- 1Department of Nursing, Hunan Traditional Chinese Medical College, Zhuzhou, China
- 2Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital and Chongqing Cancer Institute and Chongqing Cancer Hospital, Chongqing, China
- 3Key Laboratory for Biorheological Science and Technology of Ministry of Education (Chongqing University), Chongqing University Cancer Hospital and Chongqing Cancer Institute and Chongqing Cancer Hospital, Chongqing, China
Background: Polyethylene glycol (PEG) has been regarded as the primary recommendation for bowel preparation before colonoscopy. However, a conclusive conclusion has not yet been generated.
Aim: We performed this updated meta-analysis to further investigate the comparative efficacy and safety of low volume preparation based on PEG plus ascorbic acid related to 4L PEG.
Methods: A systematic search was conducted to retrieve potential randomized controlled trials (RCTs) in PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) from January 2000 to April 2018. Two independent searchers critically searched all potential citations, extracted data, and appraised risk of bias accordingly. Moreover, we used the STATA 12.0 and trial sequential analysis (TSA) 0.9 to complete all analyses.
Results: A total of 13 RCTs enrolling 3,910 patients met inclusion criteria. Meta-analysis based on PP analysis indicated that compared to standard volume PEG regime, low volume regime improved patient compliance RR = 1.01; 95% CIs = 1.00, 1.03; P = 0.143 (≥75% intake); RR = 1.07; 95% CIs = 1.00, 1.14; P = 0.046 (100% intake), the willingness to repeat the same regime (RR = 1.30; 95% CIs = 1.07, 157; P = 0.007), and patient acceptability (RR = 1.18; 95% CIs = 1.07, 1.29; P = 0.001), and decreased the overall adverse events (RR = 0.86; 95% CIs = 0.77, 0.96; P = 0.009). However, no difference was observed between these two different solutions for bowel preparation efficacy (RR = 0.98; 95% CIs = 0.95, 1.02; P = 0.340). These all results were further confirmed by TSA.
Conclusions: The effect of low volume regime was not inferior to the standard volume PEG regime, and low volume regime was associated with better compliance when subjects ingested all the solution, willingness to repeat the same regime, higher acceptability, and lower nausea in non-selected population.
Introduction
Colonoscopy has been deemed to be a critical procedure of early diagnosing lesions in the digestive tract, screening colorectal cancer as well as invasive treatment. But it is worth noting that the efficacy and safety of colonoscopy are mainly related to adequate bowel preparation and patient attendance (1–3). In practice, large volume of preparation solutions is administered to patients who are scheduled to perform colonoscopy. However, it is estimated that ~25 to 33% of patients failed to meet the optimal bowel preparations, the main reason is that the patients are intolerant to volume-related discomfort (4, 5). Published evidences suggested that inadequate bowel preparation is closely associated with lower rates of cecal intubation (6), higher operational difficulty, lower adenoma detection rates and greater financial costs (7–9).
Polyethylene glycol (PEG) remains the principle recommended laxatives for bowel cleansing prior to colonoscopy (10, 11). However, in order to obtain sufficient bowel cleaning, patients will be advised to drink 4 L of fluid, and thus the acceptance and compliance with this given regime will be weakened (12, 13). In addition, these limitations also decreased the courage of patients to be involved in the regular colonoscopy surveillance (14, 15). Therefore, reducing this volume without compromising efficacy is a further challenge. To solve this problem, researchers and practitioners shifted attention to modified products, and several studies have found that low volume PEG combined with ascorbic acid (Asc) may have the potential of improving both patient compliance and the success rate of colonoscopy (16–18). Asc is beneficial because it can enable halving the volume of the lavage solution without loss of efficacy and disgusting taste (19, 20). Several RCTs (21–23) have consistently shown that 2L PEG combined with ASC achieved a similar high degree of cleansing compared with standard volume one. Similarly, the findings from a previous meta-analysis (24) are in accordance with the aforementioned studies. However, this meta-analysis involved a quasi-randomized trial (25) and ignored the variation in adjuvants (Bisacodyl and Simethicone) (26, 27), which potentially damaged the power of summary results.
Considering the above information, we thus undertook this updated meta-analysis to further investigate the efficacy and safety of low volume PEG plus Asc related to traditional volume PEG alone comprehensively for bowel preparation before colonoscopy. We also used trial sequential analysis (TSA) to test whether a conclusive conclusion for a specific outcome can be drawn.
Methods
We finished this article in line with the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) (28) and the Cochrane Handbook for Systematic Reviews of Interventions (29). The prospective protocol for this systematic review was registered on International Prospective Register of Systematic Reviews (PROSPERO) database, and a unique identifier of CRD42018089827 was approved (30). Moreover, the protocol can be accessed in the journal of Medicine (31). The written informed consent was not to be needed, because all analyses were completed based on published data.
Selection Criteria
We pre-specified the inclusion criteria, and studies were considered if the following criteria are met: (1) Population (P): The entire population of adult patients undergoing elective colonoscopy, irrespective of outpatients and inpatients; (2) Intervention (I) and Comparison (C): The trials which investigated the comparative efficacy and safety between 2L PEG combined with Asc and 4L PEG alone were considered, and no other adjuvants was added in both groups; (3) Outcomes (O): Bowel preparation efficacy was regarded as primary outcome, and the secondary outcomes included compliance with recommend regime, willingness to repeat the same regime, acceptability to recommend regime, taste of purgative ingested, and safety; and (4) Study design (S): Only RCTs published in English and Chinese were permitted.
Articles were excluded if it conformed to at least one of the following criteria: (1) essential information which cannot be extracted and obtained from authors; (2) duplicates (derive from the same research group) with poor methodology and insufficient data.
Definition of Outcomes
The overall quality of bowel preparation was predefined as successful bowel cleansing in our study. For the purposes of the analysis, the successful preparation was reached when conformed to one of following conditions: (a) an Ottawa score of <5; (b) a Boston Bowel Preparation Scale (BBPS) score of ≥2 for all segments; (c) a grade of either excellent or good on the Aronchik scale; (d) grades A and B according to the Harefield Cleansing Scale; and (e) other non-validated 3-, 4-, or 5-point scales (excellent, good, fair, poor, very poor).
Compliance with the regimen was assessed by asking patients how much dose they have ingested. We predefined good compliance as consumption of ≥75% but <100% of the regime and excellent compliance as consumption of 100% of the regime. In terms of subjective indexes, willingness to repeat the same regime, acceptability to recommend regime, taste of purgative ingested were measured by using an unofficial questionnaire in each individual study. All adverse events related to bowel preparation were monitored and recorded during colonoscopy. All outcomes introduced above were defined by individual study.
Identification of Citations
A rigorous electronic search was performed by two independent investigators to collect any potential RCTs investigating the comparative efficacy and safety of two targeted PEG-based regimes in PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CNETRAL) from January 2000 to April 2018. Search results have been updated weekly in order to timely capture any recent studies, and the latest search was updated on August 30 2018. “colonoscopy,” “polyethylene glycols,” and “random” were used to construct search strings based on medical subject heading (MeSH) and free word which are embedded in specific files involving title, keywords and abstract. All search algorithms were designed for targeted databases, and all search algorithms were documented in Supplementary Data Sheet 2.
In addition, we also replenished the potential studies through manually checked the bibliographies of relevant articles and reviews. Two reviewers independently and critically examined citations by reading the titles, abstracts and full-texts accordingly.
Data Extraction
A predetermined data extraction table was designed. Whereafter, two reviewers independently extracted the following variables: leading author, publication year, risk of bias, age of participants, sample size, bowel preparation assessment scale, the details of regimes, and outcomes of interest. Besides, we utilized information from the www.clinicaltrials.gov and contacted authors of relevant articles to complete the results of publications when necessary. A third author rechecked all information mutually. Divergences between the two reviewers were resolved by arbitration, and consensus was accomplished after discussion.
Quality Assessment
Two reviewers independently appraised the quality (29, 32) of all included articles by adopting the modified Cochrane risk of bias assessment tool. Evaluation domains including randomization sequence generation, allocation concealment, blinding of participants, blinding of study personnel, blinding of outcome assessors, incomplete outcome data, selective reporting and other bias were assessed. Besides, these assessment results would be cross-checked. The risk of each domain were rated as “high risk of bias,” “unclear risk of bias” or “low risk of bias” according to the match level between extractive information and evaluation criteria (33). Any conflicting result was resolved by discussing with a third author.
Statistical Analysis
We used STATA software version 12.0 (Stata Corp., College Station, Texas) to perform statistical analyses. Dichotomous data was expressed as relative risk (RR) and 95% confidence intervals (CIs). Heterogeneity were qualitatively evaluated by Cochrane's Q test, and the proportion of overall variation that is attributable to between-study heterogeneity was quantitatively evaluated by I2 statistic (34, 35). We analyzed the clinical diversity and methodological comparability of every suitable study firstly according to the characteristics of the participants, research design and method, intervention regimes, and measurement and statistical analysis of outcomes. If the clinical characteristic and methodology are considered heterogeneity, qualitative analysis would be used. If not, we would use the Cochrane's Q-test to qualitatively evaluate the heterogeneity in studies in terms of each outcome (36). Moreover, the level of heterogeneity would be also quantified by the I2 statistic. If I2 is <50%, the suitable studies would be considered to be homogeneous; in contrast, the pooled results would be affected by substantial heterogeneity. We adopted random-effect model based on Mantel-Haenszel (M-H) or inverse variance (IV) approach to perform all analyses. As to the compliance with recommend regime, subgroup analyses will be planned according to the total consumption of the regime. If the number of studies analyzed in single outcome is more than 10, we detected potential publication bias by visual inspection for funnel plots asymmetry and the Egger test (37). If study with multiple-arm design is included, we will extract the data from intervention groups which are up to the inclusion criteria according to the recommendations proposed by Cochrane Collaboration (29). In order to keep the results more extract, we adopted the intention-to-treat (ITT) analysis and the per protocol (PP) analysis simultaneously in all interest outcomes which can achieve quantitative analysis. In our study, because we cannot confirm the outcome of all patients who withdrew from included trials, therefore ITT analysis defines such patients in experimental group and control group as treatment failure. Moreover, we also performed a sensitivity analysis to determine the bowel preparation efficacy including only the studies that used only split-dose regimens and those that included only outpatient.
Trials Sequential Analysis
Random error which is a contributor to false positive or negative results will result from repeated significance test of sparse and accumulated data (38–40). Thus, sequential analysis has been proposed to decrease the risk of type I errors, and modified method (TSA) has also been adopted to analyze the pooled results of meta-analysis (38). The quantification of the required information size (RIS) is a major factor to realize the TSA. In the present study, we calculated the RIS adjusted for diversity because the heterogeneity adjustment with I2 will underestimate the RIS value. The TSA was performed at the level of an overall 5% risk of a type I error and 20% of the type II error (a statistical test power of 80%) (41). If the Z-curve across the monitoring boundary, then we can draw the conclusion of getting credible conclusion before surpassing the RIS line. If the Z-curve across the futility boundary, then we can come to the conclusion of this intervention have no effect for this outcome even though the RIS was not reached. The reliable conclusion can be drawn if the adjusted monitory boundary was surpassed and/or RIS was reached. We estimated the RIS based on the empirical data autogenerated from software according to the data input (42). TSA software (version 0.9 beta) was available at http://www.ctu.dk/tsa/.
Results
Identification and Selection of Trials
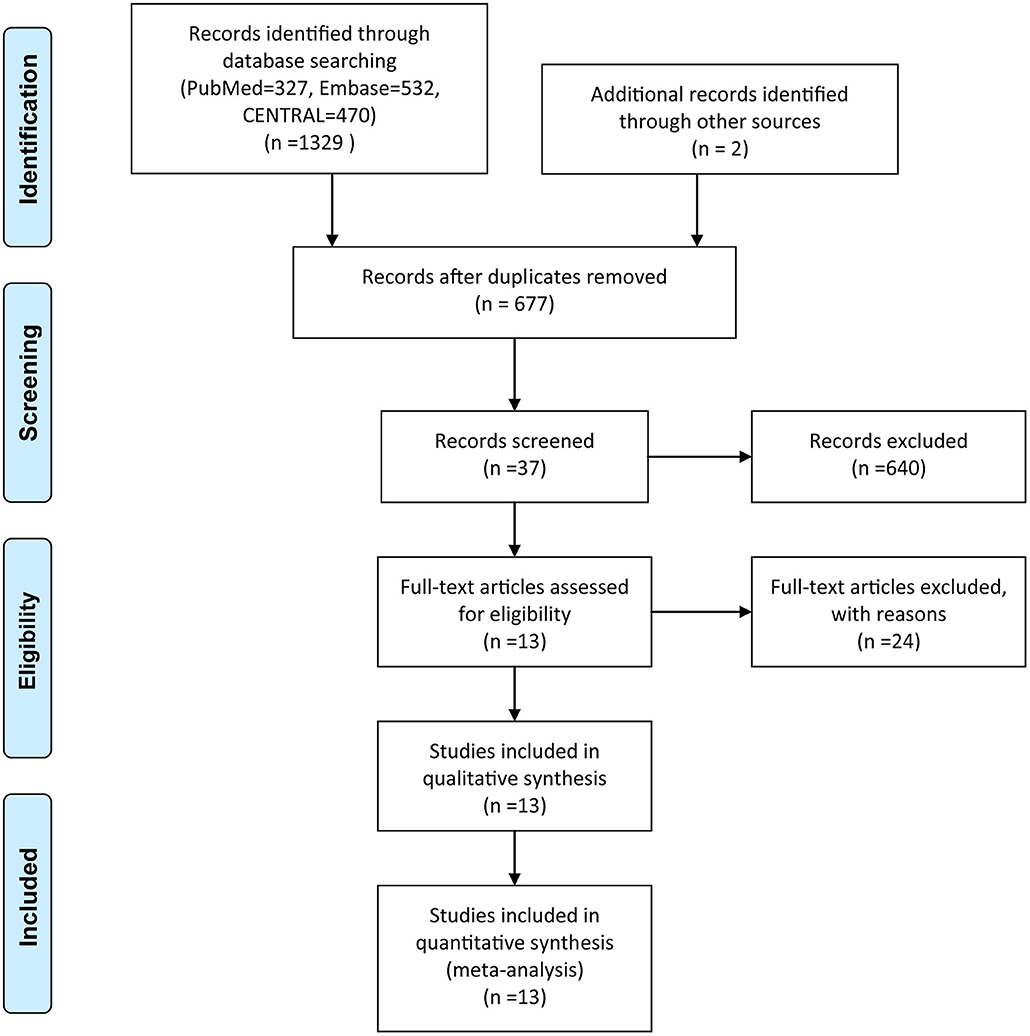
The initial literature search yielded 1,329 records, and two citations were identified through manual searching and gray literature searching. Exactly 654 articles were eliminated by using the function of duplicate checking embedded in EndNote software. After screening the title, abstract and full-text of all identified studies, 13 eligible articles including 3,910 participants ultimately met our eligibility criteria.
Among these articles, Jung et al. (43) conducted a three-arm trial, we removed a set of data from one group considering the comparability and homogeneity between groups. Besides, one trial with a four-arm study was divided into two RCTs according to the purpose of the study. A flow chart detailing the search strategy and resulting outcome was depicted in Figure 1.
Characteristics of Included Trials
The characteristics of 13 trials (14 RCTs) (13, 21–23, 43–51) incorporated into this meta-analysis are shown in Table 1. These studies were published between 2007 and 2016. Sample size of each eligible study ranges from 22 to 218.
Methodological Quality of Studies
Considering that three conference articles (47, 50, 51) only provided a simple abstract which essential information cannot be achieved, so we abandoned their assessment of risk of bias. In remaining 10 trials (13, 21–23, 43–46, 48, 49), all of them meticulously described random sequence generation. And seven articles (13, 21, 22, 43, 45, 46, 48) provided a detailed description of conducting allocation concealment. It was extremely difficult to imagine how blinding of patients could be applicable, because participants would know which bowel preparation solutions were ingested. Whereas, eight trials (13, 21, 22, 43, 45, 46, 48, 49) mentioned how to blind endoscopists who implemented this procedures. Furthermore, blinding of outcome assessment have been completed in all trials. In order to reduce attrition bias, five studies (13, 22, 23, 46, 48) adopted ITT analysis to deal with data, seven studies (13, 21, 22, 43, 46, 48, 49) reported the similar dropout rate between two groups and explained the specific reasons that are irrelevant to the study itself. All studies reported outcomes adequately, among them, half of the eligible RCTs (21, 22, 43, 44, 48) were registered in the Clinical Trials.gov., and the remaining articles that cannot provide the protocol identified this conclusion according to methods and participants section. Three trials (22, 45, 48) were funded by the pharmaceutical industry, which may introduce certain potential source of bias. We displayed the risk of bias summary for studies in Figure S1.
Bowel Preparation Efficacy
Eleven trials (13, 21, 22, 43, 45–47, 49–51) involving 2,998 participants investigated bowel preparation efficacy in PP analysis. Statistical heterogeneity was detected across the included studies [P = 0.017, I2 = 54%], and then a random-effect model was adopted to calculate estimate. Pooled result suggested that the efficacy of 2L PEG plus Asc is not inferior to the 4L PEG regimen for bowel cleansing [RR = 0.98, 95% CI is 0.95 to 1.02, P = 0.34] (Figure 2A). Six RCTs (13, 21, 22, 43, 48, 49) involving 1,857 participants reported this outcome variable in ITT analysis, which included 931 and 926 patients between groups, respectively. Substantial heterogeneity in basic clinical characteristics and methodology of eligible studies was considered [P = 0.002, I2 = 73.8%], and a random-effect model was conducted to summarize effect size. Pooled result was similar to the one in PP analysis which well-suggested that the summary effect size was robust [RR = 0.98, 95% CI is 0.91 to 1.06, P = 0.61] (Figure 2B).
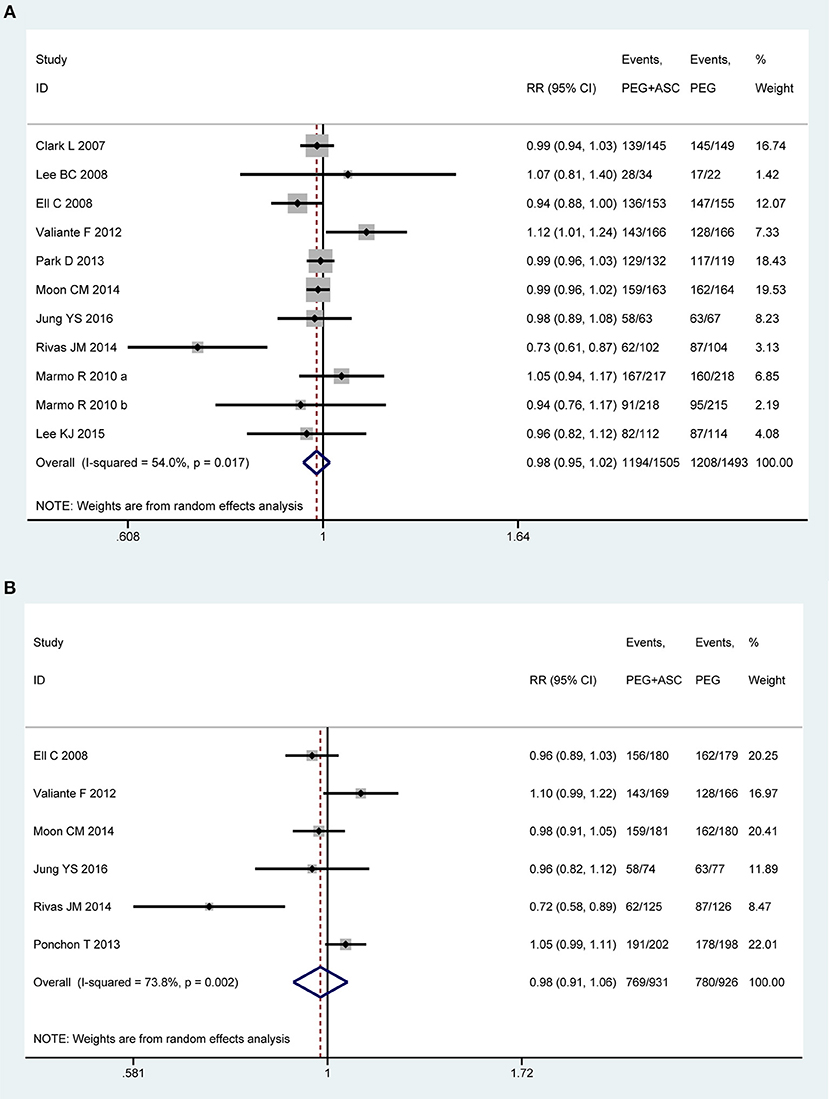
Figure 2. Meta-analysis on bowel preparation efficacy based on PP data (A) and ITT data (B). The summary effect estimate (risk ratio, RR) for individual randomized controlled trials (RCTs) are indicated by gray rectangles (the size of the rectangle is proportional to the study weight), with the black horizontal lines representing 95% confidence intervals (CIs). The overall summary effect estimate (risk ratio) and 95% confidence interval are indicated by the blue diamond below. Meta-analysis indicated no difference between 2L PEG plus Asc volume and 4L PEG regimes in terms of bowel preparation efficacy.
In order to test the heterogeneity and robust behavior in terms of bowel preparation efficacy, we also performed the sensitivity analyses according to approach of drinking solution and the type of patients. The sensitivity analysis based on outpatients was robust in terms of PP analysis [P = 0.001, I2 = 78.7%, RR = 0.971, 95% CI is 0.926 to 1.017, P = 0.213] and ITT analysis [P = 0.001, I2 = 77.5%, RR = 0.981, 95% CI is 0.891 to 1.079, P = 0.692]. Moreover, sensitivity analysis based on split-dose was also robust [PP analysis: RR = 1.005, 95% CI is 0.976 to 1.035, P = 0.731; ITT analysis: RR = 1.012, 95% CI is 0.975 to 1.051, P = 0.523] although heterogeneity for PP analysis [P = 0.140, I2 = 34.7%] and ITT analysis [P = 0.109, I2 = 47%] were all reduced.
We also undertook TSA on these data in PP analysis. The number of patients included in the meta-analysis did not exceed the RIS, but crossed below the futility boundaries. Therefore, within the set assumptions for confidence and effect size, we are therefore able to infer neither 2L PEG combined with Asc group nor 4L PEG group is more than 5% more effective than the other, and extra resources should not be wasted to plan further studies (Figure S2A).
Compliance With the Regimen
Nine trials (13, 21–23, 43, 44, 46, 49, 50) involving 2,739 participants investigated the compliance to the regimen in PP analysis. Among them, one study (50) only provided an abstract, and the concept of compliance can't be defined clearly, eventually eight trials made the quantitative analysis. We divided the eligible studies into two subgroups based on consumption of cleansing solution that participants have ingested: (1) consumption of ≥75% but < 100% of total amount recommended (≥75% intake group) and (2) consumption of 100% of total amount recommended (100% intake group). Homogeneity existed among seven RCTs that fell into ≥75% intake group [P = 0.34, I2 = 11.1%], but statistical heterogeneity is present among five studies that fell into 100% intake group [P < 0.001, I2 = 86.5%]. Therefore, a random-effects model was used. Pooled result showed that a better tendency was seen for the 2L PEG plus Asc group when patients ingested the entire solution as prescribed, while no differences in the rate of ≥75% consumption of the preparation was detected [RR = 1.01, 95% CI is 1.00 to 1.03, P = 0.143 for ≥75% intake group and RR = 1.068, 95% CI is 1.001 to 1.138, P = 0.046 for 100% intake group] (Figure 3A). Six trials (13, 21, 22, 43, 48, 49) reported the compliance to the regimen in ITT analysis, which included 931 and 930 patients between groups, respectively. No heterogeneity in basic clinical characteristics and methodology of eligible studies was found [P = 0.45, I2 = 0% for ≥75% intake group and P = 0.64, I2 = 0% for 100% intake group], and a fixed-effect model was conducted to summarize effect size. Pooled result was similar to the one in PP analysis which well-suggested that the summary effect size was robust [RR = 1.00, 95% CI is 0.98 to 1.03, P = 0.87 for ≥75% intake group and RR = 1.08, 95% CI is 1.01 to 1.15, P = 0.02 for 100% intake group] (Figure 3B).
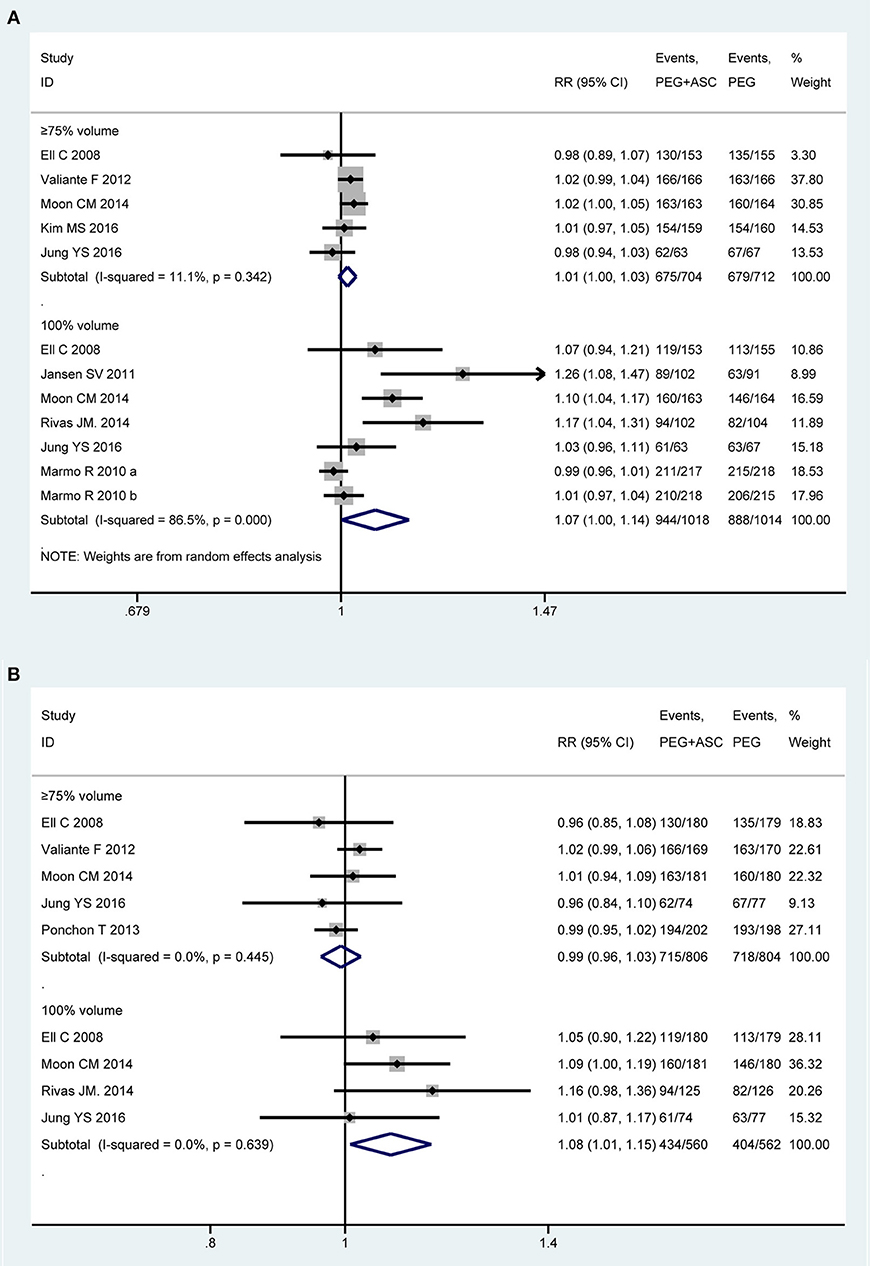
Figure 3. Meta-analysis on compliance to the regimen based on PP data (A) and ITT data (B). The summary effect estimate (risk ratio, RR) for individual randomized controlled trials (RCTs) are indicated by gray rectangles (the size of the rectangle is proportional to the study weight), with the black horizontal lines representing 95% confidence intervals (CIs). The overall summary effect estimate (risk ratio) and 95% confidence interval are indicated by the blue diamond below. Meta-analysis indicated a better compliance with recommend regime in 2L PEG plus ASC group when the full amounts of solution were ingested.
We also undertook TSA on these data in PP analysis. In ≥75% intake group, the cumulative Z-curve didn't reach the RIS, but crossed the futility boundaries, in which case, it would be inferred that the experimental intervention is not superior to the control intervention, and extra resources should not be wasted to plan further studies (Figure S2B). In 100% intake group, the cumulative Z-curve didn't exceed the RIS, but crossed the O'Brien-Fleming boundaries, in which case, it would be inferred that the experimental intervention is superior to the control intervention, and extra resources should not be wasted to plan further studies (Figure S2C).
Willingness to Retake the Same Regime
Five of all trials (43–45, 49, 50) involving 937 participants investigated the willingness to retake the same regime in PP analysis. Statistical heterogeneity was detected across the included studies [P = 0.001, I2 = 77.8%], and then a random-effect model was adopted to summarize mean effect size. Pooled result suggested that the 2L PEG plus Asc group was more likely to be willing to repeat the preparation with the same solution than the 4L PEG group [RR = 1.30, 95% CI is 1.07 to 1.57, P = 0.007] (Figure 4A). Three RCTs (43, 48, 49) reported the willingness to retake the same regime in ITT analysis, which included similar number of patients between groups, respectively. Substantial heterogeneity in basic clinical characteristics and methodology of eligible studies was considered [P < 0.001, I2 = 87.4%], and a random-effect model was conducted to summarize effect size. Pooled result was similar to the one in PP analysis which well-suggested that the summary effect size was robust [RR = 1.37, 95% CI is 1.01 to 1.84, P = 0.04] (Figure 4B).
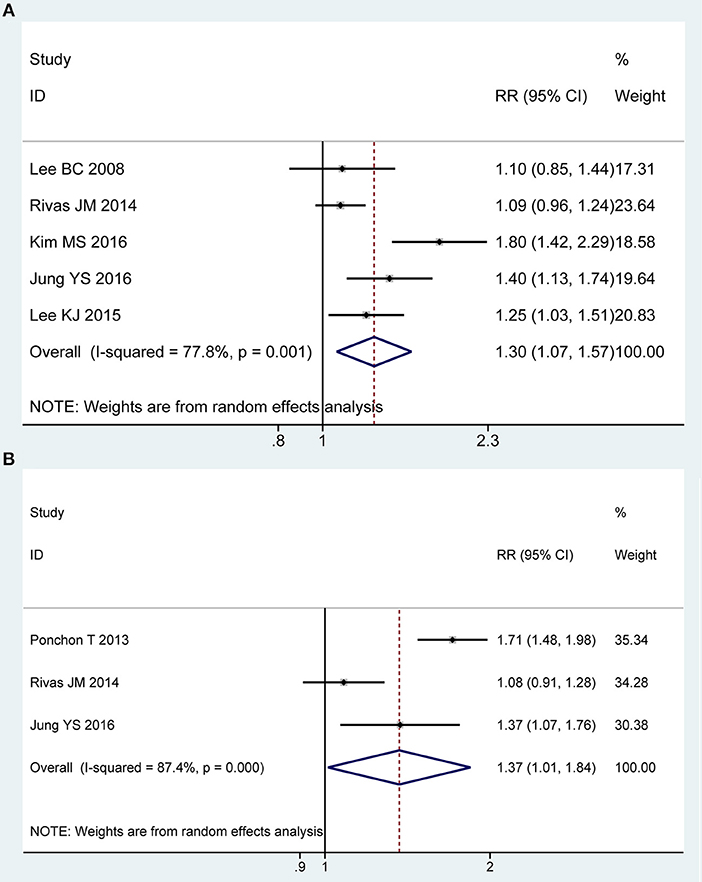
Figure 4. Meta-analysis on willingness to retake the same regime based on PP data (A) and ITT data (B). The summary effect estimate (risk ratio, RR) for individual randomized controlled trials (RCTs) are indicated by gray rectangles (the size of the rectangle is proportional to the study weight), with the black horizontal lines representing 95% confidence intervals (CIs). The overall summary effect estimate (risk ratio) and 95% confidence interval are indicated by the blue diamond below. Meta-analysis indicated a better preference to repeat the same regime when 2L PEG plus Asc vs. 4L PEG regimes.
We also undertook TSA on these data in PP analysis, the cumulative Z-curve for included trials reached the RIS, and crossed the O'Brien-Fleming boundaries, in which case, it would be inferred that willingness to repeat the same regime was higher with 2L PEG plus Asc group than with 4L PEG group, and future similar studies are futile (Figure S2D).
Acceptability to Regime
Six of all trials (13, 21, 43–45, 49) involving 1,540 participants investigated the acceptability to regime in PP analysis. Statistical heterogeneity was detected across the included studies [P = 0.008, I2 = 67.98%], and then a random-effect model was adopted to summarize mean effect size. Pooled result suggested that patient acceptability was higher for 2L PEG plus Asc group than for control group [RR = 1.18, 95% CI is 1.07 to 1.29, P = 0.001] (Figure 5A). Five RCTs (13, 21, 43, 48, 49) reported the acceptability to regime in ITT analysis, which included similar number of patients between groups, respectively. No heterogeneity in basic clinical characteristics and methodology of eligible studies was considered [P = 0.58, I2 = 0%], and a fixed-effect model was conducted to summarize effect size. Pooled result was similar to the one in PP analysis which well-suggested that the summary effect size was robust [RR = 1.12, 95% CI is 1.05 to 1.18, P < 0.001] (Figure 5B).
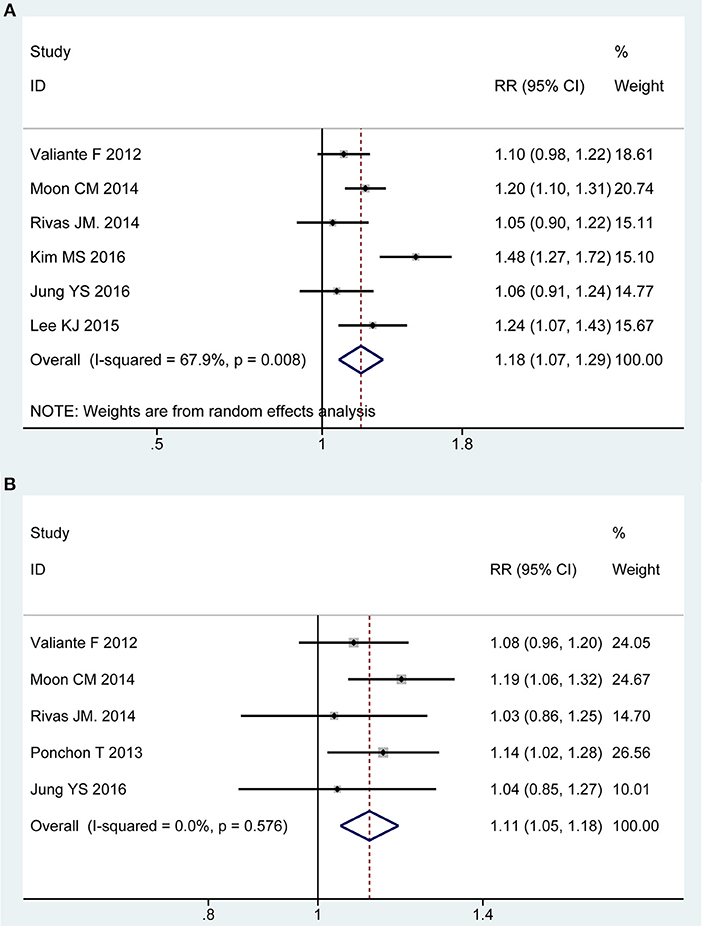
Figure 5. Meta-analysis on acceptability to regime based on PP data (A) and ITT data (B). The summary effect estimate (risk ratio, RR) for individual randomized controlled trials (RCTs) are indicated by gray rectangles (the size of the rectangle is proportional to the study weight), with the black horizontal lines representing 95% confidence intervals (CIs). The overall summary effect estimate (risk ratio) and 95% confidence interval are indicated by the blue diamond below. Meta-analysis indicated patient acceptability was higher for 2L PEG plus Asc regime than for 4L PEG regime.
We also undertook TSA on these data in PP analysis, the cumulative Z-curve for included trials reached the RIS, and crossed the O'Brien-Fleming boundaries. Therefore, we are able to infer that acceptability to regime was higher with 2L PEG plus ASC group than with 4L PEG group, and additional studies with large sample sizes should not be required in the future (Figure S2E).
Safety
Five of all trials (13, 21, 22, 43, 44) involving 1,416 participants investigated overall adverse events in PP analysis. No heterogeneity was detected across the included studies [P = 0.62, I2 = 0%], and then a fixed-effect model was adopted to summarize mean effect size. Pooled result suggested that overall adverse events was lower for 2L PEG plus Asc group than for control group [RR = 0.86, 95% CI is 0.77 to 0.96, P = 0.009] (Figure 6A). Five RCTs (13, 21, 22, 43, 44) reported the overall adverse events in ITT analysis, which included 806 and 804 patients between groups, respectively. No heterogeneity in basic clinical characteristics and methodology of eligible studies was considered [P = 0.64, I2 = 0%], and a fixed-effect model was conducted to summarize effect size. Pooled result was similar to the one in PP analysis which well-suggested that the summary effect size was robust [RR = 0.84, 95% CI is 0.77 to 0.92, P < 0.001] (Figure 6B). Treatment-related adverse effects mainly including abdominal cramps (n = 1,496), nausea (n = 1,529), and vomiting (n = 1,529) were analyzed. Pooled results are summarized in electroc Supplemetary Table 1.
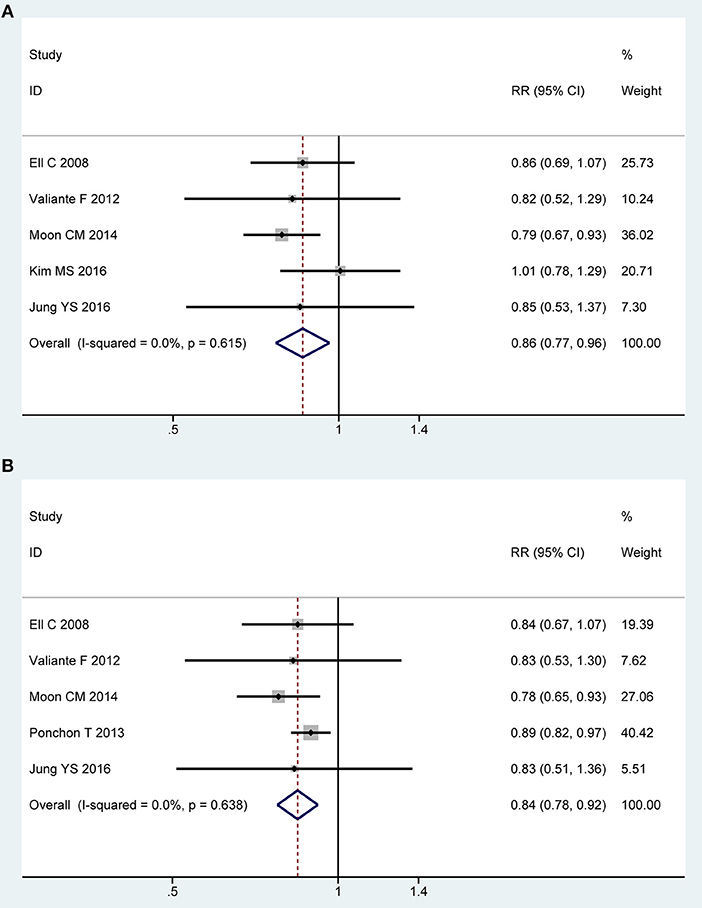
Figure 6. Meta-analysis on overall AEs based on PP data (A) and ITT data (B). The summary effect estimate (risk ratio, RR) for individual randomized controlled trials (RCTs) are indicated by gray rectangles (the size of the rectangle is proportional to the study weight), with the black horizontal lines representing 95% confidence intervals (CIs). The overall summary effect estimate (risk ratio) and 95% confidence interval are indicated by the blue diamond below. Meta-analysis indicated a significant difference when 2L PEG plus Asc vs. 4L PEG regime in terms of overall adverse events.
We only undertook TSA on these data from overall adverse events in PP analysis. For other special adverse effects, due to too little information use, boundary required sample size is ignored. Though the cumulative Z-curve for included trials didn't reach the RIS, it crossed the O'Brien-Fleming boundaries. Therefore, we are therefore able to infer that low-volume 2L PEG plus Asc showed significantly fewer overall adverse events than did 4L PEG, and extra resources should not be wasted to plan further studies (Figure S2F).
Taste of Purgative Ingested
Seven studies (22, 23, 43, 45, 46, 49, 50) reported taste of purgative ingested as an outcome. Because different non-validated scoring systems have been applied for assessing this factor in different studies, which causes difficulties in defining the cutoff of good or bad taste and analyzing the results, a descriptive analysis was performed. Five trials (22, 23, 45, 46, 50) found that participants who randomized to the 2L PEG plus Asc group rated the taste better than those who received 4L PEG solution. Conversely, in the remaining articles (43, 49), although the difference was not statistically significant, however, a trend was noted for a more pleasant taste in the 4L PEG as compared to 2L PEG plus Asc.
Publication Bias
For primary outcomes (bowel preparation efficacy), we performed a funnel plot, which often be depicted to identify the existence of publication bias. The funnel plot displayed symmetry, thereby indicating that no small study bias possibly exists. Meanwhile, the Egger's test result also revealed similar result (P = 0.383) (Figure S3). Other indicators including few studies didn't meet the conditions.
Discussion
Subjects' participation and adequate bowel cleansing are the essential requirements for a high-quality colonoscopy (1, 25, 52). Therefore, the ideal colon cleansing should be capable of evacuating the colon from all fecal material without damaging its mucosa, causing no discomfort, and minimizing fluids and electrolyte disturbances (53, 54). Traditional 4L PEG regime has been used worldwide for its high efficacy, lower price and superior safety (55, 56). But volume-related discomfort and unpleasant taste may deter the acceptability with colonoscopy, which is closely related to subject's attendance (57). Poor acceptability will impair the willingness to take the examination in the future (14, 15). Recently, low volume PEG regime shows a better toleration under the condition that its cleanliness is equal to that of traditional 4L PEG regimen (16, 21, 22, 48). Although a previous meta-analysis has confirmed low volume PEG plus Asc is an effective alternative, several limitations harmed the reliability of pooled results. Moreover, some potential RCTs have been published recently. Thus, it is essential to further determine the comparative role between low volume PEG regime and traditional large volume one in bowel preparation before colonoscopy.
Summary of Main Results
This meta-analysis found that 2L PEG plus Asc is as effective as 4L PEG solution with respect to successful bowel cleansing in non-selected population. Meanwhile, it can enhance patient willingness to repeat the same regime, acceptability, and compliance when 100% of the prescribed solution was ingested, and can decrease the overall AEs. However, whether Asc may improve palatability of PEG solution will require additional studies with large-scale to establish. Furthermore, the results of TSA confirmed the efficacy of 2L PEG plus Asc on bowel preparation efficacy, compliance, willingness, and acceptability to regime and overall AEs.
While we failed to find a difference between the two groups when patients drank ≥75% of the gut cleaning solution, significantly more patients in the 4L PEG group reflected having difficulty consuming the total amount of bowel preparation solution. There are some explanations to interpret why this discrepancy occurs: (a) despite 2L PEG plus Asc has better acceptability, researchers can partly improve the compliance through continuous education and the delivery of preparation instructions (2, 58, 59). These means addressed patient knowledge and belief barriers to quality colonoscopy preparation; (b) our subjects were patients who were more concerned about their health than the normal population. When health-care professionals counseled patients to complete intake of the solution to ensure a safe and effective procedure, they would be better able to understand the importance of compliance; and (c) several studies (22, 46) recruited hospitalized patients who may achieve higher compliance. Nonetheless, it remains obvious that a considerable proportion of patients is unable or unwilling to drink a large volume of unpalatable fluid.
Besides, it is generally believed that the addition of Asc can mask the unpleasant taste of PEG preparation (13, 22, 48), resulting in improved acceptability and patient compliance. However, contrary to our expectations, the present study failed to get it. Maybe we can speculate the palatability didn't contribute significantly to patient compliance and acceptability to regimen, in other words, there was no relationship between the taste of the cleansing agent and the probability of a positive response.
Limitations of the Present Study
There are several limitations that need to be acknowledged in our study. Firstly, there is little uniformity concerning bowel cleansing evaluation, with endoscopists using several scale tools, and criteria to definite the cutoff of adequate colon cleansing. However, all these appraisal tools place emphasis on similar aspects, including the removable volume of clear liquid and (or) fecal residue and the impact of the surplus on mucosal visibility. This point greatly reduces the incidence rate of scoring bias. Secondly, it was hard to conceive how blinding of patients could be applicable. Assessment of subjective outcomes in this article (willingness to retake the same regime, acceptability to regime, taste of purgative ingested) mainly depended on the personal judgment of participants, it is unknown if this may have introduced bias on the evaluation of these variables. Thirdly, though there was heterogeneity among comparisons regarding variations in dosing regimens (e.g., non-split or split schedule; morning or afternoon colonoscopy), in dietary restrictions, and in demographics and population types. Due to the randomization, there is a high probability that they would have been evenly distributed between the intervention arms, hence a confounding effect on the final results could be excluded. Finally, Gimeno-Garcia AZ et al found that 4L PEG based preparation is superior to 2L PEG Asc based preparation in patients with past history of poor bowel preparation (60), and thus it must be emphasized that 2L PEG plus Asc is as effective as taking 4L PEG solution in non-selected population because these results may be not generalized to hard to prepare group.
Implications for Practice and Further Research
In our review, less than half of primary studies (13, 21, 22, 48, 49) scheduled all subjects for examination in concentrated time to reduce bias related to procedure time, but didn't determine the actual time at which the preparation was finalized. Siddiqui et al. (5) reported that for every additional hour that the patient waits between the last dose of bowel preparation agents and the colonoscopy start time, the chance to achieve an inadequate cleansing in the right colon increases by up to 10%. Several previous studies have demonstrated that the sooner the procedure is conducted from ingestion, the higher the chance of achieving a clean bowel (46, 61–63), and European Society of Gastrointestinal Endoscopy further recommends (55) that the delay between the last fluid intake and colonoscopy should be no longer than 4 h (strong recommendation, moderate quality evidence). So in the design stage of study, researchers should take this factor into account to provide a more standardized, scientific, rationalized way for clinical use.
Conclusions
According to our data, 2L PEG plus Asc appears to be a more patient-friendly preparation considering both efficacy and tolerability. This 2L PEG plus Asc regimen is at least as effective as 4L PEG solution, with clear advantages in terms of patient willingness, acceptability, compliance (100% intake) and safety. However, there is no consensus regarding the effect of the taste of the cleansing agent for colonoscopy.
Author Contributions
L-JY, XT, Y-PP, and W-QC were involved with study conception and design. L-JY and X-LL were involved in data acquisition. L-JY and BS performed statistical analysis. XT and HC interpreted the data and results of the analyses. L-JY and XT drafted the manuscript, which was critically revised for intellectual content by XT and W-QC. All authors read and approved the final manuscript.
Funding
This research was supported by the Clinical Research Foundation of Chongqing University Cancer Hospital & Chongqing Cancer Institute & Chongqing Cancer Hospital with an unique approval number of LY201704.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2019.00092/full#supplementary-material
References
1. Bernstein C, Thorn M, Monsees K, Spell R, O'Connor JB. A prospective study of factors that determine cecal intubation time at colonoscopy. Gastrointes Endosc. (2005) 61:72–5. doi: 10.1016/S0016-5107(04)02461-7
2. Seo JY, Lee C, Jin EH, Yun MH, Lim JH, Kang HY, et al. Is a split-dose regimen of 2 L polyethylene glycol plus ascorbic acid tolerable for colonoscopy in an early morning visit to a comprehensive medical check-up? World J Gastroenterol. (2017) 23:1030–7. doi: 10.3748/wjg.v23.i6.1030
3. Rodriguez de Miguel C, Serradesanferm A, Lopez-Ceron M, Carballal S, Pozo A, Balaguer F, et al. Ascorbic acid PEG-2L is superior for early morning colonoscopies in colorectal cancer screening programs: a prospective non-randomized controlled trial. Gastroenterol Hepatol. (2015) 38:62–70. doi: 10.1016/j.gastrohep.2014.09.007
4. Moon W. Optimal and safe bowel preparation for colonoscopy. Clin Endoscopy. (2013) 46:219–23. doi: 10.5946/ce.2013.46.3.219
5. Siddiqui AA, Yang K, Spechler SJ, Cryer B, Davila R, Cipher D, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointes Endosc. (2009) 69:700–6. doi: 10.1016/j.gie.2008.09.047
6. Zuber-Jerger I, Kullmann F. A prospective study of factors that determine cecal intubation time at colonoscopy. Gastrointes Endosc. (2006) 63:358–9. doi: 10.1016/j.gie.2005.09.007
7. Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointes Endosc. (2011) 73:1207–14. doi: 10.1016/j.gie.2011.01.051
8. Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointes Endosc. (2012) 75:1197–203. doi: 10.1016/j.gie.2012.01.005
9. Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. (2002) 97:1696–700. doi: 10.1111/j.1572-0241.2002.05827.x
10. Yoo IK, Jeen YT, Kang SH, Lee JH, Kim SH, Lee JM, et al. Improving of bowel cleansing effect for polyethylene glycol with ascorbic acid using simethicone: a randomized controlled trial. Medicine. (2016) 95:e4163. doi: 10.1097/MD.0000000000004163
11. Saltzman JR, Cash BD, Pasha SF, Early DS, Muthusamy VR, Khashab MA, et al. Bowel preparation before colonoscopy. Gastrointes Endosc. (2015) 81:781–94. doi: 10.1016/j.gie.2014.09.048
12. Radaelli F, Meucci G, Sgroi G, Minoli G. Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. (2008) 103:1122–30. doi: 10.1111/j.1572-0241.2007.01778.x
13. Valiante F, Pontone S, Hassan C, Bellumat A, De Bona M, Zullo A, et al. A randomized controlled trial evaluating a new 2-L PEG solution plus ascorbic acid vs 4-L PEG for bowel cleansing prior to colonoscopy. Digestive Liver Dis. (2012) 44:224–7. doi: 10.1016/j.dld.2011.10.007
14. Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy - a meta-analysis. Colorectal Dis. (2006) 8:247–58. doi: 10.1111/j.1463-1318.2006.00970.x
15. Friedman S, Cheifetz AS, Farraye FA, Banks PA, Makrauer FL, Burakoff R, et al. Factors that affect adherence to surveillance colonoscopy in patients with inflammatory bowel disease. Inflammatory Bowel Dis. (2013) 19:534–9. doi: 10.1097/MIB.0b013e3182802a3c
16. Bitoun A, Ponchon T, Barthet M, Coffin B, Dugue C, Halphen M. Results of a prospective randomised multicentre controlled trial comparing a new 2-L ascorbic acid plus polyethylene glycol and electrolyte solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment Pharmacol Ther. (2006) 24:1631–42. doi: 10.1111/j.1365-2036.2006.03167.x
17. Mamula P, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, et al. Colonoscopy preparation. Gastrointes Endosc. (2009) 69:1201–9. doi: 10.1016/j.gie.2009.01.035
19. Wilson JX. Regulation of vitamin C transport. Annu Rev Nutri. (2005) 25:105–25. doi: 10.1146/annurev.nutr.25.050304.092647
20. Fujita I, Akagi Y, Hirano J, Nakanishi T, Itoh N, Muto N, et al. Distinct mechanisms of transport of ascorbic acid and dehydroascorbic acid in intestinal epithelial cells (IEC-6). Res Commun Mol Pathol Pharmacol. (2000) 107:219–31.
21. Moon CM, Park DI, Choe YG, Yang DH, Yu YH, Eun CS, et al. Randomized trial of 2-L polyethylene glycol + ascorbic acid versus 4-L polyethylene glycol as bowel cleansing for colonoscopy in an optimal setting. J Gastroenterol Hepatol. (2014) 29:1223–8. doi: 10.1111/jgh.12521
22. Ell C, Fischbach W, Bronisch HJ, Dertinger S, Layer P, Runzi M, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. (2008) 103:883–93. doi: 10.1111/j.1572-0241.2007.01708.x
23. Jansen SV, Goedhard JG, Winkens B, van Deursen CT. Preparation before colonoscopy: a randomized controlled trial comparing different regimes. Eur J Gastroenterol Hepatol. (2011) 23:897–902. doi: 10.1097/MEG.0b013e32834a3444
24. Xie Q, Chen L, Zhao F, Zhou X, Huang P, Zhang L, et al. A meta-analysis of randomized controlled trials of low-volume polyethylene glycol plus ascorbic acid versus standard-volume polyethylene glycol solution as bowel preparations for colonoscopy. PLoS ONE. (2014) 9:e99092. doi: 10.1371/journal.pone.0099092
25. Corporaal S, Kleibeuker JH, Koornstra JJ. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand J Gastroenterol. (2010) 45:1380–6. doi: 10.3109/00365521003734158
26. Parente F, Vailati C, Bargiggia S, Manes G, Fontana P, Masci E, et al. 2-Litre polyethylene glycol-citrate-simethicone plus bisacodyl versus 4-litre polyethylene glycol as preparation for colonoscopy in chronic constipation. Digestive Liver Dis. (2015) 47:857–63. doi: 10.1016/j.dld.2015.06.008
27. Zhang S, Zheng D, Wang J, Wu J, Lei P, Luo Q, et al. Simethicone improves bowel cleansing with low-volume polyethylene glycol: a multicenter randomized trial. Endoscopy. (2017) 50:412–22. doi: 10.1055/s-0043-121337
28. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
29. Higgins JPT, Green S editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration (2011). Available online at: http://handbook.cochrane.org (accessed March 2011).
30. Yi LJ, Tian X, He H. Impact of low volume versus standard volume PEG on bowel preparation efficacy prior to colonoscopy: an updated meta-analysis with trial sequential analysis. PROSPERO: International prospective register of systematic reviews. (2018).
31. Yi LJ, Tian X, Pi YP, Feng L, Chen H, Liu XL, et al. Comparative efficacy of low volume versus traditional standard volume PEG on bowel preparation before colonoscopy: protocol for an updated meta-analysis with trial sequential analysis. Medicine. (2018) 97:e0599. doi: 10.1097/MD.0000000000010599
32. Zeng X, Zhang Y, Kwong J, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
33. Deng YH, Hao XY, Zhang H, Zeng Z, Song GM. Effect of fast track surgery care on knee joint function in patients with knee joint replacement surgery: a systematic review. TMR Integr Nurs. (2019) 3:13–20. doi: 10.12032/TMRIN20180830
34. Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
35. Lu G, Ades A. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. (2004) 23:3105–24. doi: 10.1002/sim.1875
36. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
37. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
38. Jesper B, Kristian T, Christian G, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. (2008) 61:763–9. doi: 10.1016/j.jclinepi.2007.10.007
39. Brok J, Thorlund KJ, Gluud C. Apparently conclusive meta-analyses may be inconclusive–Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol. (2009) 38:287–98. doi: 10.1093/ije/dyn188
40. Kristian T, Devereaux PJ, Wetterslev J, Gordon G, Ioannidis JP, Lehana T, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. (2009) 38:276–86. doi: 10.1093/ije/dyn179
41. Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol. (2009) 9:86. doi: 10.1186/1471-2288-9-86
42. Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User Manual for Trial Sequential Analysis (TSA). Copenhagen Trial Unit. Available online at: http://www.ctu.dk/tsa/files/tsa_manual.pdf (accessed February 10, 2019).
43. Jung YS, Chang KL, Chang SE, Dong IP, Dong SH, Kim HJ. Low-volume polyethylene glycol with ascorbic acid for colonoscopy preparation in elderly patients: a randomized multicenter study. Digestion. (2016) 94:82–91. doi: 10.1159/000448887
44. Kim M, Park J, Park J, Kim H, Jang H, Joo H, et al. Does polyethylene glycol (PEG) plus ascorbic acid induce more mucosal injuries than split-dose 4-L PEG during bowel preparation? Gut Liver. (2016) 10:237–43. doi: 10.5009/gnl14439
45. Lee KJ, Park HJ, Kim HS, Baik KH, Kim YS, Park SC, et al. Electrolyte changes after bowel preparation for colonoscopy: a randomized controlled multicenter trial. World J Gastroenterol. (2015) 21:3041–8. doi: 10.3748/wjg.v21.i10.3041
46. Marmo R, Rotondano G, Riccio G, Marone A, Bianco MA, Stroppa I, et al. Effective bowel cleansing before colonoscopy: a randomized study of split-dosage versus non-split dosage regimens of high-volume versus low-volume polyethylene glycol solutions. Gastrointest Endosc. (2010) 72:313–20. doi: 10.1016/j.gie.2010.02.048
47. Park D, Moon CM, Choe YC, Yang DH, Han DS. Low-volume polyethylene glycol (PEG) plus ascorbic acid versus standard peg solution for bowel cleansing for colonoscopy-a randomized controlled study. Gastrointest Endosc. (2013) 77:Ab516. doi: 10.1016/j.gie.2013.03.850
48. Ponchon T, Boustiere C, Heresbach D, Hagege H, Tarrerias AL, Halphen M. A low-volume polyethylene glycol plus ascorbate solution for bowel cleansing prior to colonoscopy: the NORMO randomised clinical trial. Digestive Liver Dis. (2013) 45:820–6. doi: 10.1016/j.dld.2013.04.009
49. Rivas JM, Perez A, Hernandez M, Schneider A, Castro FJ. Efficacy of morning-only 4 liter sulfa free polyethylene glycol vs 2 liter polyethylene glycol with ascorbic acid for afternoon colonoscopy. World J Gastroenterol. (2014) 20:10620–7. doi: 10.3748/wjg.v20.i30.10620
50. Lee BC, Moyes DA, Mcloughlin JC, Lim PL. The efficacy, acceptability and safety of the new 2L polyethylene glycol + electrolytes + ascorbic acid (PEG + E + ASC) Vs the 4L polyethylene glycol 3350 + electrolytes (PEG + E) in patients undergoing elective colonoscopies in a UK teaching hospital. Gastrointest Endoscopy. (2008) 67:AB290. doi: 10.1016/j.gie.2008.03.836
51. Clark L, Gruss HJ, Kloess HR, Dugue C, Geraint M, Halphen M, et al. Better efficacy of a new 2 litre bowel cleansing preparation in the ascending colon. Gastrointest Endosc. (2007) 65:AB262. doi: 10.1016/j.gie.2007.03.617
52. Hwang KL, Chen WT, Hsiao KH, Chen HC, Huang TM, Chiu CM, et al. Prospective randomized comparison of oral sodium phosphate and polyethylene glycol lavage for colonoscopy preparation. World J Gastroenterol. (2005) 11:7486–93. doi: 10.3748/wjg.v11.i47.7486
53. Nelson DB, Barkun AN, Block KP, Burdick JS, Ginsberg GG, Greenwald DA, et al. Technology status evaluation report. Colonoscopy preparations. May 2001. Gastrointest Endosc. (2001) 54:829–32. doi: 10.1016/S0016-5107(01)70087-9
54. Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum. (2006) 49:792–809. doi: 10.1007/s10350-006-0536-z
55. Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. (2013) 45:142–50. doi: 10.1055/s-0032-1326186
56. Mathus-Vliegen E, Pellise M, Heresbach D, Fischbach W, Dixon T, Belsey J, et al. Consensus guidelines for the use of bowel preparation prior to colonic diagnostic procedures: colonoscopy and small bowel video capsule endoscopy. Curr Med Res Opin. (2013) 29:931–45. doi: 10.1185/03007995.2013.803055
57. Senore C, Ederle A, Fantin A, Andreoni B, Bisanti L, Grazzini G, et al. Acceptability and side-effects of colonoscopy and sigmoidoscopy in a screening setting. J Med Screen. (2011) 18:128–34. doi: 10.1258/jms.2011.010135
58. Spiegel B, Talley J, Shekelle P, Agarwal N, Snyder B, Bolus R, et al. Development and validation of a novel patient educational booklet to enhance colonoscopy preparation. Am J Gastroenterol. (2011) 106:875–83. doi: 10.1038/ajg.2011.75
59. Voiosu T, Ratiu I, Voiosu A, Iordache T, Schipor A, Baicus C, et al. Time for individualized colonoscopy bowel-prep regimens? A randomized controlled trial comparing sodium picosulphate and magnesium citrate versus 4-liter split-dose polyethylene glycol. J Gastrointest Liver Dis. (2013) 22:129–34.
60. Gimeno-Garcia AZ, Hernandez G, Aldea A, Nicolas-Perez D, Jimenez A, Carrillo M, et al. Comparison of two intensive bowel cleansing regimens in patients with previous poor bowel preparation: a randomized controlled study. Am J Gastroenterol. (2017) 112:951–8. doi: 10.1038/ajg.2017.53
61. El Sayed AM, Kanafani ZA, Mourad FH, Soweid AM, Barada KA, Adorian CS, et al. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest Endosc. (2003) 58:36–40. doi: 10.1067/mge.2003.318
62. Manno M, Pigo F, Manta R, Barbera C, Bertani H, Mirante VG, et al. Bowel preparation with polyethylene glycol electrolyte solution: optimizing the splitting regimen. Digestive Liver Dis. (2012) 44:576–9. doi: 10.1016/j.dld.2012.02.012
Keywords: colonoscopy, polyethylene glycol, bowel preparation, meta-analysis, trial sequential analysis
Citation: Yi L-J, Tian X, Shi B, Chen H, Liu X-L, Pi Y-P and Chen W-Q (2019) Low-Volume Polyethylene Glycol Improved Patient Attendance in Bowel Preparation Before Colonoscopy: A Meta-Analysis With Trial Sequential Analysis. Front. Med. 6:92. doi: 10.3389/fmed.2019.00092
Received: 16 February 2019; Accepted: 16 April 2019;
Published: 06 May 2019.
Edited by:
Enrique Quintero, Universidad de La Laguna, SpainReviewed by:
Cesare Hassan, Ospedale Nuovo Regina Margherita, ItalyAntonio Z. Gimeno Garcia, University Hospital of the Canary Islands, Spain
Copyright © 2019 Yi, Tian, Shi, Chen, Liu, Pi and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Tian, eXh0eDg4MDkxOUBob3RtYWlsLmNvbQ==
Yuan-Ping Pi, Y3F6bHl5aGxiQDE2My5jb20=
Wei-Qing Chen, Y2hlbndxMjAxNDA4MDlAMTYzLmNvbQ==
†These authors have contributed equally to this work and share joint first authorship
 Li-Juan Yi1†
Li-Juan Yi1† Xu Tian
Xu Tian Wei-Qing Chen
Wei-Qing Chen