- 1Department of Obstetrics and Gynecology, Shengjing Hospital of China Medical University, Shenyang, China
- 2Key Laboratory of Maternal-Fetal Medicine of Liaoning Province, Benxi, China
- 3Key Laboratory of Obstetrics and Gynecology of Higher Education of Liaoning Province, Benxi, China
Objective: The aim of this study was to develop a nomogram to predict the risk of placenta accreta in scarred uterus patients in China.
Methods: We retrospectively analyzed 8,371 singleton pregnancies with scarred uterus at Shengjing Hospital, affiliated with China Medical University. Two thirds of the patients were randomly assigned to the training set (n = 5,581), and one third were assigned to the validation set (n = 2,790). Multivariate logistic regression was performed by using the training set, and the nomogram was developed. Discrimination and calibration were performed by using both the training and validation sets.
Results: The multivariate logistic regression model identified number of previous cesarean section, number of vaginal bleeding, medication during pregnancy, and placenta previa as covariates associated with placenta accreta. A nomogram was developed to predict the risk of placenta accreta in the training set with a Harrell's C-index of 0.93 and 0.927 in the training set and validation set, respectively. Calibration of the nomogram predicted placenta accreta corresponding closely with the actual placenta accreta.
Conclusion: We developed a nomogram predicting the risk of placenta accreta in scarred uterus patients in China. Validation using both the training set and the validation set demonstrated good discrimination and calibration, suggesting good clinical utility.
Introduction
With the introduction of the two-child policy in China, there have been increases in numbers of women with scarred uterus, who are therefore prone to uterine rupture, postpartum hemorrhage, placenta previa, and placenta accreta (PA) (1–5).
Placenta accreta refers to abnormal adherence of placental chorionic villi to the underlying myometrium with an absence of decidua basalis. It is classified into three types based on histopathology: PA when the chorionic villi just adhere to the myometrium, placenta increta when the chorionic villi invade the myometrium, and placenta percreta when the villi invade the full thickness of the myometrium and may even penetrate the uterine serosa (6–8). PA is associated with an increased risk of maternal morbidity and mortality, preterm birth, low birth weight, perinatal mortality, recurrent PA, uterine rupture, and postpartum hemorrhage in subsequent pregnancies (9–12). Considering this, it is important to establish a risk assessment tool for use during the earlier trimester in order to improve perinatal and maternal outcomes.
The incidence of PA has increased over the past 40 years along with the increased incidence of cesarean section (CS) (13–16), and is now reported to occur in 2–90 per 10,000 births (9, 13, 14, 17). Differences in study population and diagnostic criteria may account for this wide range. Another major risk factor for PA after CS is placenta previa (10, 12, 13, 16–20). Several other risk factors have also been reported, including cryopreserved embryo transfer, older maternal age, prior uterine surgery, parity, a higher body mass index, tobacco use, coexisting hypertension or diabetes, elevated second-trimester levels of α-fetoprotein and β-human chorionic gonadotropin, and a previous retained placenta or PA (16, 18–21).
Although many risk factors for PA in women with scarred uterus have been identified, to date, there has not been an established method for determining the effects of multiple risk factors and for determining the probability of PA in an individual woman in China, especially in northeast China. The purpose of this study was to combine the risk factors associated with PA into a prediction nomogram based on the data from a single large-volume institution.
Methods
Study Population
We undertook a retrospective study of women with scarred uterus who were patients at Shengjing Hospital, China Medical University, from January 2013 to December 2017. The clinical data were evaluated, and the inclusion criteria were as follows: scarred uterus (with at least one history of previous CS or history of myomectomy), singleton pregnancies, and termination of pregnancy at 24–44 weeks via vaginal delivery or CS. The exclusion criteria were as follows: cases with twins or multiple pregnancies, and termination of pregnancy at <24 weeks or more than 44 weeks.
The diagnosis of PA was made on clinical grounds, based on the objective difficulties experienced by the attending physician in removing the placenta during CS, or retained placenta in difficult and incomplete manual removal despite active management in the third stage of labor during vaginal delivery. If there was difficulty in diagnosis, pathological examination of remaining placenta attached to the uterus following manual removal was performed. The definition of PA included all pregnancies with partially or totally adherent placenta. There were only 102 cases further diagnosed by pathological examination.
This study was approved by the Ethical Committee of Shengjing Hospital, China Medical University.
Investigated Clinical Characteristics
The clinical data came from the HIS system in our hospital and included basic patient demographics (age and body mass index), history of gestation (gravity, parity, number of previous vaginal delivery, number of previous CS, number of previous induction, and number of previous medical abortion), history of current illness, past medical history, and history of other obstetric diseases (including placenta previa, gestational diabetes, pregnancy-induced hypertension syndrome, etc.). To obtain information on any previous CS or myomectomy, we conducted a telephone follow-up of the patients (including time between last CS and pregnancy, whether previous CS was an elective CS, or whether previous CS was performed during labor).
Placenta previa was defined as the condition where the placenta, by lying in the lower uterine segment, partially or completely obstructs the internal orifice of the cervix.
Medications during pregnancy included antihypertensive drugs (labetalol, etc.), hypoglycemic drugs (insulin, etc.), hormones (progesterone, etc.), antibiotics, levothyroxine sodium, non-steroidal anti-inflammatory drugs (aspirin, etc.), and so on.
Statistical Analysis
The analyses were performed using a split-sampling strategy: the overall sample was randomly split with stratification by center into training and validation sets. The former was used for model building, and the latter was used only for model testing purposes.
For model building, statistical analyses consisted of a series of steps. First, the categorical data were expressed as proportion and analyzed by chi-square test or Fisher's exact probability test. The continuous variables were expressed as mean ± SD and analyzed by two-sample t-test and the Mann–Whitney U-test if the data were not normally distributed. Second, multivariate logistic regression analyses were performed to assess features associated with PA. Variables tested included significant variables in the first step. Covariates were then removed by backward stepwise selection method until all covariates in the final model had a value of P < 0.05, and a binary logistic regression was developed. Finally, multivariate regression coefficients of the independent predictors of PA were then used to develop a nomogram predicting the probability of PA.
Nomogram validation consisted of discrimination (Harrell's C-index) and calibration (calibration plots) by both using the training set and the validation set. In general, a C-index value >0.75 was considered to represent relatively good discrimination. The validations for risk model were performed by bootstrapping 1,000 times in the training set and the validation set.
All statistical analyses were analyzed by the SPSS 22.0 software and R software version 3.5.1 (http://www.r-project.org) with the “sampling” and “rms” packages. We considered P < 0.05 as being statistically significant in a two-tailed test.
Results
Patient Characteristics
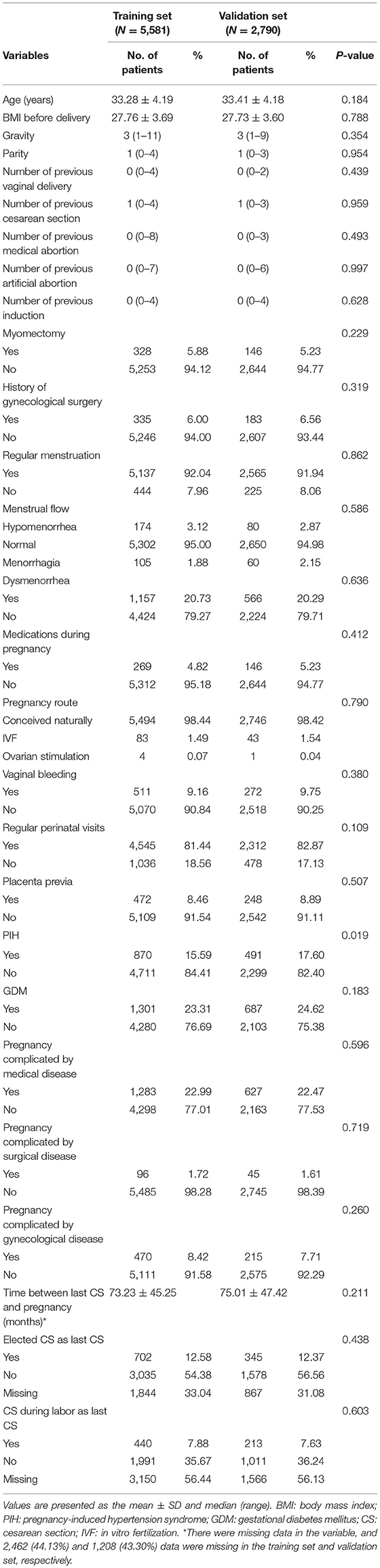
The study included 8,371 cases with scarred uterus, with the incidence of PA at 5.2%. Table 1 shows the basic characteristics of the training set (N = 5,581) and the validation set (N = 2,790). The mean ages were 33.28 ± 4.19 and 33.41 ± 4.18 years, and the times between last CS and pregnancy were 73.23 ± 45.25 and 75.01 ± 47.42 months, with 44.13% (2,463/5,581) and 43.30% (1,208/2,790) with missing data in the training set and validation set, respectively. In the training set and validation set, 90.84 and 90.25% of patients, respectively, had no bleeding during pregnancy. All in all, the patient's characteristics were similar between the training set and the validation set.
Risk Factors of PA
After examination and transformation of variables to fit the logistic regression model, variables were selected by the backward stepwise selection method (P < 0.05). The results of univariable analyses are shown in Table 2, and the multivariable logistic regression analyses are shown in Table 3. In the PA group, there were 57 (19.7%) patients with missing data in the time between last CS and pregnancy, 148 (51.0%) patients with elective CS as last CS, and 151 (52.1%) patients with CS during labor as the last CS. In the non-PA group, there were 2,406 (45.5%) with missing data in the time between last CS and pregnancy, 1,696 (32.1%) with elected CS as last CS, and 2,999 (56.7%) with CS during labor as last CS. In univariable analyses, in the presented data, the times between last CS, CS during labor as last CS and elective CS as last CS were analyzed, with the missing data overlooked. There was statistical significance only in the times between last CS and pregnancy; however, we excluded this in multivariable logistic regression due to the large percentage of missing data. The number of previous CS, number of vaginal bleeding, medications during pregnancy, and placenta previa were all found to be independent risk factors for PA (P = 0.044, P = 0.003, P = 0.003, and P < 0.001, respectively).
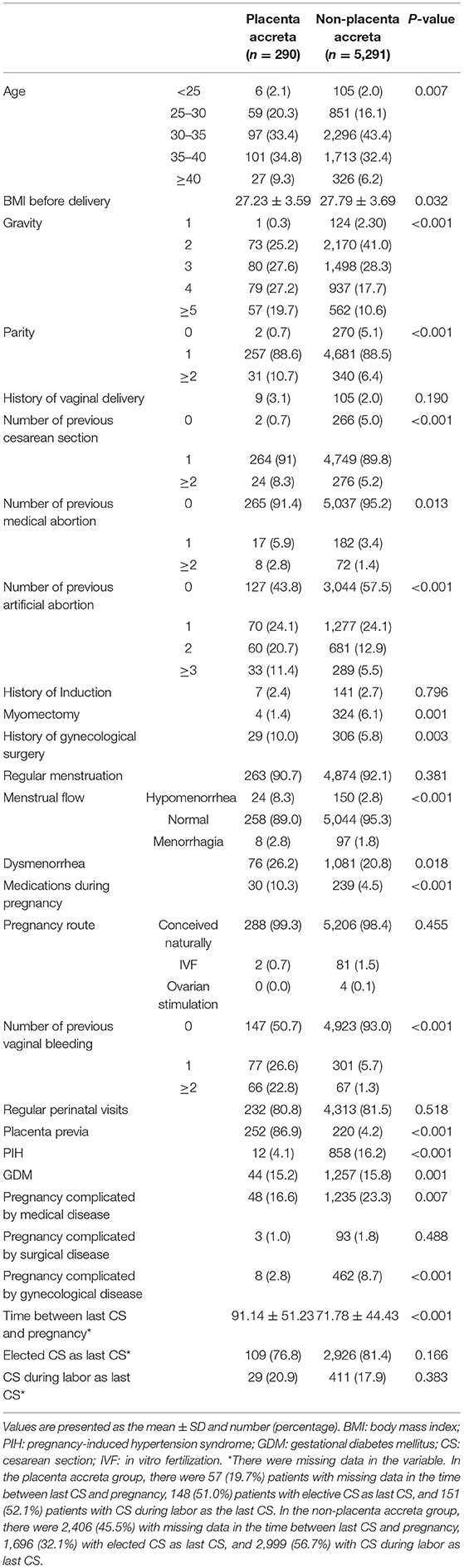
Table 2. Univariable analyses between the placenta accreta group and the non-placenta accreta group in the training set.
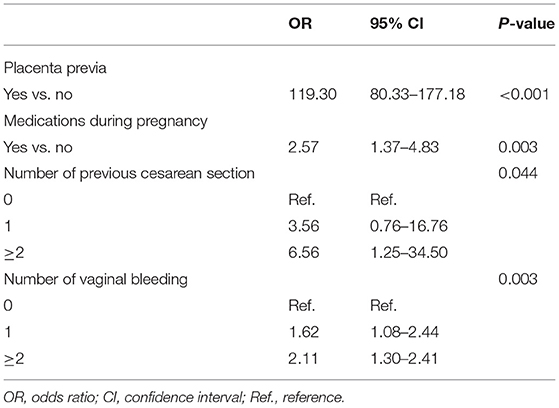
Table 3. Multivariable logistic regression analyses between the placenta accreta group and the non-placenta accreta group in the training set (N = 5,581).
Development and Validation of Nomogram
Based on the coefficients of the logistic regression model that identified independent risk factors of PA, a novel nomogram was then developed to predict the risk of PA (Figure 1). In the training set, Harrell's C-index was 0.93. Figure 2A shows the calibration plot of the nomogram in the training set. The x-axis is the predicted PA calculated by the nomogram, and the y-axis is the observed PA. Discrimination and calibration were found to be excellent in the validation set. The Harrell's C-index was 0.927. The nomogram was well-calibrated, with a good correlation between predicted and observed PA (Figure 2B). The receiver operating characteristic curves of training set and validation set are presented in Figure 3.
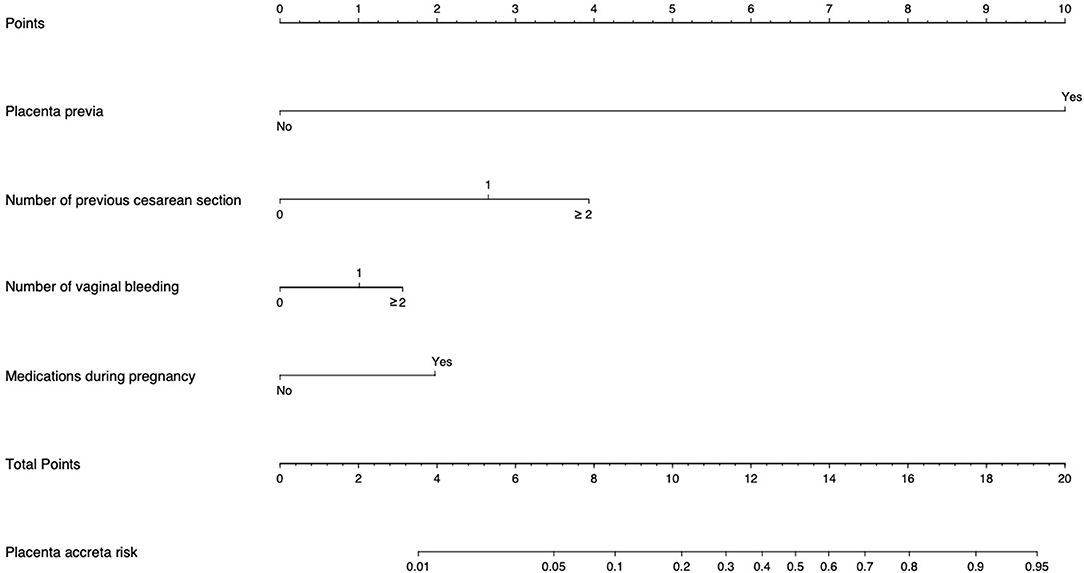
Figure 1. Placenta accreta Risk Assessment Tool. “Points” refers to point for the individual risk factor and add together to the “Total points.” “Placenta accreta risk” was calculated according to the ‘'Total points.” Example: For a patient with one previous CS (score = 2.6), with one vaginal bleeding (score = 1), with placenta previa (score = 10), and medication during pregnancy (score = 1.9), the total score is 15.5 corresponding to a 74% risk of placenta accreta (PA).
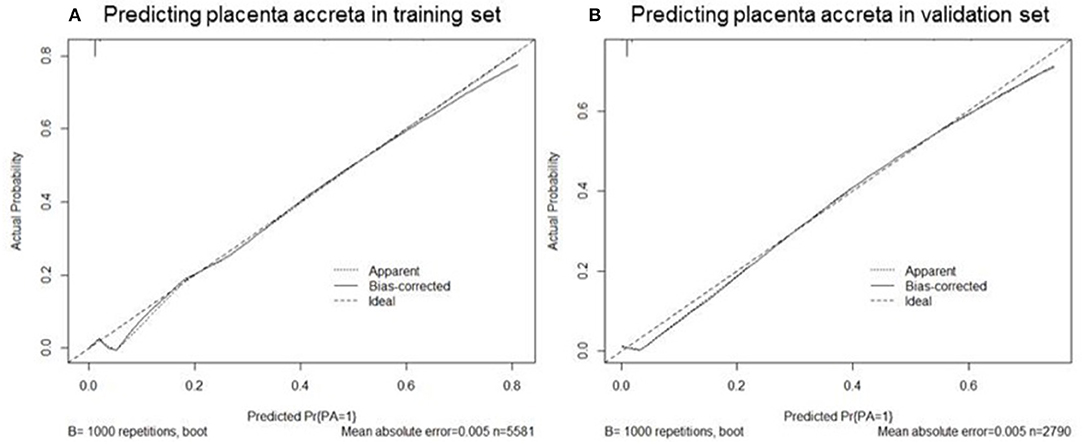
Figure 2. Calibration plots of nomogram to predict the probability of placenta accreta in the training set (A) and validation set (B). The x-axis is the predicted placenta accreta calculated by the nomogram, and the y-axis is the observed placenta accreta. The “Ideal” is the ideal curve, and the solid line “Bias-corrected” is the actual curve.
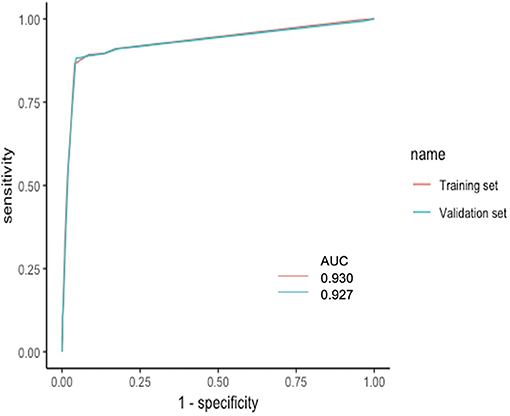
Figure 3. ROC curves of training set and validation set. The x-axis is the “1—Specificity,” and the y-axis is “Sensitivity”. AUCs were also presented with 0.930 and 0.927, respectively. AUC, area under the curve; ROC, receiver operating characteristic.
Discussion
We developed and validated a new nomogram that included number of previous CS, number of vaginal bleeding, medications during pregnancy, and placenta previa as covariates for estimating the risk of PA in pregnancy with scarred uterus.
In our study, the incidence of PA was 5.2%, which was higher than previous studies. This can be explained by the fact that in this study, the study population was limited to scarred uterus patients who had a history of CS or myomectomy.
In our study, we found number of vaginal bleeding to be an independent risk factor for PA. Rac et al. thought that vaginal bleeding decreased with advancing gestation in pregnancies with PA (22). Furthermore, women with a non-adherent low-lying placenta or placenta previa had higher rates of bleeding when compared to PA (23). In other words, women with PA may not bleed as much as their non-adherent counterparts. However, we found that number of vaginal bleeding is an independent risk factor for PA. The difference may result from the inclusion of patients in our study, while the former study is associated only with patients with prior CS and persistent placenta previa. Placenta previa was thought to be the major risk factor for PA in a review (24). Of course, in our study, placenta previa is also an independent risk factor for PA and may be related to number of vaginal bleeding.
In this study, cases were included in the medication group regardless of the type of medication used during pregnancy. In previous studies, compared to non-users, medication users were more likely to have spontaneous abortion, preterm birth, stillbirth, neonatal death, children with congenital malformations, postpartum depression, and decreased birth weight (25–27). To date there is no literature on the association between medications during pregnancy and PA. In our study, we found that all medications used during pregnancy, regardless of the type of medication, may lead to PA. In univariable analyses, pregnancies complicated by disease, including gestational diabetes mellitus and pregnancy-induced hypertension syndrome, were associated with PA, but not independently. However, medication, which may be associated with various diseases during pregnancy, is an independent risk factor for PA. Medication may be taken during pregnancy not only to treat diseases during pregnancy but for other purposes as well; for example, some patients may use antibiotics to prevent infection, or take vitamins as nutritional supplement, or take them for protection of the fetus during pregnancy (steroid hormones and Chinese herbal medicines). Moreover, some patients with these diseases may not be treated with medication during pregnancy. Therefore, there is no established correlation between medication taken during pregnancy and diseases occurring during pregnancy; nevertheless, we speculate that medication taken for disease during pregnancy is an independent risk factor for PA.
In addition, there may be other factors at work that may explain why medication is an independent risk factor. PA is associated with decidual defects, abnormal trophoblast invasion, and abnormal placental angiogenesis (28). We doubt that medication in the fetal/placental circulation affects the secretion of certain factors in the placenta, which influence trophoblast invasion and angiogenesis of the placenta. Moreover, medications such as heparin and aspirin may affect the systemic blood circulation and the secretion of cytokines, which may lead to changes in maternal systemic and endometrial hormone levels, which may in turn affect endometrial decidualization, resulting in the implantation of the embryo and PA (29, 30). However, there is currently insufficient evidence to be certain whether medication during pregnancy is an independent risk for PA. Thus, from our study, we suggest that women with a scarred uterus, when using medications during pregnancy, should be aware of the risk of PA. Since decidualization and trophoblast invasion occurred between the first and early second trimesters (31), we assumed that medication taken after the early second trimester plays a minor role in PA. Furthermore, given that the route of administration of the medication, whether oral, vaginal, or intravenous, may result in different serum or endometrial medication levels, and also given that the pharmacokinetics of many drugs are altered during pregnancy (32), the doses of medication should also change during pregnancy. A different route and a changed dose could lead to a different degree of endometrial decidualization. In the future, the time, doses, and route of medication during pregnancy should also be studied in PA patients, in order to determine the association between PA and medication during pregnancy more clearly.
In our study, we found that a history of myomectomy is a risk factor for PA, but not an independent one, which is a similar finding to that of Gyamfi-Bannerman et al. (33). Previous studies have found an increasing risk of PA with an increasing number of cesarean deliveries (12, 13, 34). We also found a history of CS to be an independent risk factor. This finding may be explained by the potentially larger area of anatomical disruption from a CS scar as opposed to the smaller myomectomy scar.
Carusi (20) and Zeng et al. (35) both considered age to be a risk factor for PA. However, after stratification in multivariate regression, age was found not to be an independent risk factor for PA as a result of a scarred uterus in older patients.
Multidisciplinary teamwork and operator experience have been shown to reduce collateral damage, with several studies demonstrating that maternal morbidity is significantly reduced by delivery in a specialist center for PA patients (36–38). However, medical institutions at or below the county level cannot identify and diagnose PA at an early stage and lack experience in treating PA, due to the uneven distribution of medical resources and the uneven development of urban and rural areas in China, which leads in turn to poor maternal and perinatal outcomes. The nomogram of this study is based solely on clinical data (except for ultrasound images), and the validation using both the training set and the validation set revealed good discrimination and calibration. The nomogram can help doctors in primary hospitals to detect the risk of PA at an early stage in order to better manage a high-risk population so they may then refer patients to a specialist center to improve the maternal and perinatal outcomes.
The advantages of our study include the development of a novel nomogram to predict the risk of PA, which can be used in primary hospitals in northeast China, and even be extended to the whole of China. Furthermore, the cases in our study were taken from Shengjing Hospital affiliated to China Medical University, which is a tertiary hospital and the Obstetrical Critical Care Center in northeast China, and they are representative of the epidemiological trends evident in northeast China. Furthermore, our study was supported by the “The National Key Research and Development Program of Reproductive Health & Major Birth Defects Control and Prevention” project. However, in the future, larger prospective investigations involving multiple centers from the project, as well as larger numbers of patients, could be undertaken with longer periods of follow-up, to validate and improve the nomogram.
Despite these strengths, some limitations of the present study should be acknowledged. First, in spite of the large sample size of both the training set and validation set, an external validation would have strengthened our findings. Second, the retrospective nature of the study does not totally rule out that several important variables were not tested, such as the time between last CS and pregnancy, whether CS took place during labor, and whether the last CS was an elected CS. Also, the number of missing data was not negligible; hence, the “not available” variables were not introduced in the nomogram. Thus, the model including these variables needs to be developed and validated in the future. Third, the diagnosis of PA was a clinical one, which may result in inaccuracies in determining the actual degree of invasion; and the surgeon's experience could also affect the accuracy. Fourth, patients with non-scarred uterus were excluded, and such patients, following intrauterine gynecological surgery and artificial abortion, may have endometrial defects and PA. Therefore, the risk of PA in non-scarred uterus patients or multiple pregnancies should also be explored in the future.
Bowman et al. (39) suggested that when evaluated by a diverse group of ultrasound providers who are blinded as to the patient's clinical history, the diagnostic performance characteristics of ultrasound may not be as high as has been previously reported. Therefore, it is important to identify where there is a high-risk population and further improve the diagnostic accuracy rate of ultrasound. Therefore, we suggest that the nomogram is routinely applied to those patients with a scarred uterus to evaluate the risk of PA, especially in a primary hospital setting.
In conclusion, we developed and validated a nomogram for estimating the risk of PA in scarred uterus patients in China. Future validation of the present model and nomogram is warranted to confirm our findings.
Data Availability Statement
The datasets generated for this study will not be made publicly available. All the clinic informations are generated from the HIS system of our hospital and followed up for a short time. It is patients' privacy and we should treat them confidentially.
Ethics Statement
The study was approved by the Ethical Committee of Shengjing Hospital and China Medical University, and patients gave signed informed consent.
Author Contributions
CQ and CL: concept. TY: development of the methodology and writing of the manuscript. NL: revision of the manuscript.
Funding
The study was supported by the project of The National Key Research and Development Program of Reproductive Health & Major Birth Defects Control and Prevention (No. 2016YFC100404 for CQ) and the Science and Technology Project of Liaoning Provincial Education Department (No. LS201611 for NL).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank CQ and CL for the study conceptualization and NL for the manuscript revision. We would like to thank all participating institutions and study participants.
References
1. Cheng KK, Lee MM. Rising incidence of morbidly adherent placenta and its association with previous caesarean section: a 15-year analysis in a tertiary hospital in Hong Kong. Hong Kong Med J. (2015) 21:511–7. doi: 10.12809/hkmj154599
2. Majeed T, Waheed F, Mahmood Z, Saba K, Mahmood H, Bukhari MH. Frequency of placenta previa in previously scarred and non-scarred uterus. Pak J Med Sci. (2015) 31:360–3. doi: 10.12669/pjms.312.6509
3. Daniel Seow Choon K, Eek Chaw T, Hester Chang Qi QL, Mor Jack NG, Wan Shi T, Kok Hian T. Incidence and contributing factors for uterine rupture in patients undergoing second trimester termination of pregnancy in a large tertiary hospital-a 10-year case series. Eur J Obstet Gynecol Reprod Biol. (2018) 227:8–12. doi: 10.1016/j.ejogrb.2018.05.016
4. Jastrow N, Vikhareva O, Gauthier RJ, Irion O, Boulvain M, Bujold E. Can third-trimester assessment of uterine scar in women with prior Cesarean section predict uterine rupture? Ultrasound Obstet Gynecol. (2016) 47:410–4. doi: 10.1002/uog.15786
5. Belachew J, Eurenius K, Muliclutvica A, Axelsson O. Placental location, postpartum hemorrhage and retained placenta in women with a previous cesarean section delivery: a prospective cohort study. Ups J Med Sci. (2017) 122:185–9. doi: 10.1080/03009734.2017.1356405
6. Luke RK, Sharpe JW, Greene RR. Placenta accreta: the adherent or invasive placenta. Am J Obstet Gynecol. (1966) 95:660–8. doi: 10.1016/S0002-9378(16)34741-X
7. Fox H. Placenta accreta: 1945–1969. Obstet Gynecol Surv. (1972) 27:475–90. doi: 10.1097/00006254-197207000-00001
8. Benirschke K, Burton GJ, Baergen RN. Pathology of the Human Placenta. 6th ed. Berlin: Springer-Verlag (2012). doi: 10.1007/978-3-642-23941-0
9. Vinograd A, Wainstock T, Mazor M, Beer-Weisel R, Klaitman V, Dukler D, et al. Placenta accreta is an independent risk factor for late pre-term birth and perinatal mortality. J Matern Fetal Neonatal Med. (2014) 28:1381–7. doi: 10.3109/14767058.2014.955004
10. Farquhar CM, Li Z, Lensen S, McLintock C, Pollock W, Peek MJ, et al. Incidence, risk factors and perinatal outcomes for placenta accreta in Australia and New Zealand: a case–control study. BMJ Open. (2017) 7:e017713. doi: 10.1136/bmjopen-2017-017713
11. Kabiri D, Hants Y, Shanwetter N, Simons M, Weiniger CF, Gielchinsky Y, et al. Outcomes of subsequent pregnancies after conservative treatment for placenta accreta. Int J Gynaecol Obstet. (2014) 127:206–10. doi: 10.1016/j.ijgo.2014.05.013
12. Eshkoli T, Weintraub AY, Sergienko R, Sheiner E. Placenta accreta: risk factors, perinatal outcomes, and consequences for subsequent births. Am J Obstet Gynecol. (2013) 208:219.e1–7. doi: 10.1016/j.ajog.2012.12.037
13. Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, Silver RM, et al. Risk factors for placenta accreta: a large prospective cohort. Am J Perinatol. (2014) 31:799–804. doi: 10.1055/s-0033-1361833
14. Garmi G, Salim R. Epidemiology, etiology, diagnosis, and management of placenta accreta. Obstet Gynecol Int. (2012) 2012:873929. doi: 10.1155/2012/873929
15. Bowman ZS, Manuck TA, Eller AG, Simons M, Silver RM. Risk factors for unscheduled delivery in patients with placenta accreta. Am J Obstet Gynecol. (2014) 210:e1–6. doi: 10.1016/j.ajog.2013.09.044
16. Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and meta-analysis. Am J Obstet Gynecol. (2017) 217:27–36. doi: 10.1016/j.ajog.2017.02.050
17. Thurn L, Lindqvist PG, Jakobsson M, Colmorn LB, Klungsoyr K, Bjarnadóttir RI, et al. Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: results from a large population-based pregnancy cohort study in the Nordic countries. BJOG. (2016) 123:1348–55. doi: 10.1111/1471-0528.13547
18. Balayla J, Bondarenko HD. Placenta accreta and the risk of adverse maternal and neonatal outcomes. J Perinat Med. (2013) 41:141–9. doi: 10.1515/jpm-2012-0219
19. Mullen C, Battarbee AN, Ernst LM, Peaceman AM. Occult placenta accreta: risk factors, adverse obstetrical outcomes, and recurrence in subsequent pregnancies. Am J Perinatol. (2019) 36:472–5. doi: 10.1055/s-0038-1669440
20. Carusi DA. The placenta accreta spectrum: epidemiology and risk factors. Clin Obstet Gynecol. (2018) 61:733–42. doi: 10.1097/GRF.0000000000000391
21. Kaser DJ, Melamed A, Bormann CL, Myers DE, Missmer SA, Walsh BW, et al. Cryopreserved embryo transfer is an independent risk factor for placenta accrete. Fertil Steril. (2015) 103:1176–84.e2. doi: 10.1016/j.fertnstert.2015.01.021
22. Rac MW, Wells CE, Twickler DM, Moschos E, McIntire DD, Dashe JS. Placenta accreta and vaginal bleeding according to gestational age at delivery. Obstet Gynecol. (2015) 125:808–13. doi: 10.1097/AOG.0000000000000674
23. Rac MW, Mcintire DD, Wells CE, Moschos E, Twickler DD. Cervical length in patients at risk for placenta accreta. J Ultrasound Med. (2017) 36:1431–6. doi: 10.7863/ultra.16.05059
24. Society of Gynecologic Oncology American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, Cahill AG, Beigi R, Heine P, Silver RM, et al. Placenta Accreta Spectrum. Am. J. Obstet. Gynecol. (2018) 219:B2–15. doi: 10.1016/j.ajog.2018.09.042
25. Bérard A, Sheehy O. Quebec Pregnancy Cohort: prevalence of medication use during gestation and pregnancy outcomes. Therapie. (2014) 69:71–81. doi: 10.1371/journal.pone.0093870
26. Sato R, Ikuma M, Takagi K, Yamagishi Y, Asano J, Matsunaga Y, et al. Exposure of drugs for hypertension, diabetes, and autoimmune disease during pregnancy and perinatal outcomes: an investigation of the regulator in Japan. Medicine. (2015) 94:e386. doi: 10.1097/MD.0000000000000386
27. Coughlin CG, Blackwell KA, Bartley C, Hay M, Yonkers KA, Bloch MH. Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstet Gynecol. (2015) 125:1224–3. doi: 10.1097/AOG.0000000000000759
28. Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. (2012) 33:244–51. doi: 10.1016/j.placenta.2011.11.010
29. Tersigni C, Marana R, Santamarìa A, Castellani R, Scambia G, Simone ND. In vitro evidences of heparin's effects on embryo implantation and trophoblast development. Reprod Sci. (2012) 19:454–62. doi: 10.1177/1933719111430994
30. Niringiyumukiza JD, Cai H, Xiang W. Prostaglandin E2 involvement in mammalian female fertility: ovulation, fertilization, embryo development and early implantation. Reprod Biol Endocrinol. (2018) 16:43. doi: 10.1186/s12958-018-0359-5
31. Duzyj CM, Buhimschi IA, Motawea H, Laky CA, Cozzini G, Zhao G. The invasive phenotype of placenta accreta extravillous trophoblasts associates with loss of E-cadherin. Placenta. (2015) 36:645–51. doi: 10.1016/j.placenta.2015.04.001
32. Tasnif Y, Morado J, Hebert MF. Pregnancy-related pharmacokinetic changes. Clin Pharmacol Ther. (2016) 100:53–62. doi: 10.1002/cpt.382
33. Gyamfi-Bannerman C, Gilbert S, Landon MB, Spong CY, Rouse DJ, Varner MW, et al. Risk of uterine rupture and placenta accreta with prior uterine surgery outside of the lower segment. Obstet Gynecol. (2012) 120:1332–7. doi: 10.1097/AOG.0b013e318273695b
34. Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. (2006) 107:1226–32. doi: 10.1097/01.AOG.0000219750.79480.84
35. Zeng C, Yang M, Ding Y, Duan S, Zhou Y. Placenta accreta spectrum disorder trends in the context of the universal two-child policy in China and the risk of hysterectomy. Int J Gynaecol Obstet. (2018) 140:312–8. doi: 10.1002/ijgo.12418
36. Shamshirsaz AA, Fox KA, Salmanian B, Diaz-Arrastia CR, Lee W, Baker BW, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. (2015) 212:218.e1–9. doi: 10.1016/j.ajog.2014.08.019
37. Shamshirsaz AA, Fox KA, Erfani H, Clark SL, Salmanian B, Baker BW, et al. Multidisciplinary team learning in the management of the morbidly adherent placenta: outcome improvements over time. Am J Obstet Gynecol. (2017) 216:612.e1–5. doi: 10.1016/j.ajog.2017.02.016
38. Silver RM, Fox KA, Barton JR, Abuhamad AZ, Simhan H, Huls CK, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. (2015) 212:561–8. doi: 10.1016/j.ajog.2014.11.018
Keywords: placenta accreta, nomogram, risk factors, scarred uterus, China
Citation: Yang T, Li N, Qiao C and Liu C (2019) Development of a Novel Nomogram for Predicting Placenta Accreta in Patients With Scarred Uterus: A Retrospective Cohort Study. Front. Med. 6:289. doi: 10.3389/fmed.2019.00289
Received: 16 July 2019; Accepted: 25 November 2019;
Published: 17 December 2019.
Edited by:
Salvatore Giovanni Vitale, University of Messina, ItalyReviewed by:
Fabio Barra, San Martino Hospital (IRCCS), ItalyAntonio Simone Laganà, University of Insubria, Italy
Copyright © 2019 Yang, Li, Qiao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chong Qiao, cWlhb2Nob25nMjAwMkBob3RtYWlsLmNvbQ==; Caixia Liu, bGl1Y3hzaGltZW5AMTYzLmNvbQ==
 Tian Yang1,2,3
Tian Yang1,2,3 Caixia Liu
Caixia Liu