- 1Medical School of Chinese PLA, Beijing, China
- 2SICU, The Eighth Medical Center of PLA General Hospital, Beijing, China
- 3ICU, Hebei Yanda Hospital, Langfang, China
- 4Feng Tai District Center for Disease Control and Prevention, Beijing, China
- 5NICU, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Purpose: To investigate physicians' perception of patients' tolerance levels regarding sedation, which could affect sedation practice for mechanically ventilated (MV) patients.
Methods: This is a questionnaire survey combined with a 24 h cross-sectional study. The physician's propensity score for light sedation (PS-LS) was estimated by his/her response to the given answers for each item of the questionnaire, which tested the levels of interviewee's desire to manage MV patient with light sedation. Thereby, the mean physicians' PS-LS of each participating ICU (ICU-meanPS-LS) was calculated. The practical measurements of all variables listed on the questionnaire were used to semi-quantitatively assess stimulus intensity of what the recruited patients suffered (i.e., semi-quantitative stimulus intensity, SSI). Sedation depth was assessed by Richmond Agitation Sedation Scale (RASS).
Results: 555 of 558 (99.5%) physicians from 102 ICUs were concerned with patients' tolerance levels regarding sedation while titrating sedation depth. The physician's PS-LS was non-normally distributed with median (IQR) of 3 (0–5). ICU-meanPS-LS was calculated in 92 out of 102 ICUs participating in the cross-sectional study, which was ranged from −5 to 7 with a median (IQR) of 2.37 (0.16–4.33). A significant increasing trend in prevalence of light sedation was observed over increasing ICU-meanPS-LS quartiles (from Q1 to Q4, χ2-test for trend, p = 0.002). Moreover, odds ratio for probability of light sedation remained significant in MV patients from Q4 ICUs vs. Q1 ICUs, adjusted by APACHE II score (OR, 2.332; 95% CI: 1.463–3.717; p < 0.001) or SSI score (OR, 2.445; 95% CI: 1.468–4.074; p = 0.001). Notably, adjusted OR for mortality was significant in deeply sedated MV patients (OR, 2.034; 95% CI: 1.435–2.884; p < 0.001).
Conclusions: ICU physician's individualized perception for patients' tolerance levels regarding sedation, in light sedation affected sedation practice for MV patients.
Introduction
While deep sedation has been associated with morbidity and mortality in mechanically ventilated (MV) patients (1–3), increasing data suggest that targeting lighter levels of sedation (RASS −2 ~ +1, Richmond Agitation Sedation Scale) could be beneficial to reduce ICU stay and ventilator days (4–6). The strategy of maintaining light rather than deep sedation was therefore recommended as a standard care for critically ill adult patients worldwide (7–9). However, recently published studies demonstrated that up to 65% MV patients continued to score deep sedation (RASS ≤ −3) (3, 10–12), which suggested that the recommendation for promoting lighter sedation is far from being implemented well.
Low adherence to lightening sedation strategy remained under interpreted, although frequent deep sedation was previously linked to inadequate assessments, lack of multidisciplinary cooperation, and even misperception as well (13–16). Interestingly, Rose et al. recently reported that most ICU nurses and physicians were concerned about agitation and agitated events, which affected their willingness to decrease sedation significantly (17). In fact, agitation was common in MV patients (18, 19). Moreover, agitation or agitated adverse events were observed more frequently in the arm of patients who were sedated at the lighter target than the usual care in several published randomized control trials (RCTs) (4–6, 20). Meanwhile, evidence regarding ICU physicians' concerns about patients' tolerance levels in light sedation remain limited. Notably, it was under investigated whether ICU physicians' concerns affected their behaviors in sedation titration. Therefore, a questionnaire survey combined with a cross-sectional study were conducted, hypothesizing that ICU physician's perception for patient's tolerance levels in light sedation was individualized, which impacted decision-making on implementation of minimizing sedation strategy for MV patients.
Methods
A total of 102 Chinese ICUs, where members of the standing committees of Chinese Association of Critical Care Physicians or Chinese Society of Critical Care Medicine worked, were involved in this study. A questionnaire combined with a 24 h cross-sectional study was simultaneously conducted in each participating ICU on May 11th, 2016, approved by the Ethics Committees of each hospital. Owing to the absence of additional interventions, obtaining informed written consent for the individual patients was waived. The study protocol was registered on the website of www.chictr.org.cn (registration number: ChiCTR-EOC-16008444).
Questionnaire Survey
A questionnaire named “Concerns of ICU physicians for lightening the sedation depth in MV patients” (Cronbach's Alpha = 0.737, Table 1) was developed by a modified Delphi method. The major components of the questionnaire were based on results of a three-round Delphi processing in a panel of 15 experts and testing in 63 doctors. Consequently, 10 stimuli-derived events (with agreement rate > 80%, Table S1) were selected, comprising two domains, ventilator setting and vital organ dysfunction. The ventilator settings included four stimuli-derived events, the ventilation mode (Mode), positive end-expiratory pressure (PEEP), plateau pressure (Pplat), and fraction of inspiration O2 (FiO2) as well. In the vital organ dysfunction domain, we examined the PaO2/FiO2 (P/F), respiratory rate (RR), minute ventilation (Min-V), plasma lactate level (Lac), Glasgow Coma Scale (GCS), and dosage of norepinephrine (NE) or equal-effect dose of other vasopressors. We allowed three response options (A, B, C), leading to the categorization of the response from high to low. An additional answer D was used to define an event that was not considered important in the decision-making for sedation depth. For conducting this questionnaire, up to six on-duty physicians (no more than two physicians with title of either senior, attending or resident, respectively) from each of participating ICUs were interviewed face-to-face by a well-trained clinical research coordinator on May 11th, 2016. All questionnaires were collected directly after interviewing and incomplete questionnaires were excluded from this study.
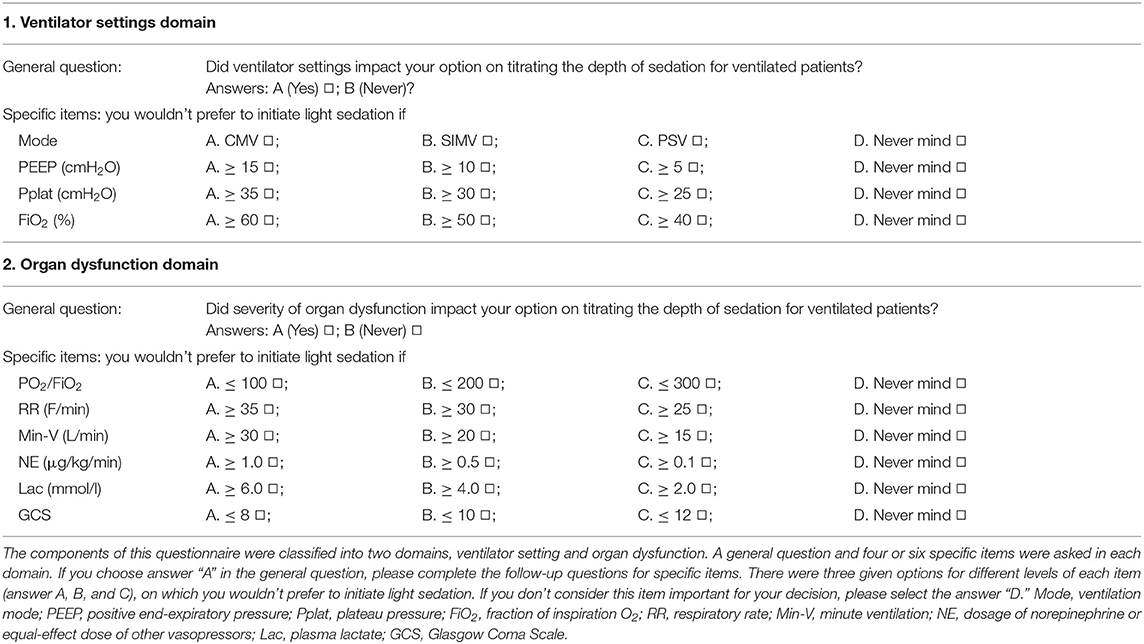
Table 1. Questionnaire items to address the ICU physicians concerns regarding lightening the sedation depth for MV patients.
Specifically, answer “A” was defined as high desire to manage MV patient with light sedation, regardless the levels of ventilator setting or the severity of organ dysfunction; answer “C” was defined as low desire to use light sedation for MV patients even if the level of ventilator settings or the severity of organ dysfunction was evidently low; and answer “B” was defined as the desire for using light sedation depending on the clinical condition. Based on suggestion of a panel of experts, answers A, B, and C were scored 1, 0, and −1 to represent physician's desire for light sedation from high to low, respectively. The sum of score for each specific item in the questionnaire was used to evaluate the physician's propensity score for light sedation (PS-LS, theoretically ranged from −10 to 10). Thereby, ICU mean physician's PS-LS (ICU-meanPS-LS, i.e., the sum of physicians' PS-LS divided by the number of the interviewed physicians in the ICU) was used to estimate the overall propensity for light sedation of each participating ICU where the cross-sectional study was successfully completed.
Design of the Cross-Sectional Study
A 24 h cross-sectional study was conducted in the ICUs participating questionnaire survey on May 11th, 2016. Inclusion criteria were adult MV patients equal to or older than 18 years old and the predicted ICU stay over 24 h. Exclusion criteria included MV patients <18 years old or GCS = 3 and pregnant females. In addition to baseline and demographic data, we collected information regarding the previous MV days, the original disease, Acute Physiology and Chronic Health Evaluation (APACHE II) score and the clinical outcome. Variables related to mechanical ventilation and organ dysfunction listed on the questionnaire were recorded at 6:00 a.m., 14:00 p.m., and 22:00 p.m., respectively while RASS was assessed. RASS ≥ +2 and RASS −2 to 1 were used to define agitation and light sedation. Patients with 1 record of RASS ≤ -3 were categorized into the poorly maintained at light sedation group while those with 3 records of RASS ≥-2 were allocated into well-maintained at light sedation group.
In literature, there are lack of criteria to evaluate nociceptive stimulus. Naturally, intensity of stimulus might change with the variety of stimulus sources, such as the 10 events listed on this questionnaire. Therefore, the practical measurements of all these variables were used to semi-quantitatively assess stimulus intensity of what the recruited patients suffered, named as semi-quantitative stimulus intensity (SSI) score. We predefined SSI as 2, 1, and 0 if the observed value of each specific item was equal to or above the level of answer “A,” “B,” or “C,” respectively.
Statistical Analysis
All data were double logged and proofread by Epidata 3.1 and a database was developed. Quantitative data were described using mean (SD) or median (IQR) and categorical variables using frequencies and percentages. Normal distribution of data was analyzed by Kolmogorov-Smirnova test or Shapiro-Wilk test. We analyzed relevant covariates that might associate with the probability of being well-maintained at light sedation and mortality in MV patients with univariable and multivariable binary logistic regression and reported odds ratios with 95% CIs and p-values. Chi-square test was used for trend of the prevalence of patients well-maintained at light sedation in ICU-meanPS-LS quartiles. The relationship between probability of well-maintaining patients at light sedation and the estimated SSI score was analyzed using Pearson correlation. All statistical analyses were performed using SPSS software (V.18.0, Chicago, IIIinois, USA). A p-value below 0.05 (2-sided significance testing) was considered statistically significant.
Results
Demographic Characters of the Interviewed Physicians and the Recruited Patients
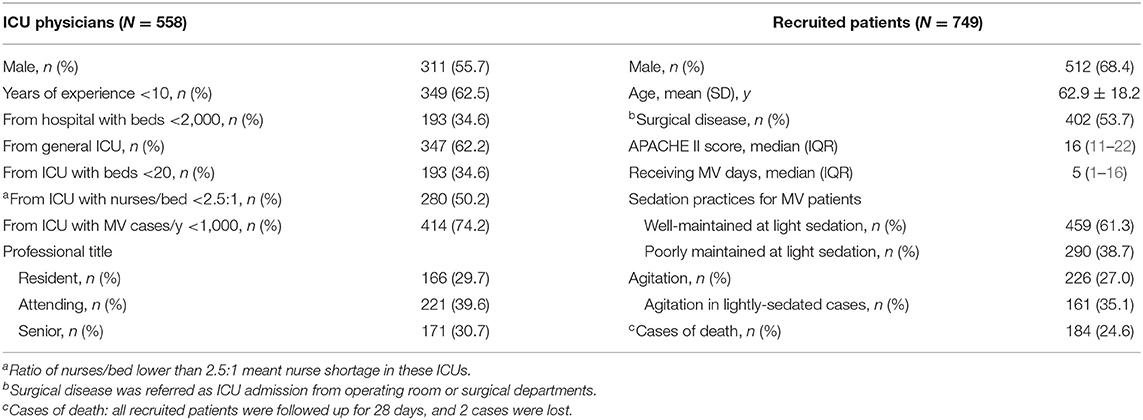
The questionnaire was successfully completed by 558 out of 576 (96.9%) interviewed physicians, comprising of 166 (29.7%) residents, 221 (39.6%) attending, and 171 (30.7%) senior physicians in 102 ICUs among 77 hospitals located in 26 Chinese cities. Demographic data of physicians and characteristics of the ICUs and hospitals are summarized in Table 2. Out of these 102 participating ICUs, however, 10 ICUs were excluded owing to the absence of adult MV patients (n = 7), all patients with GCS = 3 (n = 1) and two or less physicians of the ICU completing the questionnaire (n = 2) in this cross-sectional study. A total of 749 MV patients were included from the remaining 92 ICUs, with age of 62.9 ± 18.2 years old, APACHE II score of 16 (11–22) and 5 (1–16) days receiving MV before the study day (Table 2).
Concern of ICU Physician on Light Sedation
The majority of the recruited ICU physicians were concerned of ventilator setting [520/558 (93.2%)] and disease severity [554/558 (99.3%)] when titrating the sedation depth. Only three physicians (0.5%) answered that neither ventilator settings nor organ dysfunction severity impacted their sedation practices. For each item in this questionnaire, 66.4–93.7% of the physicians chose the answer of A, B, or C. That is, only 6.3–33.6% of them chose answer D (Figure 1).
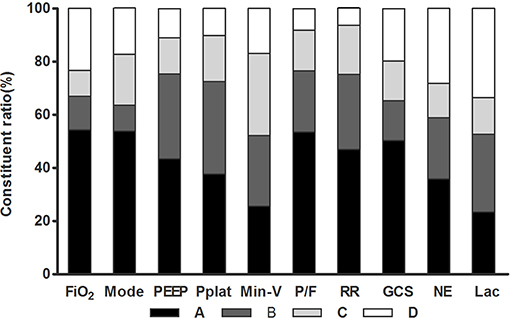
Figure 1. Constituent ratio of physician's options for the 10 items in the questionnaire. FiO2, fraction of inspiration O2; Mode, ventilation mode; PEEP, positive end-expiratory pressure; Pplat, plateau pressure; Min-V, minute ventilation; P/F, PaO2/FiO2; RR, respiratory rate; GCS, Glasgow Coma Scale; NE, dosage of norepinephrine or equal-effect dose of other vasopressors; Lac, plasma lactate.
ICU Physician's PS-LS and ICU-meanPS-LS
For each specific item, the physician's response varied greatly (Figure 1). In particular, answer “A” was selected by over 50% of the physicians in four events: “Mode,” “FiO2,” “PaO2/FiO2,” and GCS, respectively. On the other hand, choosing answer “C” varied from 9.8% (FiO2) to 31.1% (Min-V).
The median (IQR) of physicians' PS-LS was 3 (0–5) ranging from −10 to 10 (Figure 2). Among the 558 interviewees, only 46 (8.2%) received PS-LS ≤ −5 (i.e., who selected answer C for five specific events at least), while 192 physician (34.4%) had PS-LS ≥5 (i.e., who selected answer A for five specific events at least). Most physicians (320, 57.4%) had no intense propensity for the sedation level. Among all analyzed characters, only the gender (female vs. male, β = 0.713; 95%CI: 0.016–1.411; p = 0.045) was significantly associated with PS-LS (Table S2). Interestingly, the physician seniority (residents, attending, or senior physician) did not affect distribution of ICU physicians in different PS-LS (p = 1.000, Figure S1).
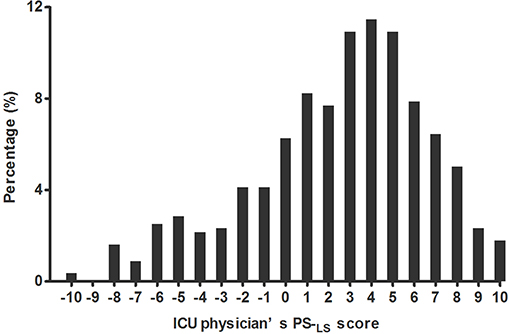
Figure 2. Distribution of ICU physicians at different propensity scores for light sedation (PS-LS). The physician's PS-LS ranged from −10 to 10 with median (IQR) 3 (0–5), which were non-normally distributed by Kolmogorov-Smirnova test (P < 0.001). The larger PS-LS score was, the more interviewees preferred light sedation.
Based on PS-LS of the interviewed physicians, ICU-meanPS-LS was calculated in 92 ICUs participating in cross-sectional study, which was normally distributed (p = 0.189), ranged from −5 to 7 with a median (IQR) of 2.37 (0.16–4.33) as shown on Figure S2. Accordingly, the 92 ICUs were divided into quartiles by ICU-meanPS-LS (Q1: −5–0.16, 17 ICUs; Q2: 0.16–2.37, 26 ICUs; Q3: 2.37–4.33, 28 ICUs; Q4: 4.33–7, 21 ICUs).
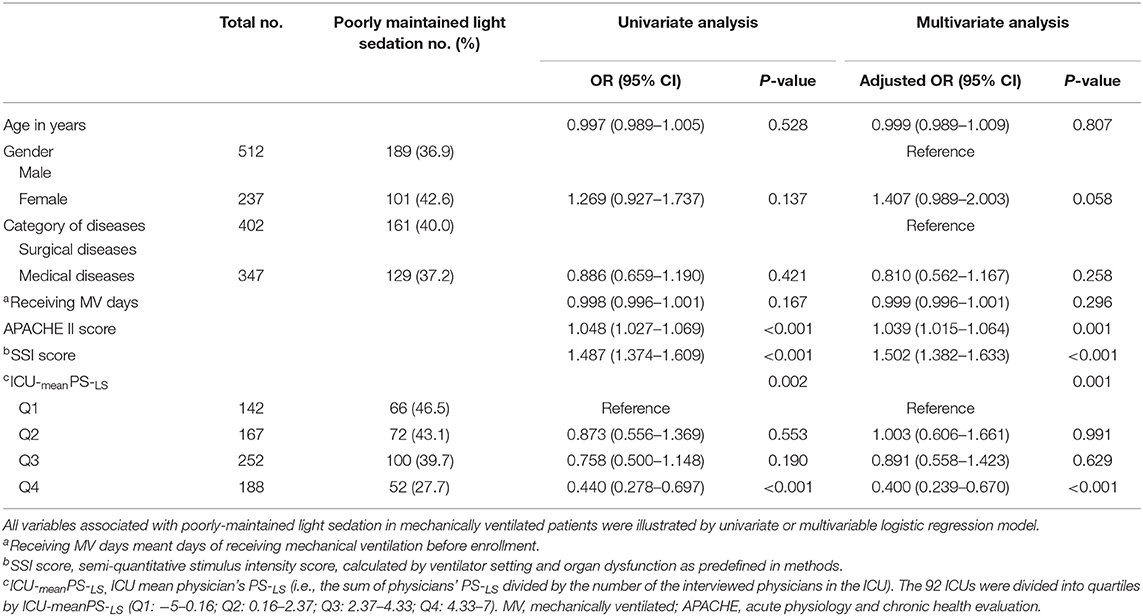
Risk Factors for Poorly Maintaining MV Patients at Light Sedation
Among the enrolled patients (n = 749), 459 (61.3%) patients were well-maintained at light sedation levels (RASS ≥ −2). Meanwhile, 38.7% (290/749) patients were recorded with RASS ≤ -3 at least once within the 24 h observational period (Table 2). Multivariable binary logistic regression demonstrated that risk factors for poorly maintaining MV patients at light sedation included APACHE II score (OR, 1.039; 95% CI: 1.015–1.064; p = 0.001), SSI score (OR, 1.502; 95% CI: 1.382–1.633; p < 0.001), and ICU-meanPS-LS (referred to Q1, Q4 OR, 0.400, 95% CI: 0.239–0.670, p < 0.001, Table 3). In addition, the prevalence of well-maintaining MV patients at light sedation was negatively correlated with SSI scores in this cross-sectional study (r = −0.967, p < 0.001, Figure S3).
Impact of ICU-meanPS-LS on Implementation of Light Sedation in Mechanically Ventilated Patients
A significant increasing trend in prevalence of MV patients being well-maintained at light sedation was observed over increasing ICU-meanPS-LS quartiles (from Q1–Q4, x2 test for trend, p = 0.002). Moreover, odds ratio for probability of being well-maintained at light sedation remained significant in MV patients from Q4 ICUs vs. Q1 ICUs, adjusted by APACHE II score (OR, 2.332; 95% CI: 1.463–3.717; p < 0.001) and SSI score (OR, 2.445; 95% CI: 1.468–4.074; p = 0.001, Figure 3). In subgroup analysis determined by SSI, furthermore, OR for probability of well-maintained at light sedation in the highest vs. the lowest ICU-meanPS-LS quartile was significant in subgroups of low SSI scores (SSI = 0–2, OR, 2.385; 95% CI: 1.025–5.549; p = 0.044, Q4 vs. Q1) and middle SSI scores (SSI = 3–5, OR, 2.784; 95% CI: 1.343–5.774; p = 0.006, Q4 vs. Q1), but not with high SSI scores (SSI = 6–11, OR, 2.017; 95% CI: 0.649–6.271; p = 0.226, Figure S4).
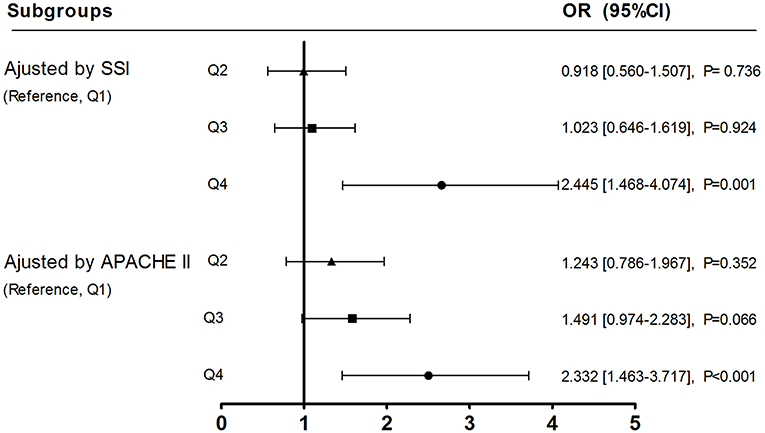
Figure 3. Adjusted odds ratios for ICU−meanPS-LS affecting probability of well-maintaining MV patients at light sedation. All the 92 ICUs were divided into quartiles (Q1–4) by ICU−meanPS-LS marked as ▴ for Q2, □ for Q3 and • for Q4, respectively. Referred to Q1, OR (95% CI) for probability of well-maintaining MV patients at light sedation was analyzed over increasing ICU-meanPS-LS quartiles (subgroups of Q2, 3, and 4), adjusted by SSI or APACHE II score.
Risk of Death in MV Patients Poorly vs. Well-Maintained at Light Sedation
By a regression model, adjusted OR for mortality was significant in patients characterized with high APACHE II score (OR, 1.072; 95% CI: 1.047–1.097; p < 0.001) and receiving deep sedation once at least during 24 h observation period (OR, 2.034; 95% CI: 1.435–2.884; p < 0.001). In addition, an association between poorly maintained at light sedation and mortality remained significant in subgroup of MV patients with low SSI (p = 0.009) as well as middle SSI (p = 0.023), but not in those with high SSI scores (p = 0.808, Figure 4).
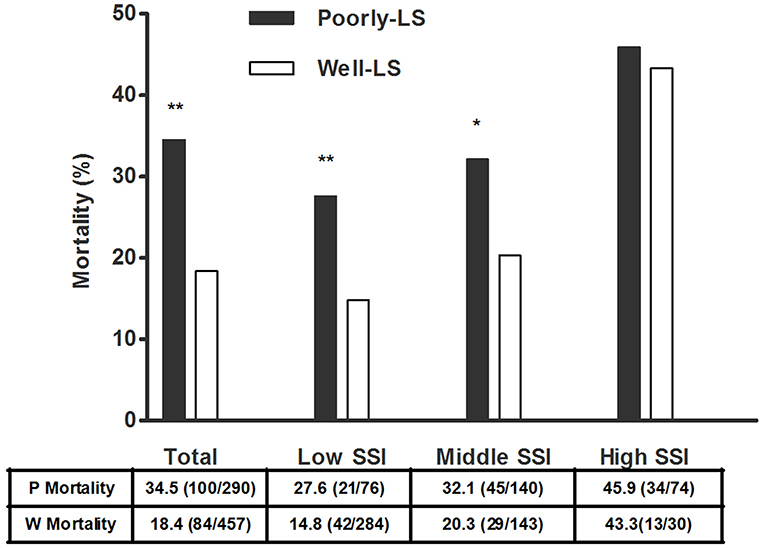
Figure 4. Mortality in MV patients poorly vs. well-maintained at light sedation. Based on the quartiles of SSI scores, 749 MV patients were stratified into low SSI (quartile 1, SSI = 0–2), middle SSI (quartile 2, SSI = 3–5) and high SSI (quartile 3 and 4, SSI = 6–11) subgroups (Patients distributed in quartile 3 and quartile 4 of SSI scores were assigned into high SSI scores subgroup owing to a small number of MV patients in both quartiles). Mortality between MV Patients poorly and well-maintained at light sedation was analyzed by Chi-square test. Poorly-LS meant “poorly-maintained at light sedation” group and Well-LS meant “well-maintained at light sedation” group. **p < 0.01 and *p < 0.05 compared to group Well-LS. SSI, semi-quantitative stimulus intensity; MV, mechanically ventilated.
Discussion
In this study, we found that almost all of the interviewed physicians were concerned about patients' tolerance levels regarding sedation, while titrating sedation depth for MV patients. Estimated by the responses to all items of the questionnaire, meanwhile, their propensity score for light sedation (PS-LS) was highly varied. Importantly, a significant increasing trend in prevalence of MV patients being maintained at light sedation was observed over increasing ICU-meanPS-LS quartiles (from Q1 to Q4). Moreover, adjusted odds ratio for well-maintaining MV patients at light sedation remained significant in the highest referred to the lowest ICU-meanPS-LS quartile, adjusted by APACHE II score and SSI score. These findings suggested that ICU physician's perception for the tolerance levels regarding sedation of MV patients in light sedation was transferred into clinical practice.
In recent years, the pain, agitation, and delirium guidelines (PAD) (7) and early comfort using analgesia, minimal sedatives and maximal humane care (eCASH) concepts encouraged ICU physicians to maintain the MV patients at lighter sedation levels (21, 22). Timely monitoring RASS and CPOT/BPS (Critical care Pain Observation Tool/Behavior Pain Scale) was recommended to appropriately evaluate patients' tolerance levels regarding sedation toward intensive cares and to effectively avoid excessive sedatives and analgesics (7, 21, 23). Our finding of a shift in distribution of physicians toward preferring light sedation (Figure 2) added additional evidence regarding the impact of guidelines on changes in clinical habits. However, this change remained limited. Prevalence of deep sedation was continually high in MV patients. Similar to other recently published results (10, 11, 24), 38.7% of MV patients were poorly maintained at light sedation in this cross-sectional study (one record of deep sedation at least in 24 h, Table 2). It was noted that the decreased prevalence of deep sedation was predominantly documented in previous RCTs (4–6, 19) rather than in real clinical practices as reported by observational studies (10–12, 25). These data suggested that implementation of the light sedation strategy challenges what intensive care currently delivers.
This study provided the new information that the ICU physicians' concern about patients' tolerance levels in light sedation was an important barrier to implementation of minimizing sedation strategy for MV patients. Generally, titration of analgesics and sedatives was used to regulate patients' tolerance levels regarding sedation against nociceptive stimuli (26). Meanwhile, the actual necessity for the regulation of patients' tolerance levels regarding sedation with analgesics and sedatives remained unclear owing to the lack of criteria to scale stimulus. Light sedation is only contraindicated for distinctive types of patients who are evidenced with extremely high intensity of stimuli, such as patients with serious Acute Respiratory Distress Syndrome (ARDS) or patients presented with severe traumatic brain injury (TBI), etc. (27–29). ICU physicians are not clearly guided to titrate levels of sedation, adapting to various stimuli in the vast majority of MV patients. Faced with frequent agitation in MV patients (Table 2) (18, 19), ICU physicians' concerns about patient tolerance levels regarding sedation against stimuli became inevitable. Moreover, their concerns were highly varied because of individualized estimates of stimuli. Indeed, we found that most physicians (320/558, 57.4%) had no intense propensity for light levels of sedation while 8.2% (46/558) of ICU physicians showed an unjustifiable low desire to use light sedation for MV patients (Figure 2). Therefore, the development of a reliable and valid tool to scale patient's suffering stimuli is necessary for avoiding physicians' concerns about patients' tolerance levels regarding sedation as well as for optimizing the levels of sedation objectively and appropriately. We attempted to scale stimulus what MV patients suffered by a semi-quantitative assessment of the suspected stimulus-derived events, named as semi-quantitative stimuli (SSI) in this cross-sectional study. It was interestingly demonstrated that probability of light sedation was negatively correlated with SSI scores of MV patients (r = −0.967, p < 0.001, Figure S3). However, further research is needed to promote validity of this tool in stimulus assessment for optimizing our sedation practices.
A strength of this study was the combination of the questionnaire survey with the cross-sectional study, which determined the association between ICU physicians' perception for the tolerance levels regarding sedation of MV patients and his/her decision making for the use of light sedation. In fact, there was previously little evidence regarding whether ICU physicians' beliefs drove sedation practices (30). In this study, a significantly increased prevalence of lightly sedated MV patients was found in ICUs stratified into the highest vs. the lowest ICU-meanPS-LS quartile (Table 3). Moreover, this finding was further convinced by adjusting SSI score (Figure 3). These results suggested that ICU physicians' perception of the tolerance levels regarding sedation of MV patients in light sedation impact sedation practice for mechanically ventilated patients was an important factor in the poor implementation of light sedation strategy. Therefore, the development of a valid assessment of nociceptive stimulus to predict rather than to concern patients' tolerance levels regarding sedation against stimuli individually would be helpful to ICU physicians delivering necessity-based sedation depth for MV patients.
Consistent with previous reports, the risk potential of death was significantly increased in deeply sedated patients (1–3). Interestingly, subgroup analysis demonstrated that there was no significant difference in the mortality of MV patients between poorly and well-maintained at light sedation in the high SSI group (SSI ≥ 6). However, poorly maintaining MV patients at light sedation was shown to be harmful for the majority of MV patients, who were estimated with low and middle SSI. Notably, the impact of ICU-meanPS-LS on the prevalence of light sedation was significant in these two subgroups of MV patients. ICU physicians' concern of patients' tolerance levels regarding sedation in light sedation seemed to be associated with outcomes.
There were several limitations in this study. Firstly, the 10 specific events of the questionnaire were identically weighted to analyze PS-LS. However, the answer “D” (an issue considered unimportant for sedation depth decision-making) could partially, at least, diminish the influence of this limitation. Secondly, the MV patients' suffering stimuli were multiple and complex. Only few variables regarding ventilator settings and vital organ dysfunction were considered in this study. However, those 10 events were derived from a Delphi processing and were approved by the interviewees. Thirdly, we didn't investigate the association of physicians PS-LS with their decision making on depth of sedation for MV patients, for whom they were responsible. Indeed, it was too difficult to be performed reliably. Despite this, this study provided a window to look at the perception of ICU physicians on light-sedated patients' tolerance levels regarding sedation vs. their clinical behaviors.
Conclusions
Owing to lack of criteria to scale stimulus, ICU physicians' perception for patients' tolerance levels regarding sedation in light sedation was highly individualized. Importantly, the fact that a significantly increasing prevalence of well-maintaining MV patients at light sedation was observed over increasing ICU-meanPS-LS quartiles suggested that ICU physicians' perception for the tolerance levels regarding sedation of MV patients in light sedation affected decision making on sedation depth for MV patients. As the consequence of ICU physicians' individualized concerns, poorly maintenance at light sedation was frequent, and was significantly associated with an increased mortality. Therefore, to develop a valid assessment for nociceptive stimuli rather than to individually analyze concerns about patients' tolerance levels regarding sedation would be helpful for ICU physicians delivering necessity-based sedation depth for MV patients. SSI score was an attempt to scale stimulus in this study. Further research is needed to promote its reliability and validity.
Data Availability Statement
All datasets generated for this study are included in the manuscript/Supplementary Files.
Ethics Statement
The study was conducted in accordance with the Helsinki Declaration and approved by the Ethics Committees of each participating hospital. Owing to the absence of additional interventions, the requirement for written informed consent was waived by the ethics committees of all participating hospitals. The study protocol was registered on the website of www.chictr.org.cn (registration number: ChiCTR-EOC-16008444).
Author Contributions
PM contributed to the study concept and design, data interpretation, and article drafting. YG contributed to the literature search, questionnaire drafting, data collection, data analyzation, and article drafting. HY and JL participated in questionnaire drafting, data collection, and information organization. JX participated in the study design and data statistical analyzation. JZ participated in the study design. All authors read and approved the final manuscript.
Funding
This study was supported by Chinese Medical Association (grant number: 13010010386). The study sponsors have had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2019.00226/full#supplementary-material
Abbreviations
MV, mechanically ventilated; RASS, Richmond Agitation Sedation Scale; RCTs, randomized control trials; Mode, ventilation mode; PEEP, positive end-expiratory pressure; Pplat, plateau pressure; FiO2, fraction of inspiration; RR, respiratory rate; Min-V, minute ventilation; P/F, PaO2/FiO2; Lac, plasma lactate level; GCS, Glasgow Coma Score; NE, norepinephrine; PS-LS, propensity score for light sedation; ICU-meanPS-LS, ICU mean physician's PS-LS; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; SSI, semi-quantitative stimuli Intensity; PAD, Pain, Agitation, and Delirium; eCASH, early Comfort using Analgesia, minimal Sedatives and maximal Humane care; CPOT/BPS, Critical care Pain Observation Tool/Behavior Pain Scale; ARDS, Acute Respiratory Distress Syndrome; TBI, Traumatic brain injury.
References
1. Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. (2012) 186:724–31. doi: 10.1164/rccm.201203-0522OC
2. Shehabi Y, Chan L, Kadiman S, Alias A, Ismail WN, Tan MA, et al. Sedation depth and long-term mortality in MV critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. (2013) 39:910–8. doi: 10.1007/s00134-013-2830-2
3. Shehabi Y, Bellomo R, Kadiman S, Ti LK, Howe B, Reade MC, et al. Sedation intensity in the first 48 hours of mechanical ventilation and 180-day mortality: a multinational prospective longitudinal cohort study. Crit Care Med. (2018) 46:850–9. doi: 10.1097/CCM.0000000000003071
4. Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT. Efficacy and safety of a paired sedation and ventilator weaning protocol for MV patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. (2008) 371:126–34. doi: 10.1016/S0140-6736(08)60105-1
5. Strom T, Martnussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. (2010) 375:475–80. doi: 10.1016/S0140-6736(09)62072-9
6. Treggiari MM, Romand JA, Yanez ND, Deem SA, Goldberg J, Hudson L, et al. Randomized trial of light versus deep sedation on mental health after critical illness. Crit Care Med. (2009) 37:2527–34. doi: 10.1097/CCM.0b013e3181a5689f
7. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. (2013) 41:263–306. doi: 10.1097/CCM.0b013e3182783b72
8. DAS-Taskforce 2015, Baron R, Binder A, Biniek R, Braune S, Buerkle H, et al. Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-Guideline 2015) - short version. Ger Med Sci. (2015) 13:Doc19. doi: 10.3205/000223
9. Celis-Rodríguez E, Birchenall C, de la Cal MÁ, Castorena Arellano G, Hernández A, Ceraso D, et al. Clinical practice guidelines for evidence-based management of sedoanalgesia in critically ill adult patients. Med Intensiva. (2013) 37:519–74. doi: 10.1016/j.medine.2013.04.002
10. Tanaka LM, Azevedo LC, Park M, Schettino G, Nassar AP, Réa-Neto A, et al. Early sedation and clinical outcomes of MV patients: a prospective multicenter cohort study. Crit Care. (2014) 18:R156. doi: 10.1186/cc13995
11. Balzer F, Weiß B, Kumpf O, Treskatsch S, Spies C, Wernecke KD, et al. Early deep sedation is associated with decreased in-hospital and two-year follow-up survival. Crit Care. (2015) 19:197. doi: 10.1186/s13054-015-0929-2
12. SRLF Trial Group. Impact of oversedation prevention in ventilated critically ill patients: a randomized trial-the AWARE study. Ann Intensive Care. (2018) 8:93. doi: 10.1186/s13613-018-0425-3
13. Jackson DL, Proudfoot CW, Cann KF, Walsh TS. The incidence of sub-optimal sedation in the ICU: a systematic review. Crit Care. (2009) 13:R204. doi: 10.1186/cc8212
14. Mehta S, McCullagh, Burry L. Current sedation practices: lessons learned from international surveys. Anesthesiol Clin. (2011) 29:607–24. doi: 10.1016/j.anclin.2011.09.003
15. Peitz GJ, Balas MC, Olsen KM, Pun BT, Ely EW. Top 10 myths regarding sedation and delirium in the ICU. Crit Care Med. (2013) 41S9:46–56. doi: 10.1097/CCM.0b013e3182a168f5
16. Sneyers B, Laterre PF, Perreault MM, Wouters D, Spinewine A. Current practices and barriers impairing physicians' and nurses' adherence to analgo-sedation recommendations in the intensive care unit - a national survey. Crit Care. (2014) 18:655. doi: 10.1186/s13054-014-0655-1
17. Rose L, Fitzgerald E, Cook D, Kim S, Steinberg M, Devlin JW, et al. Clinician perspectives on protocols designed to minimize sedation. J Crit Care. (2015) 30:348–52. doi: 10.1016/j.jcrc.2014.10.021
18. Jaber S, Chanques G, Altairac C, Sebbane M, Vergne C, Perrigault PF, et al. A prospective study of agitation in a medical-surgical ICU: incidence, risk factors, and outcomes. Chest. (2005) 128:2749–57. doi: 10.1378/chest.128.4.2749
19. Burk RS, Grap MJ, Munro CL, Schubert CM, Sessler CN. Agitation onset, frequency, and associated temporal factors in critically ill adults. Am J Crit Care. (2014) 23:296–304. doi: 10.4037/ajcc2014186
20. Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B. Early goal-directed sedation versus standard sedation in MV critically ill patients: a pilot study. Crit Care Med. (2013) 41:1983–91. doi: 10.1097/CCM.0b013e31828a437d
21. Vincent JL, Shehabi Y, Walsh TS, Pandharipande PP, Ball JA, Spronk P. Comfort and patient-centred care without excessive sedation: the eCASH concept. Intensive Care Med. (2016) 42:962–71. doi: 10.1007/s00134-016-4297-4
22. Wang J, Peng ZY, Zhou WH, Hu B, Rao X, Li JG. A National multicenter survey on management of pain, agitation, and delirium in intensive care units in China. Chin Med J (Engl). (2017) 130:1182–88. doi: 10.4103/0366-6999.205852
23. Kerson AG, DeMaria R, Mauer E, Joyce C, Gerber LM, Greenwald BM, et al. Validity of the richmond agitation-sedation scale (RASS) in critically ill children. J Intensive Care. (2016) 4:65. doi: 10.1186/s40560-016-0189-5
24. Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, et al. Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. (2019) 380:2506–17. doi: 10.1056/NEJMoa1904710
25. Luetz A, Balzer F, Radtke FM, Jones C, Citerio G, Walder B, et al. Delirium, sedation and analgesia in the intensive care unit: a multinational, two-part survey among intensivists. PLoS ONE. (2014) 9:e110935. doi: 10.1371/journal.pone.0110935
26. Jacobi J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. (2002) 30:119–41. doi: 10.1097/00003246-200201000-00020
27. Murray MJ, DeBlock H, Erstad B, Gray A, Jacobi J, Jordan C, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. (2016) 44:2079–103. doi: 10.1097/CCM.0000000000002027
28. Abou El, Fadl MH, O'Phelan KH. Management of Traumatic Brain Injury: An Update. Neurosurg Clin N Am. (2018) 29:213–21. doi: 10.1016/j.nec.2017.11.002
29. Oddo M, Crippa IA, Mehta S, Menon D, Payen JF, Taccone FS, et al. Optimizing sedation in patients with acute brain injury. Crit Care. (2016) 20:128. doi: 10.1186/s13054-016-1294-5
Keywords: physician's perception, light sedation, stimulus intensity, sedation practice, mechanical ventilation
Citation: Gong Y, Yang H, Xie J, Liu J, Zhou J and Ma P (2019) ICU Physicians' Perception of Patients' Tolerance Levels in Light Sedation Impacts Sedation Practice for Mechanically Ventilated Patients. Front. Med. 6:226. doi: 10.3389/fmed.2019.00226
Received: 29 July 2019; Accepted: 30 September 2019;
Published: 18 October 2019.
Edited by:
Zhongheng Zhang, Zhejiang University, ChinaReviewed by:
Yoshinori Ohta, Hyogo College of Medicine, JapanDusica Simic, University of Belgrade, Serbia
Copyright © 2019 Gong, Yang, Xie, Liu, Zhou and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Penglin Ma, bWFwZW5nbGluMUAxNjMuY29t
 Yichun Gong
Yichun Gong Huilong Yang
Huilong Yang Junqing Xie4
Junqing Xie4 Penglin Ma
Penglin Ma