
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Med. , 25 September 2018
Sec. Translational Medicine
Volume 5 - 2018 | https://doi.org/10.3389/fmed.2018.00241
This article is part of the Research Topic Innovations in Modeling and Simulation to Advance Translational Science View all 15 articles
Protecting and promoting public health is the mission of the U.S. Food and Drug Administration (FDA). FDA's Center for Devices and Radiological Health (CDRH), which regulates medical devices marketed in the U.S., envisions itself as the world's leader in medical device innovation and regulatory science–the development of new methods, standards, and approaches to assess the safety, efficacy, quality, and performance of medical devices. Traditionally, bench testing, animal studies, and clinical trials have been the main sources of evidence for getting medical devices on the market in the U.S. In recent years, however, computational modeling has become an increasingly powerful tool for evaluating medical devices, complementing bench, animal and clinical methods. Moreover, computational modeling methods are increasingly being used within software platforms, serving as clinical decision support tools, and are being embedded in medical devices. Because of its reach and huge potential, computational modeling has been identified as a priority by CDRH, and indeed by FDA's leadership. Therefore, the Office of Science and Engineering Laboratories (OSEL)—the research arm of CDRH—has committed significant resources to transforming computational modeling from a valuable scientific tool to a valuable regulatory tool, and developing mechanisms to rely more on digital evidence in place of other evidence. This article introduces the role of computational modeling for medical devices, describes OSEL's ongoing research, and overviews how evidence from computational modeling (i.e., digital evidence) has been used in regulatory submissions by industry to CDRH in recent years. It concludes by discussing the potential future role for computational modeling and digital evidence in medical devices.
The mission of the U. S. Food and Drug Administration (FDA) is to protect and promote public health, and it does so by ensuring the safety, effectiveness and security of FDA-regulated products1 These products include, but are not limited to, medical devices, drugs for humans and animals, and biological products such as vaccines and the blood supply, each of which are managed by separate Centers within the Agency. The FDA accomplishes its mission by performing pre-market clearance, approval and post-market monitoring of the performance and safety of products, enforcing, and ensuring compliance to manufacturing processes and quality control, and conducting regulatory science research. The latter, although less well-known in the scientific community, is fundamental to support science-based regulatory decision-making by FDA. Regulatory science encompasses the development of new methods, standards, and approaches to assess the safety, efficacy, quality, and performance of FDA-regulated products and products under development. Each Center in the FDA is committed to advancing these efforts, which have accelerated the product development pathway and regulatory review cycle so that new, innovative products can be made available to the American public.
The FDA faces many challenges (1), such as new and evolving public health threats; rapid scientific breakthroughs and emerging technologies resulting in novel products that may raise unique testing and safety issues; globalization of public health, science, manufacturing and supply chains; and providing timely, accurate and useful consumer information in an age of information overload. To enable the Agency to meet today's public health needs and to be fully prepared for the challenges and opportunities of tomorrow, FDA leadership developed a strategic plan identifying nine target areas, stating that investment in these areas is essential to mission success2 Of those nine, four priority areas identified an important role for computational modeling3, see Table 1. These priorities also have relevant aspects related to medical devices4. regulated by the Center for Devices and Radiological Health (CDRH)5, as mentioned by the FDA Commissioner in a blog posted in July 20176.
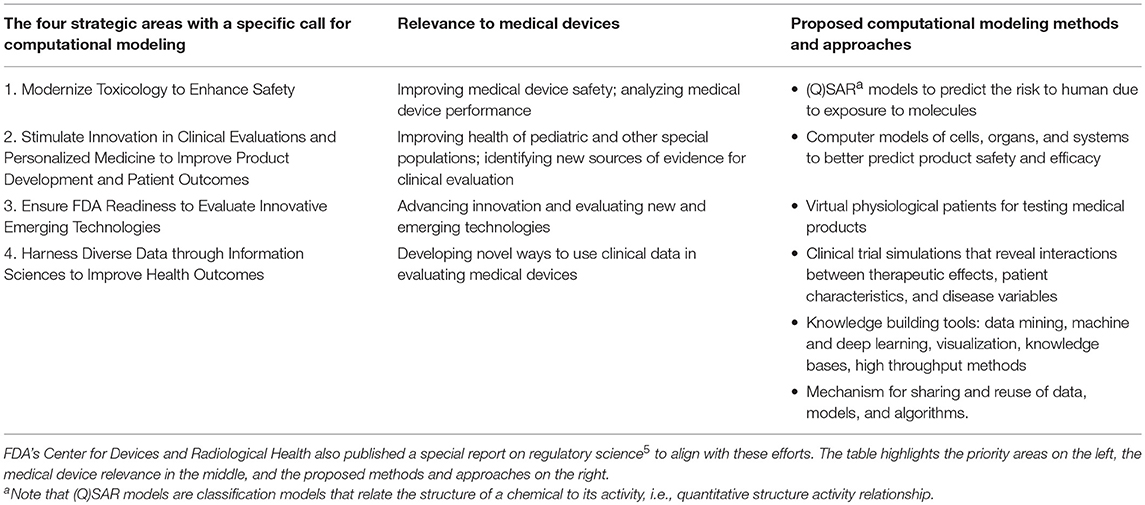
Table 1. In 2011, FDA identified an important role for computational modeling in its strategic priorities.
CDRH's mission goes beyond protecting and public health; with a vision to be the world's leader in medical device innovation, they provide consumers, patients, their caregivers, and providers with understandable and accessible science-based information about the products it oversees, and facilitate innovation by advancing regulatory science. Science-based regulatory decisions are made with evidence collected from four different models: animal, bench, computational7, and human (i.e., clinical trials), see Figure 1A. While each model has its advantages and limitations for evaluating different aspects of medical device performance (3), computational modeling is a promising one for supporting the future of medical devices and healthcare. FDA's Office of Science and Engineering Laboratories (OSEL) has committed significant resources for transforming computational modeling from a valuable scientific tool to a valuable regulatory tool because of its potential for significant cost-savings in evaluating medical devices, simulating performance under scenarios that may not be possible with human use or that could more effectively be evaluated with simulation.
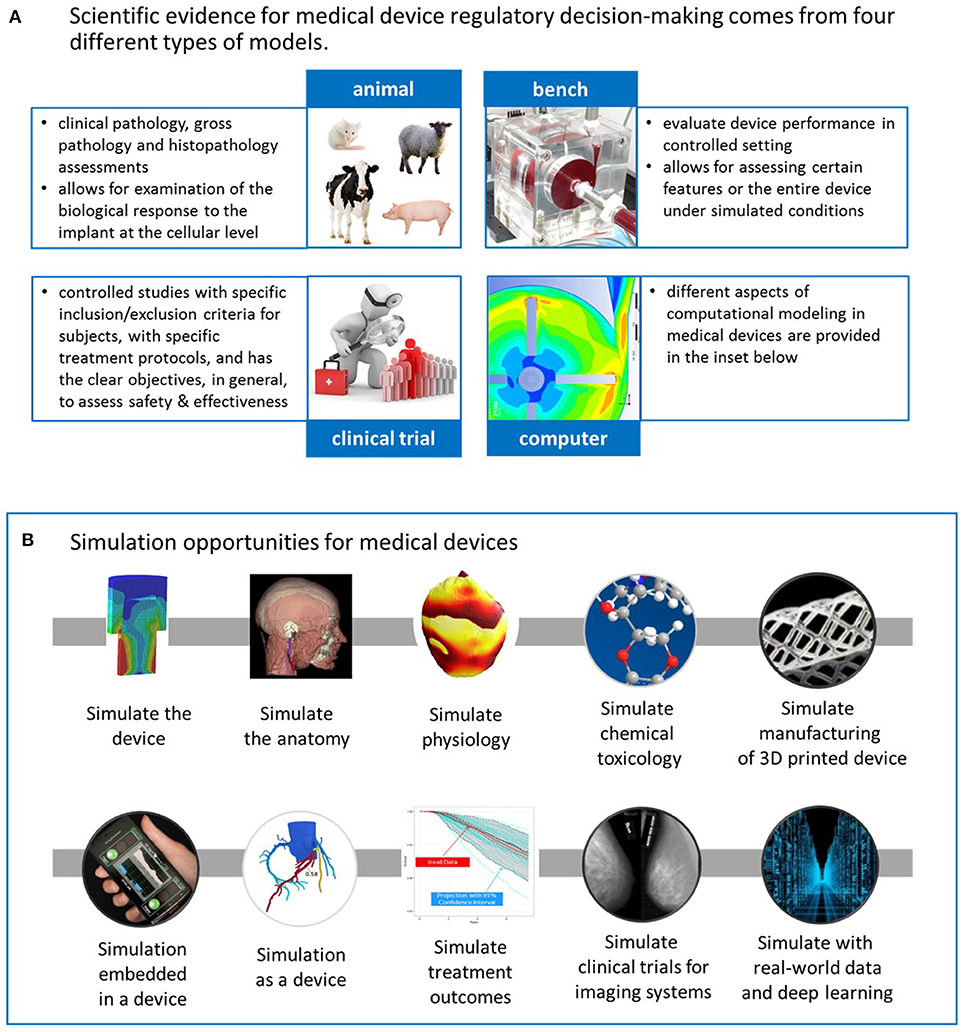
Figure 1. (A) CDRH's science-based regulatory decisions about medical devices are made with evidence collected from four different models: animal, bench, computational, and human (i.e., clinical trials). (B) Computational modeling has the potential to transform medical device design and evaluation in several ways. The upper row consists of applications that typically support the design or evaluation of the physical device. The lower row represents other applications, such as those embedded in a device or simulation as a medical device. Moreover, computational modeling can also simulate treatment outcomes or simulate the clinical trial for imaging systems. Lastly, it can play a critical role in the development of data-driven models from real-world data. See the text for more details.
OSEL has a unique role in medical device regulation serving as the research arm for CDRH. OSEL's expertise spans a variety of scientific, engineering, and mathematical disciplines8, with a diverse group of 130 full time scientists and engineers (supported by numerous post-doctoral fellows and interns) that provide expert support internally to the regulatory teams and externally to industry, clinical and the scientific communities. They conduct cutting-edge research, ensure readiness for emerging and innovative medical technologies, develop evaluation strategies and testing standards, create accessible and understandable public health information, deliver timely decisions for products across their life cycle, and readily share data and engage with stakeholders to advance regulatory science. The growing area of computational modeling is fully supported by OSEL and CDRH through research and development of methods and tools, serving as expert consultants by reviewing and assessing computational modeling submitted by medical device companies, i.e., sponsors, in regulatory submissions, and publicly sharing computational modeling that supports regulatory decision-making. Moreover, OSEL houses a high-performance computing center which supports scientific computing needs for CDRH and other Centers across FDA9
Because OSEL scientists have different roles to support CDRH, we designed and conducted a 35-question survey to better understand computational modeling in research and regulatory domains, including goals and objectives on the use of computational modeling in research, reliance of evidence from computational modeling and simulation (i.e., digital evidence) in regulatory submissions, and opportunities for the future with computational modeling and simulation. Thirty-six OSEL scientists with direct involvement in computational modeling projects and initiatives were interviewed, their responses were transcribed in a database and then shared with the scientists for fact checking. This perspective will present the results from that survey and highlight the different roles that computational modeling has and can play in medical devices, and discuss the potential future for digital evidence and simulation in medical devices.
Computational modeling can be used to simulate and better understand medical devices in several ways, as depicted in Figure 1B. Starting with the upper row, the simplest and most common implementation of computational modeling for medical devices is simply to simulate the device under a variety of conditions that mimic some aspect of the clinical or use environment to investigate some aspect of the device's performance. Computational modeling applications also include simulating the anatomy or serving as computational human phantoms for medical imaging systems or as a platform for assessing implanted devices; simulating physiology, such as electrophysiology during arrhythmias in the heart, or of pancreatic function; simulating chemical toxicology (using (Q)SAR models), which can support our ability to understand whether or not compounds released from medical devices, such as from dyes and coatings, are harmful; simulating the additive manufacturing process to optimize a 3D-printed product or simulating the substrate on which a 3D-printed product will be manufactured. The applications in the upper row of Figure 1B typically support design or evaluation of a physical, medical device. Other applications, depicted in the lower row, include computational algorithms embedded in a medical device or serving as the medical device, i.e., software as a medical device (4). An example of the former is embedded control algorithms in glucose monitors, which have the potential for advancing modern artificial pancreas systems10 used in glucose regulation for patients with diabetes (5). The models for the artificial pancreas have been used to replace in vivo animal studies to initiate clinical studies for these closed-loop devices (6). An example of computational modeling as a medical device is the use of personalized simulation to indicate whether a patient is a candidate for a medical device or a pharmaceutical, for example, to simulate an invasive clinical procedure or dosage effect to predict an outcome before the therapy is selected.
Computational modeling can also be used to simulate treatment outcomes. Statistical models have long been used to simulate clinical trial design and interpret results. An evolving concept is that of “virtual patients,” and new statistical models to augment clinical trial design with virtual patients to predict treatment outcomes (7, 8). It is important to note that a “virtual patient” is not necessarily a digitized patient; it is an approach that allows previously collected evidence (such as digital evidence or other historical clinical evidence typically referred to as “external evidence”) to inform the collection of new evidence from a clinical trial using Bayesian methodologies. Thus, computational modeling can enable a pathway to expose fewer patients to experimental therapies by relying on other sources of evidence. It can offer an opportunity to address questions that we cannot address clinically due to financial or ethical considerations, and investigate aspects of device performance in many more clinically-relevant cases (hundreds of thousands as compared to hundreds). Computational modeling can facilitate the exploration of using a medical device in populations that cannot be investigated clinically, such as in patients with rare diseases or pediatric patients, without harm. Computational modeling has also enabled the complete “in silico” simulation of clinical trials for medical imaging systems. By this we mean the implementation of different computational models to simulate the entire clinical evaluation of an imaging system, creating a “virtual clinical trial,” where no patients are physically exposed to the imaging system–more on this later. Lastly, knowledge-base tools can be harnessed to develop data-driven models from big data sources, such as real-world data, and employ deep learning methods to gain relevant insights about medical device use and performance. Computational modeling for medical devices has a broad scope impacting many facets of the product lifecycle, and scientists from OSEL are leading or closely collaborating with leaders in the field in each of the aforementioned categories.
The research conducted in OSEL is directly motivated by regulatory needs, scientific questions arising from the review of regulatory submissions and anticipating future direction of industry needs through technology forecasting. Moreover, the vision of CDRH is to ensure patients in the U.S. have access to innovative medical devices first in the world, and computational modeling is one tool to support faster more efficient regulatory approvals without sacrificing patient safety or the confidence in regulatory decisions. Some companies have stated that the cost for clinical trials may soon outpace revenue (9), and industry will therefore need other relevant and reliable data sources for demonstrating safety and effectiveness of medical devices; computational modeling is a practical and viable method for gathering clinical information to augment clinical trials (10). More details on this are provided in the closing section.
There is a broad range of modeling disciplines that OSEL scientists are using in their medical device-driven research, including photon transport, fluid dynamics, heat transfer, electromagnetism, solid mechanics, acoustics and optics, along with anatomical, physiological, and mechanistic modeling. Other include (Q)SAR models for assessing molecular carcinogenicity (11), deep learning methods and artificial intelligence for analyzing and synthesizing real-world data. Within this diverse range, OSEL has been advancing different areas of computational modeling for medical devices. The following examples provide a glimpse of the many computational modeling applications in OSEL.
Scientists have developed computational models of medical devices for investigating a specific approach or consideration about the medical device. It is important to emphasize that the computational studies below are not of a specific manufacturer's device, but of generic devices where the study results have broad impact in that device domain and are translatable to other domains. These models include implantable cardiovascular stents for assessing different methods to calculate fatigue safety factor (12); heart valves implanted with non-circular configurations (13) to assess the impact on stresses and strains; inferior vena cava filters to demonstrate a new method for computing embolus transport (14); hip implants for evaluating the impact of the design on contact mechanics (15); radiofrequency coils for MRI systems (16, 17) to investigate the design parameters on the electromagnetic field; surgical facemasks (18) for evaluating aerosol leakage of different designs; blood pump (19) for assessing the ability to predict hemolysis using computational fluid dynamics; and electrical stimulation of implanted lead wires (20) to investigate local heating. They have also developed new methods for simulating photon transport of x-ray emitters (21) and compressive sensing for imaging systems (22). Another computational effort was the development of a complex constitutive models for absorbable polymers used in medical implants (23).
Computational models of anatomy or physiology include improved drug delivery in the cornea with ultrasound energy (24); physiological models of heart cells (25), renal circulation (26), hemodynamic responses to blood volume perturbations (27), left bundle branch block (28), gas dynamics in the retina (29), coupled electrical and mechanical activity in the heart (30); energy absorption in patients with deep-brain stimulators (31–34), breast tissue expanders (35), in pregnant women and fetus during MRI exams (36); subthalamic nucleus (37), the breast (38), cancellous bone (39), the head (40) and whole body models (41, 42).
A part of OSEL's mission is to improve CDRH's ability to evaluate medical devices and support the regulatory approval of innovative medical devices more efficiently without sacrificing safety. Therefore, some of the research efforts involve demonstrating through examples that the output from computational modeling is a viable source of regulatory-grade evidence, i.e., sufficiently-credible digital evidence that can support regulatory applications (43–45); developing frameworks (46, 47) and metrics (48) for assessing the trustworthiness of models, and studying workflows for creating reproducible models11 (48), and identifying considerations for computational patient models for autonomous medical devices (49).
Other computational tools to assess specific aspects of device performance or safety that industry can employ include a simulator for high-intensity focused ultrasound (HIFU) beams and heating effects (50, 51), benchmarks models for computational fluid dynamics (19), patient-specific workflows for assessing clot trapping efficiency in IVC filters (52), surrogate models for predicting device-specific and species-specific hemolysis (Craven et al., under review), optical-thermal light-tissue interactions for photoacoustic breast imaging (53), and an online app for assessing the safety of color additives (54).
Additional efforts are pushing the state of the art of simulation for medical devices, including fluid-structure-interaction of deformable blood clots, computational human phantoms for active implants (37), lesion insertion and image reconstruction (55), computational patient models for closed-loop control devices (27), whole-heart modeling for electrophysiology devices (30), computational modeling for determining hemolysis levels in patients supported by blood-circulating medical devices (56), evaluating exposure risk from nickel leaching devices (57) and risk assessment for framing policy and deciding on the stockpile of personal protective equipment for wide-spread outbreaks or virus epidemics (58).
Lastly, as previously mentioned, one team is developing and validating a framework for streamlining the market entry of imaging systems relying solely on simulation in place of clinical trials. The VICTRE project (virtual imaging of clinical trials for regulatory evaluation) approach involves simulating the anatomical structure of the breast (with or without a neoplastic lesion), the radiological transmission (i.e., imaging system) and reconstructed images, and the clinical reader studies. By simulating each component of the clinical trial process, there is the potential for minimizing the need for clinical trials and thus the regulatory review of imaging systems (59). Note that the VICTRE project uses statistical analysis tools that evaluate the diagnostic performance of radiologists (virtual or human). An important aspect of the statistical analyses is that they account for radiologist variability and case variability. Such analyses are not trivial and have been developed by OSEL scientists (60–63) with validation based on sophisticated simulation tools (64).
OSEL scientists share their models and data with the public to facilitate the use and broader adoption of these computational tools and approaches. For example, anatomical models of the head (the MIDA model) and whole body models (the Virtual Family) can be downloaded from the IT'IS Foundation's website12, 13 The experimental and computational data from an FDA-led multi-laboratory study for fluid dynamics on generic medical devices can be found here;14 the simulation and statistical tools for the VICTRE project are here15 and here (65, 66); the HIFU simulator here16 Other software applications are being shared through Github17, such as the design of a generic inferior vena cava filter18 and a risk assessment tool for assessing color additives19
The OSEL scientists also serve as expert consultants on regulatory submissions. The review and decision about a medical device regulatory application requires a team of experts led by the regulatory offices in CDRH. OSEL scientists serve as specialized, technical experts on the regulatory teams. More than 2500 consulting reviews were completed by all scientists in OSEL in 2017, and about 500 were completed by the 36 scientists interviewed for this perspective. Of the 500 consults performed by the scientists surveyed, 220 (44%) included computational modeling and digital evidence in the submission. (Note that therefore 9% of all expert consults performed by OSEL for the regulatory offices in 2017 involved computational modeling). Of these 220, the submission-type breakdown is as follows: 36% were for premarket 510(k) notifications (for moderate risk devices), 25% for clinical trial applications, 24% for pre-submissions, 13% for premarket approval applications (for high risk devices), with only a handful for other submission types. With respect to medical areas, the largest number of consults in 2017 were for neurological devices, followed by cardiovascular and orthopedic devices, imaging systems, and surgical devices.
The survey results indicated that the primary use of computational modeling in regulatory submissions was to identify the appropriate bench testing configurations, such as worst-case or clinically challenging conditions, for cardiovascular, orthopedic, and surgical implants. The second most common use of computational modeling was to provide evidence supporting the safety assessment of patients with and without implanted devices when exposed to the radiofrequency (RF) fields of an MR system. A noteworthy example of the latter is the recent clearance20 of the first 7 Tesla MRI system (Siemens Magnetom)21, where the Virtual Family (41) and the MIDA head model (40) were used to predict aspects of safety and effectiveness of the new system. Examples of the former include RF safety evaluation for patients with implanted electrically passive (e.g., joint replacement, stents) or electrically active devices (e.g., neurostimulators, pacemakers, cochlear implants). Other modeling examples include therapeutic ultrasound systems where simulation results of the ultrasound energy delivered to in vivo locations have been used in regulatory submissions as justification for system parameters, or the recent clearance of Compressed Sensing GRASP-VIBE® to support high-resolution dynamic abdominal imaging under free-breathing. From the 510(k) summary22, “A comparison of the functionality was performed between the new feature and the device feature by detailed simulations with a numerical [computational] phantom”.
In general, computational modeling can be part of a regulatory submission in two ways. The first is when simulation results serve as supporting (digital) evidence in a marketing application for a medical device. The second is when simulation is a medical device, such as for clinical decision support; this is “software as a medical device.” Virtually all consults regarding computational modeling were of the former; the latter, with just a handful of submissions, is a new growth area for CDRH, especially with the release of the FDA guidance that describes the clinical evaluation for these software application, and the new program area on Digital Health Technologies23 Two examples of software as a medical device that have received FDA clearance used patient-specific computational models generated from CT imaging data to non-invasively predict clinically-relevant quantities for treatment selection. Heartflow® generates a personalized 3D model of the patient's coronary arteries and simulates blood flow to predict fractional flow reserve24 The CardioInsight® Mapping System generates a personalized model of the patient's heart and torso, then simulates the electrical activity on the heart surface from body surface potential recordings25 For more information on these and other patient-specific cardiovascular models see (67).
As indicated by the relatively large number of pre-submissions (approximately five dozen) that contain computational modeling, many sponsors are using this mechanism to discuss with FDA how their computational approaches will be used in different regulatory pathways. The pre-submission26 process enables interaction between companies and FDA to discuss issues (e.g., outstanding regulatory deficiencies) or present new technology or regulatory approaches. Pre-submissions might include details describing how computational modeling might support device performance, augment clinical trials, or be a part of a software as a medical device, but might also include the introduction of innovative devices for informational purposes or for strategic regulatory planning. The mechanism for early interaction is called the Information Meeting, found on page 22 of the guidance.
In this section, we have presented some success stories of computational modeling being used to support medical device regulatory review. However, there remain hurdles for broader adoption of computational modeling. FDA is using its leadership role to help overcome some of these hurdles; one in particular is on communication. The regulatory review process is typically dominated by the review of tens (sometime hundreds) of test reports. CDRH reviewers do not conduct or run simulations for specific regulatory submissions, and consequently rely on the details of a report to understand what was accomplished. Detailed and comprehensive reporting of computational modeling can thus substantively improve the acceptance of digital evidence submitted to CDRH. In 2016, FDA published a guidance document (68) on the details that should be provided to CDRH if computational modeling is used in a regulatory application. The scope of this guidance document, however, does not address the adequacy of the evidence, and therefore adherence to the guidance may not always result in a sufficiently credible digital evidence to support the device safety and/or effectiveness claims. Reasons for this failure include the lack of appropriate scope of use for the computational model or that the verification and validation (V&V) results provided do not support using the model for the specified use. Industry has communicated to FDA that what remains unclear is the V&V evidentiary bar and lack of standards for computational modeling studies. Therefore, FDA has been working closely with the ASME V&V40 Standards Subcommittee on a new standard (44) that will be published in Summer 2018. It presents a risk-informed credibility assessment framework that will help decision-makers determine the V&V evidence needed to support using a computational model for a specific context of use. Other device-specific modeling standards are being developed through ASTM and IEEE. Moreover, FDA actively engages with stakeholders about computational modeling efforts by hosting yearly workshops and conferences with co-sponsors such as the NIH & NSF27 and the Biomedical Engineering Society (69), recorded webinars28, 29, 30 and training seminars (70) on these documents. The use of simulation and digital evidence is rapidly evolving so FDA hopes industry will connect early and often through the pre-submission process to discuss potential opportunities for their computational modeling approaches.
The rapid advance of technology has drastically changed the power and availability of computational modeling tools. Increased storage capacity via the cloud, the acceleration of the graphics processing unit (GPU), parallelization, multicore machines, and high performance computing have transformed and facilitated the building of higher fidelity models and models with more complexity through multiscale and multiphysics applications, and improved the resolution and capability for enhanced visualization. With this increased capability and power, evidence from computational modeling has the potential to replace traditional, more burdensome data collection from other models. Notable are the simulations of radiofrequency energy absorption that have replaced confirmatory clinical trials for the MR safety assessment of implants previously discussed (10). Wanting to find more opportunities to minimize the burden of animal and human studies, CDRH will continue to promote the use of computational modeling in medical device development, applications and regulatory submissions and is committed to ensuring the appropriate research, methodologies and expertise is available to make such advances. For example, the FDA has been exploring the use of computational modeling as a possible way to facilitate better reprocessing for reusable medical devices31 Computational fluid dynamics models have the capability to isolate high risk regions in new device designs and can provide appropriate cleaning protocols to eliminate the risk of infection in reusable devices (71).
Another demonstrative example of FDA's commitment to facilitating innovation in medical devices is the collaboration between CDRH and industry through the Medical Device Innovation Consortium (MDIC) using the mechanism of a “mock submission” to demonstrate the regulatory process and evidence collection that could be submitted to FDA when using a new framework to augment clinical trials with virtual patients32. The mock submission approach has been a successful means to gather input from industry and FDA about new and innovative approaches for medical device evaluation (72). The MDIC, a non-profit in the U.S., was established to advance medical device regulatory science for patient benefit. The “Clinical Trials Informed by Bench and Simulation” working group included members from industry and FDA; their main objective was to establish and implement a framework, called the virtual patient discount model (VPDM), for incorporating virtual patients from simulation with real patient data from a clinical study through statistical simulations. Due to its novelty, the members of the working group formed a “sponsor team,” with members from industry and FDA, and prepared the mock submission proposing to initiate a clinical trial with a reduced number of actual patients by harnessing virtual patients; the goal was to present and evaluate the VPDM. The VPDM (73) harnesses Bayesian methods in conjunction with FDA guidance (74) on the use of Bayesian statistics in medical device clinical trials. The mock submission highlighted two key themes. First, early communication between the sponsor and regulatory teams is important to identify areas for detailed discussion, education, or that raise issues of concern, particularly due to the intersection of clinical statistics and engineering simulation. Second, model credibility, suitability, and context of use should be integral to the model development work, as they will heavily influence the success of the approach. All mock submission documents and FDA's formal response are publicly available on the MDIC website for the Virtual Patient Project.
The aforementioned VICTRE project for imaging systems also exemplifies other advances FDA is considering for augmenting clinical trials. The power of this approach lies in the ability to replace expensive and lengthy clinical trials for new imaging systems with completely in silico trials. The physics for these systems are well-known and all aspects of the evaluation cycle can be simulated with sufficient confidence to replace each step with simulation. The main objective of this approach is to achieve the same regulatory conclusion with the virtual clinical trial as with a large 600-patient clinical trial (75). The success of this approach relies on statistical models, deep learning and artificial intelligence (AI); these advanced analysis and statistical methods, which are comparable with mechanistic, predictive engineering tools, have the potential to dramatically transform medical devices (76). In fact, CDRH just permitted the marketing of the first medical device with AI33.
The device uses deep learning algorithms (77) to analyze images of the eye taken with a retinal camera for detecting diabetic retinopathy.
In other industries, computational modeling has long been recognized as a crucial scientific tool for getting innovative products into the hands of consumers34 (78) and facilitating design excellence through product lifecycle management (PLM). “PLM is a business solution which aims to streamline the flow of information about the product and related processes throughout the product's lifecycle such that the right information in the right context at the right time can be made available (79)”. If the medical device industry were to embrace PLM, they could more fully harness the power of simulation in each phase of the product's lifecycle and utilize AI tools to implement knowledge gained from real-world data to enhance their understanding of performance, support continuous improvement, and inform new designs and therapies. FDA also believes that computational modeling is poised to become a critical tool for accelerating regulatory decision-making. Continued adoption will be essential for advancing FDA's mission, improving our ability to evaluate medical devices more efficiently, reducing regulatory burden for sponsors, and accelerating the introduction of innovative technologies to the U.S. market for the benefit of patients.
TM led this study, designed the survey with PP and MA, analyzed results, organized and gathered references, drafted perspective article, organized multiple reviews and connected with authors cited in the article ensure accuracy. MA conducted the survey and interviews, collated responses in a database, and analyzed results. PP co-designed the survey, analyzed results, provided input and edited article. EM provided input on survey, analyzed results, provided input and edited article.
The mention of commercial products, their sources, or their use in connection with material reported herein is not to be constructed as either an actual or implied endorsement of such products by the Department of Health and Human Services or the Food and Drug Administration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer AH declared a past co-authorship with one of the authors TM to the handling Editor.
The reviewer AH and handling Editor declared their shared affiliation.
We thank OSEL colleagues Leonardo Angelone, Genevieve McRae and Donna Lochner for reviewing this article; we value their perspective and input. We would also like to acknowledge Ms. Lochner for her support of advancing computational modeling as a regulatory tool, and for serving as a focal point for bringing academic, government and industry stakeholders together to tackle important issues during her tenure at the FDA.
2. ^http://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/
3. ^Computational modeling is the process of representing a real-world system by means of a computer and then running the simulation by implementing a numerical scheme.
4. ^The FDA definition of medical devices excludes drugs, which achieve their effects through chemical action within or on the body. The vast majority of medical device manufacturers are classified by the U.S. Department of Commerce in five industries: x-ray and electromedical equipment, surgical and medical instruments, surgical appliances and supplies, dental equipment, and ophthalmic goods (2)
5. ^https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHReports/ucm274152.htm
6. ^https://blogs.fda.gov/FDAvoice/index.php, post July 7, 2017.
7. ^Evidence from algorithms, and computer-based modeling and simulation will be referred to as digital evidence.
8. ^https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHOffices/ucm115989.htm
9. ^https://scl-wiki-01.fda.gov/wiki/index.php/Main_Page
10. ^https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/HomeHealthandConsumer/ConsumerProducts/ArtificialPancreas/default.htm
11. ^NIBIB. R01EB024573, https://simtk.org/projects/kneehub
12. ^The MIDA Model: https://www.itis.ethz.ch/virtual-population/regional-human-models/mida-model/,
13. ^The Virtual Family: https://www.itis.ethz.ch/virtual-population/virtual-population/overview/
14. ^https://nciphub.org/wiki/FDA_CFD
15. ^https://github.com/didsr/victre
16. ^https://www.mathworks.com/matlabcentral/fileexchange/30886-high-intensity-focused-ultrasound-simulator.
18. ^https://github.com/kenaycock/Generic-IVC-Filter
19. ^https://dsaylor.github.io/CHRIS/
20. ^510(k) Summary: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K170840.pdf
21. ^https://usa.healthcare.siemens.com/news/magnetomterrafdaclearance.html
22. ^510(k) Summary: https://www.accessdata.fda.gov/cdrh_docs/pdf17/K173617.pdf
23. ^https://www.fda.gov/medicaldevices/digitalhealth/
24. ^https://www.accessdata.fda.gov/cdrh_docs/pdf15/K152733.pdf
25. ^https://www.accessdata.fda.gov/cdrh_docs/pdf16/k162440.pdf
26. ^FDA Final Guidance, Software as a Medical Device (SAMD): Clinical Evaluation. (2017). Available from: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm311176.pdf
27. ^https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-meddev-gen/documents/document/ucm364603.pdf
28. ^ORS Webinar: FDA - Modeling and Simulation Initiatives at CDRH on Vimeo (2017): https://vimeo.com/194712010,
29. ^ORS Webinar: FDA - Assessing the Predictive Capability of Computational Modeling for Medical Device Submissions on Vimeo (2017): https://vimeo.com/221479130,
30. ^Xtalks Webinar (2018) FDA Perspectives on Computer Simulations in the Evaluation of Medical Devices, https://xtalks.com/webinars/fda-perspectives-on-computer-simulations-in-the-evaluation-of-medical-devices/
31. ^https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofReusableMedicalDevices/ucm252952.htm
32. ^Virtual Patient Website, http://mdic.org/computer-modeling/virtual-patients/
33. ^https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm604357.htm
34. ^UL Science news: https://newscience.ul.com/articles/computational-modeling-of-lithium-ion-batteries
2. Institute of Medicine. Assessing Medical Technologies. Washington, DC: The National Academies Press (1985).
3. Morrison TM, Dreher ML, Nagaraja S, Angelone LM, Kainz W. The role of computational modeling and simulation in the total product life cycle of peripheral vascular devices. J Med Devices (2017) 11:024503. doi: 10.1115/1.4035866
4. International Medical Device Regulators Forum. Final Guidance, Software as a Medical Device, IMDRF/SaMD WG/N10 FINAL. Available online at: http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-131209-samd-key-definitions-140901.pdf (2013)
5. Zavitsanou S, Chakrabarty A, Dassau E, Doyle FJ III. Embedded control in wearable medical devices: application to the artificial pancreas. Processes (2016) 4:35. doi: 10.3390/pr4040035
6. Cobelli C, Renard E, Kovatchev B. Artificial pancreas: past, present, future. Diabetes (2011) 60:2672–82. doi: 10.2337/db11-0654
7. Viceconti M, Cobelli C, Haddad T, Himes A, Kovatchev B, Palmer M. In silico assessment of biomedical products: The conundrum of rare but not so rare events in two case studies. Proc Inst Mech Eng H. (2017) 231:455–66. doi: 10.1177/0954411917702931
8. Himes A, Haddad T, Bardot D. Augmenting a clinical study with virtual patient models: food and drug administration and industry collaboration. J Med Devices (2016) 10:030947. doi: 10.1115/1.4033870
9. Kramer JM, Schulman KA. Institute of Medicine (US). Envisioning a Transformed Clinical Trials Enterprise in the United States: Establishing an Agenda for 2020: Workshop Summary. Washington (DC): National Academies Press (US) (2012). Appendix, F., Discussion Paper: Transforming the Economics of Clinical Trials. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK114653/
10. Faris O, Shuren J. An FDA viewpoint on unique considerations for medical-device clinical trials. NEJM (2017) 376:1350–7. doi: 10.1056/NEJMra1512592
11. Brown R, White S, Goode J, Pradeep P, Merrill S. Use of QSAR modeling to predict the carcinogenicity of color additives. In: ASME 2013 Conference on Frontiers in Medical Devices: Applications of Computer Modeling and Simulation (2013). p. 2. doi: 10.1115/FMD2013-16161
12. Marrey R, Baillargeon B, Dreher M, Weaver JD, Nagaraja S, Rebelo N, et al. Validating Fatigue safety factor calculation methods for cardiovascular stents. J Biomech. Eng. (2018) 140:9. doi: 10.1115/1.4039173
13. Duraiswamy N, Weaver JD, Ekrami Y, Retta SM, Wu C. A parametric computational study of the impact of non-circular configurations on bioprosthetic heart valve leaflet deformations and stresses: possible implications for transcatheter heart valves. Cardiovasc Eng Technol (2016) 7:126–38. doi: 10.1007/s13239-016-0259-9
14. Aycock KI, Campbell RL, Manning KB, Craven BA. A resolved two-way coupled CFD/6-DOF approach for predicting embolus transport and the embolus-trapping efficiency of IVC filters. Biomech Model Mechanobiol. (2017) 16:851–69. doi: 10.1007/s10237-016-0857-3
15. Donaldson FE, Nyman EJr, Coburn JC. Prediction of contact mechanics in metal-on-metal Total Hip Replacement for parametrically comprehensive designs and loads. J Biomech. (2015) 48:1828–35. doi: 10.1016/j.jbiomech.2015.04.037
16. Lucano E, Liberti M, Lloyd T, Apollonio F, Wedan S, Kainz W, et al. A numerical investigation on the effect of RF coil feed variability on global and local electromagnetic field exposure in human body models at 64 MHz. Magn Reson Med. (2018) 79:1135–44. doi: 10.1002/mrm.26703
17. Lucano E, Liberti M, Mendoza G, Lloyd T, Iacono MI, Apollonio F, et al. Assessing the electromagnetic field generated by a radiofrequency body coil at 64 MHz: defeaturing vs. accuracy. IEEE Trans Biomed Eng. (2016) 63:1591–601. doi: 10.1109/TBME.2015.2506680
18. Guha S, McCaffrey B, Hariharan P, Myers MR. Quantification of leakage of sub-micron aerosols through surgical masks and facemasks for pediatric use. J Occup Environ Hyg. (2017) 14:214–23. doi: 10.1080/15459624.2016.1237029
19. Malinauskas RA, Hariharan P, Day SW, Herbertson LH, Buesen M, Steinseifer U, et al. FDA benchmark medical device flow models for CFD validation. ASAIO J. (2017) 63:150–60. doi: 10.1097/MAT.0000000000000499
20. Bassen HI, Angelone LM. Evaluation of unintended electrical stimulation from MR gradient fields: simplified computational models vs. experimental measurements for an implanted lead wire in a human tissue-equivalent phantom. Front Biosci E (2012) 4:1731–42. doi: 10.2741/e494
21. Badal A, Badano A. Accelerating Monte Carlo simulations of photon transport in a voxelized geometry using a massively parallel graphics processing unit. Med Phys. (2009) 36:4878–80. doi: 10.1118/1.3231824
22. Graff CG, Sidky EY. Compressive sensing in medical imaging. Appl Optics (2015) 54:C23–44. doi: 10.1364/AO.54.000C23
23. Dreher ML, Nagaraja S, Bergstrom J, Hayman D. Development of a flow evolution network model for the stress-strain behavior of poly(L-lactide). J Biomech Eng. 139:091002. doi: 10.1115/1.4037071
24. Hariharan P, Gouza GD, Horner M, Morrison TM, Malinauskas RA, Myers MR. Use of the FDA nozzle model to illustrate validation techniques in computational fluid dynamics (CFD) simulations. PloS ONE (2017) 12:e0178749. doi: 10.1371/journal.pone.0178749
25. Gray RA, Pathmanathan PP. A Parsimonious model of the rabbit action potential elucidates the minimal physiological requirements for alternans and spiral wave breakup. PLoS Comput Biol. (2016) 12:e1005087. doi: 10.1371/journal.pcbi.1005087
26. Scully CG, Mitrou N, Braam B, Cupples WA, Chon KH. Detecting interactions between the renal autoregulation mechanisms in time and space. IEEE Trans Biomed Eng. (2017) 64:690–8. doi: 10.1109/TBME.2016.2569453
27. Bighamian R, Parvinian B, Scully CG, Kramer G, Hahn JO. Control-oriented physiological modeling of hemodynamic responses to blood volume perturbation. Control Eng Practice (2018) 73:149–60. doi: 10.1016/j.conengprac.2018.01.008
28. Galeotti L, van Dam PM, Loring Z. Evaluating strict and conventional left bundle branch block criteria using electrocardiographic simulations. Europace (2013) 15:1816–21 doi: 10.1093/europace/eut132
29. Hall SK, Williamson TH, Guillemaut JY, Goddard T, Baumann AP, Hutter JC. Modeling the dynamics of tamponade multicomponent gases during retina reattachment surgery. AIChE J. (2017) 9:3651–62. doi: 10.1002/aic.15739
30. Gurev V, Pathmanathan P, Fattebert JL, Wen HF, Magerlein J, Gray RA, et al. A high-resolution computational model of the deforming human heart. Biomech Model Mechanobiol. (2015) 14:829–49. doi: 10.1007/s10237-014-0639-8
31. Serano P, Angelone LM, Katnani H, Eskandar E, Bonmassar G. A novel brain stimulation technology provides compatibility with MRI. Sci Rep. (2015) 5:9805. doi: 10.1038/srep09805
32. Guerin B, Serano P, Iacono MI, Herrington TM, Widge AS, Dougherty DD, et al. Realistic modeling of deep brain stimulation implants for accurate electromagnetic MRI safety studies. Phys Med Biol. (2018) 63: 095015. doi: 10.1088/1361-6560/aabd50
33. Golestanirad L, Angelone LM, Iacono MI, Liang L, Katnani H, Wald LL, et al. Local SAR near deep brain stimulation (DBS) electrodes for realistic vs. simplified lead trajectories in patient-specific human head models. Magn Reson Med. (2017) 78:1558–1565. doi: 10.1002/mrm.26535
34. Golestanirad L, Iacono MI, Keil B, Angelone LM, Bonmassar G, Fox M, et al. Construction and modeling of a reconfigurable MRI coil for lowering SAR in patients with deep brain stimulation implants. Neuroimage (2017) 147:577–88. doi: 10.1016/j.neuroimage.2016.12.056
35. Park BS, Razjouyan A, Angelone LM, McCright B, Rajan SS. RF safety evaluation of a breast tissue expander device for mri: numerical simulation and experiment. IEEE Trans Electromagn Compat. (2017) 59:1390–9. doi: 10.1109/TEMC.2017.2678201
36. Razjouyan A, Park BS, Rajan SS, Kainz W, McCright B, Angelone LM. MRI in Pregnant Women: A Systematic Analysis of Radiofrequency Safety Assurance depending on Landmark Positions at 64/128 MHz. Phys Med Biol. (2018) 77: 2048–56. doi: 10.1002/mrm.26268
37. Iacono MI, Neufeld E, Bonmassar G, Akinnagbe E, Jakab A, Cohen E, et al. A computational model for bipolar deep brain stimulation of the subthalamic nucleus. In: 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Chicago, IL (2014). p. 6258–61. doi: 10.1109/EMBC.2014.6945059
38. Graff CG. A new, open-source, multi-modality digital breast phantom. Phys Med Imaging (2016) 9783:978309. doi: 10.1117/12.2216312
39. Wear KA, Nagaraja S, Dreher ML, Sadoughi S, Zhu S, Keaveny TM. Relationships among ultrasonic and mechanical properties of cancellous bone in human calcaneus in vitro. Bone (2017) 103:93–101. doi: 10.1016/j.bone.2017.06.021
40. Iacono MI, Neufeld E, Akinnagbe E, Wolf J, Oikonomidis IV, Sharma D, et al. MIDA: a multimodal imaging-based detailed anatomical model of the human head and neck. PLoS ONE (2015) 10:e0124126. doi: 10.1371/journal.pone.0124126
41. Christ A, Kainz W, Hahn EG, Honegger K, Zefferer M, Neufeld E, et al. The Virtual Family–development of surface-based anatomical models of two adults and two children for dosimetric simulations. Phys Med Biol. (2010) 55:N23–38. doi: 10.1088/0031-9155/55/2/N01
42. Gosselin MC, Neufeld E, Moser H, Huber E, Farcito S, Gerber L, et al. Development of a new generation of high-resolution anatomical models for medical device evaluation: the Virtual Population 3.0. Phys Med Biol. (2014) 59:5287–303. doi: 10.1088/0031-9155/59/18/5287
43. Aycock KI, Rebelo N, Craven C. Code verification for solid mechanics problems including superelastic nitinol. In: ASME 2018 V&V Symposium (2018).
44. ASME V and V40. ANSI Standard, Assessing the Credibility of Computational Models for Medical Devices. Available online at: http://go.asme.org/VnV40Committee (2018).
45. Morrison TM, Aycock KI, Weaver JD, Craven B. A mock submission to initiate a clinical trial in the U.S. with modeling and simulation. In: Proceedings Virtual Physiological Human Institute Conference. (Zaragosa) (2018).
46. Pathmanathan P, Gray RA, Romero VJ, Morrison TM. Applicability analysis of validation evidence for biomedical computational models. J. Verif. Valid. Uncert. (2017) 2:021005-021005-11. doi: 10.1115/1.4037671
47. Pathmanathan P, Gray RA. Validation and trustworthiness of multiscale models of cardiac electrophysiology. Front Physiol. (2018) 9:106. doi: 10.3389/fphys.2018.00106
48. Hariharan P, Nabili M, Guan A, Zderic V, Myers M. Model for porosity changes occurring during ultrasound-enhanced transcorneal drug delivery. Ultrasound Med Biol. (2017) 43:1223–36. doi: 10.1016/j.ultrasmedbio.2017.01.013
49. Halloran JP, Besier TF, Imhauser CW, Laz P, Morrison TM, Shelburne K, et al. A collaborative strategy to establish reproducibility in simulation-based prediction of natural knee mechanics. In: Proceedings Virtual Physiological Human Institute Conference 2018. (Zaragosa) (2018).
50. Parvinian B, Scully C, Wiyor H, Kumar A, Weininger S. Regulatory considerations for physiological closed-loop controlled medical devices used for automated critical care: food and drug administration workshop discussion topics. Anesth Analg. (2017) 126:1916–25. doi: 10.1213/ANE.0000000000002329
51. Soneson J. A user-friendly software package for hifu simulation. AIP Conf Proc. (2009) 1113:165. doi: 10.1063/1.3131405
52. Soneson JE. Extending the utility of the parabolic approximation in medical ultrasound using wide-angle diffracztion modeling. IEEE Trans Ultrason Ferroelectr Freq Control. (2017) 64:679–87. doi: 10.1109/TUFFC.2017.2654125
53. Aycock KI, Campbell RL, Lynch FC, Manning KB, Craven BA. Computational predictions of the embolus-trapping performance of an IVC filter in patient-specific and idealized IVC geometries. Biomech Model Mechanobiol. (2017) 16:1957–69. doi: 10.1007/s10237-017-0931-5
54. Gould T, Wang Q, Pfefer TJ. Optical-thermal light-tissue interactions during photoacoustic breast imaging. Biomedical Opt Express (2014) 5:832–47. doi: 10.1364/B.O.E.5.000832
55. Chandrasekar V, Janes DW, Forrey C, Saylor DM, Bajaj A. et al. Improving risk assessment of color additives in medical device polymers. J Biomed Mater Res. (2018) 106:310–9. doi: 10.1002/jbm.b.33845
56. Ghanian Z, Pezeshk A, Petrick N, Sahiner B. Towards the use of computationally inserted lesions for mammographic CAD assessment. In medical imaging 2018: image perception, observer performance, and technology assessment. Int Soc Opt Photonics (2018) 10577:105770L. doi: 10.1117/12.2293800
57. Saylor D, Buehler P, Brown R, Malinauskas R. Predicting plasma free hemoglobin levels in patients due to medical device related hemolysis. ASAIO J. (2018). doi: 10.1097/MAT.0000000000000801. [Epub ahead of print].
58. Saylor DM, Craven BA, Chandrasekar V, Simon DD, Brown RP, Sussman EM. Predicting patient exposure to nickel released from cardiovascular devices using multi-scale modeling. Acta Biomater. (2018) 70:304–14. doi: 10.1016/j.actbio.2018.01.024
59. Myers MR, Hariharan P, Guha S, Yan J. A mathematical model for assessing the effectiveness of protective devices in reducing risk of infection by inhalable droplets. Math Med Biol A J IMA (2018) 35:1–23. doi: 10.1093/imammb/dqw018
60. Badano A, Badal A, Glick S, Graff CG, Samuelson F, Sharma D, et al. In silico imaging clinical trials for regulatory evaluation: initial considerations for VICTRE, a demonstration study. In: Medical Imaging 2017: Physics of Medical Imaging, Thomas GF, Joseph YL, Taly GS, editors. Vol. 10132. International Society for Optics and Photonics (2017). p. 1013220.
61. Gallas BD. One-shot estimate of MRMC variance: AUC. Acad Radiol. (2006) 13:353–62. doi: 10.1016/j.acra.2005.11.030
62. Gallas BD, Pennello GA, Myers KJ. Multireader multicase variance analysis for binary data. J Opt Soc Am A Special Issue Image Qual. (2007) 24:B70–80. doi: 10.1364/JOSAA.24.000B70
63. Gallas BD, Brown DG. Reader studies for validation of CAD systems. Neural Netw Special Conf Issue (2008) 21:387–97. doi: 10.1016/j.neunet.2007.12.013
64. Gallas BD, Bandos A, Samuelson F, Wagner RF. A framework for random-effects roc analysis: biases with the bootstrap and other variance estimators. Commun Stat A Theory (2009) 38:2586–603. doi: 10.1080/03610920802610084
65. Gallas BD, Hillis SL. Generalized Roe and Metz ROC Model: analytic link between simulated decision scores and empirical AUC variances and covariances. J Med Img (2014) 1:031006. doi: 10.1117/1.JMI.1.3.031006
66. Gallas BD. iROEMETZ v2.1: Application for Modeling and Simulating MRMC Reader Studies.In: Division of Imaging and Applied Mathematics. Silver Spring, MD: CDRH, FDA. Available onlin at: https://github.com/DIDSR/iMRMC/releases.Computational Fluid Dynamics at the National Cancer Institute (NCI), National Institutes of Health (NIH) (2013).
67. Gallas BD. iMRMC v4.0: Application for Analyzing and Sizing MRMC Reader Studies. In: Division of Imaging, Diagnostics, and Software Reliability, Silver Spring, MD: OSEL/CDRH/FDA. Available onlin at: https://github.com/DIDSR/iMRMC/releases, https://cran.r-project.org/web/packages/iMRMC/index.html (2017).
68. Gray RA, Pathmanathan P. Patient-specific cardiovascular computational modeling: diversity of personalization and challenges. J Cardiovasc Trans Res. 11:80–8. doi: 10.1007/s12265-018-9792-2
69. Morrison TM. FDA Final Guidance. Reporting on Computational Modeling Studies for Medical Device Submissions. Issued September 21, 2016. Available online at: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/Ucomputational modeling381813.pdf (2016).
70. Morrison TM, Angelone LM, Bestelmeyer A, Bischoff JE. Innovations in modeling and simulation: patient-centered healthcare. Ann Biomed Eng. (2016) 44:3719–49. doi: 10.1007/s10439-016-1710-7
71. Morrison T, Angelone L, Myers M, Soneson J, Wang Q, Weaver J, et al. FDA seminar on computational modeling for medical devices. Figshare (2017). doi: 10.6084/m9.figshare.5018783.v3
72. Hariharan P, Paruchuri SS, Topoleski LDT, Rinaldi J, Casamento JP, Myers MR, et al. A test method to assess the contribution of fluid shear-stress to the cleaning of reusable device surfaces. J Biomed Mat Res B. (in press).
73. Regnier FE, Skates SJ, Mesri M, Rodriguez H, TeŽak Ž, Kondratovich MV, et al. Protein-based multiplex assays: mock presubmissions to the US Food and Drug Administration. Clin Chem (2010) 56:165–71. doi: 10.1373/clinchem.2009.140087
74. Haddad T, Himes A, Thompson L, Irony T, Nair R, MDIC Computer Modeling and Simulation Working Group Participants. Incorporation of stochastic engineering models as prior information in Bayesian medical device trials. J Biopharma Stats (2017) 6:1089–103. doi: 10.1080/10543406.2017.1300907
75. Campbell G. FDA Final Guidance. Guidance for the Use of Bayesian Statistics in Medical Device Clinical Trials. Available online at: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071121.pdf (2010).
76. Badano A. Open-source, In-Silico clinical trial evaluating digital breast tomosynthoesis as replacement for mammography, (2018).
77. Hamidian S, Sahiner B, Petrick N, Pezeshk A. 3D convolutional neural network for automatic detection of lung nodules in chest CT. Proc SPIE Int Soc Opt Eng. (2017) 10134:1013409. doi: 10.1117/12.2255795
78. Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA (2016) 316:2402–10. doi: 10.1001/jama.2016.17216
79. Miller L. Product Innovation Through Computational Prototypes and Supercomputing. Comput Sci Eng. (2017) 19:9–17. doi: 10.1109/MCSE.2017.3301223
Keywords: medical devices, computational modeling, regulatory science, virtual patients, virtual clinical trials, FDA
Citation: Morrison TM, Pathmanathan P, Adwan M and Margerrison E (2018) Advancing Regulatory Science With Computational Modeling for Medical Devices at the FDA's Office of Science and Engineering Laboratories. Front. Med. 5:241. doi: 10.3389/fmed.2018.00241
Received: 30 April 2018; Accepted: 08 August 2018;
Published: 25 September 2018.
Edited by:
Markus Reiterer, Medtronic, United StatesReviewed by:
Marco Viceconti, Insigneo Institute of in silico Medicine, United KingdomCopyright © 2018 Morrison, Pathmanathan, Adwan and Margerrison. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tina M. Morrison, dGluYS5tb3JyaXNvbkBmZGEuaGhzLmdvdg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.