- 1Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Neurosurgery, Suining Municipal Hospital of TCM, Suining, China
Background: Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease with different clinical and pathophysiological characteristics. Cumulative evidence shows that eosinophil levels may be connected to the therapeutic effects and phenotype of COPD. However, the prevalence of eosinophilic inflammation in COPD and the baseline characteristics of eosinophilic COPD remain unknown. Our study investigated the prevalence of COPD with eosinophil levels of >2% and the characteristics of eosinophilic COPD.
Methods: We searched the Cochrane Central Library, Medline, Embase, and the Web of Science for trials of eosinophil and COPD published from database inception to May 1, 2019.
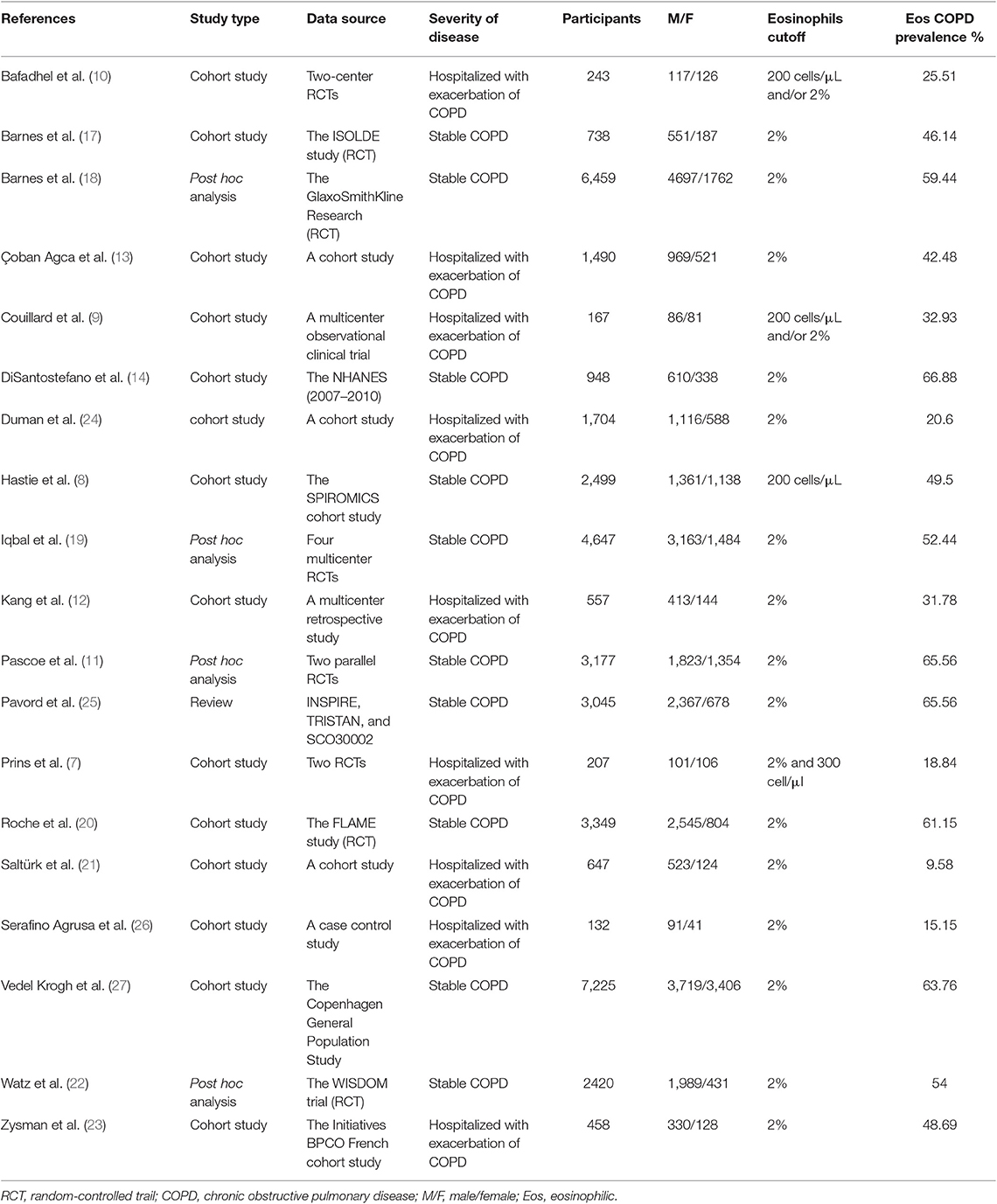
Results: In total, 40,112 COPD patients that were involved in 19 trials were included in the final analysis. The prevalence of eosinophilic COPD ranged from 18.84 to 66.88%, with an average prevalence of 54.95% across all studies. We found that men, ex-smokers, individuals with a history of ischemic heart disease, and individuals with a higher body mass index (BMI) were at higher risk of eosinophilic COPD (OR 1.36, 95% CI 1.26–1.46, P < 0.00001; OR 1.23, 1.12–1.34, P < 0.0001; OR 1.31, 1.14–1.50, P = 0.001; MD 0.70, 0.27–1.12, P = 0.001). There was, however, a lower proportion of GOLD stage I patients among those with eosinophilic COPD (OR 0.84, 0.73–0.96, P = 0.01). No significant differences were found in terms of age, current smoker status, pack-years smoked, percent of predicted forced expiratory volume in 1 s, hypertension, diabetes, or other GOLD stages between the two groups (P > 0.05).
Conclusions: Our analysis suggests that eosinophilic inflammation is prevalent in COPD. Eosinophilic COPD was more likely to occur in men, ex-smokers, those with a higher BMI, and those with a high risk of some comorbidity; however, a lower proportion of patients with eosinophilic COPD experienced mild airflow limitations.
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous disease with a variety of features and characteristics. Identification of COPD phenotypes may allow targeted therapeutic strategies. Eosinophilic inflammation is generally believed to be characteristic of asthma, whereas neutrophilic inflammation is considered to be a typical sign of COPD. However, recent reports have shown that eosinophilic inflammation occurs in COPD, in both the exacerbation and stable phases (1, 2). Growing evidence suggests that eosinophil levels may be related to the therapeutic effect and phenotypes of COPD, even after asthma patients are carefully excluded (3–6).
A sputum eosinophil level of >3% is a recognized sign of airway eosinophilic inflammation (4, 6). It was reported that blood eosinophil levels of >2% are indicative of a higher sensitivity in identifying airway eosinophil levels of >3% during COPD exacerbation (1). An alternative cut-off level (≥200 cells per μL or 300 cells per μL) has been used in some studies in addition to the 2% cut-off (7–10). Research has shown that blood eosinophil is a clinically reliable predictor of the inflammatory phenotype. We conclude from these studies that the blood eosinophil level is of reasonable importance in patients with COPD and, as such, is a promising biomarker to guide disease management.
A number of studies have investigated the prevalence and baseline clinical characteristics of patients with eosinophilic COPD. The prevalence of eosinophilic COPD, however, has differed wildly between studies. In one study, 2,083 patients (66%) had eosinophil levels of ≥2% in a post-hoc analysis that included 3,177 patients (11). In a retrospective multicenter study enrolling 605 hospitalized patients, 177 patients (29%) had blood eosinophil levels of >2% (12). In a retrospective analysis of a randomized clinical trial, 18.8% of patients had eosinophil levels of >2% (7).
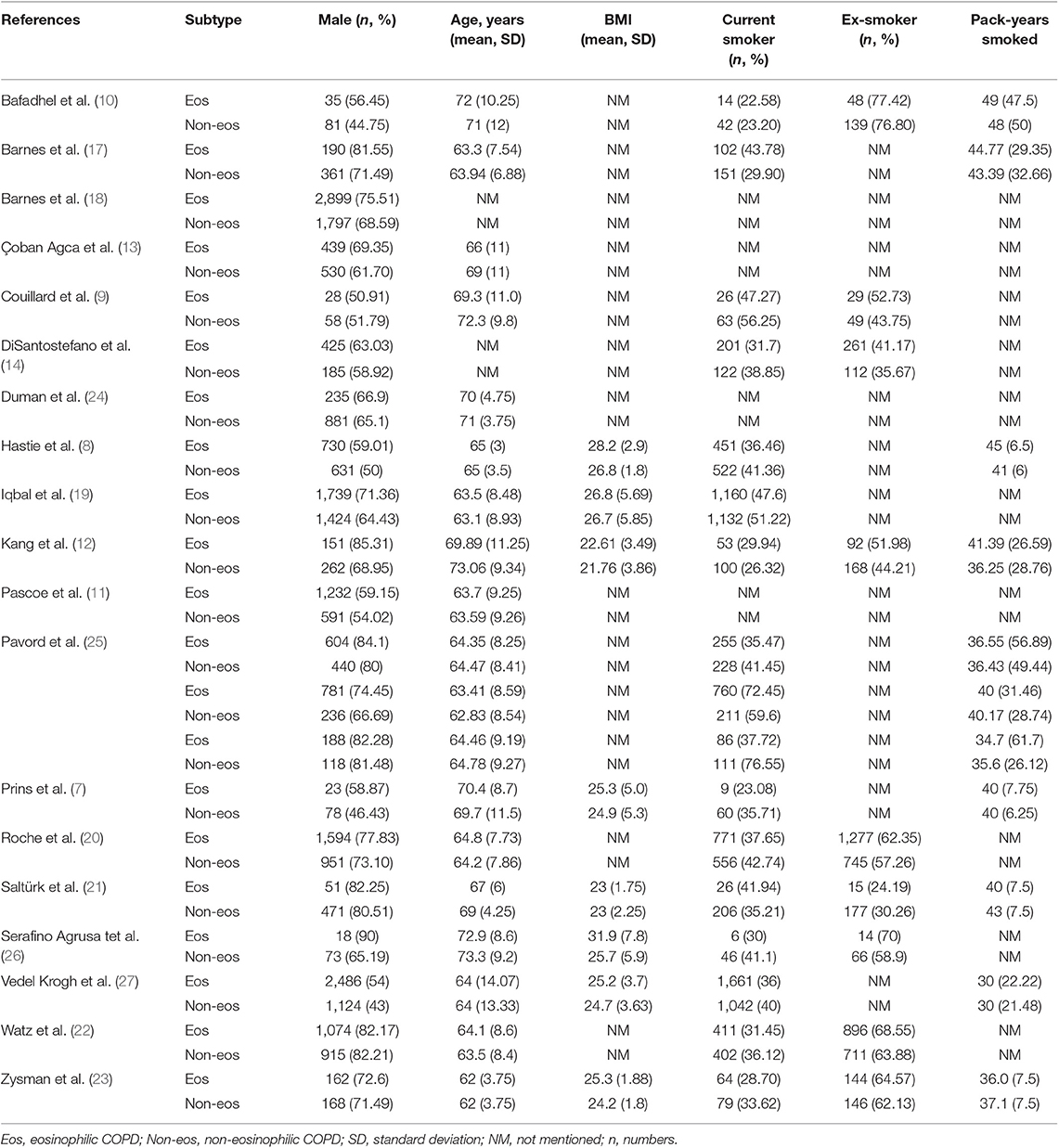
Nonetheless, the baseline clinical characteristics of eosinophilic COPD remain unclear. An analysis of the ECLIPSE cohort study showed that COPD patients with eosinophil levels that were persistently >2% were older, were more likely to be male, were less likely to be a current smoker, had a lower fat-free mass index, and had a higher percent of predicted forced expiratory volume in 1 s (ppFEV1) compared with the other COPD groups (2). An observational cohort study suggested that significantly higher numbers of male and young patients were found in the eosinophilic COPD group (13). In a national survey, being male and older in age and having congestive heart failure were significantly associated with eosinophil levels of >2% in COPD (14). In an analysis of the SPIROMICS study, significant differences were found in terms of age, sex, genus, body mass index (BMI), smoking history (pack-years), and current smoker status, but there was no evidence of a difference in the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage between patients with lower eosinophil (<200 cells per μL) and higher eosinophil (≥200 cells per μL) levels. A significantly lower ppFEV1 and FEV1: FVC percentage were found in the higher eosinophilic group (8). Couillard et al. (9), however, reported that there was no significant difference between the two phenotypes of COPD in sex, age, smoking status, home oxygen use, comorbidity, lung function, GOLD stage, or hospitalization for COPD in the previous year.
The aim of this study was to evaluate published studies that investigated the prevalence and baseline characteristics of eosinophilic COPD and apply standard meta-analysis methods to gain a more precise result.
Methods
Search Strategies
We searched the Cochrane Central Register of Controlled Trials, Medline, the Web of Science, and Embase for studies with the keywords “Eosinophil” and “Chronic obstructive pulmonary disease,” not limited to any language, publication type, or time. We searched for reports published up to May 1, 2019. In order to minimize bias and errors, we also retrieved the reference articles of all included studies. Keywords in related conference articles were also used to retrieve studies. This study was registered with PROSPERO. The findings are reported in compliance with the PRISMA guidelines.
Inclusion and Exclusion Criteria
Studies matching the following criteria were considered suitable for inclusion: (1) randomized controlled trials (RCTs), as well as observational, cohort, case control, and retrospective studies; (2) trials conducted in patients with COPD aged >40 years; and (3) trials reporting data on the prevalence or baseline clinical characteristics of COPD according to an eosinophil cut-off level of 2% in the blood. Patients admitted due to other medical problems; those with a history of asthma, interstitial pulmonary disease, active pulmonary tuberculosis, or lung cancer; those with other diseases that could influence eosinophil count (eosinophilic pneumonia, allergic diseases, parasitic infections); and individuals with severe dysfunction of other organs or systems or malignant tumors were excluded. Conference articles and trials conducted in pregnant subjects were omitted.
Outcomes
The prevalence and baseline clinical characteristics of COPD according to eosinophil levels were the primary and the secondary outcomes, respectively. The baseline clinical characteristics of COPD included demographic characteristics (sex, age, and BMI), smoking status (current-smoker, ex-smoker, and pack-years smoked), lung function (ppFEV1), comorbidity (ischemic heart disease, hypertension, and diabetes), and GOLD stage.
Study Selection
Two phases were performed by two separate researchers to verify the studies that met the eligibility criteria. Duplicated studies were first discarded by checking titles and abstracts. Suitable studies were then identified by assessing the full text. Trials reporting data on the prevalence or baseline clinical characteristics of COPD and using an eosinophil cut-off level of 2% in the blood were included.
Data Extraction
Two researchers extracted suitable information from the included studies following the criteria suggested by Cochrane (15). Corresponding authors were emailed for any missing data.
Quality Assessment
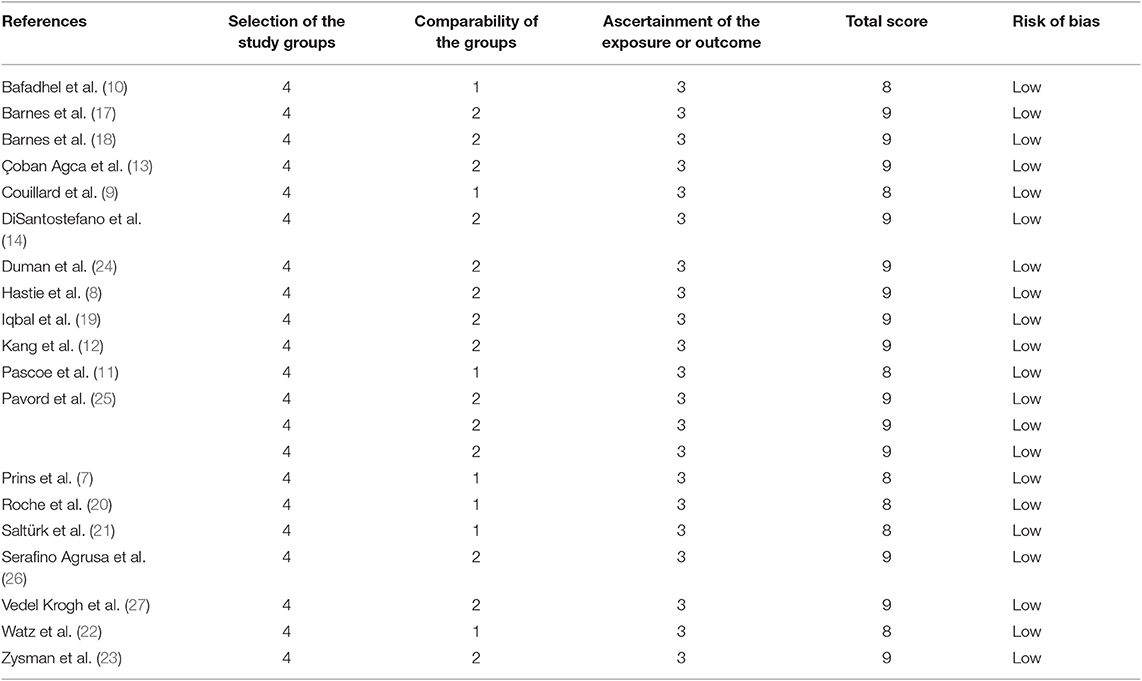
The Newcastle–Ottawa Scale (NOS) was utilized to evaluate the quality of non-randomized studies (16). Two investigators conducted the quality assessment. A third investigator was consulted to resolve any discrepancies.
Statistical Analysis
The statistical analysis was performed using the Cochrane systematic review software, Review Manager (RevMan; Version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, 2014). The Mantel-Haenszel test was used to adjudicate statistical significance at a z-value and P-value < 0.05, as well as evaluate the hypothesis. The outcomes are shown in forest plots. The outcomes of continuous and dichotomous variables are expressed as mean differences (MD) and odds ratios (OR), respectively. The χ2 test with P < 0.1 and I2 > 50% was used to determine significance in the test for heterogeneity. The sensitivity analysis was performed to substitute ranges of values or alternative decisions. A random-effects model was used in case of statistical heterogeneity; otherwise, a fixed-effects model was applied. Any disagreement was resolved by a third investigator reaching a mutual consensus.
Results
Study Description
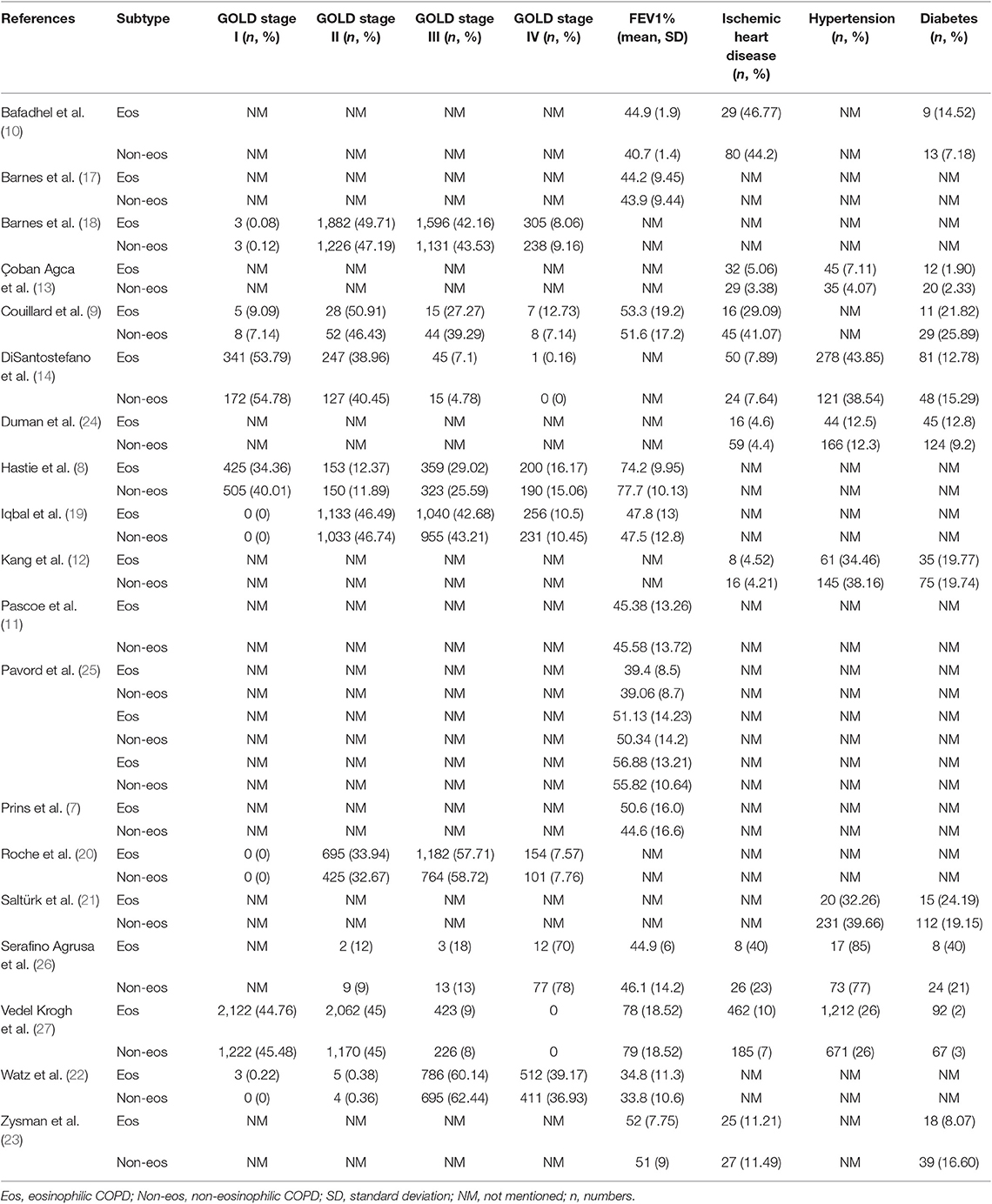
We searched 192 studies, of which nineteen studies (7–14, 17–27) with 40,112 participants were included in the final analysis (Figure 1). According to the cut-off level of 2% eosinophil in the blood, 22,043 and 18,069 patients were classified as having eosinophilic and non-eosinophilic COPD, respectively. The prevalence of eosinophilic COPD ranged from 9.58 to 66.88%, with a mean of 54.95% among all subjects. The male/female ratios were 15,084:6,959 and 11,363:6,706 in the eosinophilic and non-eosinophilic COPD groups, respectively. The mean age of participants was 62–72 years in the eosinophilic COPD group and 60–73.06 years in the non-eosinophilic COPD group. Regarding the outcomes evaluated, 19 studies (7–14, 17–27) reported data regarding sex, 17 (7–13, 19–27) reported age, 8 (8, 12, 19, 21–23, 26, 27) reported BMI, 15 (7–11, 14, 18, 19, 21–23, 25–27) reported smoking status, 13 (7–11, 17–20, 22, 23, 25–27) reported lung function data, 10 (9, 10, 12–14, 21, 23, 24, 26, 27) reported comorbidities, and 9 reported GOLD stage (9, 10, 12–14, 21, 23, 24, 26, 27). Details of participants' characteristics and outcomes are shown in Tables 1–3. No study was omitted for low quality. The risk of bias assessment is detailed in Table 4.
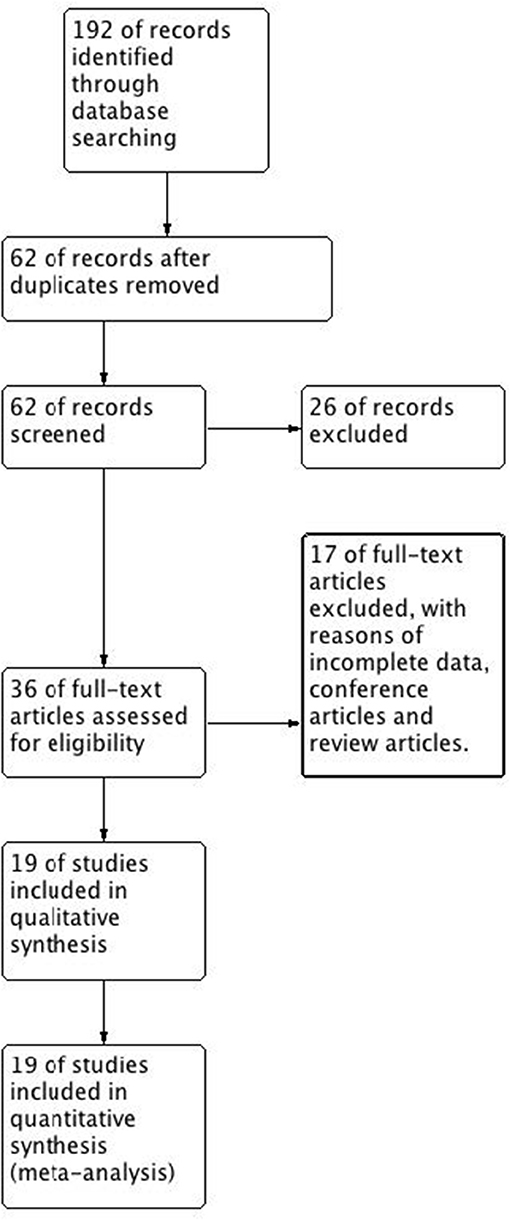
Figure 1. Flow diagram. CENTRAL, Cochrane Central Register of Controlled Trials; RCT, randomized controlled trial.
Heterogeneity
No heterogeneity was observed regarding sex, ex-smoker status, ischemic heart disease, or GOLD stage. In contrast, significant statistical heterogeneities were found in the analysis of age, BMI, current-smoker, pack-years smoked, ppFEV1, hypertension, and diabetes (I2 = 76%, MD −0.33, −0.73–0.07, P = 0.10; I2 = 91%, MD 0.70, 0.27–1.12, P = 0.001; I2 = 96%, OR 0.78, 0.59–1.02, P = 0.07; I2 = 92%, MD 0.52, −1.62–2.67, P = 0.63; I2 = 96%, MD 0.34, −1.03–1.71, P = 0.62; I2 = 51%, OR 1.10, 0.91–1.33, P = 0.32; I2 = 60%, OR 0.99, 0.75–1.30, P = 0.93) (Figures 1, 3, 4; Appendix Figures S1, S3, S4, S6, S7). Sensitivity analysis was performed to assess whether any study biased the overall results. The overall effect and summary MDs or ORs were recalculated after removing each study one at a time. This analysis revealed the constancy of the results of age, BMI, current-smoker, pack-years smoked, ppFEV1, and hypertension, as the sum MDs or ORs were uniform and without obvious variation, and the total effects (P-values) did not reveal a statistically significant difference (range of recalculated summary MDs or ORs: −0.14 to −0.41; 0.57–0.81; 0.69–0.86; −0.48–0.71; 1.04–1.15; 0.92–1.06). The heterogeneity was clearly reduced for hypertension when the study of Çoban Agca et al. (13) was removed. A non-significant difference was found in the analysis of hypertension after recalculation (I2 = 28, OR 1.04, 0.89–1.21, P = 0.63) (Appendix Figure S14).
Outcomes
Primary Outcome
The prevalence of eosinophilic COPD ranged from 18.84 to 66.88% and the mean prevalence across all studies was 54.95%.
Secondary Outcome
Demographic Characteristics
There was a significantly higher rate of male patients and higher BMI in the eosinophilic COPD group (OR 1.36, 95% CI 1.26–1.46, P < 0.00001; MD 0.70, 0.27–1.12, P = 0.001) (Figures 2, 3). There was no statistically significant difference in age between the two groups (MD −0.33, −0.73–0.07, P = 0.10) (Figure 4).
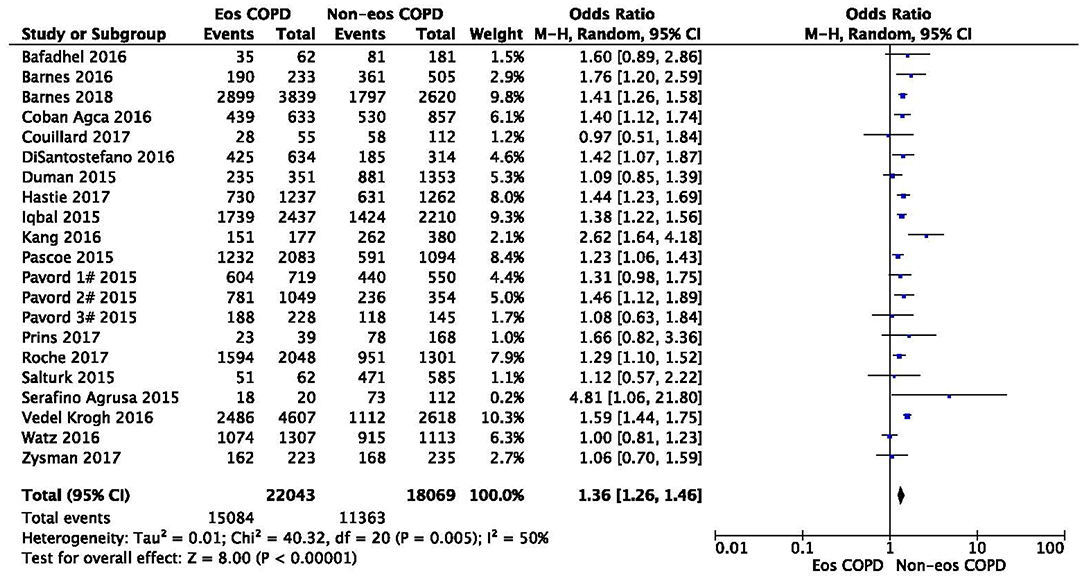
Figure 2. Comparison of gender character between eosinophilic and non-eosinophilic COPD. M.-H., Mantel-Haenszel; CI, confidence interval; Eos, eosinophilic; Non-eos, non-eosinophilic; COPD, chronic obstructive pulmonary disease.
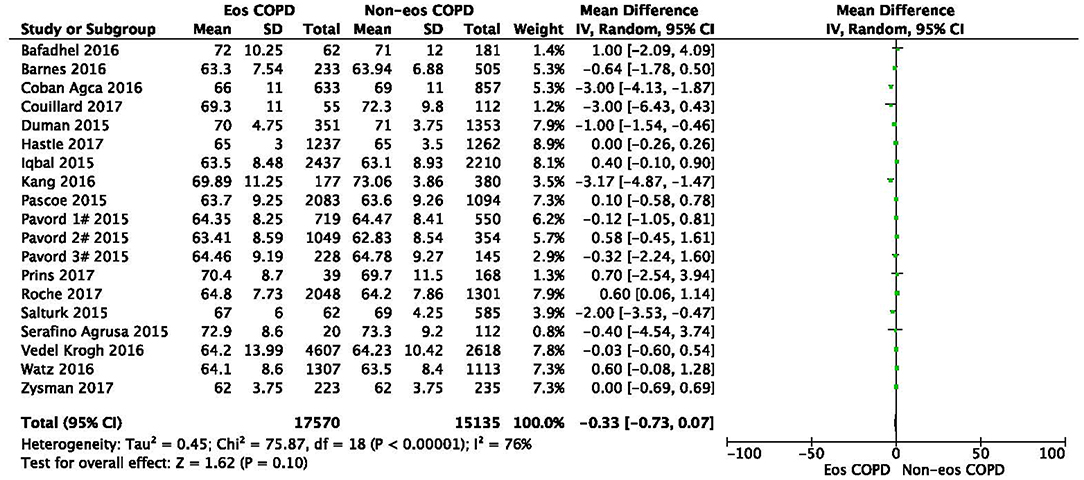
Figure 3. Comparison of age character between eosinophilic and non-eosinophilic COPD. SD, standard derivation; IV, Inverse Variance; CI, confidence interval; Eos, eosinophilic; Non-eos, non-eosinophilic; COPD, chronic obstructive pulmonary disease.
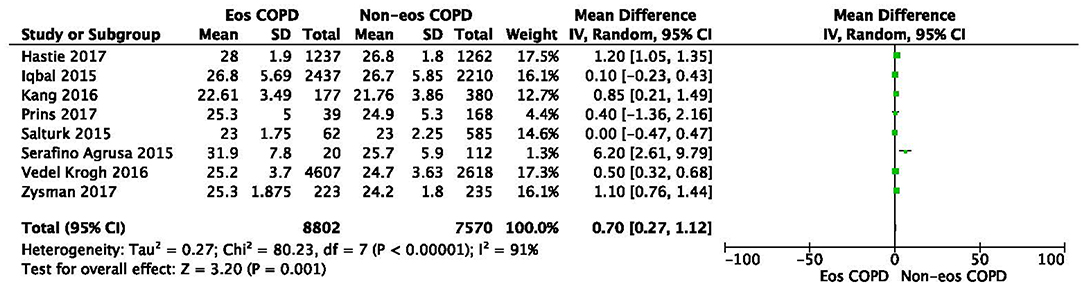
Figure 4. Comparison of BMI character between eosinophilic and non-eosinophilic COPD. SD, standard derivation; IV, Inverse Variance; CI, confidence interval; BMI, body-mass index; Eos, eosinophilic; Non-eos, non-eosinophilic; COPD, chronic obstructive pulmonary disease.
Smoking Status
We found a significantly higher rate of ex-smokers in the eosinophilic group (OR 1.23, 1.12–1.34, P < 0.0001) (Appendix Figure S2), but no difference was found in the proportion of current smokers or pack-years smoked (OR 0.78, 0.59–1.02, P = 0.07; MD 0.52, −1.62–2.67, P = 0.63) (Appendix Figures S1, S3).
Lung Function
With regard to lung function, no significant difference was found in the ppFEV1 between the two groups (MD 0.34, −1.03–1.71, P = 0.62) (Appendix Figure S4).
Comorbidity
A significantly higher rate of ischemic heart disease was found in the eosinophilic COPD group (OR 1.31, 1.14–1.50, P = 0.001) (Appendix Figure S5). However, there was no significant difference in hypertension or diabetes between the groups (OR 1.10, 0.91–1.33, P = 0.32; OR 0.99, 0.75–1.30, P = 0.93) (Appendix Figures S6, S7).
GOLD Stage
A significantly lower rate of GOLD stage I was found in the eosinophilic COPD group (OR 0.84, 0.73–0.96, P = 0.01) (Appendix Figure S8). No significant difference was found in the proportion of subjects with GOLD stage II, III, or IV between the two groups (OR 1.04, 0.98–1.09, P = 0.17; OR 0.99, 0.94–1.04, P = 0.67; OR 1.01, 0.92–1.10, P = 0.89) (Appendix Figures S9–S11).
Subgroup Analysis
When restricted to different disease statuses, a significantly higher proportion of male patients was observed in both the stable and acute exacerbation phases of COPD in the eosinophilic group (n = 34,507, OR 1.36, 95% CI 1.26–1.47, P < 0.00001; n = 5605, OR 1.39, 95% CI 1.11–1.73, P = 0.004) (Appendix Figure S12). The subgroup analysis found that subjects in the eosinophilic group were significantly younger when restricting the analysis to the acute exacerbation phase. No difference was found between groups in the stable phase (n = 5605, MD −1.38, −2.34 to −0.42, P < 0.0001; n = 27,100, MD 0.16, −0.02–0.33, P = 0.08) (Appendix Figure S13).
Discussion
This comprehensive systematic review and meta-analysis investigated the prevalence and baseline clinical characteristics of eosinophilic COPD. The prevalence of eosinophilic COPD ranged from 18.84 to 66.88%, with an average prevalence of 54.95% across all studies. The prevalence of COPD varied greatly owing to differences in diagnostic criteria, as well as survey and analytical methods. Reasons for the large range in the prevalence of eosinophilic COPD may be similar, except for the effect of different races, regions, and countries (28). In Japan, there was a tendency to exclude patients with any feature of asthma from the diagnosis of COPD, especially in younger patients with milder forms of the disease. This then leads to a low diagnostic rate of eosinophilic COPD (29–32).
In this study, we found that male patients are more at risk for eosinophilic COPD (OR 1.36, 95% CI 1.26–1.46, P < 0.00001) (Figure 2). Sex is one of the most fundamental and defining features of subpopulations in human beings. A higher absolute eosinophil count and eosinophil percentage were observed in men in an observational study; however, the number of participants enrolled was relatively low (476) (33). This may suggest that men are prone to having higher eosinophil levels, and that eosinophilic inflammation increases the risk of progression to COPD in men. More trials are needed, however, to verify this hypothesis. A higher BMI was also observed in the eosinophilic group (MD 0.70, 0.27–1.12, P = 0.001) (Figure 3). Our result was consistent with the result of a longitudinal analysis, which revealed that COPD patients with persistent eosinophil levels of >2% had fat-free mass (2). No significant difference in age was found between the two groups (MD −0.33, −0.73–0.07, P = 0.10) (Figure 4). Considering that the primary analysis in our study was aimed at identifying the characteristics of the subtypes of COPD, there is no prior relevant information that can be referenced. The mechanisms for these differences remain unclear.
Regarding smoking status, we found a significant difference in the proportion of ex-smokers between the two groups (OR 1.23, 1.12–1.34, P < 0.0001) (Appendix Figure S2), but no difference in the proportion of current smokers or in pack-years smoked (OR 0.78, 0.59–1.02, P = 0.07; MD 0.52, −1.62–2.67, P = 0.63) (Appendix Figures S1, S3). Pooled analysis showed that the prevalence of ex-smokers was higher in patients with eosinophilic COPD. The inflammation detected in the respiratory tract may be a modified inflammatory response to chronic irritants, such as cigarette smoke. The presence of persistent lung inflammation after smoking cessation remains unknown; even perturbations and autoantigens in the lung microbiome may play a role (34, 35). We hypothesize that smoking may induce eosinophilic inflammation and that the inflammation persists even after smoking cessation, although more research is needed to confirm this hypothesis.
In terms of lung function, no significant difference was found in the ppFEV1 (MD 0.34, −1.03–1.71, P = 0.62) (Appendix Figure S4). A significantly lower prevalence of GOLD stage I was, however, found in the eosinophilic COPD group (OR 0.84, 0.73–0.96, P = 0.01) (Appendix Figure S8). We believe that the mild severity of airflow limitations is more common in non-eosinophilic COPD and rarer in eosinophilic COPD. Our analysis was not consistent with previous findings. In the ECLIPSE cohort study, patients with COPD with persistent eosinophil levels of >2% had a significantly higher ppFEV1 (2). In the SPIROMICS study, patients with a lower baseline eosinophil level (<1%) were prone to severe COPD (36). There is no definitive explanation, however, for this problem. Additional studies are needed to further investigate the relationship between eosinophil and FEV1 in COPD patients.
There was a significantly lower prevalence of chronic heart failure in the eosinophilic COPD group (OR 0.81, 0.68–0.97, P = 0.02) (Appendix Figure S5). No difference in the prevalence of hypertension or diabetes between groups was found. COPD patients often have important concomitant illnesses. The SPIROMICS study suggested a higher incidence of comorbidities (prior heart attack, anemia, diabetes, and chronic heart failure) among COPD patients with eosinophil levels of ≤2% (36). The comorbidities in COPD may be caused by original genetic variances in response to the inhalation of poisonous particles, particularly during smoking (37). More rigorous trials are needed to clarify this issue.
Significant variability in blood eosinophil levels has been shown throughout the course of COPD (38, 39). To investigate the stability of blood eosinophilic inflammation (≥2%), subjects were classified into predominantly (PE), intermittently (IE), and rarely (RE) eosinophilic groups in one study (40). The PE group was characterized by an increased risk of eosinophilic inflammation during exacerbation. The PE group at stable visits and eosinophilia during exacerbation were associated with a minor risk of bacterial infection during exacerbation. Bacterial infection during exacerbation was higher in winter in the PE group. Blood eosinophil counts in the stable status could predict the nature of inflammation during future exacerbations. When combined with an understanding of seasonal variation, this may also provide a basis for the development of new therapy. More research, however, is warranted.
Although blood eosinophil is considered to be a promising biomarker, eosinophil-guided treatment of acute exacerbation of COPD remains an issue. Bafadhel et al. (41) showed that systemic corticosteroid use in a low eosinophil (<2%) group was associated with less improvement in chronic respiratory questionnaire scores and higher treatment failure when compared to the placebo group. On the contrary, Sivapalan et al. (42) reported that, when compared to standard therapy in patients hospitalized for COPD, eosinophil-guided therapy did not lead to a difference in the number of days alive, number of patients discharged from the hospital within 14 days of recruitment, or the risk of treatment failure at 30 days. Future studies on eosinophil-guided therapies are needed.
This study has several strengths. First, it is a comprehensive systematic review and meta-analysis to analyze the prevalence and baseline clinical characteristics of eosinophilic COPD. Additionally, the studies that were included were of high quality. All data were collected at the very beginning of each study, protecting against subsequent interference. Our results are thus highly credible. This study has some limitations. First, the studies that were included were not RCTs. Nonetheless, the extracted data were obtained from RCTs that enrolled a large number of patients with COPD and classified according to eosinophilic and non-eosinophilic inflammation status. Second, given that this analysis is the first to verify the baseline clinical characteristics of eosinophilic COPD, the underlying mechanisms remain unclear. Finally, a proportion of patients had already been treated with corticosteroids and antibiotics in the community. It remains unclear whether and to what extent these therapies affect the eosinophil count. Further research is therefore warranted.
Conclusions
In conclusion, eosinophilic inflammation is prevalent in COPD. Eosinophilic COPD was more common in men, ex-smokers, subjects with higher BMI, and in those with a high risk of some comorbidity. The group also included a low proportion of patients with mild airflow limitations. Future rigorous prospective trials are needed, particularly in basic research, to further identify the relationship between eosinophil levels and COPD. Additional studies should explore the exact mechanisms that are responsible for the characteristics of eosinophilic COPD.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
H-XW and D-YC initiated and coordinated the study. H-XW and K-QZ were responsible for the data collection and data analysis. Studies were reviewed by D-YC. H-XW wrote the first draft of the manuscript. All the authors were involved in the interpretation of the analyses and gave input to the final manuscript.
Funding
This study was partially funded by the Science and Technology support project of Sichuan province (2015SZ0234-3).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all the people who participated in the primary trials and the teams who did them.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2019.00282/full#supplementary-material
Abbreviations
COPD, chronic obstructive pulmonary disease; BMI, body-mass index; ppFEV1, percent of predicted forced expiratory volume in 1 s; GOLD, global initiative for chronic obstructive lung disease.
References
1. Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. (2011) 184:662–71. doi: 10.1164/rccm.201104-0597OC
2. Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R, et al. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. (2014) 44:1697–700. doi: 10.1183/09031936.00162414
3. Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax. (2005) 60:193–8. doi: 10.1136/thx.2004.032516
4. Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. (2000) 356:1480–5. doi: 10.1016/S0140-6736(00)02872-5
5. Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. (2006) 27:964–71. doi: 10.1183/09031936.06.00072105
6. Pizzichini E, Pizzichini MM, Gibson P, Parameswaran K, Gleich GJ, Berman L, et al. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. (1998) 158:1511–7. doi: 10.1164/ajrccm.158.5.9804028
7. Prins HJ, Duijkers R, Lutter R, Daniels JM, van der Valk P, Schoorl M, et al. Blood eosinophilia as a marker of early and late treatment failure in severe acute exacerbations of COPD. Respir Med. (2017) 131:e124. doi: 10.1016/j.rmed.2017.07.064
8. Hastie AT, Martinez FJ, Curtis JL, Doerschuk CM, Hansel NN, Christenson S, et al. Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med. (2017) 5:956–67. doi: 10.1016/S2213-2600(17)30432-0
9. Couillard S, Larivée P, Courteau J, Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. (2017) 151:366–73. doi: 10.1016/j.chest.2016.10.003
10. Bafadhel M, Greening NJ, Harvey-Dunstan TC, Williams JE, Morgan MD, Brightling CE, et al. Blood eosinophils and outcomes in severe hospitalized exacerbations of COPD. Chest. (2016) 150:320–8. doi: 10.1016/j.chest.2016.01.026
11. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. (2015) 3:435–42. doi: 10.1016/S2213-2600(15)00106-X
12. Kang HS, Rhee CK, Kim SK, Kim JW, Lee SH, Yoon HK, et al. Comparison of the clinical characteristics and treatment outcomes of patients requiring hospital admission to treat eosinophilic and neutrophilic exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. (2016) 11:2467–73. doi: 10.2147/COPD.S116072
13. Çoban Agca M, Aksoy E, Duman D, Özmen I, Yildirim E, Güngör S, et al. Does eosinophilia and neutrophil to lymphocyte ratio affect hospital re-admission in cases of COPD exacerbation? Tuberk Toraks. (2017) 65:282–90. doi: 10.5578/tt.57278
14. DiSantostefano RL, Hinds D, Le HV, Barnes NC. Relationship between blood eosinophils and clinical characteristics in a cross-sectional study of a US population-based COPD cohort. Respir Med. (2016) 112:e96. doi: 10.1016/j.rmed.2016.01.013
15. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Cochrane (2019). Available online at: www.training.cochrane.org/handbook
16. Cota GF, de Sousa MR, Fereguetti TO, Rabello A. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed February 15, 2018).
17. Barnes N, Ishii T, Hizawa N, Midwinter D, James M, Hilton E, et al. The distribution of blood eosinophil levels in a Japanese COPD clinical trial database and in the rest of the world. Int J Chron Obstruct Pulmon Dis. (2018) 13:433–40. doi: 10.2147/COPD.S144108
18. Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. (2016) 47:1299–303. doi: 10.1183/13993003.01370-2015
19. Iqbal A, Barnes NC, Brooks J. Is blood eosinophil count a predictor of response to bronchodilators in chronic obstructive pulmonary disease? Results from post hoc subgroup analyses. Clin Drug Investig. (2015) 35:685–8. doi: 10.1007/s40261-015-0322-6
20. Roche N, Chapman KR, Vogelmeier CF, Herth FJF, Thach C, Fogel R, et al. Blood eosinophils and response to maintenance COPD treatment: data from the FLAME trial. Am J Respir Crit Care Med. (2017) 195:1189–97. doi: 10.1164/rccm.201701-0193OC
21. Saltürk C, Karakurt Z, Adiguzel N, Kargin F, Sari R, Celik ME, et al. Does eosinophilic COPD exacerbation have a better patient outcome than non-eosinophilic in the intensive care unit? Int J Chron Obstruct Pulmon Dis. (2015) 10:1837–46. doi: 10.2147/COPD.S88058
22. Watz H, Tetzlaff K, Wouters EF, Kirsten A, Magnussen H, Rodriguez-Roisin R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post hoc analysis of the WISDOM trial. Lancet Respir Med. (2016) 4:390–8. doi: 10.1016/S2213-2600(16)00100-4
23. Zysman M, Deslee G, Caillaud D, Chanez P, Escamilla R, Court-Fortune I, et al. Relationship between blood eosinophils, clinical characteristics, and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis. (2017) 12:1819–24. doi: 10.2147/COPD.S129787
24. Duman D, Aksoy E, Agca MC, Kocak ND, Ozmen I, Akturk UA, et al. The utility of inflammatory markers to predict readmissions and mortality in COPD cases with or without eosinophilia. Int J Chron Obstruct Pulmon Dis. (2015) 10:2469–78. doi: 10.2147/COPD.S90330
25. Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, et al. Blood eosinophils and inhaled corticosteroid/longacting β-2 agonist efficacy in COPD. Thorax. (2016) 71:118–25. doi: 10.1136/thoraxjnl-2015-207021
26. Serafino-Agrusa L, Scichilone N, Spatafora M, Battaglia S. Blood eosinophils and treatment response in hospitalized exacerbations of chronic obstructive pulmonary disease: a case–control study. Pulmon Pharmacol Ther. (2016) 37:89–94. doi: 10.1016/j.pupt.2016.03.004
27. Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG. Blood eosinophils and exacerbations in COPD: the Copenhagen General Population Study. Am J Respir Crit Care Med. (2016) 193:965–74. doi: 10.1164/rccm.201509-1869OC
28. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
29. Takahashi T, Ichinose M, Inoue H, Shirato K, Hattori T, Takishima T. Underdiagnosis and undertreatment of COPD in primary care settings. Respirology. (2003) 8:504–8. doi: 10.1046/j.1440-1843.2003.00501.x
30. Fukuchi Y, Nishimura M, Ichinose M, Adachi M, Nagai A, Kuriyama T, et al. COPD in Japan: the Nippon COPD epidemiology study. Respirology. (2004) 9:458–65. doi: 10.1111/j.1440-1843.2004.00637.x
31. Omori H, Nonami Y, Mihara S, Marubayashi T, Morimoto Y, Aizawa H. Prevalence of airflow limitation on medical check-up in Japanese subjects. J UOEH. (2007) 29:209–19. doi: 10.7888/juoeh.29.209
32. Onishi K, Yoshimoto D, Hagan GW, Jones PW. Prevalence of airflow limitation in outpatients with cardiovascular diseases in Japan. Int J Chron Obstruct Pulmon Dis. (2014) 9:563–8. doi: 10.2147/COPD.S59962
33. Wongkrajang P, Chinswangwatanakul W, Mokkhamakkun C, Chuangsuwanich N, Wesarachkitti B, Thaowto B, et al. Establishment of new complete blood count reference values for healthy Thai adults. Int J Lab Hem. (2018) 40:478–83. doi: 10.1111/ijlh.12843
34. Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD–implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. (2014) 9:1207–24. doi: 10.2147/COPD.S51226
35. Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, et al. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: the role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med. (2009) 180:1196–207. doi: 10.1164/rccm.200903-0324OC
36. Weir M, Zhao H, Han MK, Kanner R, Pirozzi CS, Scholand MB, et al. Eosinophils in chronic obstructive pulmonary disease, the SPIROMICS cohort. Am J Respir Crit Care Med. (2014) 189:A5902. doi: 10.1164/rccm.201308-1541PP
37. Armani C, Landini L, Leone A. Interactive effect of cigarette smoking and gene variants for predisposing to cardiovascular disease. Curr Pharm Des. (2010) 16:e2538. doi: 10.2174/138161210792062885
38. Schumann DM, Tamm M, Kostikas K, Stolz D. Stability of the blood eosinophilic phenotype in stable and exacerbated COPD. Chest. (2019) 156:456–65. doi: 10.1016/j.chest.2019.04.012
39. Vogelmeier CF, Kostikas K, Fang J, Tian H, Jones B, Morgan CL, et al. Evaluation of exacerbations and blood eosinophils in UK and US COPD populations. Respir Res. (2019) 20:178. doi: 10.1186/s12931-019-1130-y
40. Kim VL, Coombs NA, Staples KJ, Ostridge KK, Williams NP, Wootton SA, et al. Impact and associations of eosinophilic inflammation in COPD: analysis of the AERIS cohort. Eur Respir J. (2017) 50:1700853. doi: 10.1183/13993003.00853-2017
41. Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, et al. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-placebo-controlled trial. Am J Respir Crit Care Med. (2012) 186:48–55. doi: 10.1164/rccm.201108-1553OC
42. Sivapalan P, Lapperre TS, Janner J, Laub RR, Moberg M, Bech CS, et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): a multicenter, randomised, controlled, open-label, non-inferiority trial. Lancet Respir Med. (2019) 7:699–709. doi: 10.1016/S2213-2600(19)30176-6
Keywords: eosinophil, chronic obstructive pulmonary disease, biomarkers, inflammation, smoking, airflow
Citation: Wu H-X, Zhuo K-Q and Cheng D-Y (2019) Prevalence and Baseline Clinical Characteristics of Eosinophilic Chronic Obstructive Pulmonary Disease: A Meta-Analysis and Systematic Review. Front. Med. 6:282. doi: 10.3389/fmed.2019.00282
Received: 23 July 2019; Accepted: 19 November 2019;
Published: 10 December 2019.
Edited by:
Hsiao-Chi Chuang, Taipei Medical University, TaiwanReviewed by:
Shu-Chuan Ho, Taipei Medical University, TaiwanRafael A. Calderon-Candelario, University of Miami, United States
Le Thuong Vu, Ho Chi Minh City Medicine and Pharmacy University, Vietnam
Copyright © 2019 Wu, Zhuo and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Yun Cheng, Y2hlbmdkZXl1bnNjdUAxNjMuY29t
†These authors have contributed equally to this work
 Hong-Xia Wu
Hong-Xia Wu Kai-Quan Zhuo
Kai-Quan Zhuo De-Yun Cheng
De-Yun Cheng