
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Med. , 14 February 2019
Sec. Translational Medicine
Volume 6 - 2019 | https://doi.org/10.3389/fmed.2019.00023
This article is part of the Research Topic The Silent Cry: How to Turn Translational Medicine Towards Patients and Unmet Medical Needs View all 20 articles
This article is a correction to:
Controlled Human Infections As a Tool to Reduce Uncertainty in Clinical Vaccine Development
A Corrigendum on
Controlled Human Infections As a Tool to Reduce Uncertainty in Clinical Vaccine Development
Roestenberg, M., Kamerling, I. M. C., and de Visser, S. J. (2018). Front. Med. 5:297. doi: 10.3389/fmed.2018.00297
In the original article, there was a mistake in “Figure 2C” as published. In the figure legend, the incorrect number was used to indicate the second-best route and should have been “5.22 €M” instead of “5.97 €M”. The corrected Figure 2C appears below.
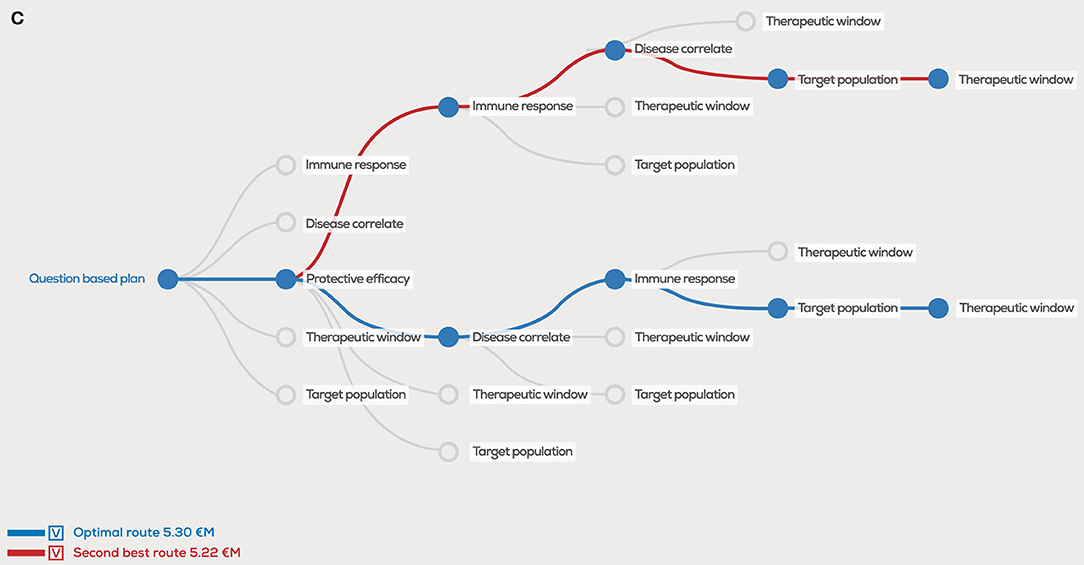
Figure 2. Decision tree showing the optimal order of questions to generate the highest project value, given estimated probability of success (PoS) and costs (million euro, €M) per scientific question. In the case PoS and costs for every question are equal, the order does not matter for the project value (A), however unequal distributions will clearly show different project values for the optimal order in blue, second best order in red and user defined order in green (B). A 1 €M additional investment in “correlates” has a major effect on the optimal order and project value (C).
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Keywords: vaccine, malaria, product development (PD) process, clinical development, low-income access
Citation: Roestenberg M, Kamerling IMC and de Visser SJ (2019) Corrigendum: Controlled Human Infections As a Tool to Reduce Uncertainty in Clinical Vaccine Development. Front. Med. 6:23. doi: 10.3389/fmed.2019.00023
Received: 09 January 2019; Accepted: 25 January 2019;
Published: 14 February 2019.
Edited and reviewed by: Berent Prakken, Utrecht University, Netherlands
Copyright © 2019 Roestenberg, Kamerling and de Visser. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meta Roestenberg, bS5yb2VzdGVuYmVyZ0BsdW1jLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.