- Department Experimental Pain Research, CBTM, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
Itching can result from activity of specialized primary afferent neurons (“pruriceptors”) that have been shown to express certain molecular markers such as B-type natriuretic peptide and several members of the Mrgpr-family in rodents. On the other hand, neurons involved in pain processing (“nociceptors”) can also provoke itching when the activation site is restricted to an isolated tiny spot within the epidermis. Individuals classified as having sensitive skin report increased itching and pain sensations upon weak external stimuli that are not painful or itchy in the control group. Numerous possible factors could contribute to sensitive skin along the pathway of transduction of the external stimuli into peripheral neuronal signals, followed by neuronal processing, finally resulting in the perception: (a) reduced local protective factors leading to impaired skin barrier function, (b) increased production of excitatory skin mediators, (c) sensitized peripheral neurons, (d) facilitated spinal and central processing, and (e) reduced descending inhibition from the central nervous system. For all of those pathophysiological mechanisms there are clinical examples such as atopic dermatitis (a,b,c), neuropathic itching (c,e), and restless leg syndrome (d,e). However, none of these factors have been directly linked to the occurrence of sensitive skin. Moreover, individuals reporting sensitive skin are heterogeneous and a subpopulation with defined pathophysiology has not yet been identified. Given that the condition is reported in about 50% of women, and thereby includes many healthy individuals, it appears problematic to assign a definitive pathophysiological mechanism to it.
Itch Processing and Sensitive Skin
Sensitive skin has recently been defined “by the occurrence of unpleasant sensations (stinging, burning, pain, pruritus, and tingling sensations) in response to stimuli that normally should not provoke such sensations. These unpleasant sensations cannot be explained by lesions attributable to any skin disease. The skin can appear normal or be accompanied by erythema” (1). Concerning the occurrence of itching in sensitive skin, possible contributing factors could interact all the way between barrier function, peripheral neuronal activation, central processing, and descending inhibition. In this manuscript the neurophysiology of itch processing will be summarized and possible interactions in sensitive skin will be discussed.
Molecular Markers for Itch Processing Neurons
Specific pathways have been identified in rodents that are involved in encoding non-histaminergic itching. These include functional markers for primary pruriceptive afferent neurons in rodents (MrgprA1, MrgprC11, MrgprD) and human (MrgprX1) (2), peripheral mediators that are primarily linked to itching rather than pain behavior (interleukin [IL] 13, IL-31, autotaxin, lysophosphatidic acid [LPA], thymic stromal lymphopoietin [TSLP], cathepsin S) (3) and central transmitters and pathways for itch processing (B-type natriuretic peptide [BNP], gastrin releasing peptide [GRP]) (4, 5). Spinal neurons containing gastrin releasing peptide have been found critical for itching, but not for pain (6) suggesting specificity, even though they are also activated by nociceptive stimuli (7). However, it is important to avoid over-simplification as many of the itch-related mediators, receptors or markers are also found in nociceptive pathways. This is obvious for the neurokinin 1 receptor (NK1R) (8), sodium channel subtypes, such as NaV1.7 (9, 10) and LPA (11). Moreover, MrgprD positive neurons originally described as nociceptors (12) have recently been implicated in neuropathic pain (13) and neurons carrying the TSLP receptor were described as mediating “itch and/or pain” (14).
Specificity
About 20 years ago, mechanoinsensitive (“silent”) histamine-sensitive C-nociceptors in human (15) and spinothalamic projection neurons in a cat (16) were identified as part of a specific pruritic pathway. More recently, molecular markers of non-histaminergic itch-specific neurons were identified in rodents, such as B-type natriuretic peptide (BNP) (17, 18) and members of the mas-related G-protein receptor family (mrgprA3, C11) (19–21) in primary afferent neurons, but also the gastrin releasing peptide (GRP) (5, 7, 22) in dorsal horn neurons. Non-histaminergic itch signaling has received major interest when mas-related G-protein coupled receptors (Mrgprs) were identified on presumably itch-related neurons in mice, i.e., MrgprA3 (23), D (24), and C11 (25). Also, BAM8-22, an activator of MrgrpC11 induces itching in the human skin (26). Similarly, an intracutaneous injection of beta-alanine, an activator of MrgprD, provokes mainly itching, but also pain in humans (24, 27). Chloroquine has often been used in mice to elicit itch-behavior via the activation of MrgprA3 (23, 28, 29). Thus, the plethora of new information on pathways and mediators for itching in rodents as described above might imply that the “labeled line” theory for itching has finally been verified.
If itching in sensitive skin was based on the activation of specific “pruriceptors” the peripheral pruritic mediators released in sensitive skin and the neuronal pathway needs to be identified. One could hypothesize that like in neuronal degeneration, inflammatory mediators (30), such as interleukin-31 (IL-31), IL-33 (30), lysophosphatidic acid (LPA), and cathepsin S are released (31, 32) from degenerating axons, which could selectively activate pruriceptors as schematically shown in Figure 1A. Although such a scenario appears plausible, LPA (34), cathepsin S (31), and IL-33 (35) have also been linked to chronic pain conditions, and thus, might not be optimally suited as itch-specific mediators. Only IL-31 would remain as mediator, mainly associated to itching rather than pain, which would be in line with human data on antipruritic effects of IL-31 receptor antibodies (36), but also the co-expression of the IL-31 receptor and natriuretic peptide B in a subpopulation of dorsal root ganglion neurons (37). Thus, for inflammatory itching with high levels of TH-derived IL-31, such as atopic dermatitis, human data also suggests a simple itch pathway in which peripheral pruritogens activate a specific pruriceptive subpopulation of neurons.
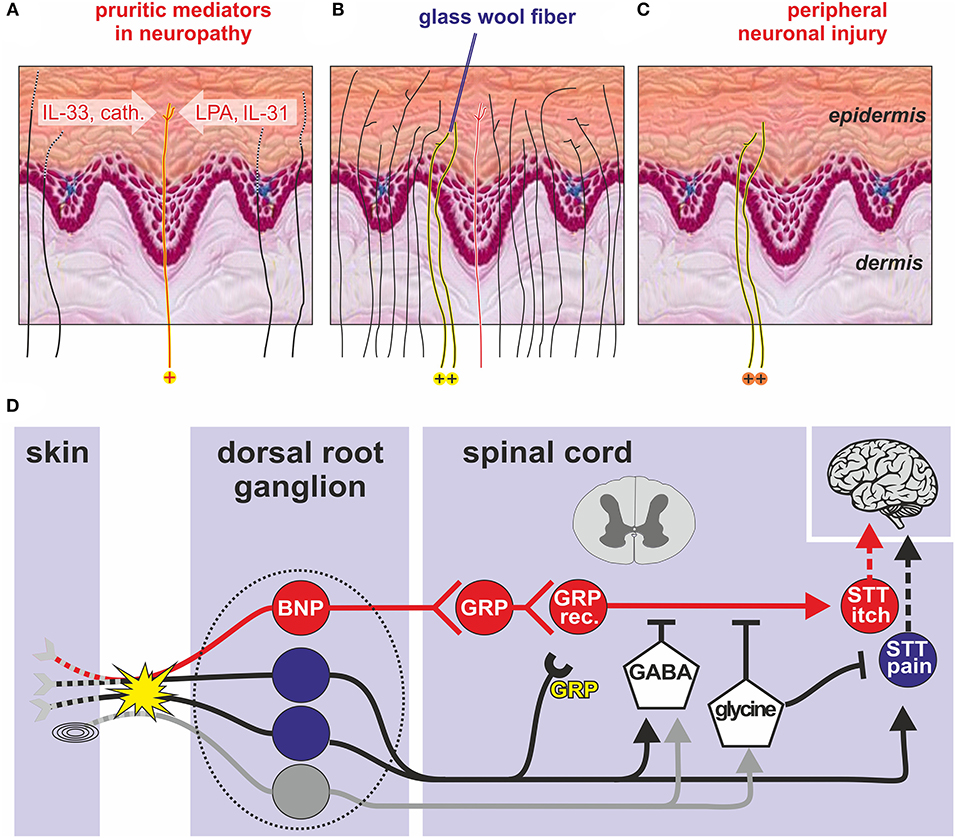
Figure 1. Transduction of an itch in the skin (A-C). (A) Degenerating peripheral nerve fibers (dotted lines) due to peripheral sensorineural injury may release inflammatory mediators such as LPA, IL-31, and IL33 (30) that activate itch-specific pruriceptors (red line, labeled yellow with “+” at bottom). The pruriceptors cause itching via activation of itch-specific pathways (red “labeled line”). (B) In healthy skin, itching can be caused when punctate stimuli (e.g., a glass wool fiber) activate only a few adjacent nociceptive fibers within the epidermis (labeled yellow with “+” at bottom) whereas directly adjacent fibers, including specific pruriceptors (red) remain silent. If instead activated en masse (e.g., by trauma) their combined activation will cause pain. (C) The same localized activation can be mimicked after peripheral neuronal injury when spontaneous action potentials from the few remaining abnormal epidermal nociceptors (labeled yellow with “+” at bottom) reproduce the discharge profile of non-lesioned skin (“spatial contrast mechanism” of itching). (D) Spinal processing of itching based on animal data: Skin and mucosal BNP primary sensory neurons (red) with cell bodies in the dorsal root ganglion (dotted line) stimulate GRP-releasing interneurons in the dorsal horn of the spinal cord that stimulate GRP-receptive interneurons (GRP rec.) and finally projection neurons (STT itch) that send itching signals to the brain via the contralateral STT. Pain neurons (blue) and touch neurons (gray) can inhibit ascending itch signals via GABAergic interneurons, whereas glycinergic interneurons inhibit both itching and pain processing. Peripheral nerve injury (yellow explosion) can induce GRP de-novo synthesis that might facilitate spinal itch processing (yellow “GRP”). BNP, B-type natriuretic peptide; cath., cathepsin S; DRG, dorsal root ganglion; GABA, gamma aminobutyric acid; GRP, gastrin related peptide; IL-31, interleukin 31; IL-33, interleukin33; LPA, lysophosphatidic acid; SST, spinothalamic tract, Pacinian corpuscles (symbol before the yellow explosion) modified from Steinhoff et al. (33).
Spatial Contrast Type of Itch
Electrophysiological data from rodents and monkeys did not support a “labeled line” for itching (38–40) as no specific subpopulation of itch neurons was found. The results rather support the pattern theory of itching according to which nociceptors can signal an itch or pain based on the combination of activated fibers, resulting in population coding (41). Moreover, very focal activation of nociceptors in the skin can explain itching without a “labeled line”: noxious stimulation that is directed only to a few sensory endings within the epidermis elicits itching in human skin (42), even when the stimulus is the algogen capsaicin. It has been suggested that local activation of only few epidermal nociceptors can cause itching by a “mismatch signal” (43) or “spatial contrast” (44), provided by few activated and many non-activated nociceptive endings innervating the same skin site, for example by a minute glass wool fiber (Figure 1B). Such a discharge pattern indicates that a noxious event is minute and localized within the epidermis.
In peripheral neuropathy the skin is partially denervated leaving some isolated sensory endings within the epidermis (Figure 1C). Clinically, we used to associate such depletion of skin innervation to reduced sensory function. However, if there is some local inflammation or spontaneous activity of these isolated remaining nerve branches, the resulting discharge pattern would equal the one just described as “spatial contrast.” Thus, a combination of ongoing or evoked activity from sparsely surviving or newly regenerating nerve branches could generate neuropathic itching via the “spatial contrast” mechanism.
It is of clinical interest that the same spatial arrangement of isolated epidermal sensory nerve fibers is generated by neurons reinnervating scar tissue, for example after burns (45). The combination of spatial arrangement and spontaneous activity of regenerating sprouts might underlie the development of an itch in this condition. Of note, scratching itself can also lead to the reduction of epidermal nerve fiber density (46, 47); however, it remains unclear to which extent scratch-induced axotomy might exaggerate chronic inflammatory itching conditions.
It is remarkable that it took only about 10 years to identify an itch-specific spinal pathway in mice: B-type natriuretic peptide (BNP) skin afferents in the periphery, synapse onto BNP receptor positive neurons in the superficial dorsal horn that contain gastrin releasing peptide (GRP). The third neuron expresses GRP-receptors and excites pruriceptive neurons that ascend the spinothalamic tract (48) (Figure 1D). Itchiness is one of the most common side-effects of administering drugs targeting μ-opioid receptors, and this seems to be related to cross-activation of spinal GRP receptors (49).
In mice, peripheral nerve injuries incite broad de-novo GRP expression in DRG neurons (50). This response might contribute to neuropathic itching as nociceptors that typically inhibit itching may undergo a phenotypic switch by this de-novo expression. When they become spontaneously active, they could release their GRP in the dorsal horn and contribute to neuropathic itching via volume transmission (Figure 1D, yellow “GRP”). Thus, in addition to the spatial contrast mechanism described above, peripheral nerve injury could also provoke neuropathic itching via de-novo expression of GRP in primary afferent nociceptors.
Combination of Itch Theories
Basic itching mechanisms are generally discussed in their “pure” form. However, as shown above, there is evidence that physiologic itch processing may combine elements from different basic theories traditionally regarded as mutually exclusive. Under experimental conditions, more of such combinations appear consistent with experimental data: activation of subpopulations of C-afferents, such as MrgprA3 positive nociceptors (6, 51), might generate itching not only via the assumed specificity, but will also activate a subset of nociceptors innervating a given skin site. Such a combination of active and non-active nociceptors from the same skin site will thereby mimic a spatial contrast pattern. Another example would be a high number of spontaneously active nociceptors that signal pain; upon reduction of this number, for example by analgesic therapy, the chances increase to create a spatial contrast pattern by the still active nociceptors. This would represent a combination of a spatial contrast theory and the old intensity theory. Interestingly, clinical observations indeed support such a development: in patients with postherpetic neuralgia, resolving pain may be combined with an increase in itching (52, 53). Thus, based on defined experimental models, we have successfully developed basic theories that can explain differentiation between itching and pain based on specificity in a “labeled line” or the discharge pattern in its temporal or spatial expression (Figure 2, lower part). These approaches provide us with powerful tools when trying to explain a clinical neuropathic itch. However, rather than assuming that in pathological conditions there is a mutually exclusive explanation for itching purely based on one theory of itching, we might rather adapt our conceptual framework and include mechanisms that contribute elements from several theories (Figure 2, upper part).
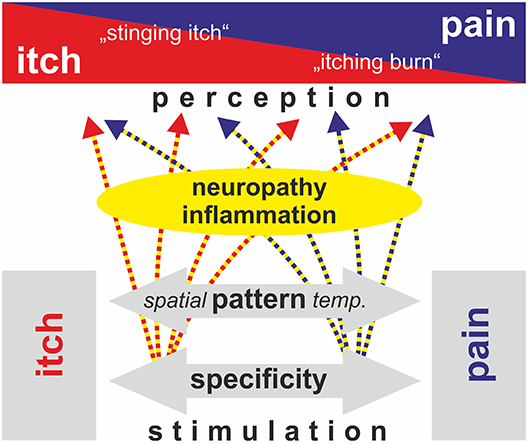
Figure 2. Schematic view of mechanisms that have been proposed to explain the generation of an itch (specificity, pattern, intensity; black and white boxes). Neuropathy and inflammatory processes can modulate primary afferent discharge and spinal processing such that spatial and temporal pattern are changed (gray arrow). Thereby discharge that would genuinely be processed as distinct itching may be perceived as partly painful (“stinging itch”) and vice versa (“itching burn”). Rather than strictly following only one of the above theories, the generation of neuropathic itching in patients thereby may be based on a combination of spatial/temporal pattern and specificity. Modified from Steinhoff et al. (54).
Potential Links Between Sensitive Skin and Facilitated Itching
Even though there is a correlation with sensitive skin and a dry skin type (55) it is not included in the definition of sensitive skin (1). Dry skin models of itching (56) can therefore not be directly linked to sensitive skin. Thus, hypotheses related to neurons have to be discussed: sensitized peripheral neurons alone or in combination with facilitated spinal processing could underlie sensitive skin. Alternatively, central processing alone or in combination with reduced descending inhibition from the central nervous system could be key mechanisms.
Peripheral Neuropathy and Sensitive Skin
Peripheral neuropathy is a common cause of neuropathic pain and itching (33, 54) with postherpetic neuralgia and diabetes being abundant examples. The diagnosis of small fiber neuropathy can be based on reduced epidermal innervation density (57) and functional impairment as measured by quantitative sensory testing (QST) (58). There is ample evidence for a clear correlation between altered sensory thresholds and epidermal nerve fiber density: very high correlations (up to 0.75) were found between QST parameters, intraepidermal nerve fiber density and sensory scores (59) and in general, QST has proven to be a sensitive and highly useful tool for early detection of small fiber impairment (without pain) in diabetes (60). On the one hand the link between sensory thresholds and itching or pain is more problematic. The severity of small fiber neuropathy does not predict painfulness or itching in the patients (61) as not a single item from the QST battery was helpful in differentiating neuropathic patients with severe pain from those without pain (62). Even without this clear link it is remarkable that individuals with sensitive skin had lower innervation densities as compared to control subjects (17 vs. 15 fibers per mm) (63), but innervation density was still in the normal range; for small fiber neuropathy one would expect more pronounced reductions below six fibers per mm (64). Functional tests revealed sensitized heat pain thresholds in sensitive skin individuals (65) and links between sensitive skin and small fiber neuropathy were proposed (63, 66–68). However, based on the negative correlation between epidermal innervation density and heat pain thresholds small fiber neuropathy would be expected to increase rather than decrease heat pain thresholds (61). Thus, based on functional and structural results, there is no evidence for small fiber neuropathy in individuals with sensitive skin.
Central Processing and Descending Inhibition
It has been noted that patients with sensitive skin also report a sensitive cornea (69) and irritable bowel syndrome (70), that is also linked to interstitial cystitis (71), and fibromyalgia (72). Increased pain sensitivity is observed in all these conditions and central sensitization has generally been assumed as a common mechanism (73, 74). In this respect it is interesting that definitive small fiber neuropathy with pathologically reduced intraepidermal nerve fiber density has been reported in patients with fibromyalgia (75, 76) possibly indicating a special subpopulation with peripheral neuropathy. In chronic pain the importance of catastrophizing has been established in recent years (77) with catastrophizing also correlating to ratings of induced pain (78). Based on the overlap between sensitive skin and the above-mentioned chronic pain conditions, one might expect that there is also some overlap in terms of higher scores for catastrophizing. Recent data in mice even provide direct evidence for an overlap between itching and anxiety-like behavior when histamine-responsive central neurons in the amygdala were stimulated optogenetically (79). Unfortunately, there is currently no data available on catastrophizing or anxiety in individuals reporting sensitive skin. Moreover, when separating peripheral vs. central neuronal processing we over-simplified and did not take into consideration interactions that exist between central stress responses and cutaneous neuro-immune interactions (80).
Increased evoked mechanical pain ratings were also found in restless leg syndrome (81), a condition in which reduced descending inhibition has been assumed (82). In primary restless leg syndrome typical mechanical pinprick stimuli are felt more intensely, but there is no evidence for small fiber impairment such as heat hypoalgesia (83, 84). Interestingly, centrally acting analgesics such as gabapentin have been successfully used for treatment (85). In summary, centrally mediated hypersensitivity has been established as a contributing factor in a number of chronic pain conditions. Thus, reduced descending inhibition or broadly increased central processing can lead to hypersensitivity to noxious stimulation. However, it is unclear to which degree such a phenomenon contributes to sensitive skin.
Perspectives
Given the fast progress in our understanding of itch mediators, pathways and processing we might feel to be close to link those mechanisms to sensations reported in sensitive skin. However, to date there is no clear evidence for any specific changes in the itching pathway that could explain itching in sensitive skin. The link between sensitive skin and irritable bowel syndrome might suggest that sensitized central processing or reduced descending inhibition similarly contributes to both conditions. It is important to note that research on sensitive skin has mainly focused on peripheral mechanisms whereas it is established that central processing is highly important for chronic pain or itch conditions. Moreover, in the absence of objective pathologic findings, such a common complaint should not be regarded as abnormal or diseased, but as within the normal range. Given that a high percentage (about 50%) of the population reporting sensitive skin, we would expect a high degree of heterogeneity and therefore studies should include parameters affecting central processing of sensory information such as catastrophizing.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the German Research Foundation project FOR2690.
References
1. Misery L, Stander S, Szepietowski JC, Reich A, Wallengren J, Evers AW, et al. Definition of sensitive skin: an expert position paper from the special interest group on sensitive skin of the international forum for the study of itch. Acta Derm Venereol. (2017) 97:4–6. doi: 10.2340/00015555-2397
2. LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. (2014) 15:19–31. doi: 10.1038/nrn3641
3. Kremer AE, Feramisco J, Reeh PW, Beuers U, Oude Elferink RP. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim Biophys Acta. (2014) 1842:869–92. doi: 10.1016/j.bbadis.2014.02.007
4. Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci. (2014) 17:175–82. doi: 10.1038/nn.3619
5. Mu D, Deng J, Liu KF, Wu ZY, Shi YF, Guo WM, et al. A central neural circuit for itch sensation. Science. (2017) 357:695–99. doi: 10.1126/science.aaf4918
6. Albisetti GW, Pagani M, Platonova E, Hosli L, Johannssen HC, Fritschy JM, et al. Dorsal horn gastrin-releasing peptide expressing neurons transmit spinal itch but not pain signals. J Neurosci. (2019) 103:102–17.e5. doi: 10.1523/JNEUROSCI.2559-18.2019
7. Sun S, Xu Q, Guo C, Guan Y, Liu Q, Dong X. Leaky gate model: intensity-dependent coding of pain and itch in the spinal cord. Neuron. (2017) 93:840–53 e5. doi: 10.1016/j.neuron.2017.01.012
8. Stander S, Siepmann D, Herrgott I, Sunderkotter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS ONE. (2010) 5:e10968. doi: 10.1371/journal.pone.0010968
9. Lee JH, Park CK, Chen G, Han Q, Xie RG, Liu T, et al. A monoclonal antibody that targets a NaV1.7 channel voltage sensor for pain and itch relief. Cell. (2014) 157:1393–404. doi: 10.1016/j.cell.2014.03.064
10. Devigili G, Eleopra R, Pierro T, Lombardi R, Rinaldo S, Lettieri C, et al. Paroxysmal itch caused by gain-of-function Nav1.7 mutation. Pain. (2014) 155:1702–7. doi: 10.1016/j.pain.2014.05.006
11. Velasco M, O'Sullivan C, Sheridan GK. Lysophosphatidic acid receptors (LPARs): potential targets for the treatment of neuropathic pain. Neuropharmacology. (2017) 113:608–17. doi: 10.1016/j.neuropharm.2016.04.002
12. Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. (2005) 45:17–25. doi: 10.1016/j.neuron.2004.12.015
13. Wang C, Gu L, Ruan Y, Geng X, Xu M, Yang N, et al. Facilitation of MrgprD by TRP-A1 promotes neuropathic pain. FASEB J. (2019) 33:1360–73. doi: 10.1096/fj.201800615RR
14. Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. (2013) 155:285–95. doi: 10.1016/j.cell.2013.08.057
15. Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjörk HE. Specific C-receptors for itch in human skin. J Neurosci. (1997) 17:8003–8. doi: 10.1523/JNEUROSCI.17-20-08003.1997
16. Andrew D, Craig AD. Spinothalamic lamina 1 neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci. (2001) 4:72–7. doi: 10.1038/82924
17. Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. (2013) 340:968–71. doi: 10.1126/science.1233765
18. Aresh B, Freitag FB, Perry S, Blumel E, Lau J, Franck MCM, et al. Spinal cord interneurons expressing the gastrin-releasing peptide receptor convey itch through VGLUT2-mediated signaling. Pain. (2017) 158:945–61. doi: 10.1097/j.pain.0000000000000861
19. Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. (2008) 28:4331–5. doi: 10.1523/JNEUROSCI.0716-08.2008
20. Bader M, Alenina N, Andrade-Navarro MA, Santos RA. MAS and its related G protein-coupled receptors, Mrgprs. Pharmacol Rev. (2014) 66:1080–105. doi: 10.1124/pr.113.008136
21. Buddenkotte J, Steinhoff M. Pathophysiology and therapy of pruritus in allergic and atopic diseases. Allergy. (2010) 65:805–21. doi: 10.1111/j.1398-9995.2010.01995.x
22. Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. (2009) 325:1531–4. doi: 10.1126/science.1174868
23. Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. (2009) 139:1353–65. doi: 10.1016/j.cell.2009.11.034
24. Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, et al. Mechanisms of itch evoked by beta-alanine. J Neurosci. (2012) 32:14532–7. doi: 10.1523/JNEUROSCI.3509-12.2012
25. Liu Q, Weng HJ, Patel KN, Tang Z, Bai H, Steinhoff M, et al. The distinct roles of two GPCRs, MrgprC11 and PAR2, in itch and hyperalgesia. Sci Signal. (2011) 4:ra45. doi: 10.1126/scisignal.2001925
26. Sikand P, Dong X, LaMotte RH. BAM8-22 peptide produces itch and nociceptive sensations in humans independent of histamine release. J Neurosci. (2011) 31:7563–7. doi: 10.1523/JNEUROSCI.1192-11.2011
27. Wooten M, Weng HJ, Hartke TV, Borzan J, Klein AH, Turnquist B, et al. Three functionally distinct classes of C-fibre nociceptors in primates. Nat Commun. (2014) 5:4122. doi: 10.1038/ncomms5122
28. Ru F, Sun H, Jurcakova D, Herbstsomer RA, Meixong J, Dong X, et al. Mechanisms of pruritogen-induced activation of itch nerves in isolated mouse skin. J Physiol. (2017) 595:3651–66. doi: 10.1113/JP273795
29. Akiyama T, Tominaga M, Davoodi A, Nagamine M, Blansit K, Horwitz A, et al. Cross-sensitization of histamine-independent itch in mouse primary sensory neurons. Neuroscience. (2012) 10. doi: 10.1016/j.neuroscience.2012.09.019
30. Chandran V, Coppola G, Nawabi H, Omura T, Versano R, Huebner EA, et al. A systems-level analysis of the peripheral nerve intrinsic axonal growth program. Neuron. (2016) 89:956–70. doi: 10.1016/j.neuron.2016.01.034
31. Zhang X, Wu Z, Hayashi Y, Okada R, Nakanishi H. Peripheral role of cathepsin S in Th1 cell-dependent transition of nerve injury-induced acute pain to a chronic pain state. J Neurosci. (2014) 34:3013–22. doi: 10.1523/JNEUROSCI.3681-13.2014
32. Reddy VB, Sun S, Azimi E, Elmariah SB, Dong X, Lerner EA. Redefining the concept of protease-activated receptors: cathepsin S evokes itch via activation of Mrgprs. Nat Commun. (2015) 6:7864. doi: 10.1038/ncomms8864
33. Steinhoff M, Schmelz M, Szabo IL, Oaklander AL. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. (2018) 17:709–20. doi: 10.1016/S1474-4422(18)30217-5
34. O'Brien MS, Philpott HTA, McDougall JJ. Targeting the Nav1.8 ion channel engenders sex-specific responses in lysophosphatidic acid-induced joint neuropathy. Pain. (2019) 160:269–78. doi: 10.1097/j.pain.0000000000001399
35. Huang SJ, Yan JQ, Luo H, Zhou LY, Luo JG. IL-33/ST2 signaling contributes to radicular pain by modulating MAPK and NF-kappaB activation and inflammatory mediator expression in the spinal cord in rat models of noncompressive lumber disk herniation. J Neuroinflammation. (2018) 15:12. doi: 10.1186/s12974-017-1021-4
36. Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti-interleukin-31 receptor a antibody for atopic dermatitis. N Engl J Med. (2017) 376:826–35. doi: 10.1056/NEJMoa1606490
37. Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, et al. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. (2016) 26:967. doi: 10.1038/cr.2016.90
38. Davidson S, Moser H, Giesler G. Ascending pathways for itch. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment, Chapter 22. Boca Raton, FL: CRC Press (2014). 373–390. doi: 10.1201/b16573-23
39. Ringkamp M, Meyer R. Pruriceptors. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment, Chapter 9. Boca Raton, FL: CRC Press (2014). 129–142. doi: 10.1201/b16573-10
40. Akiyama T, Iodi CM, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS ONE. (2011) 6:e22665. doi: 10.1371/journal.pone.0022665
41. Handwerker HO. Itch hypotheses: from pattern to specificity and to population coding. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment, Chapter 1. Boca Raton, FL: CRC Press (2014). 1–8. doi: 10.1201/b16573-2
42. Sikand P, Shimada SG, Green BG, LaMotte RH. Similar itch and nociceptive sensations evoked by punctate cutaneous application of capsaicin, histamine and cowhage. Pain. (2009) 144:66–75. doi: 10.1016/j.pain.2009.03.001
43. Schmelz M. Itch and pain. Neurosci Biobehav Rev. (2010) 34:171–6. doi: 10.1016/j.neubiorev.2008.12.004
45. Kwa KAA, Pijpe A, Rashaan ZM, Tuinebreijer WE, Breederveld RS, van Loey NE Course and predictors of pruritus following burns: a multilevel analysis. Acta Derm Venereol. (2018) 98:636–40. doi: 10.2340/00015555-2935
46. Schuhknecht B, Marziniak M, Wissel A, Phan NQ, Pappai D, Dangelmaier J, et al. Reduced intraepidermal nerve fibre density in lesional and nonlesional prurigo nodularis skin as a potential sign of subclinical cutaneous neuropathy. Br J Dermatol. (2011) 165:85–91. doi: 10.1111/j.1365-2133.2011.10306.x
47. Pereira MP, Pogatzki-Zahn E, Snels C, Vu TH, Uceyler N, Loser K, et al. There is no functional small-fibre neuropathy in prurigo nodularis despite neuroanatomical alterations. Exp Dermatol. (2017) 26:969–71. doi: 10.1111/exd.13343
48. Mishra SK, Hoon MA. Transmission of pruriceptive signals. Handbook Exp Pharmacol. (2015) 226:151–62. doi: 10.1007/978-3-662-44605-8_8
49. Liu XY, Liu ZC, Sun YG, Ross M, Kim S, Tsai FF, et al. Unidirectional cross-activation of GRPR by MOR1D uncouples itch and analgesia induced by opioids. Cell. (2011) 147:447–58. doi: 10.1016/j.cell.2011.08.043
50. Solorzano C, Villafuerte D, Meda K, Cevikbas F, Braz J, Sharif-Naeini R, et al. Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J Neurosci. (2015) 35:648–57. doi: 10.1523/JNEUROSCI.2955-14.2015
51. Huang CC, Yang W, Guo C, Jiang H, Li F, Xiao M, et al. Anatomical and functional dichotomy of ocular itch and pain. Nat Med. (2018) 24:1268–76. doi: 10.1038/s41591-018-0083-x
52. Ishikawa R, Iseki M, Koga R, Inada E. Investigation of the correlation between postherpetic itch and neuropathic pain over time. Pain Res Manag. (2018) 2018:9305126. doi: 10.1155/2018/9305126
53. Kramer S, Baeumler P, Geber C, Fleckenstein J, Simang M, Haas L, et al. Somatosensory profiles in acute herpes zoster and predictors of postherpetic neuralgia. Pain. (2019) 160:882–94. doi: 10.1097/j.pain.0000000000001467
54. Steinhoff M, Oaklander AL, Szabo IL, Stander S, Schmelz M. Neuropathic itch. Pain. (2019) 160 (Suppl. 1):S11–6. doi: 10.1097/j.pain.0000000000001551
55. Misery L, Jourdan E, Huet F, Brenaut E, Cadars B, Virassamynaik S, et al. Sensitive skin in France: a study on prevalence, relationship with age and skin type and impact on quality of life. J Eur Acad Dermatol Venereol. (2018) 32:791–5. doi: 10.1111/jdv.14837
56. Miyamoto T, Nojima H, Shinkado T, Nakahashi T, Kuraishi Y. Itch-associated response induced by experimental dry skin in mice. Jpn J Pharmacol. (2002) 88:285–92. doi: 10.1254/jjp.88.285
57. Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, et al. European federation of neurological societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European federation of neurological societies and the peripheral nerve society. Eur J Neurol. (2010) 17:903–9. doi: 10.1111/j.1468-1331.2010.03023.x
58. Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. (2010) 150:439–50. doi: 10.1016/j.pain.2010.05.002
59. Themistocleous AC, Ramirez JD, Shillo PR, Lees JG, Selvarajah D, Orengo C, et al. The Pain in Neuropathy Study (PiNS): a cross-sectional observational study determining the somatosensory phenotype of painful and painless diabetic neuropathy. Pain. (2016) 157:1132–45. doi: 10.1097/j.pain.0000000000000491
60. Blankenburg M, Kraemer N, Hirschfeld G, Krumova EK, Maier C, Hechler T, et al. Childhood diabetic neuropathy: functional impairment and non-invasive screening assessment. Diabetic Med. (2012) 29:1425–32. doi: 10.1111/j.1464-5491.2012.03685.x
61. Karlsson P, Hincker AM, Jensen TS, Freeman R, Haroutounian S. Structural, functional, and symptom relations in painful distal symmetric polyneuropathies: a systematic review. Pain. (2019) 160:286–97. doi: 10.1097/j.pain.0000000000001381
62. Raputova J, Srotova I, Vlckova E, Sommer C, Uceyler N, Birklein F, et al. Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. Pain. (2017) 158:2340–53. doi: 10.1097/j.pain.0000000000001034
63. Buhe V, Vie K, Guere C, Natalizio A, Lheritier C, Le Gall-Ianotto C, et al. Pathophysiological study of sensitive skin. Acta Derm Venereol. (2016) 96:314–8. doi: 10.2340/00015555-2235
64. McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. (1998) 55:1513–20. doi: 10.1001/archneur.55.12.1513
65. Huet F, Dion A, Batardiere A, Nedelec AS, Le Caer F, Bourgeois P, et al. Sensitive skin can be small fibre neuropathy: results from a case-control quantitative sensory testing study. Br J Dermatol. (2018) 179:1157–62. doi: 10.1111/bjd.17082
66. Saint-Martory C, Sibaud V, Theunis J, Mengeaud V, Lauze C, Schmitt AM, et al. Arguments for neuropathic pain in sensitive skin. Br J Dermatol. (2015) 172:1120–1. doi: 10.1111/bjd.13466
67. Brenaut E, Marcorelles P, Genestet S, Menard D, Misery L. Pruritus: an underrecognized symptom of small-fiber neuropathies. J Am Acad Dermatol. (2015) 72:328–32. doi: 10.1016/j.jaad.2014.10.034
68. Misery L, Bodere C, Genestet S, Zagnoli F, Marcorelles P. Small-fibre neuropathies and skin: news and perspectives for dermatologists. Eur J Dermatol. (2014) 24:147–53. doi: 10.1684/ejd.2013.2189
69. Misery L, Cochener B, Brenaut E, Seite S, Taieb C. Association of sensitive skin with sensitive corneas and sensitive eyelids. J Eur Acad Dermatol Venereol. (2019) 33:1358–62. doi: 10.1111/jdv.15595
70. Misery L, Duboc H, Coffin B, Brenaut E, Huet F, Taieb C. Association between two painful and poorly understood conditions: irritable bowel and sensitive skin syndromes. Eur J Pain. (2019) 23:160–6. doi: 10.1002/ejp.1296
71. Alagiri M, Chottiner S, Ratner V, Slade D, Hanno PM. Interstitial cystitis: unexplained associations with other chronic disease and pain syndromes. Urology. (1997) 49:52–7. doi: 10.1016/S0090-4295(99)80332-X
72. Gracely RH, Schweinhardt P. Programmed symptoms: disparate effects united by purpose. Curr Rheumatol Rev. (2015) 11:116–30. doi: 10.2174/1573397111666150619095125
73. Lowenstein L, Kenton K, Mueller ER, Brubaker L, Heneghan M, Senka J, et al. Patients with painful bladder syndrome have altered response to thermal stimuli and catastrophic reaction to painful experiences. Neurourol Urodyn. (2009) 28:400–4. doi: 10.1002/nau.20676
74. Harte SE, Schrepf A, Gallop R, Kruger GH, Lai HH, Sutcliffe S, et al. Quantitative assessment of non-pelvic pressure pain sensitivity in urological chronic pelvic pain syndrome: a MAPP research network study. Pain. (2019). doi: 10.1097/j.pain.0000000000001505
75. Uceyler N, Sommer C. Pain: from new perspectives to novel treatments. Lancet Neurol. (2015) 14:22–3. doi: 10.1016/S1474-4422(14)70296-0
76. Doppler K, Rittner HL, Deckart M, Sommer C. Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain. (2015) 156:2319–25. doi: 10.1097/j.pain.0000000000000285
77. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. (2009) 9:745–58. doi: 10.1586/ern.09.34
78. Terry MJ, Moeschler SM, Hoelzer BC, Hooten WM. Pain catastrophizing and anxiety are associated with heat pain perception in a community sample of adults with chronic pain. Clin J Pain. (2016) 32:875–81. doi: 10.1097/AJP.0000000000000333
79. Sanders KM, Sakai K, Henry TD, Hashimoto T, Akiyama T. A subpopulation of amygdala neurons mediates the affective component of itch. J Neurosci. (2019) 39:3345–56. doi: 10.1523/JNEUROSCI.2759-18.2019
80. Peters EM, Michenko A, Kupfer J, Kummer W, Wiegand S, Niemeier V, et al. Mental stress in atopic dermatitis–neuronal plasticity and the cholinergic system are affected in atopic dermatitis and in response to acute experimental mental stress in a randomized controlled pilot study. PLoS ONE. (2014) 9:e113552. doi: 10.1371/journal.pone.0113552
81. Stiasny-Kolster K, Pfau DB, Oertel WH, Treede RD, Magerl W. Hyperalgesia and functional sensory loss in restless legs syndrome. Pain. (2013) 154:1457–63. doi: 10.1016/j.pain.2013.05.007
82. Lanza G, Bachmann CG, Ghorayeb I, Wang Y, Ferri R, Paulus W. Central and peripheral nervous system excitability in restless legs syndrome. Sleep Med. (2017) 31:49–60. doi: 10.1016/j.sleep.2016.05.010
83. Bachmann CG, Rolke R, Scheidt U, Stadelmann C, Sommer M, Pavlakovic G, et al. Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain. (2010) 133:762–70. doi: 10.1093/brain/awq026
84. Stiasny-Kolster K, Magerl W, Oertel WH, Moller JC, Treede RD. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. (2004) 127:773–82. doi: 10.1093/brain/awh079
Keywords: neuropathic itch, sensitization, pattern theory, itch pathway, degeneration, inflammation
Citation: Schmelz M (2019) Itch Processing in the Skin. Front. Med. 6:167. doi: 10.3389/fmed.2019.00167
Received: 14 May 2019; Accepted: 08 July 2019;
Published: 19 July 2019.
Edited by:
Laurent Misery, Université de Bretagne Occidentale, FranceReviewed by:
Mitsutoshi Tominaga, Juntendo University, JapanIrina Khamaganova, Pirogov Russian National Research Medical University, Russia
Earl Carstens, University of California, Davis, United States
Copyright © 2019 Schmelz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Schmelz, bWFydGluLnNjaG1lbHpAbWVkbWEudW5pLWhlaWRlbGJlcmcuZGU=
 Martin Schmelz
Martin Schmelz