirteen articles were selected, and their full text was retrieved (15–27). No additional records were found screening the reference list of the retrieved articles. Four articles were excluded from the analysis because both 18F-FDG PET and PET/CT were performed, but sufficient information about the findings of the 18F-FDG PET/CT subgroup were not provided (15–18). Finally, nine articles including 791 patients who underwent 18F-FDG PET/CT were eligible for the systematic review (19–27). Six articles about change of binary SCLC staging using 18F-FDG PET/CT in 277 patients were included in the meta-analysis (6, 20–22, 24, 27). The characteristics of the included studies are presented in Tables 1–3. The overall quality assessment of these studies is reported in Figure 2.
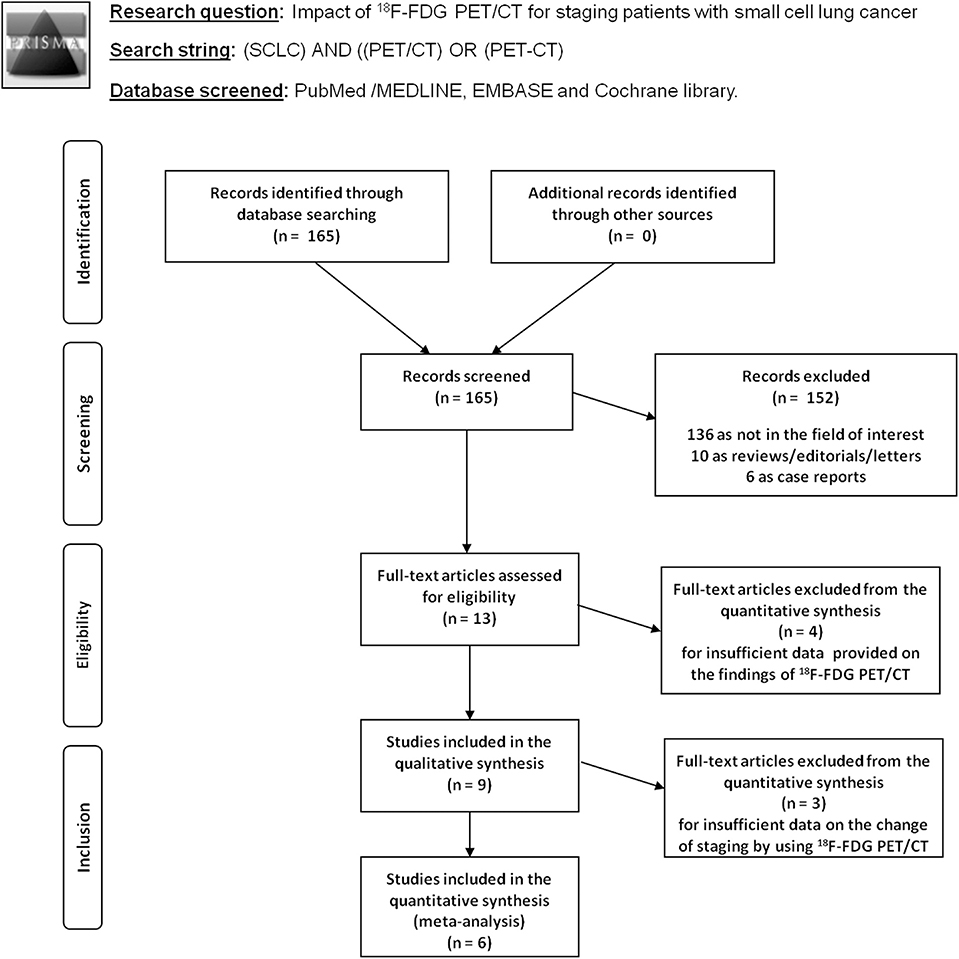
Figure 1. Flow chart of the search for eligible studies on the impact of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) for small cell lung cancer (SCLC) staging.
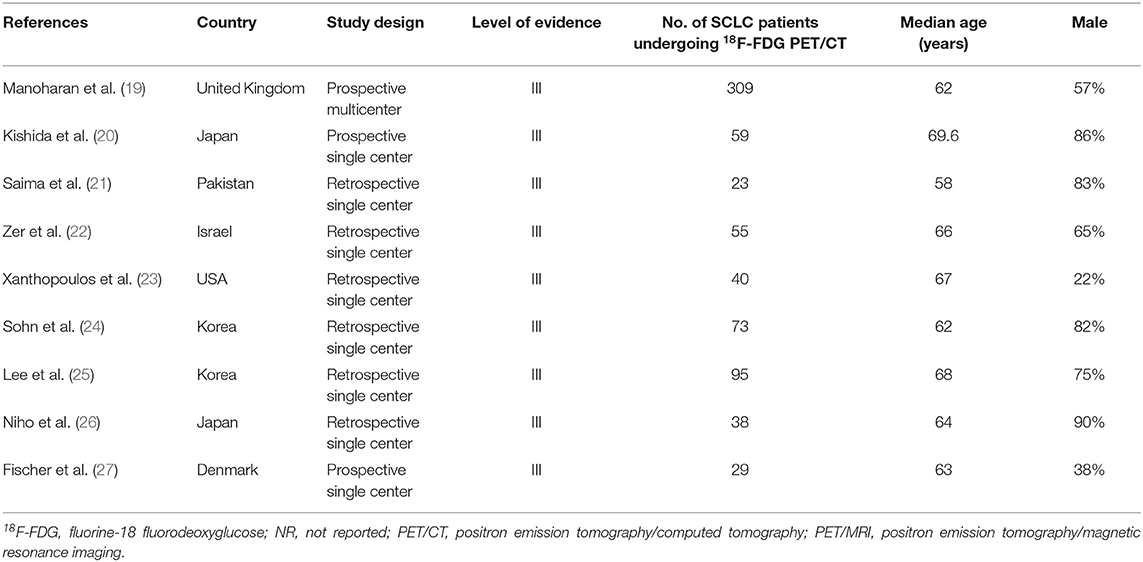
Table 1. Basic study and patient characteristics on the impact of 18F-FDG PET/CT in staging patients with small cell lung cancer.
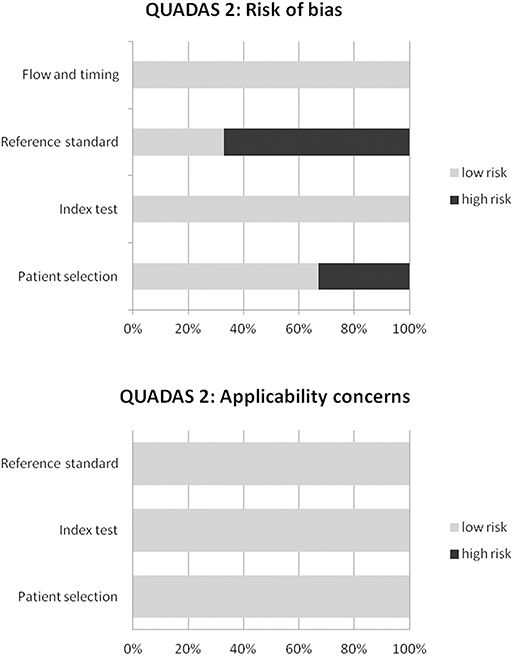
Figure 2. Overall quality assessment of the studies included in the systematic review according to the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.
Nine articles including data on the impact of 18F-FDG PET/CT in staging SCLC were selected (Table 1) (19–27). The included studies were published from 2007 to 2019. Patients from several countries and different continents were represented. One-third of the studies were prospective, and most of them were single-center studies (89%). The median age of the SCLC patients ranged from 58 to 69.6 years. The percentage of male patients widely ranged from 22 to 90%.
The technical aspects about 18F-FDG PET/CT were heterogeneous (Table 2). The hybrid imaging modality was PET/CT using low-dose CT in most of the cases and contrast-enhanced CT in 22% of the studies. Mean injected radiotracer activity were quite different among the included studies. The mean time between radiopharmaceutical injection and image acquisition was 60 min. The analysis of PET images was carried out using visual/qualitative analysis in all studies. In some studies, a further analysis using the calculation of semiquantitative parameters, for instance the maximal standardized uptake value (SUVmax), was performed. A composite reference standard including histology, further imaging, or clinical/biochemical follow-up was used in the included studies. 18F-FDG PET/CT findings were compared with CT or bone scintigraphy findings in most of the articles.
Three studies evaluated the diagnostic accuracy of 18F-FDG PET/CT staging compared to conventional staging without 18F-FDG PET/CT in patients with SCLC (20, 21, 27).
In the prospective study of Fischer et al., the diagnostic performance of 18F-FDG PET/CT in correctly staging LD-SCLC and ED-SCLC was 95% (19 out of 20 SCLC correctly staged), and it was higher compared to that of conventional imaging methods (85%; 17 out of 20 patients correctly staged) (27). The better diagnostic performance of 18F-FDG PET/CT in correctly staging LD-SCLC and ED-SCLC was also confirmed by another prospective study of Kishida et al. who found a diagnostic accuracy of 96.6% (57/59 patients correctly staged as ED-SCLC or LD-SCLC) for 18F-FDG PET/CT compared to 91.5% (54/59 patients correctly staged as ED-SCLC or LD-SCLC) for conventional staging, even if this difference was not statistically significant (20).
Six studies assessed the change of binary SCLC staging using 18F-FDG PET/CT compared to conventional staging (upstaging from LD-SCLC to ED-SCLC or downstaging from ED-SCLC to LD-SCLC, respectively) reporting percentages ranging from 5.1 to 21.7% (20–22, 24, 26, 27).
About the comparison among 18F-FDG PET/CT and bone scintigraphy for SCLC staging, the study of Lee et al. demonstrated that, in patients with SCLC, 18F-FDG PET/CT showed higher detection rate of bone metastases than bone scintigraphy. In 95 SCLC performing both methods for detecting bone metastases, the sensitivity of 18F-FDG PET/CT was 100 and 87% on a per-patient- and on a per-lesion-based analysis, respectively; conversely, the sensitivity of bone scintigraphy was 37 and 29% on a per-patient- and on a per-lesion-based analysis, respectively. Based on these findings, 18F-FDG PET/CT should replace bone scintigraphy for staging SCLC (25).
On the other hand, for detecting brain metastases of SCLC, 18F-FDG PET/CT cannot substitute brain MRI due to the limited detection rate of brain metastases by 18F-FDG PET/CT (20); the main concern about the use of 18F-FDG PET for evaluating brain lesions is the high background 18F-FDG uptake in normal brain.
About the impact of 18F-FDG PET/CT staging on outcome of SCLC patients, the survival outcomes were similar in patients staged with or without 18F-FDG PET/CT, according to the recently published analysis of the CONVERT randomized controlled trial; however, this analysis cannot support the omission of 18F-FDG PET/CT for SCLC staging due to some study limitations, as reported by the authors (19).
Xanthopoulos et al. found that LD-SCLC patients staged with 18F-FDG PET/CT exhibited improved disease control and survival when compared with LD-SCLC patients staged without 18F-FDG PET/CT. The median overall survival from diagnosis in patients staged with 18F-FDG PET/CT was 32 vs. 17 months in patients staged without PET/CT (p = 0.03), and 3-year survival was 47 vs. 19%, respectively. Median time-to-distant failure was 29 vs. 12 months, respectively (p = 0.04); median time-to-local failure was not reached vs. 16 months, respectively (p = 0.04). On multivariable analysis, 18F-FDG PET/CT staging was associated with survival (odds ratio = 0.24; p = 0.04) (23).
Six studies (277 SCLC patients) were selected for the meta-analysis on the change of binary SCLC staging using 18F-FDG PET/CT (20–22, 24, 26, 27). The pooled percentage of change of binary SCLC stage using 18F-FDG PET/CT (upstaging from LD-SCLC to ED-SCLC or downstaging from ED-SCLC to LD-SCLC, respectively) was 15% (95% CI, 9–21%) (Figure 3). A moderate heterogeneity among the included studies was found (I2 = 50%). The Egger's test did not demonstrate a significant publication bias (p = 0.08).
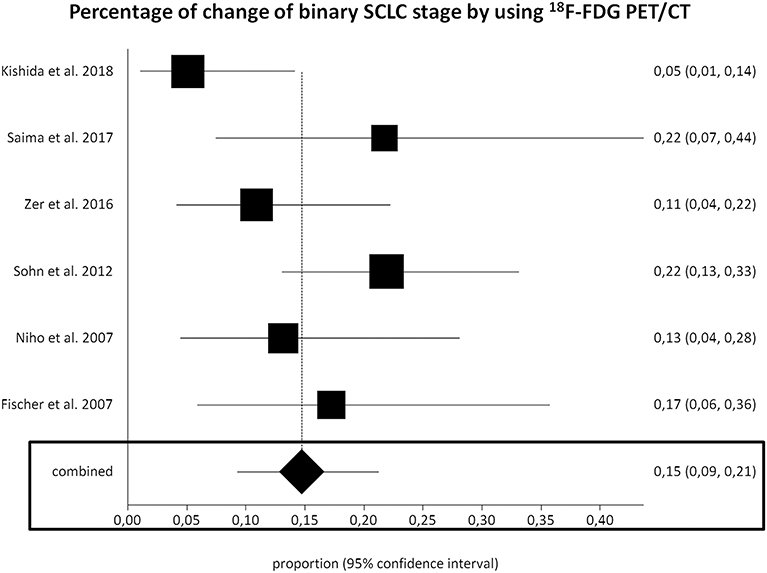
Figure 3. Plots of individual studies and pooled percentage of change of binary small cell lung cancer (SCLC) stage using fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), including 95% confidence intervals (95% CI). The size of the squares indicates the weight of each study.
Performing a subgroup analysis based on the different type of study design, the percentage of change of binary SCLC staging using 18F-FDG PET/CT in prospective and retrospective studies was 11% and (95% CI, 2–25%) and 17% (95% CI, 12–23%), respectively.
This is the first meta-analysis focused on the impact of hybrid 18F-FDG PET/CT for staging of SCLC patients. In fact, previous published evidence-based documents included mostly articles about the use of 18F-FDG PET only in staging SCLC (28–32). As the current state of the art of PET imaging in oncology is hybrid 18F-FDG PET/CT, we have provided timely and updated information selecting only articles that have evaluated the impact of SCLC staging using this hybrid imaging modality.
Several studies have used 18F-FDG PET/CT for staging SCLC patients; however, most of these studies have limited statistical power, due to their relatively small patient population. We have pooled data from the published studies to provide more robust estimates on the impact of 18F-FDG PET/CT for staging SCLC patients.
The most relevant information provided by our systematic review and meta-analysis is that 18F-FDG PET/CT has an impact in staging SCLC patients, as this method may change the binary SCLC stage (from LD-SCLC to ED-SCLC or vice versa) in ~15% of patients. This change of binary SCLC stage automatically leads to a change of therapeutic management, as only LD-SCLC may have benefit of a radiation therapy for curative intent (4).
The change of binary SCLC stage may be related to the better diagnostic accuracy of 18F-FDG PET/CT compared to conventional imaging methods in detecting SCLC metastases in different organs (except for brain metastases), as functional abnormalities detected by 18F-FDG PET usually precede morphological alterations revealed by conventional imaging methods (5). To this regard, in a previous meta-analysis, the pooled sensitivity, specificity, and positive and negative likelihood ratio (LR+ and LR–) of 18F-FDG PET or PET/CT in diagnosing ED-SCLC were 97.5% (95%CI, 94.2–99.2%), 98.2% (95% CI, 94.9–99.6%), 19.86 (95% CI, 9.79–40.3), and 0.06 (95% CI, 0.03–0.1), respectively (29). There were no significant differences in diagnostic accuracy between 18F-FDG PET/CT and PET alone in the detection of ED-SCLC, but limited data about 18F-FDG PET/CT were available (29).
Current National Comprehensive Cancer Network guidelines suggest to perform 18F-FDG PET/CT for staging SCLC only if LD-SCLC is suspected (4). Furthermore, the American College of Radiology appropriateness criteria recommend the use of 18F-FDG PET/CT in patients with suspicious LD-SCLC being considered for treatment with curative intent, whereas the use of 18F-FDG PET/CT for further staging is considered optional if ED-SCLC is established (33). Evidence-based data provided by our meta-analysis strengthen the current recommendations on the use of 18F-FDG PET/CT for staging SCLC patients.
Some limitations of our analysis should be underlined. The first limitation is the low number of included studies on the impact of 18F-FDG PET/CT in staging SCLC. Another limitation is the heterogeneity among the included studies likely based on differences of patient characteristics, methodological aspects, and study quality. Conversely, we did not detect a significant publication bias in our meta-analysis.
Based on the data reported in our systematic review, we would like to suggest to perform more large multicentric and prospective studies on the impact of 18F-FDG PET/CT for SCLC staging to strengthen its role in this setting.
Furthermore, more prospective studies to clarify the impact of 18F-FDG PET/CT staging on the survival outcomes of SCLC patients would be useful to support its routine clinical use for SCLC staging. Unfortunately, the available data to this regard are not univocal (19, 23). The major advantages of 18F-FDG PET/CT staging in SCLC may lie in avoiding unnecessary toxicity in ED-SCLC and in suggesting the appropriate addition of thoracic radiation therapy in LD-SCLC with potential better patient survival (5). It has been recently demonstrated by a retrospective study in a mixed SCLC patient population performing both 18F-FDG PET and PET/CT that 18F-FDG PET staging in patients with SCLC was associated with greater overall survival and lung-cancer-specific survival, likely reflecting stage migration and stage-appropriate therapy (17).
Lastly, cost-effectiveness analyses on the use of 18F-FDG PET/CT in staging SCLC are currently lacking in the literature. One article performed a cost analysis in a European setting, taking into account the overall costs of staging and therapy of SCLC, using two different strategies for staging (staging with 18F-FDG PET/CT compared to conventional staging without 18F-FDG PET/CT), reporting a non-significant difference in overall costs among these strategies (27). Another cost analysis in an Australian setting demonstrated that the initial costs of the two staging strategies, with or without 18F-FDG PET/CT, were not significantly different. 18F-FDG PET/CT staging may reduce healthcare costs for SCLC patients through avoidance of inappropriate thoracic radiation therapy (31).
18F-FDG PET/CT is a very useful imaging method in staging SCLC patients, as it may change the binary SCLC stage, resulting in change of therapeutic management, in ~15% of cases. Further well-designed studies on the use of this hybrid imaging method for SCLC staging are needed.
The datasets generated for this study are available on request to the corresponding author.
All authors contributed to the manuscript. Different tasks are described here below. FM and GT: conceptualization. GT and MP: methodology. FM, MP, MV, GP, PF, LG, and AR: data curation. FM and GT: writing. MV, GP, PF, LG, and AR: review and editing.
This meta-analytic project was funded by the Advisory Board of Research of Ente Ospedaliero Cantonale (ABREOC) (Principal investigator: FM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors are grateful to the staff of the Clinical Trial Unit of Ente Ospedaliero Cantonale (CTU-EOC) for their support.
1. Lu T, Yang X, Huang Y, Zhao M, Li M, Ma K, et al. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag Res. (2019) 11:943–53. doi: 10.2147/CMAR.S187317
2. Kalemkerian GP, Schneider BJ. Advances in small cell lung cancer. Hematol Oncol Clin North Am. (2017) 31:143–56. doi: 10.1016/j.hoc.2016.08.005
3. Oronsky B, Reid TR, Oronsky A, Carter CA. What's new in SCLC? A review. Neoplasia. (2017) 19:842–7. doi: 10.1016/j.neo.2017.07.007
4. Kalemkerian GP, Loo BW, Akerley W, Attia A, Bassetti M, Boumber Y, et al. NCCN guidelines insights: small cell lung cancer, version 2.2018. J Natl Compr Canc Netw. (2018) 16:1171–82. doi: 10.6004/jnccn.2018.0079
5. Sager O, Dincoglan F, Demiral S, Uysal B, Gamsiz H, Elcim Y, et al. Utility of molecular imaging with 2-deoxy-2-[fluorine-18] fluoro-dglucose positron emission tomography (18F-FDG PET) for Small cell lung cancer (SCLC): a radiation oncology perspective. Curr Radiopharm. (2019) 12:4–10. doi: 10.2174/1874471012666181120162434
6. Lococo F, Treglia G, Cesario A, Paci M, Filice A, Versari A, et al. Functional imaging evaluation in the detection, diagnosis, and histologic differentiation of pulmonary neuroendocrine tumors. Thorac Surg Clin. (2014) 24:285–92. doi: 10.1016/j.thorsurg.2014.04.004
7. Lococo F, Cesario A, Paci M, Filice A, Versari A, Rapicetta C, et al. PET/CT assessment of neuroendocrine tumors of the lung with special emphasis on bronchial carcinoids. Tumour Biol. (2014) 35:8369–77. doi: 10.1007/s13277-014-2102-y
8. Treglia G, Kroiss AS, Piccardo A, Lococo F, Santhanam P, Imperiale A. Role of positron emission tomography in thyroid and neuroendocrine tumors. Minerva Endocrinol. (2018) 43:341–55. doi: 10.23736/S0391-1977.17.02742-0
9. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, Clifford T, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. (2018) 319:388–96. doi: 10.1001/jama.2017.19163
10. Sadeghi R, Treglia G. Systematic reviews and meta-analyses of diagnostic studies: a practical guideline. Clin Transl Imaging. (2017) 5:83–7. doi: 10.1007/s40336-016-0219-2
11. Treglia G, Sadeghi R. Meta-analyses and systematic reviews on PET and PET/CT in oncology: the state of the art. Clin Transl Imaging. (2013) 1:73–5. doi: 10.1007/s40336-013-0013-3
12. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2 group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
13. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
14. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. (2006) 25:3443–57. doi: 10.1002/sim.2380
15. Azad A, Chionh F, Scott AM, Lee ST, Berlangieri SU, White S, et al. High impact of 18F-FDG-PET on management and prognostic stratification of newly diagnosed small cell lung cancer. Mol Imaging Biol. (2010) 12:443–51. doi: 10.1007/s11307-009-0295-z
16. Hillner BE, Siegel BA, Shields AF, Liu D, Gareen IF, Hunt E, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the national oncologic PET registry. J Nucl Med. (2008) 49:1928–35. doi: 10.2967/jnumed.108.056713
17. Hong JC, Boyer MJ, Spiegel DY, Williams CD, Tong BC, Shofer SL, et al. Increasing PET use in small cell lung cancer: survival improvement and stage migration in the VA central cancer registry. J Natl Compr Canc Netw. (2019) 17:127–39. doi: 10.6004/jnccn.2018.7090
18. Kamel EM, Zwahlen D, Wyss MT, Stumpe KD, von Schulthess GK, Steinert HC. Whole-body (18)F-FDG PET improves the management of patients with small cell lung cancer. J Nucl Med. (2003) 44:1911–7.
19. Manoharan P, Salem A, Mistry H, Gornall M, Harden S, Julyan P, et al. (18)F-Fludeoxyglucose PET/CT in SCLC: analysis of the CONVERT randomized controlled trial. J Thorac Oncol. (2019) 14:1296–305. doi: 10.1016/j.jtho.2019.03.023
20. Kishida Y, Seki S, Yoshikawa T, Itoh T, Maniwa Y, Nishimura Y, et al. Performance comparison between (18)F-FDG PET/CT plus brain MRI and conventional staging plus brain MRI in staging of small cell lung carcinoma. AJR Am J Roentgenol. (2018) 211:185–92. doi: 10.2214/AJR.17.18935
21. Saima R, Humayun B, Khalid NI. Triage of limited versus extensive disease on (18)F-FDG PET/CT scan in small cell lung cancer. Asia Ocean J Nucl Med Biol. (2017) 5:109–13. doi: 10.22038/aojnmb.2017.8751
22. Zer A, Domachevsky L, Rapson Y, Nidam M, Flex D, Allen AM, et al. The Role of 18F-FDG PET/CT on staging and prognosis in patients with small cell lung cancer. Eur Radiol. (2016) 26:3155–61. doi: 10.1007/s00330-015-4132-2
23. Xanthopoulos EP, Corradetti MN, Mitra N, Fernandes AT, Kim M, Grover S, et al. Impact of PET staging in limited-stage small-cell lung cancer. J Thorac Oncol. (2013) 8:899–905. doi: 10.1097/JTO.0b013e31828e8996
24. Sohn BS, Lee DH, Kim EK, Yoon DH, Kim HO, Ryu JS, et al. The role of integrated 18F-FDG PET-CT as a staging tool for limited-stage small cell lung cancer: a retrospective study. Onkologie. (2012) 35:432–8. doi: 10.1159/000341073
25. Lee JW, Lee SM, Lee HS, Kim YH, Bae WK. Comparison of diagnostic ability between (99m)Tc-MDP bone scan and (18)F-FDG PET/CT for bone metastasis in patients with small cell lung cancer. Ann Nucl Med. (2012) 26:627–33. doi: 10.1007/s12149-012-0622-3
26. Niho S, Fujii H, Murakami K, Nagase S, Yoh K, Goto K, et al. Detection of unsuspected distant metastases and/or regional nodes by FDG-PET scan in apparent limited-disease small-cell lung cancer. Lung Cancer. (2007) 57:328–33. doi: 10.1016/j.lungcan.2007.04.001
27. Fischer BM, Mortensen J, Langer SW, Loft A, Berthelsen AK, Petersen BI, et al. A prospective study of PET/CT in initial staging of small-cell lung cancer: comparison with CT, bone scintigraphy and bone marrow analysis. Ann Oncol. (2007) 18:338–45. doi: 10.1093/annonc/mdl374
28. Kalemkerian GP, Gadgeel SM. Modern staging of small cell lung cancer. J Natl Compr Canc Netw. (2013) 11:99–104. doi: 10.6004/jnccn.2013.0012
29. Lu YY, Chen JH, Liang JA, Chu S, Lin WY, Kao CH. 18F-FDG PET or PET/CT for detecting extensive disease in small-cell lung cancer: a systematic review and meta-analysis. Nucl Med Commun. (2014) 35:697–703. doi: 10.1097/MNM.0000000000000122
30. Mitchell MD, Aggarwal C, Tsou AY, Torigian DA, Treadwell JR. Imaging for the pretreatment staging of small cell lung cancer: a systematic review. Acad Radiol. (2016) 23:1047–56. doi: 10.1016/j.acra.2016.03.017
31. Ruben JD, Ball DL. The efficacy of PET staging for small-cell lung cancer: a systematic review and cost analysis in the Australian setting. J Thorac Oncol. (2012) 7:1015–20. doi: 10.1097/JTO.0b013e31824fe90a
32. Thomson D, Hulse P, Lorigan P, Faivre-Finn C. The role of positron emission tomography in management of small cell lung cancer. Lung Cancer. (2011) 73:121–6. doi: 10.1016/j.lungcan.2011.03.013
Keywords: positron emission tomography/CT, molecular imaging, staging, lung cancer, small cell lung cancer
Citation: Martucci F, Pascale M, Valli MC, Pesce GA, Froesch P, Giovanella L, Richetti A and Treglia G (2020) Impact of 18F-FDG PET/CT in Staging Patients With Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Med. 6:336. doi: 10.3389/fmed.2019.00336
Received: 27 September 2019; Accepted: 23 December 2019;
Published: 29 January 2020.
Edited by:
Ronan Abgral, Centre Hospitalier Regional Universitaire (CHU) de Brest, FranceReviewed by:
Xavier Palard-Novello, University of Rennes 1, FranceCopyright © 2020 Martucci, Pascale, Valli, Pesce, Froesch, Giovanella, Richetti and Treglia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giorgio Treglia, Z2lvcmdpby50cmVnbGlhQGVvYy5jaA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.