
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 17 October 2019
Sec. Translational Medicine
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00225
This article is part of the Research Topic Biobanks as Essential Tools for Translational Research: The Belgian Landscape View all 12 articles
 Ligia Craciun1,2*
Ligia Craciun1,2* Selim Alex Spinette1,2
Selim Alex Spinette1,2 Marc Rassy2
Marc Rassy2 Roberto Salgado2
Roberto Salgado2 Alexandre de Wind2
Alexandre de Wind2 Pieter Demetter2
Pieter Demetter2 Laurine Verset2
Laurine Verset2 Maria Gomez-Galdon2
Maria Gomez-Galdon2 Marie Chintinne2
Marie Chintinne2 Nicolas Sirtaine2
Nicolas Sirtaine2 Nicolas de St Aubain2
Nicolas de St Aubain2 Ioanna Laios2
Ioanna Laios2 Francoise Roy2
Francoise Roy2 Denis Larsimont1,2
Denis Larsimont1,2Introduction: Tumor banks make a considerable contribution to translational research. Using emerging molecular tests on frozen material facilitates the development of new diagnostic and therapeutic strategies, especially in rare cases. However, standard quality control schemes are lacking in the current literature.
Methods: In 2017, we have conducted a robust quality control test on 100 of 15,000 fresh frozen samples collected between 2000 and 2013 at the Jules Bordet Tumor Bank (Brussels). RNA and DNA extraction was done. The quality of RNA, DNA and proteins were evaluated, respectively by measuring RNA Integrity Number (RIN), by checking Electrophoretic Integrity (EI) and by performing Immunohistochemistry staining (IHC). A score, ranging from poor (1) to excellent (4), was attributed based on technical analysis.
Results: RNA purity was scored 4 in 97% of the cases, 3 in 2%, and 2 in 1%. RIN scores were similarly 4 in 89%, 3 in 10%, and 2 in 1% of the cases. DNA purity was scored 4 in 94% and 3 in 6%, EI was scored 4 in 100% of the cases. Despite morphology loss after freezing, HER2, ER, and Ki67 IHC stainings yielded a score of 4 in the majority of samples. Furthermore, participating in the ISBER Proficiency Testing helped us validate our techniques and the technician's work. Seven processing schemes were carried out, the scores obtained were very satisfactory (20/27) or satisfactory (7/27).
Conclusion: Tumor Banks can be precious for translational research. Nevertheless, firm quality controls should be applied to ensure high quality material delivery. Only then can biobanks contribute to diagnostics, biomarkers discovery and reliable molecular test development.
Collecting samples for research is an old concept among pathologists and researchers. Nevertheless, biobanking is only fairly recent (1), as well as legislation concerning collection of human tissue and data protection (EU Data Protection Directive—Directive 95/46/EC). Controlling the quality of collected material in a biobank is crucial before providing tissue specimens for research. Quality control procedures must be in place to evaluate the samples and the effects of long-term storage. The lack of reproducibility in gene signatures is often associated with tissue heterogeneity due to, among other things, the lack of standardization of collection procedures (2). A good level of molecular integrity is essential to avoid variability in the results of research projects. The quality of nucleic acids is of major importance for several techniques used in genetic analysis.
Convenient quality control procedures must check the validity of final products (samples or derivatives) for different applications of end-use in research, irrespective of the used extraction method. Scores and cutoffs are to be adopted to determine the quality acceptance limits.
The first phase, termed pre-analytical phase, summarizes all steps from tissue sampling to the start of the desired end-use application. Each of these steps can affect the quality of the sample, the quality of the results, and their reproducibility (3, 4). Once the critical pre-analytical steps (medications, anesthesia, warm and cold ischemia time) for an application are known, researchers will only examine samples that meet the pre-analytical conditions previously defined. Quality control procedures (QC) are used to either confirm tissue quality from known pre-analytical conditions or investigate tissue quality from unknown pre-analytical condition(s). The ideal quality control “biomarkers” should be ubiquitous, measurable by accessible methods and leading to a dichotomous response to a specific pre-analytic variation.
Validation of clinically appropriate biomarkers should take into account the potential impact of pre-analytical variation on each of them. This validation process is the key to research using bio-resources (a requalified tissue sample for research and its associated data). Rapid stabilization of tissues by snap freezing immediately can reduce artifactually altered gene expression. Moreover, unlike FFPE tissue, the RNA and DNA from frozen tissue are of high molecular weight, lack cross-linking modifications and are therefore better candidates for the “next-generation” testing.
Good QC tools aim to test the molecular integrity and protein quality. They must also be compatible with genomic, epigenomic, transcriptomic, proteomic, and metabolomic tests.
Moreover, histological control of stored tissue is a crucial step. Generally, 10% of the frozen samples are unsuitable for the molecular analysis mainly because of insufficient quantity of malignant cells or necrosis (5).
The purpose of this work is to establish the quality limits of the tissues stored in tumor banks by independently evaluating the morphological (proteins) and molecular (DNA and RNA) characteristics on randomized selected frozen tumor samples.
In parallel, we have used the Biospecimen Proficiency Testing (PT) programme launched by IBBL, as an external quality assessment tool to verify the precision and accuracy of the in house biospecimens testing methods. Seven processing and testing schemes were performed: DNA Extraction from FFPE Material, DNA Extraction from Frozen Tissue, DNA Quantification and Purity, Total RNA Extraction from Frozen Tissue, RNA Integrity, RNA Quantification and Purity, and Tissue Histology.
Ethical approval, concerning the biobank activities and objectives, was granted by the ethics committee of the Institut Jules Bordet (CE1891 and CE2897). Of note, and according to the Belgian laws (2008-12-19/44 and 2018-01-09/14), the ethics committee of the Institut Jules Bordet approved the study protocol and waived the requirement of patients written consent.
One hundred biobank frozen samples (52 breasts, 13 thyroids, 12 lymph nodes, 9 endometrium, 3 ovaries, 2 sarcomas, 2 kidney, 2 colon, 1 prostate, 1 lung, 1 small intestine, 1 spleen, 1 uterus) originating from 100 patients were tested for DNA, RNA and protein quality. Selected samples dated from 2000 to 2013. Tumor samples were embedded in Tissue-Tek® O.C.T.™ Compound (by Sakura®) and frozen at −80°C. This method allows sectioning on a cryostat without residues during the staining procedure. Indeed, frozen sections were performed on a cryostat (by Leica Biosystems®). The first slide was stained by H&E (Hematoxylin and Eosin). Twenty serial sections were collected in RNase free Eppendorf tubes. Four additional sections were used for the IHC staining. Of note, all necessary material for sections handling was cooled on dry ice, to preserve the cold chain and avoid temperature fluctuations. The tumor morphology and cellularity were last reviewed by a pathologist.
DNA and RNA were extracted from frozen specimens using AllPrep DNA/RNA Micro Kit (Qiagen) according to the manufacturer's instructions. DNA and RNA were finally eluted in a volume of 20 μL.
A good DNA quality is important for studies on genomic DNA and CGH analyses. Two parameters were evaluated: concentration and purity, measured by the OD and DNA integrity by electrophoresis gel. The ratio for pure DNA should be between 1.8 and 2.1: a lower ratio is indicative of protein contamination, while a higher ratio indicates a degradation of the DNA. This ratio is only an indication of purity of nucleic acids and does not necessarily reflect the integrity of the nucleic acids.
A visual analysis on electrophoresis gel was done to estimate the sample integrity. The degree of DNA degradation was examined using electrophoresis in a 2% agarose gel (Reliant™ Gel System, 2% SeaKem® Gold Agarose, Lonza, USA). Intact genomic DNA appears as a compact, high-molecular-weight band with no scanty low-molecular-weight smears. 1 kb DNA ladder from Solis Biodyne was used as molecular marker. A quality score could be assigned (Table 1).
The yield and purity of total DNA and RNA were determined using a spectrophotometer (Nanodrop TM ND-1000, Thermo Fisher Scientific). A 260/280 OD ratio >1.8 was considered an indicator of acceptably pure RNA, relatively free of protein.
RNA was examined on the Agilent 2100 bioanalyzer, based on microfluidic capillary electrophoresis. RNA 6000 Nano LabChip kits were used. For each sample, 1 μL of extracted RNA was analyzed. RIN scores, ranging from 1 to 10, were retrieved. A RIN between 7 and 10 was associated with intact RNA.
Based on the 260/280 OD ratio and on the RIN, a score was assigned for each case, as shown in Table 2.
Consecutive frozen tissue sections (4 μm) were immunohistochemically (IHC)-stained using a BenchMark XT IHC/ISH automated slide stainer (Ventana Mediated Systems, by Roche®). The following antibodies were used: anti-HER2/NEU (rabbit monoclonal antibody, clone 4B5, Roche® Ventana®); anti-Estrogen Receptor (ER) (rabbit monoclonal antibody, clone SP1, Roche® Ventana®); and anti-Ki-67 (mouse monoclonal antibody, clone MIB-1, Agilent®). Breast tumor samples (n = 52) were tested with anti-HER2/NEU and anti-ER antibodies. Non-breast tumor samples (n = 48) were tested with anti-Ki67 antibody.
For this study, H&E staining allowed the evaluation of cellular integrity and morphology, while immunohistochemistry staining (Ki67, HER2, and ER) provided a practical evaluation of proteins quality control.
A quality score, based on visual evaluation of quality staining, was assigned. All scoring systems were based on two separate components: the specificity and the intensity of staining. Technical sensitivity and specificity cannot be accurately calculated when IHC is used as a qualitative test because it is merely a descriptive test. The relation between the staining and the protein availability isn't linear. Calibration controls aren't either available. Scoring was blindly done by two independent pathologists. While scoring, routine sections from FFPE (formalin fixed and paraffin embedded) blocks were used as reference (Table 3).
The material used for this scheme was a Jurkat cell line. We received one tube containing 2 FFPE sections of 20 μm. We extracted the DNA following our usual routine silica membrane-based DNA extraction method. The extracted DNA sample was shipped back to the PT provider. The total DNA yield per 20 μm section, DNA purity, DNA integrity (DIN), DNA functionality and amplifiability (cross-linking assessment) and DNA quality (ENZO score) of all extracted DNA were assessed.
The material used for this scheme was a pig (Sus) liver. We received one tube containing one CryoXtract core of 10 to 20 mg. We performed the DNA extraction following our usual routine silica membrane-based DNA extraction method. The extracted DNA sample was shipped back to the PT provider. The total DNA yield per mg of tissue, the DNA purity (A260/A280), the double-stranded DNA yield per mg of tissue, the DNA integrity (DIN) and the presence of PCR inhibitors using a SPUD assay were assessed by IBBL.
The DNA used for this scheme was extracted from whole blood. We received three different Test Items containing DNA at a different concentration and 260/280 ratio (i.e., Tube A, Tube B, and Tube C). For each Test Item (Tube A, Tube B, and Tube C), we measured the DNA concentration (μg/ml) and 260/280 ratio by spectrophotometry.
The material used for this scheme was a pig (Sus) liver. In this scheme, we received one single “Processing Item” (one tube containing one CryoXtract core of 10 to 20 mg). The RNA was extracted following our usual routine silica membrane-based RNA extraction method. The extracted RNA sample was shipped back to the PT provider. The total RNA yield per mg of tissue, the RNA purity (A260/A280) and the RNA integrity (RIN) were assessed by IBBL.
The RNA used for this scheme was extracted from a Jurkat cell line by a silica-based method. Three different Test Items containing RNA at a different level of integrity (i.e., Tube A, Tube B, and Tube C) were received. For each Test Item (Tube A, Tube B, and Tube C), we measured the RNA Integrity (RIN) on the Agilent® 2100 Bioanalyzer System.
The RNA used for this scheme was extracted from a Jurkat cell line by a silica-based method. Three different Test Items containing RNA at a different concentration and 260/280 ratio (i.e., Tube A, Tube B, and Tube C) were received. For each Test Item (Tube A, Tube B, and Tube C), we measured the RNA concentration (μg/ml) and 260/280 ratio by spectrophotometry.
The Test Items were pictures of human colon adenocarcinoma (Test Items A) and human breast adenocarcinoma (Test Item B, Test Item C, Test Item D, and Test Item E). The tissue characterization/mapping was done through the assessment of the percentage of uninvolved tissue areas (Test Item A, Test Item B, and Test Item C) and of viable tumor areas (Test Item D and Test Item E).
For each test, the scoring system was based on deviation from the assigned value. A consensus score was established as follow: below 1 standard deviation: 0 (very satisfactory); below 2 standard deviations: 1 (satisfactory); above 2 standard deviations: 2 (questionable); and above 3 standard deviations: 3 (requiring action). The results were reported through the website http://biospecimenpt.ibbl.lu.
The morphology was successfully determined in the majority of samples. Two samples were tumor free and one has been totally consumed through sectioning.
Based on 260/280 ratio, the majority of tested samples were evaluated with a score of 4 (94%) (Figure 1), and 6 samples were scored with 3. The 260/230 ratio were used as a secondary measure of nucleic acid purity. The data is available but doesn't provide any supplementary information. No contamination by salt or organic compounds was noted. The EI was estimated at score 4 for all the tested samples.
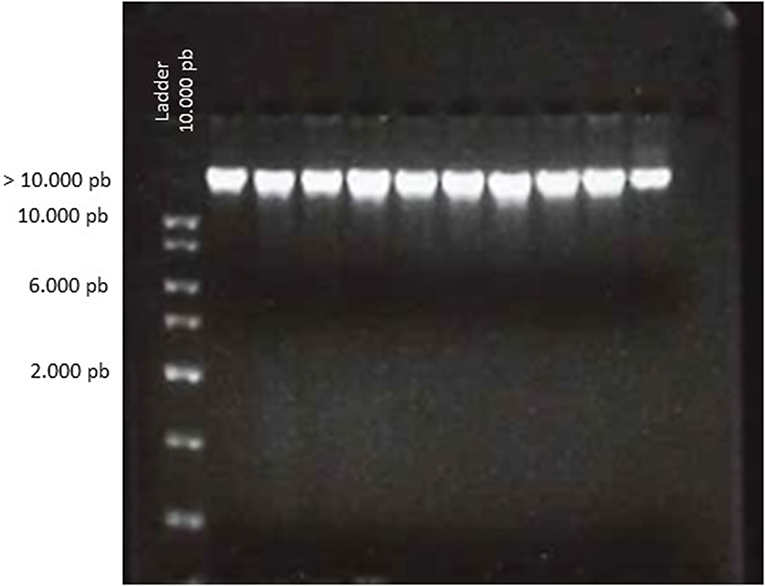
Figure 1. Electrophoretic analysis of genomic DNA from biobanked frozen tumor samples. DNA (5 μL) was loaded on a 2% agarose gel and visualized by ethidium bromide staining. The gel shows the result of 10 representative samples. Compact bands of DNA were observed for all samples at a high molecular weight according to the ladder. The absence of smearing favors the absence of DNA degradation.
Upon optical density (OD) measurement of extracted RNA, most samples were evaluated with a score of 4 (97%), two samples were scored with 3 and one sample was unusable due to insufficient RNA amount. In the latter, the tissue fragment was mainly fibrotic on microscopic examination. RIN values (Figure 2) were classified as of sufficient quality: score of 4 (89%) and score of 3 (10%); the sample characterized by a score of 2 (1%) was considered inadequate for further analysis.
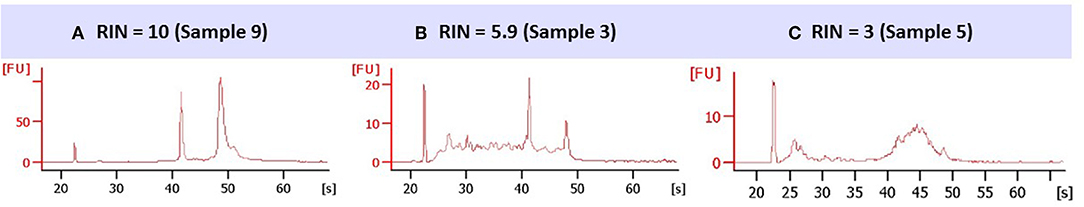
Figure 2. Representative electropherogram for different RIN classes. 1 μL of sample RNA was charged in the microfabricated chips. (A) RIN = 10, from one representative sample classified as score 4; the different regions (pre-, 5S-, fast-, inter-, precursor-, post-region) and peaks (marker, 18S, 28S) are correctly presented. (B) RIN = 5.9, from one representative sample classified as score 3; intermediate peaks appear on the zone 5S and fast-regions, pointing to RNA degradation. (C) RIN = 3, from one representative sample classified as score 2; peaks of ribosomal subunits, 18S and 28S, are absent.
All tested samples were characterized by a good histologic quality control. The percentage of area of the tissue involved with tumor was considered acceptable despite the presence of freezing artifacts in almost all cases (Figure 3). The majority of screened samples were scored with 3 or 4 (Table 4).

Figure 3. Frozen breast tumor sample. (A) Hematoxylin/eosin staining (30x); (B) ER IHC staining; (C) Her2 IHC staining. The tumor was characterized as ductal carcinoma, ER/Her2 positive, by the pathologist in 2001 and confirmed on the 16-year-old frozen sample. The pathologist evaluation is based on four criteria: the intensity of staining, the percentage of positive cells, background, and the localization of hybridized antibody (membrane, cytoplasm).
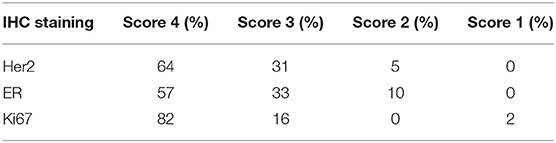
Table 4. Summary of the IHC scores assigned to samples of the study cohort based on the visual evaluation.
Our results (16,990 ng/20 μm slice) were compared to all the results' average (7,453.22 ng/20 μm slice) and silica membrane-based (8,141.92 ng/ 20 μm slice). They have been designated as “accurate” or “very satisfactory,” consensus score “0.” The ratio 260/280 (1.95) has been designated as “accurate” or “very satisfactory,” consensus score “0.” The DIN (5.40) was also designated as “accurate” or “very satisfactory,” score 0, compared to all results average (4.49). The ENZO score was qualified as good-excellent and the level of PCR inhibitors were qualified as compatible with CGH assay.
Our results (1,733.60 ng/mg tissue) were compared to all results average (1,865.18 ng/mg tissue) and silica membrane-based (2,045.98 ng/mg tissue). They have been designated as “accurate” or “very satisfactory,” consensus score “0.” The double-stranded DNA yield per mg tissue were 1,134.60 ng/mg tissue. It was considered “very satisfactory” when compared with the mean of all results (955.83 ng/mg tissue). The ratio 260/280 (1.90) has been designated as “accurate” or “very satisfactory,” consensus score “0.” The DIN (6.30) was also designated as “accurate” or “very satisfactory,” score 0, compared to all results average (5.67).
The accuracy of our measurements was qualified as “very satisfactory,” consensus score “0” when compared with mean values: Tubes A/B/C, 246.1/117.6/ 31.5 vs. 248.5/119.5/32.7 μg/ml. DNA purity was evaluated as “very satisfactory,” consensus score “0”: obtained values were 1.71/1.30/1.83 compared with expected ratio values: 1.72/1.32/1.92.
The average of all expected results was 1,843.68 ng/mg tissue, our result was 572.9 ng/mg tissue, designated “acceptable” or “satisfactory,” consensus score “1.” RNA purity was evaluated as “very satisfactory,” consensus score “0”: obtained ratio value was 2.1 compared with expected ratio value 2.03. The obtained RIN value was 6.8 instead 6.55 mean all values considered “very satisfactory,” consensus score “0.”
The three obtained values (2.73/4.33/9.47) were slightly different from expected values (2.55/4.74/9.43), yielding a consensus score of “0”/“1”/“0.”
The three obtained values (93.6/60.1/33.4 μg/ml) were slightly different from expected values (90.5/60.1/33.4), yielding a consensus score of “0”/“0”/“1.” RNA purity was evaluated as “satisfactory” and “very satisfactory,” data not shown.
Regarding tissue histology, our consensus score required revision for the slide A, colon adenocarcinoma, and was satisfactory or very satisfactory for slides B and C, breast carcinoma. Evaluating viable tumor tissue was satisfactory or very satisfactory for slides D and E.
The Institut Jules Bordet tumor bank is completely integrated in the Pathological Department. The pathologist and technician pathologist are critical to identify the presence and type of tumor lesion and are responsible for ensuring diagnostic use prior to releasing tissue for research. The proximity of the operating room allows specimens quick handling reducing pre-analytical biases. Some samples stored in our biobank are already more than 20 years old.
Medical research projects are dependent on biobanked tissue of high quality because the gene expression analysis is affected by the quality of extracted RNA and DNA (6). Different factors influence the quality of nucleic acids and proteins including pre-analytical variables, transport, duration of processing at ambient temperature, necrosis, temperature and freezing products, size and number of aliquots and storage duration. The long-term storage temperature could impact the tissue quality. We currently use the OCT embedding medium on cryovials, followed by −80°C storage temperature for the solid tumors. The OCT embedding medium acts as cryoprotector from the freeze-thaw effects and gives the possibility to verify the histology after frozen sectioning and H&E staining.
Histologic quality control must be performed on biological samples. Different percentage cut-offs of tumor nuclei are required for each downstream use of the sample. The percentage of viable tumor cells is very important to perform NGS. It can range from 2% (7) to 80% (8). In our opinion, it is crucial to inform the researcher on the histologic quality before performing sensitive and expensive techniques.
RNA preservation is particularly important for gene expression analysis. RNA is known to be quickly degraded by ubiquitous RNase enzymes. OD reading is useful for determining the amount and purity of nucleic acids. Ribosomal RNA integrity is often used to reflect all RNAs physical integrity. In our study, RIN values were evaluated at score 4, even for the oldest samples. In addition to rRNA which represent 80% of total RNA, the messenger RNA (mRNA) and microRNA (miRNA) which constitute a small class of coding and, respectively, noncoding cellular RNA are the most interesting target for research. The stability of mRNA is better despite complete degradation of rRNA (9). Once again, the researcher together with biobank staff has to establish the tissue quality requirements before starting the research project. If the rare tumors are concerned by the research, too stringent criteria must be revised.
We have determined the suggested applications based on the value of the RIN (Table 5) (10).
At our institute, optical density and gel electrophoresis are commonly adopted for quick evaluation of extracted DNA purity and integrity. On a 2% agarose gel, intact genomic DNA appears as a compact, high-molecular-weight band with no low-molecular-weight smears. The scores assigned to our 2017 QC were mostly of 4, demonstrating an excellent quality of stored frozen tissues. Amplifying a specific sequence by PCR could give an information about usability of DNA for downstream molecular applications. Low amounts of PCR products can be attributed to poor quality DNA or poor quality tissues.
Investigation of different surface proteins can yield useful information on pathological pathways or biomarkers related to a particular disease. Specific immunohistochemical stains can be performed to evaluate specific antigens. This technique is routinely used on FFPE diagnostic blocs.
Assessing protein integrity is important since the freezing process may result in proteolysis and protein degradation (11, 12). Nevertheless, an accessible comprehensive way to assess protein quality is not available for frozen samples. Histologic evaluation of the tissue by the pathologist can provide a preliminary screening of degraded tissues. Our method fits with our lab equipment. Evaluating proteins quality by IHC of frozen tissue is really challenging because of cell structure freezing-related modifications. Training or experience is required for the pathologist scoring the stained slides. Mass spectrometry has become a crucial technique for almost all proteomics experiments, it should be considered for further analysis. It represents, indeed, the gold standard technique to test the protein quality, this technique is judicious when available. Using one or other technique is depending on the laboratory equipment and possibilities.
External quality tests, such as the ISBER Biorepository proficiency testing (13), allow both validation and improvement of protocols. Every failed QC item is deeply analyzed and corrected. If necessary, a dialogue with the external partner is established for additional information. Data can be exchanged regarding the test performance or the technician's work. The protocol deviation is then registered and corrective action adopted.
In conclusion, we proposed in this paper an easy quality control schema of biobank stored frozen samples with different ages, different tissue types and different types of morphology. Quality control for RNA, DNA and proteins might be performed periodically on a subset of samples in a biobank. The quality of our tumor samples was very satisfactory and adapted to a large panel of “next-generation” technologies. Our methods and techniques were validated by the external ISBER Proficiency testing program. Based on easy scoring procedures, the biobanks can give indications for downstream molecular biology application (14).
The datasets generated for this study are available on request to the corresponding author.
LC conceived and designed the experiment. SS performed the techniques. LC, SS, MR, RS, AW, PD, LV, MG-G, MC, NS, NSA, and DL analyzed the data. IL and FR quality management supervisor. LC and MR wrote the paper. DL oversaw the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
1. Vaught JB. Biorepository and biospecimen science: a new focus for CEBP. Cancer Epidemiol Biomarkers Prev. (2006) 15:1572–3. doi: 10.1158/1055-9965.EPI-06-0632
2. Freedman LP, Cockburn IM, Simcoe TS. The economics of reproducibility in preclinical research. PLoS Biol. (2015) 13:e1002165. doi: 10.1371/journal.pbio.1002165
3. Aktas B, Sun H, Yao H, Shi W, Hubbard R, Zhang Y, et al. Global gene expression changes induced by prolonged cold ischemic stress and preservation method of breast cancer tissue. Mol Oncol. (2014) 8:717–27. doi: 10.1016/j.molonc.2014.02.002
4. Hewitt SM, Lewis FA, Cao Y, Conrad RC, Cronin M, Danenberg KD, et al. Tissue handling and specimen preparation in surgical pathology: issues concerning the recovery of nucleic acids from formalin-fixed, paraffin-embedded tissue. Arch Pathol Lab Med. (2008) 132:1929–35. doi: 10.1043/1543-2165-132.12.1929
5. Boudou-Rouquette P, Touibi N, Boëlle PY, Tiret E, Fléjou JF, Wendum D. Imprint cytology in tumor tissue bank quality control: an efficient method to evaluate tumor necrosis and to detect samples without tumor cells. Virchows Arch. (2010) 456:443–7. doi: 10.1007/s00428-010-0889-5
6. Talaat AM, Howard ST, Hale W IV, Lyons R, Garner H, Johnston SA. Genomic DNA standards for gene expression profiling in Mycobacterium tuberculosis. Nucleic Acids Res. (2002) 30:e104. doi: 10.1093/nar/gnf103
7. Rui H, ZhenXing Y, MingHui Li, HuaSheng X. Impact of the next-generation sequencing data depth on various biological result inferences. Sci China Life Sci. (2013) 56:104–9. doi: 10.1007/s11427-013-4441-0
8. Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. (2008) 455:1061–8. doi: 10.1038/nature07385
9. Eikmans M, Rekers NV, Anholts JD, Heidt S, Claas FH. Blood cell mRNAs and microRNAs: optimized protocols for extraction and preservation. Blood. (2013) 121:e81–9. doi: 10.1182/blood-2012-06-438887
10. Opitz L, Salinas-Riester G, Grade M, Jung K, Jo P, Emons G, et al. Impact of RNA degradation on gene expression profiling. BMC Med Genomics. (2010) 3:36. doi: 10.1186/1755-8794-3-36
11. Andreasson A, Kiss NB, Juhlin CC, Höög A. Long-term storage of endocrine tissues at - 80°C does not adversely affect RNA quality or overall histomorphology. Biopreserv Biobank. (2013) 11:366–70. doi: 10.1089/bio.2013.0038
12. Kelly R, Albert M, de Ladurantaye M, Moore M, Dokun O, Bartlett JMS. RNA and DNA integrity remain stable in frozen tissue after long-term storage at cryogenic temperatures: a report from the ontario tumour bank. Biopreserv Biobank. (2019) 17:282–7. doi: 10.1089/bio.2018.0095
13. Betsou F, Sobel ME. The ISBER biorepository proficiency testing program: two successful years already, and new features to come. Biopreserv Biobank. (2013) 11:255–6. doi: 10.1089/bio.2013.1143
Keywords: biobank, electrophoretic integrity, quality control, DNA, RNA, morphology, quality scores
Citation: Craciun L, Spinette SA, Rassy M, Salgado R, de Wind A, Demetter P, Verset L, Gomez-Galdon M, Chintinne M, Sirtaine N, de St Aubain N, Laios I, Roy F and Larsimont D (2019) Tumor Banks: A Quality Control Scheme Proposal. Front. Med. 6:225. doi: 10.3389/fmed.2019.00225
Received: 21 June 2019; Accepted: 30 September 2019;
Published: 17 October 2019.
Edited by:
Annelies Debucquoy, Belgian Cancer Registry, BelgiumReviewed by:
Jing He, Guangzhou Women and Children's Medical Center, ChinaCopyright © 2019 Craciun, Spinette, Rassy, Salgado, de Wind, Demetter, Verset, Gomez-Galdon, Chintinne, Sirtaine, de St Aubain, Laios, Roy and Larsimont. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ligia Craciun, bGlnaWEuY3JhY2l1bkBib3JkZXQuYmU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.