- 1Department of Otolaryngology, The University of Tokyo, Tokyo, Japan
- 2Department of Otolaryngology, The University of California, Davis, Davis, CA, United States
Introduction: Multiple system atrophy (MSA) has detrimental effects on swallowing function. The swallowing function of patients with MSA has not been systematically characterized and the underlying pathophysiological mechanisms of dysphagia remain poorly understood.
Objectives: To investigate the characteristics of swallow function in MSA using high-resolution manofluorography (HRMF).
Methods: We conducted a retrospective review of twenty-five MSA patients who underwent HRMF from 2016 to 2017. HRMF was utilized on patients with only oral diet (Functional Oral Intake Scale (FOIS) >3). Pharyngoesophageal and proximal esophageal pressure profiles were evaluated and compared to established normative data. The frequency and characteristics of upper esophageal sphincter (UES) and proximal esophageal abnormalities during rest and swallow were calculated.
Results: The ages of patient cohort in our study ranged from 48–81 years (median 65 years) with male predominance (68%). We observed a distinct abnormal deglutitive proximal esophageal contraction (ADPEC) in 14 (56% of patients), which appears to reflect a discoordinated response of the striated muscle esophagus. Deficient UES relaxation duration, impaired UES relaxation, hypertensive resting UES pressure and hypotensive resting UES pressure were detected in 8 patients (32%), 3 patients (12%), 1 patient (4%), and 11 patients (44%) respectively.
Conclusions: In patients with MSA, abnormal UES resting pressure is common. A discoordinated proximal esophageal pressure response was identified and may be a pathognomonic manometry finding for MSA. These findings may serve as indications of early stage swallowing dysfunction in patients with MSA.
Introduction
Multiple system atrophy (MSA) is a neurodegenerative disorder that causes systemic degeneration of the cerebellar, extrapyramidal and autonomic nervous systems in various combinations (1) and is generally classified as parkinsonism predominance (MSA-P) and/or cerebellar ataxia (MSA-C) (2).
Deterioration of swallowing function is one of the manifestations of MSA that dramatically affects quality of life and prognosis of patients (3) and dysphagia- related complications such as respiratory infections (mostly aspiration pneumonia) and malnutrition are most the common causes of death (4, 5). Delayed oral and pharyngeal phases of swallowing, in combination with impaired airway protection and esophageal sphincter contraction disturbances, may lead to acute aspiration and pneumonia (6). Impaired relaxation of the upper esophageal sphincter (UES) has been reported regardless of MSA phenotype (7). It is hypothesized that neurodegenerative changes in the brain stem and vagus nerve might contribute to a dysfunctional cricopharyngeous muscle, resulting in impairment of UES opening and bolus residue in the pyriform sinuses (3, 6, 7). Despite the prevalence of swallow dysfunction in persons with MSA, swallowing function in MSA has not been investigated systematically, and the underlying pathophysiological mechanisms of dysphagia, especially the incipient swallowing changes in MSA, remain poorly understood. Early detection of dysphagia, prevention of aspiration pneumonia and possible intervention can increase quality of life and survival time of MSA patients.
Technological advancements and utilizations of high-resolution manometry (HRM) have led to a better understanding of pharyngeal and esophageal disorders. HRM has been utilized to characterize swallowing dysfunction in patients with neuromuscular disease, such as Parkinson's disease but not in MSA. Moreover, high-resolution manofluorography (HRMF), an armamentarium combining high-resolution manometric and videofluoroscopic swallowing examinations, improves the accuracy of diagnosis of esophageal disorders and facilitates dysphagia management (8, 9). The purpose of this investigation was to evaluate the characteristic pharyngoesophageal and cervical esophageal findings of HRMF in patients with MSA.
Methods
Ethics
This study was approved by the Human Ethics Committee of the University of Tokyo (No. 2487). Written informed consent was obtained from every patient and patients' anonymities were preserved.
Patients
Twenty-five MSA patients whose diagnosis was made at the Department of Neurology in our institute between the years 2016 to 2017 were included. Patients' diagnoses were confirmed according to the Consensus Statement of diagnostic criteria and excluded from Gilman's criteria (2).
Methodology
We conducted a retrospective review of patients with MSA who underwent HRMF at the University of Tokyo Hospital. Clinical and demographic profiles were analyzed, including age, gender, type, and severity stage of MSA (10), presence of vocal fold immobility, Functional Oral Intake Scale (FOIS) (11), Penetration Aspiration Scale (PAS) score (12) in thin liquid intake (10 mPa•s) and thickened liquid intake (200 mPa•s) and HRMF parameters.
MSA severity was designated from Stage 1 to 5 as previously reported (10). FOIS scores (11), and PAS scores (12) were assigned as Level 1–7 (normal level: 7) and Score 1–8 (most severe score: 8) separately (Table 1).
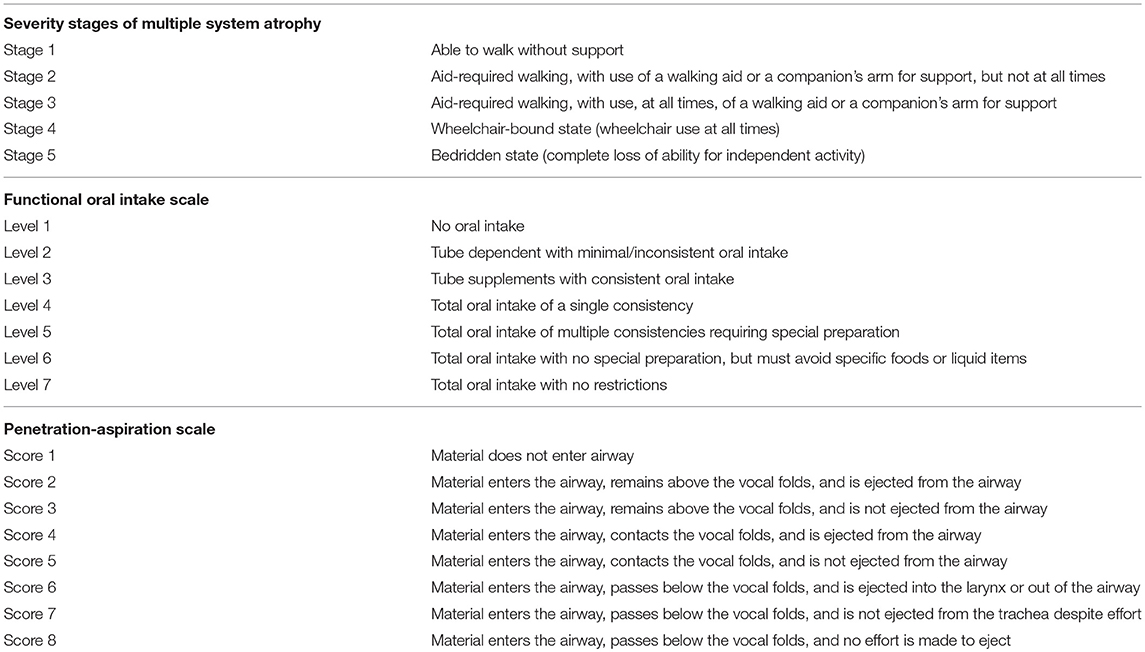
Table 1. Details of severity stages of Multiple System Atrophy (10), a Functional Oral Intake Scale (11), and a Penetration Aspiration Scale (12).
High Resolution Manofluorographic Study and Measures
HRMF studies were performed on all MSA patients who were on an oral diet (FOIS >3) in the upright position. The manometric catheter was lubricated with 4% viscous lidocaine and inserted transnasally. The videofluoroscopic swallow study (VFSS) was conducted in the lateral view simultaneously. The protocol consisted of a 5-min baseline recording, followed by 3 dry swallows and 3 wet swallows of 5 cc thickened contrast agent (iohexol: Omnipaque®, Daiichi-Sankyo, Tokyo, Japan). A solid-state high-resolution manometer (Starlet, Star Medical, Tokyo, Japan) was used for all data acquisition. The manometric catheter (Unisensor, Portsmouth, NH, USA) has an outer diameter of 4 mm and 20 circumferential pressure sensors spaced 1 cm apart. The data acquisition frequency was 40 Hz for each sensor. The system is calibrated to record pressures between −50 and 300 mmHg. Video was recorded at a rate of 30 frames/s and analyzed by Star.exeve 8.1 (Star Medical, Tokyo, Japan). Pharyngeal and proximal esophageal measures were obtained on all patients.
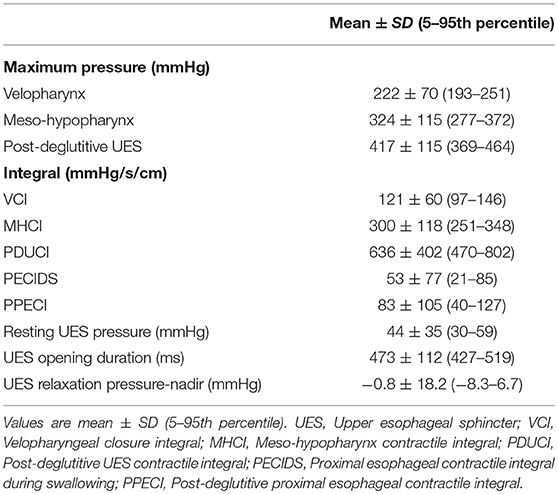
The following parameters were obtained from the manometric output: maximum swallowing pressures (velopharynx, meso-hypopharynx, post-deglutitive UES pressure), integrals [velopharyngeal closure integral, meso-hypopharynx contractile integral, post-deglutitive UES contractile integral, proximal esophageal contractile integral during UES relaxation (early response), proximal esophageal contractile integral], UES relaxation duration, UES relaxation pressure (nadir) and UES resting pressure (Figure 1).
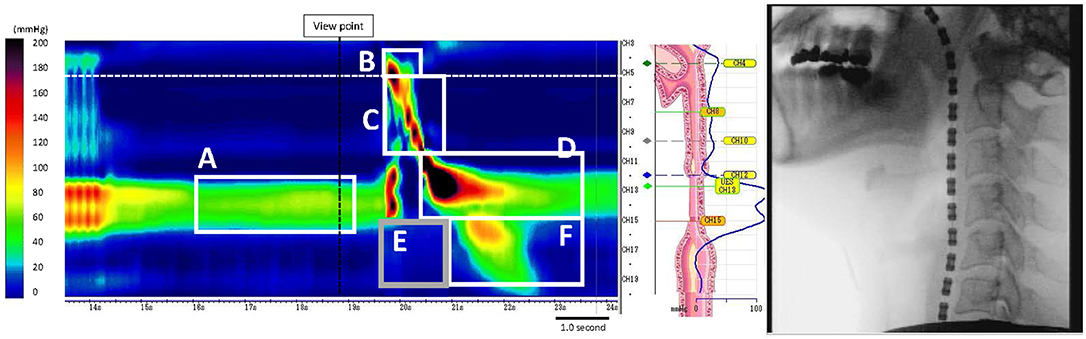
Figure 1. Measurement of UES pressure and each integral (in a healthy subject). (A) Resting UES pressure, (B) VCI, Velopharyngeal closure integral, (C) MHCI, Meso-hypopharynx contractile integral, (D) PDUCI, Post-deglutitive UES contractile integral, (E) PECIDS, Proximal esophageal contractile integral during swallowing, (F) PPECI, Post-deglutitive proximal esophageal contractile integral.
Abnormal HRMF metrics in the UES and proximal esophagus were identified using the following criteria: impaired UES relaxation pressure/duration (relaxation pressure >12 mmHg/<0.45 s), hypertensive resting UES pressure (>104 mmHg), and hypotensive resting UES pressure (<34 mmHg) (13, 14). Proximal esophageal contraction integral above 0 mmHg during UES opening (an early response occurring concurrently with UES opening) was also defined as abnormal, a phenomenon observed in this study among MSA patients. The frequency of abnormal hypertensive and discoordinated abnormal proximal esophageal contraction during swallowing (ADPEC), deficient UES relaxation duration, UES abnormalities, and other sub-types of UES abnormal presentations on manometry were calculated. Finally, subjects were divided into two groups based on presence or absence of ADPEC and intergroup comparison of HRMF parameters were made and correlations between ADPEC and clinical features between groups were evaluated.
Statistical Analysis
Statistical comparisons between groups were performed by using Mann–Whitney U-tests implemented in GraphPad Prism (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA). Demographic variables (type of MSA, vocal cord immobility) were compared by Fisher's exact test between patients with or without ADPEC. P < 0.05 was considered statistically significant.
Results
Demographic Data
Data from the charts of 25 patients with MSA were abstracted. The age of the cohort (Table 2) ranged from 48 to 81 years (median 65 years). Sixty-eight percent (17/25) was male. Seven out of 25 patients were diagnosed with MSA-P (Parkinsonian features predominant type MSA), and the remaining 18 patients were diagnosed with MSA-C (cerebellar symptoms predominant type MSA). Regarding MSA severity, 7 patients were stage 1 or 2, 10 patients were stage 3 and 8 patients were designated stage 4. Bilateral vocal fold immobility was detected in 8 patients (32%). Total oral nutritional dependence was noted in all patients.13 patients (52%) had no restrictions (FOIS 7), 8 patients (32%) had minimal dietary restrictions (FOIS 6), and 4 patients (16%) had moderate diet modification (FOIS 5). For thin liquids, 15 patients (60%) had PAS 1-3, 5 patients (20%) had a PAS of 4-6, and 5 patients (20%) had a PAS of 7 or 8. For thickened liquids, 20 patients (80%) had a normal PAS (PAS 1), 3 patients (12%) had a PAS of 2, and 2 patients (8%) had a PAS of 6.
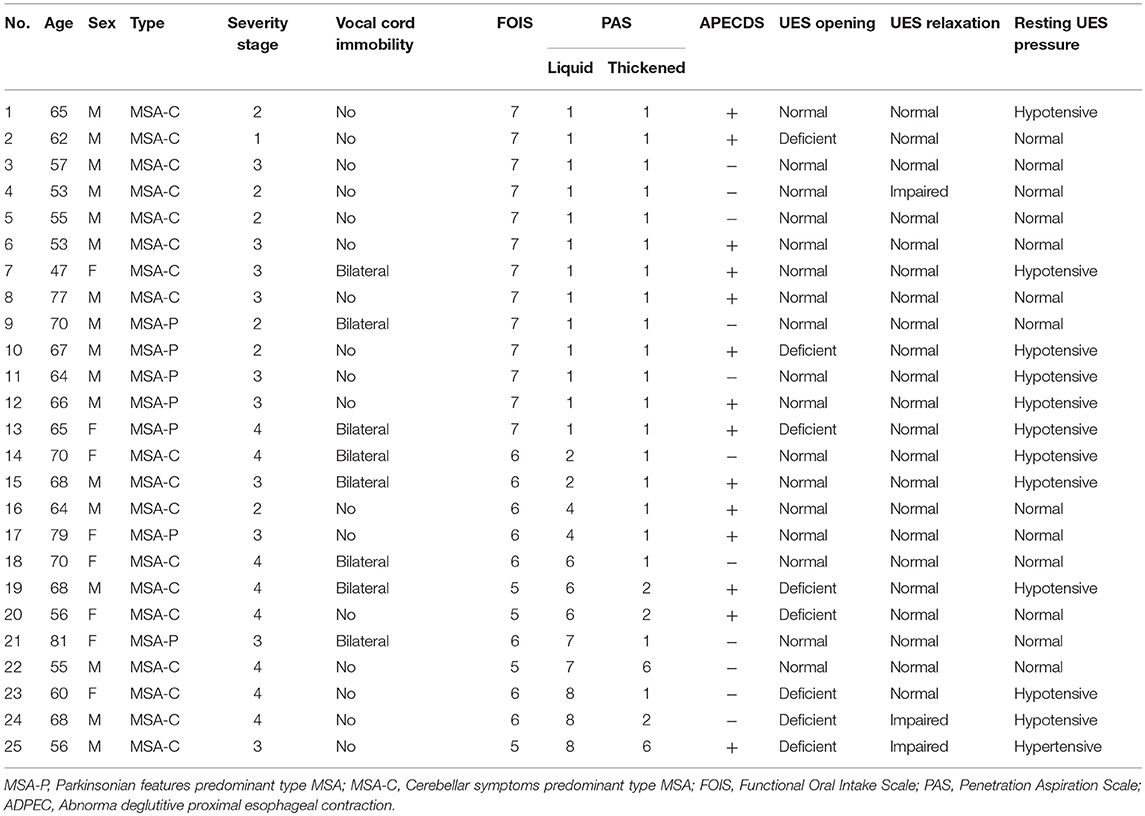
Table 2. Demographic data and high-resolution manofluorography (HRMF) findings of upper esophageal sphincter (UES) and proximal esophageal metrics in multiple system atrophy (MSA) patients.
HRMF Pharyngeal and Proximal Esophageal Metrics in MSA Patients
Mean, standard deviation and the measured ranges for each pharyngeal and proximal esophageal metric from patients with MSA included in this study are shown in Table 3. The maximum swallowing pressures and integrals of each segment, UES relaxation duration, resting UES pressure and UES relaxation pressure (nadir) were included. Pharyngeal contraction and UES status were verified for each study by VFSS in order to minimize measurement errors.
Abnormal HRMF Findings of UES and Proximal Esophageal Metrics
HRMF measurements of UES and proximal esophageal metrics on pressure topography are illustrated in Figure 1. HRMF revealed a pattern of abnormal hypertensive and discoordinated proximal esophageal contraction during swallowing (ADPEC) (Figure 2) in 14 patients (56%). Deficient UES relaxation duration, impaired UES relaxation, hypertensive resting UES pressure and hypotensive resting UES pressure were detected in 8 patients (32%), 3 patients (12%), 1 patient (4%), and 11 patients (44%), respectively.
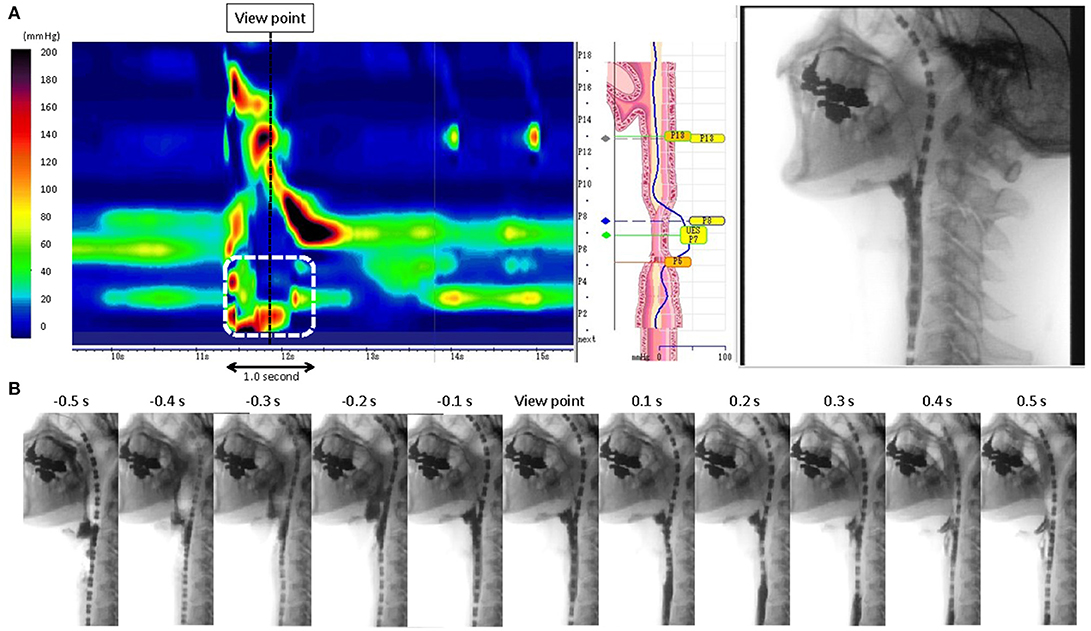
Figure 2. (A) Representative pattern of abnormal hypertensive and discoordinated proximal esophageal contraction during swallowing (ADPEC: The area surrounded by a white broken line). A black broken line shows the view point of the static fluoroscopic image. (B) 1.0 s time series images of fluoroscopic examination.
Comparison of Clinical Features and HRMF Values Between Groups With and Without Abnormal Hypertensive and Discoordinated Proximal Esophageal Contraction During Swallowing
There were no statistically significant differences in any of the HRMF values between groups with or without ADPEC. Similarly, no significant associations were found between the presence of ADPEC and types of MSA (OR 0.94; 95% CI 0.16–5.46; p = 1.00) or presence of vocal fold immobility (OR 0.70; 95% CI 0.13–3.79; p = 0.69). There was no significant difference in severity stages of MSA between groups with and without ADPEC (Table 4).
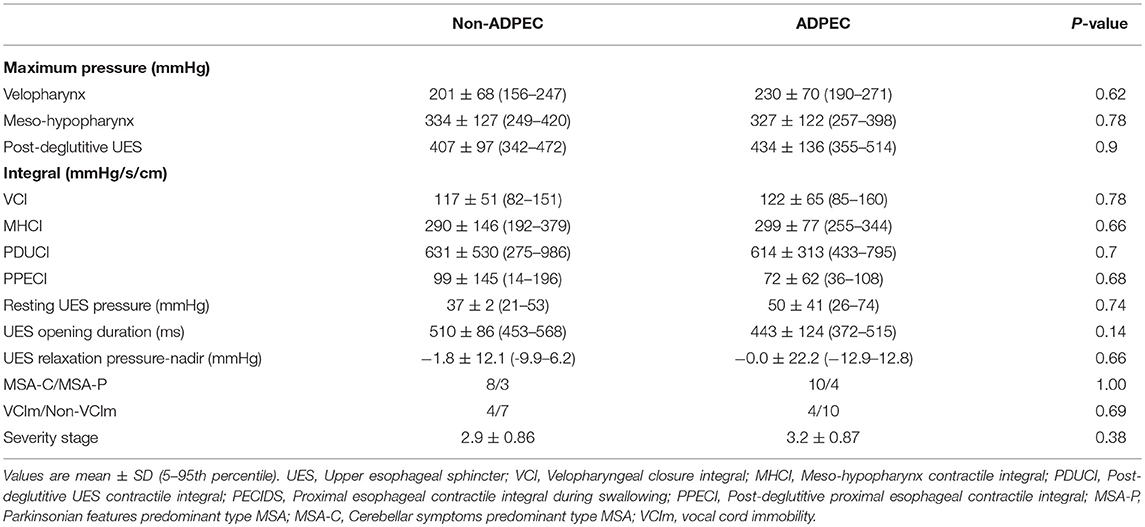
Table 4. Comparison of HRMF values between groups with/without abnormal discoordinated deglutitive proximal esophageal contraction (ADPEC) and associations of ADPEC and type of MSA, vocal cord immobility.
Discussion
Swallowing dysfunction is common in patients with MSA. Appropriate evaluations for dysphagia may prevent or delay complications such as aspiration pneumonia and sudden death. In this study, abnormal UES pressures and a pattern of discoordinated proximal esophageal contraction pressure during swallowing and resting were identified in MSA patients with oral nutritional status (FOIS score >3). These findings did not correlate with the severity stage, types of MSA, or concurrent vocal fold immobility.
MSA is a progressive neurodegenerative disease and can affect swallowing function variably with disease progression. The disordered oral stage of swallowing manifests in delayed bolus transport to the pharynx and worsens progressively due to bradykinesia and disrupted tongue coordination (15). Diminished oropharyngeal and hypopharyngeal manometric swallowing pressures of various degrees have been reported (7). The current investigation supports these data and suggests that pharyngeal pressures on HRMF are variable. In addition, another study showed 23.1% of MSA patients had incomplete relaxation of the UES (7). Deficient UES relaxation duration and impaired UES relaxation were also found in certain MSA patients in this study, however, the pathophysiology of how MSA affected the UES remains incompletely understood.
MSA can also cause esophageal stage impairments such as dysmotility (16). It has been proposed that preganglionic cholinergic dysfunction and specifically loss of the dorsal vagal nucleus innervating the digestive system lead to food stagnation within the esophagus (17) and occur more prevalently in MSA patients with dysphagia (16). Thus, evaluation of the esophageal phase is also indicated.
Complex sequences of swallowing are controlled and regulated by the medullary swallowing center, which is also known as the central pattern generators (CPGs) (18). The finding of ADPEC in MSA patients in this study may be an example of abnormal or discoordinated motility stemming from central nervous system dysfunction. Additional investigations are necessary to obtain a better understanding of the mechanism of ADPEC in patients with MSA and other neurodegenerative disease.
Vocal fold immobility is another frequent complication of MSA (3). Swallowing dysfunction and vocal fold immobility are related, as both the thyropharyngeal and cricopharyngeal muscles are innervated by the vagus nerve (3, 7). Few studies have evaluated the relationship between vocal fold immobility and dysphagia in MSA. We previously reported that vocal fold abductor paralysis tended to precede dysphagia in MSA-C patients (19) and the MSA-C patients with severe vocal fold abductor paralysis also suffered from severe dysphagia (3). Impaired UES opening and bolus stasis at the pyriform sinuses have been reported as associated with vocal fold abductor paralysis in MSA (7). In this study, however, no significant association was found between ADPEC and vocal fold immobility. This could result from the restriction of patients with only mild or moderate severity MSA in our cohort. An association between ADPEC and vocal fold immobility may be more common in patients with high severity MSA.
HRMF can improve understanding and advance our capabilities of evaluating the pharyngo-esophageal region. In this study, concurrent UES and proximal esophageal abnormalities, possibly due to neuromuscular dysfunction, were frequently noted even in mild MSA patients, and ADPEC identified on HRMF may be suggestive of MSA. Our findings suggest that HRMF may assist with the earlier detection of deglutitive abnormalities before the symptom of dysphagia develops. However, it is not fully clear whether the ADPEC is an early indication of pathophysiologic and neurodegenerative changes in MSA patients, an indicator of MSA severity, or possibly a compensatory proximal esophageal contraction in response to ongoing pharyngo-esophageal dysmotility. Longitudinal observations are required to elucidate the clinical significance of ADPEC in patients with MSA.
MSA affects the autonomic nervous system and swallowing function progressively. Pulmonary complications, malnourishments, or even mortality is related to clinical deterioration. The data suggest that HRMF may be beneficial for the early detection of swallowing dysfunction in MSA patients. Further investigation is required to confirm the accuracy of HRMF in the early detection of MSA and assess the effect of early detection on wellness and quality of life.
Conclusions
UES pressure anomalies and a discoordinated proximal esophageal pressure response are common manometric findings in persons with MSA. Further investigation is required to investigate the mechanisms underlying this pressure pattern and validate the application of HRMF as a diagnostic tool for MSA.
Author Contributions
RU developed the concept, designed and performed the experiments, and analyzed the data. TN, PB, and TY developed the concept, designed the experiments. TG, TS, NN-Z, and SS performed the experiments, and analyzed the data. All authors contributed to interpretation of the data and writing of the manuscript.
Funding
This work was supported by a Japan Society for the Japan Society Grant-in-Aid for Scientific Research (number 16KT0190).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. (1999) 163:94–8. doi: 10.1016/S0022-510X(98)00304-9
2. Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology (2008) 71:670–6. doi: 10.1212/01.wnl.0000324625.00404.15
3. Ueha R, Nito T, Sakamoto T, Yamauchi A, Tsunoda K, Yamasoba T. Post-operative swallowing in multiple system atrophy. Eur J Neurol. (2016) 23:393–400. doi: 10.1111/ene.12880
4. Papapetropoulos S, Tuchman A, Laufer D, Papatsoris AG, Papapetropoulos N, Mash DC. Causes of death in multiple system atrophy. J Neurol Neurosurg Psychiatry (2007) 78:327–9. doi: 10.1136/jnnp.2006.103929
5. Zhang L, Cao B, Zou Y, Wei QQ, Ou R, Liu W, et al. Causes of death in Chinese patients with multiple system atrophy. Aging Dis. (2018) 9:102–8. doi: 10.14336/AD.2017.0711
6. Higo R, Nito T, Tayama N. Swallowing function in patients with multiple-system atrophy with a clinical predominance of cerebellar symptoms (MSA-C). Eur Arch Otorhinol. (2005) 262:646–50. doi: 10.1007/s00405-004-0883-0
7. Higo R, Tayama N, Watanabe T, Nitou T, Ugawa Y. Videofluoroscopic and manometric evaluation of swallowing function in patients with multiple system atrophy. Ann Otol Rhinol Laryngol. (2003) 112:630–6. doi: 10.1177/000348940311200710
8. Nativ-Zeltzer N, Kahrilas PJ, Logemann JA. Manofluorography in the evaluation of oropharyngeal dysphagia. Dysphagia (2012) 27:151–61. doi: 10.1007/s00455-012-9405-1
9. Nativ-Zeltzer N, Logemann JA, Zecker SG, Kahrilas PJ. Pressure topography metrics for high-resolution pharyngeal-esophageal manofluorography-a normative study of younger and older adults. Neurogastroenterol Motil. (2016) 28:721–31. doi: 10.1111/nmo.12769
10. Watanabe H, Saito Y, Terao S, Ando T, Kachi T, Mukai E, et al. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain (2002) 125:1070–83. doi: 10.1093/brain/awf117
11. Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil. (2005) 86:1516–20. doi: 10.1016/j.apmr.2004.11.049
12. Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia (1996) 11:93–8. doi: 10.1007/BF00417897
13. Mathews SC, Ciarleglio M, Chavez YH, Clarke JO, Stein E, Chander Roland B. Upper esophageal sphincter abnormalities are strongly predictive of treatment response in patients with achalasia. World J Clin Cases (2014) 2:448–54. doi: 10.12998/wjcc.v2.i9.448
14. Park CH, Lee YT, Yi Y, Lee JS, Park JH, Yoon KJ. Ability of high-resolution manometry to determine feeding method and to predict aspiration pneumonia in patients with dysphagia. Am J Gastroenterol. (2017) 112:1074–83. doi: 10.1038/ajg.2017.81
15. Fernagut PO, Vital A, Canron MH, Tison F, Meissner WG. Ambiguous mechanisms of dysphagia in multiple system atrophy. Brain (2012) 135:e205. doi: 10.1093/brain/awr185
16. Taniguchi H, Nakayama H, Hori K, Nishizawa M, Inoue M, Shimohata T. Esophageal involvement in multiple system atrophy. Dysphagia (2015) 30:669–73. doi: 10.1007/s00455-015-9641-2
17. Benarroch EE, Schmeichel AM, Sandroni P, Low PA, Parisi JE. Involvement of vagal autonomic nuclei in multiple system atrophy and Lewy body disease. Neurology (2006) 66:378–83. doi: 10.1212/01.wnl.0000196638.98781.bb
18. Amri M, Car A. Projections from the medullary swallowing center to the hypoglossal motor nucleus: a neuroanatomical and electrophysiological study in sheep. Brain Res. (1988) 441:119–26. doi: 10.1016/0006-8993(88)91389-3
Keywords: multiple system atrophy, high resolution manofluorography, swallowing, upper esophageal sphincter, proximal esophageal abnormality, pharyngoesophageal abnormality, abnormal deglutitive proximal esophageal contraction
Citation: Ueha R, Goto T, Sato T, Nativ-Zeltzer N, Shen SC, Nito T, Belafsky PC and Yamasoba T (2018) High Resolution Manofluorographic Study in Patients With Multiple System Atrophy: Possible Early Detection of Upper Esophageal Sphincter and Proximal Esophageal Abnormality. Front. Med. 5:286. doi: 10.3389/fmed.2018.00286
Received: 27 June 2018; Accepted: 17 September 2018;
Published: 05 October 2018.
Edited by:
Yeong Yeh Lee, University of Science, Malaysia, MalaysiaReviewed by:
Askin Erdogan, Augusta University, United StatesMousumi Chaudhury, Arkansas Children's Nutrition Center, United States
Copyright © 2018 Ueha, Goto, Sato, Nativ-Zeltzer, Shen, Nito, Belafsky and Yamasoba. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rumi Ueha, VUVIQVItT1RPQGgudS10b2t5by5hYy5qcA==
 Rumi Ueha
Rumi Ueha Takao Goto1
Takao Goto1 Taku Sato
Taku Sato Tatsuya Yamasoba
Tatsuya Yamasoba