- 1Department of Public Health and Infectious Diseases, Sapienza University of Rome, Rome, Italy
- 2Dani Di Giò Foundation–Onlus, Rome, Italy
- 3Istituto Zooprofilattico Sperimentale del Lazio e della Toscana Mariano Aleandri, Rome, Italy
- 4Vascular and Endovascular Surgery Division, Department of Surgery Paride Stefanini, Policlinico Umberto I, Sapienza University of Rome, Rome, Italy
- 5Laboratory Affiliated to Istituto Pasteur Italia-Fondazione Cenci Bolognetti, Department of Molecular Medicine, Sapienza University of Rome, Rome, Italy
We report a case of Yersinia enterocolitica septicemia in a 63-year-old patient admitted to the Vascular Surgery Department of Umberto I Hospital (Rome, Italy) for an abdominal aortic aneurysm. The microorganism, recovered from both peripheral blood cultures and aneurysmatic aortic wall specimens, was identified as Y. enterocolitica using matrix-assisted laser desorption ionization–time of flight analysis (MALDI-TOF MS) and 16S rDNA gene sequencing. The isolate responsible for septicemia belonged to the O:9 serotype (biogroup 2). A genetic screening of the isolate made it possible to detect the presence of both the yst and ail genes, encoding a heat-stable enterotoxin and a protein involved in invasion/adherence and serum resistance, respectively. Our case contributes in enriching epidemiological data concerning Y. enterocolitica infections, which might represent severe complications in patients suffering from cardiovascular diseases. Moreover, this study, together with the others, should be regarded as valuable and useful tools for monitoring the rate of infections worldwide.
Introduction
Yersinia enterocolitica is a non-spore Gram-negative bacterium belonging to Yersinia genus and to the Enterobacteriaceae family. Among Yersinia species, only Y. pestis, Y. pseudotuberculosis, and some Y. enterocolitica strains are pathogenic for humans, whereas others are environmental species that might be opportunistic pathogens. Y. enterocolitica is a heterogeneous group of bacterial strains, classified into 6 biogroups (1A, 1B, 2, 3, 4, and 5) based on phenotypic characteristics, human/animal pathogenicity, and ecologic and geographic distribution (1). Serologically, Y. enterocolitica is further distinguished into more than 57 O serogroups, depending on their lipopolysaccharide-O-antigens. Only isolates belonging to biogroup 1A may be considered avirulent so far. Among the other 5 biogroups, strains belonging to serogroups O:3 (biogroup 4), O:5,27 (biogroups 2 and 3), O:8 (biogroup 1B), and O:9 (biogroup 2) are most commonly isolated from human samples worldwide (2, 3). However, the most prevalent Y. enterocolitica serogroup in many European countries is serogroup O:3 followed by O:9, whereas the serogroup O:8 is mainly detected in the United States (1, 3). Despite a significant decrease between 2008 and 2016, human Yersiniosis represents the third most commonly reported bacterial food-borne zoonosis in all EU countries (https://ecdc.europa.eu/).
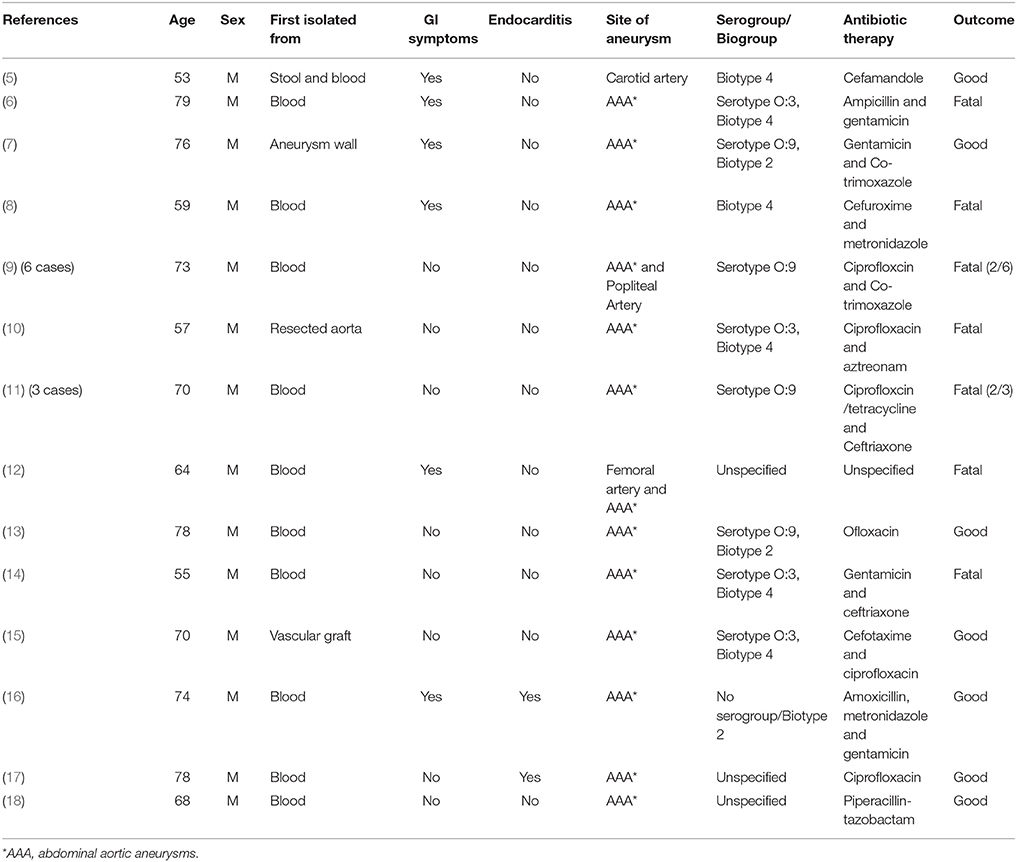
As a foodborne pathogen, Y. enterocolitica causes acute terminal ileitis and mesenteric Iymphadenitis. Depending on both patient conditions and bacterial serogroup, it can spread at the systemic level (3, 4). Several septic complications have been described that can evolve, eventually, into endocarditis or infected aortic aneurysm, also known as mycotic aneurysm (3). At present, only 14 papers associated with Y. enterocolitica have been reported in literature (Table 1), including 13 cases of infected abdominal aortic aneurysms (AAA). The main pathogenic properties of Y. enterocolitica are due to the presence of the two chromosomally encoded genes, ail and yst (1, 19, 20). The ail gene encodes for the membrane-associated protein Ail, involved in adhesion to and invasion into eukaryotic cells, as well as serum resistance. Conversely, the yst gene encodes for thermostable enterotoxin Yst, whose presence is related with diarrheal illness, although its role remains largely controversial (21). Being the most common virulence genes of Y. enterocolitica, both are considered excellent PCR targets for bacterial detection and subtyping as well as for its pathogenicity potential (22).
Herein, we describe a case of Y. enterocolitica septicemia in a patient suffering from an AAA Microbiological and molecular analyses have demonstrated the presence of a strain belonging to the O:9 serotype, carrying both the ail and yst genes and resistant to amoxicillin/clavulanate.
Case Report
Ethics Statement
The subject gave a written informed consent in accordance with the Declaration of Helsinki.
A 63-year-old patient was admitted to the Vascular Surgery Department of Umberto I Hospital (Rome, Italy) for an AAA.
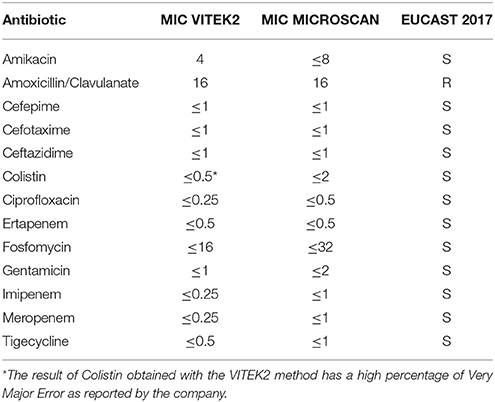
At admission, the patient presented with low-grade fever (37.2°C) and reported a history of asthenia and weight loss in the previous months. No abdominal symptoms, vomiting or diarrhea were referred. In September 2017, the patient performed an abdominal ultrasound and blood tests, including three sets of peripheral blood culture that were sent to the Microbiology laboratory. Blood tests showed an erythrocyte sedimentation rate of 48 mm/h, a white blood cell (WBC) count of 13.94 × 109/L and a C-reactive protein (CRP) level of 185 mg/L. An empirical therapy was therefore started with the intravenous administration of daptomycin (500 mg die), ertapenem (1 g die), and fluconazole (400 mg die). Abdominal ultrasound identified described an abdominal aortic aneurysm with a diameter of 6.42 cm. The computed tomography (CT) scan confirmed the presence of the AAA with a periaortic inflammation (Figure 1). Moreover, transthoracic echocardiogram ruled out active endocarditis. The patient therefore underwent endovascular repair. After 24 h of incubation in the automatic Virtuo BacT/ALERT (bioMérieux, Inc. France), two sets of blood cultures became positive. Microscopic examination revealed motile Gram-negative coccobacilli. Colonies on MacConkey agar were small, round and non-lactose fermenting. Colonies were subcultured onto CIN agar plates (Cefsulodin Irgasan Novobiocin) (bioMérieux, Inc. France), selective for Yersinia spp. After 24 h of incubation at 28°C, the colonies displayed the typical red bull's eye appearance, characterized by a red center with a colorless translucent rim (Figure 2). Biochemical tests of the colonies, using VITEK 2 System (bioMérieux, Inc. France), identified Y. enterocolitica/frederiksenii with 99% probability. Accordingly, MALDI-TOF MS System confirmed the identification of Y. enterocolitica, with a score of 2.265 (23) (Figure 3). Finally, the 16S rDNA gene sequence analysis confirmed the Y. enterocolitica identification. Antibiotic susceptibility tests with VITEK 2 System (bioMérieux, Inc. France) and MICROSCAN WalkAway System 96 Plus (Beckman Coulter S.r.l.) revealed that the Y. enterocolitica isolate was susceptible to all antibiotics tested, except to amoxicillin/clavulanate (Table 2). Serogrouping analysis using specific Y. enterocolitica antisera evidenced that the Y. enterocolitica isolate belonged to the O:9 serotype (biogroup 2). Finally, the presence of both ail and yst genes in the Y. enterocolitica isolate was detected using Real-time PCR.
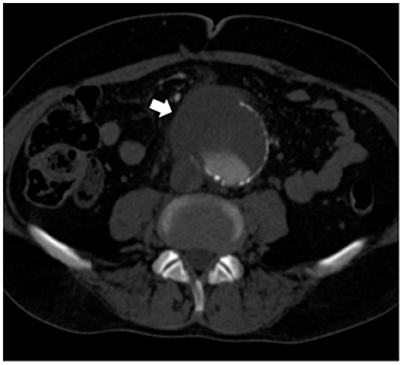
Figure 1. Preoperative CT scan showing an abdominal aortic aneurysm surrounded by inflammation/fluid collection (white arrow).
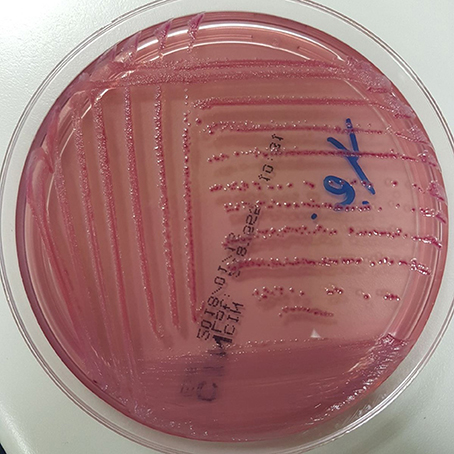
Figure 2. Y. enterocolitica on CIN agar: small colonies with the typical red bull's eye appearance, namely a red center with colorless translucent rim.
Figure 3. Peptide mass fingerprinting of Y. enterocolitica from peripheral blood cultures (A) and from aneurysm specimens (B) by MALDI-TOF MS.
Two weeks after the endovascular procedure, the patient underwent open surgical repair. During the procedure, the surgeon reported a strong foul necrotic smell and noticed tight adhesions between the inflamed small intestine and the aneurysm. Specimens were collected from the aneurysmatic aortic wall and sent to the Microbiology laboratory. These aneurysm specimens were, firstly, incubated in brain heart infusion (BHI) broth and, subsequently, plated on different microbiological media, including CIN agar plates. Again, microbiological, biochemical, molecular, antibiotic susceptibility, and serogrouping analyses identified the clinical isolate as Y. enterocolitica O:9 serotype (biogroup 2). Therefore, we concluded that the same Y. enterocolitica isolate was colonizing the patient. The antibiotic therapy was modified; the patient suspended daptomycin and fluconazole and was treated intravenously with ertapenem (1 g die) and tigecycline (100 mg for first day and 50 mg for following days) for 4 weeks. Over this period, all the sets of blood culture were negative. The patient was discharged home 8 weeks after surgery.
Materials and Methods
Peripheral blood culture bottles, for both aerobic and anaerobic microorganisms, were incubated for 24 h in the automatic Virtuo BacT/ALERT (bioMérieux, Inc. France). Aneurysm specimens were incubated in BHI at 37°C for 24 h. Clinical isolates were examined under light microscope (400x) for motility and upon Gram staining. In parallel, isolates were seeded on Blood agar, MacConkey agar, Extended Spectrum Beta-Lactamase (ESBL) agar (bioMérieux, Inc. France), and CIN agar plates and incubated at 28°C for 24 h. Colonies were identified by VITEK 2 System (bioMérieux, Inc. France), by MALDI-TOF MS System (Bruker Daltonics, Inc.) and by 16S rDNA gene sequencing, as previously described respectively in Febbraro et al. (24) and in Yu et al. (25). Antimicrobial susceptibility testing was performed by VITEK 2 System (bioMérieux, Inc. France) and by MICROSCAN WalkAway System 96 Plus (Beckman Coulter S.r.l.). The resulting minimum inhibitory concentration (MIC) values were classified into clinical categories of susceptible, intermediate or resistant, following the EUCAST recommendations (26). Serogrouping analysis was performed by slide agglutination using O:3, O:5, O:8, and O:9 antisera (Biogenetics, s.r.l. Italy) following the recommendation of the manufacturer. Real-time PCR was performed as previously described (21). Both analyses were carried out at the Experimental Zooprophylactic Institute of Lazio and Tuscany “Mariano Aleandri” (Rome, Italy).
Discussion
There has been an increasing body of evidence showing that Y. enterocolitica serogroups O:3 and O:9 are a cause of mycotic aneurysm and septicaemia, mainly in males over 50 years, with risk factors for cardiovascular disease (10, 14). Herein, we report a case of Y. enterocolitica serogroup O:9 (biogroup 2) septicemia in a patient with an AAA. It is important to note that this is the first study reporting the identification of Y. enterocolitica both in the peripheral blood and in the specimens collected from the aneurysmatic aortic wall.
It has been reported that, following ingestion, virulent Y. enterocolitica crosses the intestinal mucus layer and binds to the M cells overlying Peyer's Patches. Thereafter, bacteria penetrate these cells to gain access to and multiply in subjacent tissue. Among others, attachment and invasion of M cells is promoted by the membrane-associated Ail protein, encoded by the chromosomal invasion ail gene, thereby facilitating the spread to several tissues, as well as bloodstream (1). Also in this environment, Ail provides strong protection to complement killing bactericidal activity, thereby allowing survival of bacteria in the blood and increasing its spreading (1). Due to Y. enterocolitica predilection for vascular tissues, it is highly likable that bacteria colonize the aneurysmatic aorta during bacteremia. On the other hand, the thermostable enterotoxin Yst, encoded by the yst gene, is mainly related with diarrheal illness (21). Although its role remains largely controversial, it has been reported in an animal model that Yst can cause weight loss without the occurrence of diarrhea, possibly depending on the degree of host immune competency.
Our clinical and microbiological data support the hypothesis that Y. enterocolitica firstly colonized the patient's intestine. Accordingly, the patient referred a weight loss in the previous months. Thereafter, the inflammatory process of the small intestine, in tightly conjunction to the aneurysm, allowed Y. enterocolitica translocation into the aneurysm, thereby shortening the period of bacteraemia. Y. enterocolitica septicemia occurs commonly in patients suffering from underlying disease(s) (1). Although our patient did not seem to have predisposing factors, the high degree of inflammation of the small intestine might have even favored Y. enterocolitica translocation into the aneurysm.
Concluding Remarks
Our case contributes in enriching epidemiological data concerning Y. enterocolitica infections, which might represent severe complications in patients suffering from cardiovascular diseases. The most common site of infections in these patients is the aortic aneurysm, causing the mycotic aneurysm. Therefore, colonization of the aneurysmatic aorta during bacteraemia can lead to vessel rupture due to inflammation and necrosis, but also to endocarditis, due to the contiguity of the infection site (1, 18). Finally, together with the previous reports, our case underlines the importance of the microbiological surveillance of Y. enterocolitica. Regretfully, no routine screening test for detecting Y. enterocolitica for cardiovascular disease patients is available at the moment. No reliable data of monitoring circulating Yersinia spp. on food and animals are available, since it is not mandatory for Zooprophylactic Institutes to report data (27). Therefore, all case reports on Y. enterocolitica involved in cardiovascular diseases should be regarded as valuable and useful tools to monitor the rate of infections worldwide.
Author Contributions
DR, AB, CA, MT, and VP contributed equally to this work. DR, AB, MT, and VP collected clinical data and guided the testing and experiments. DS and CA guided the 16S rDNA gene analyses for bacterial identification. RT conducted the serogroup analysis using specific antisera and Real-time PCR to detect virulence genes. WM and FS collected clinical samples and provided clinical data. All of authors discussed the results and implications. DR, AB, MT, DS, CA, and VP wrote the manuscript. All the authors revised and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MF declared a shared affiliation, though no other collaboration, with several of the authors DR, AB, CA, DS, MT, and VP to the handling Editor.
Acknowledgments
This work was supported by the Ricerca Scientifica Sapienza to GA.
References
1. Bottone EJ. Yersinia enterocolitica: overview and epidemiologic correlates. Microbes Infect. (1999) 1:323–33. doi: 10.1016/S1286-4579(99)80028-8
2. Asplund K, Johansson T, Siitonen A. Evaluation of pulsed-field gel electrophoresis of genomic restriction fragments in the discrimination of Yersinia enterocolitica O:3. Epidemiol Infect. (1998) 121:579–86. doi: 10.1017/S0950268898001629
3. Fàbrega A, Vila J. Yersinia enterocolitica: pathogenesis, virulence and antimicrobial resistance. Enferm Infect Microbiol Clin. (2012) 30:24–32. doi: 10.1016/j.eimc.2011.07.017
4. Fredriksson-Ahomaa M, Stolle A, Korkeala H. Molecular epidemiology of Yersinia enterocolitica infections. FEMS Immunol Med Microbiol. (2006) 47:315–29. doi: 10.1111/j.1574-695X.2006.00095.x
5. Plotkin GR, O'Rourke JN, Jr. Mycotic aneurysm due to Yersinia enterocolitica. Am J Med Sci. (1981) 281:35–42. doi: 10.1097/00000441-198101000-00006
6. Verhaegen J, Dedeyne G Vansteenbergen W, Vandepitte J. Rupture of vascular prosthesis in a patient with Yersinia enterocolitica bacteremia. Diagn Microbiol Infect Dis. (1985) 3:451–4.
7. Van Noyen R, Peeters P, Van Dessel F, Vandepitte J. Mycotic aneurysm of the aorta due to Yersinia enterocolitica. Contrib Microbiol Immunol. (1987) 9:122–6.
8. Knudsen JD, Arpi M, Jensen KT. A fatal case of Yersinia enterocolitica septicemia associated with mycotic aortic aneurysm. Infection (1993) 21:338–9.
9. Prentice MB, Fortineau N, Lambert T, Voinnesson A, Cope D. Yersinia entercolitica and mycotic aneurysm. Lancet (1993) 341:1535–6. doi: 10.1016/0140-6736(93)90672-4
10. Hagensee ME. Mycotic aortic aneurysm due to Yersinia enterocolitica. Clin Infect Dis. (1994) 19:801–2. doi: 10.1093/clinids/19.4.801
11. Mercie P, Morlat P, N'Gako A, Pheline P, Bezian MC, Gorin G, et al. Aortic aneurysms due to Yersinia enterocolitica: three new cases and a review of the literature. J Mal Vasc. (1996) 21:68–71.
12. Donald K, Woodson J, Hudson H, Menzoian JO. Multiple mycotic pseudoaneurysms due to Yersinia entercolitica: report of a case and review of the literature. Ann Vasc Surg. (1996) 10:573–7. doi: 10.1007/BF02000446
13. La Scola B, Musso D, Carta A, Piquet P, Casalta JP. Aortoabdominal aneurysm infected by Yersinia enterocolitica serotype O:9. J Infect. (1997) 35:314–5. doi: 10.1016/S0163-4453(97)93536-2
14. Tame S, Wit D, Meek A. Yersinia enterocolitica and mycotic aneurysm. Aust N Z J Surg. (1998) 68:813–4. doi: 10.1111/j.1445-2197.1998.tb04689.x
15. Abad C, Ponce G, Elcuaz R. Early vascular graft infection due to Yersinia enterocolitica after repair of an abdominal aortic aneurysm. J Cardiovasc Surg. 45:161–2.
16. Kumar P, Zhang J, Miller S, Wilton G. Yersinia enterocolitica: a rare cause of infected aortic aneurysm successfully treated with antibiotics and endovascular repair. JMM Case Rep. (2014). doi: 10.1099/jmmcr.0.002626
17. Mason JC, Lal P, Torella F, Sharma A, Cooke R, Anson J. Yersinia entercolitica: a rare cause of infective endocarditis and mycotic aneurysm. Clin Microbiol Newsl. (2014) 36:2. doi: 10.1016/j.clinmicnews.2014.01.002
18. Kumar HR, Eskandari MK. The benefit of early repair for a mycotic aortic aneurysm due to Yersinia enterocolitica infection. J Vasc Surg Cases (2015) 1:61–4. doi: 10.1016/j.jvsc.2014.12.001
19. Miller VL, Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. (1988) 56:1242–8.
20. Pierson DE, Falkow S. The ail gene of Yersinia enterocolitica has a role in the ability of the organism to survive serum killing. Infect Immun. (1993) 61:1846–52.
21. Peruzy MF, Murru N, Perugini AG, Capuano F, Delibato E, Mercogliano R, et al. Evaluation of virulence genes in Yersinia enterocolitica strains using SYBR Green real-time PCR. Food Microbiol. (2017) 65:231–5. doi: 10.1016/j.fm.2017.03.004
22. Petsios S, Fredriksson-Ahomaa M, Sakkas H, Papadopoulou C. Conventional and molecular methods used in the detection and subtyping of Yersinia enterocolitica in food. Int J Food Microbiol. (2016) 237:55–72. doi: 10.1016/j.ijfoodmicro.2016.08.015
23. Stephan R, Cernela N, Ziegler D, Pflüger V, Tonolla M, Ravasi D, et al. Rapid species specific identification and subtyping of Yersinia enterocolitica by MALDI-TOF mass spectrometry. J Microbiol Methods (2011) 87:150–3. doi: 10.1016/j.mimet.2011.08.016
24. Febbraro F, Rodio DM, Puggioni G, Antonelli G, Pietropaolo V, Trancassini M. MALDI-TOF MS versus VITEK®2: comparison of systems for the identification of microorganisms responsible for bacteremia. Curr Microbiol. (2016) 73:843–50. doi: 10.1007/s00284-016-1121-x
25. Yu J, Zhou XF, Yang SJ, Liu WH, Hu XF. Design and application of specific 16S rDNA-targeted primers for assessing endophytic diversity in Dendrobium officinale using nested PCR-DGGE. Appl Microbiol Biotechnol. (2013) 97:9825–36. doi: 10.1007/s00253-013-5294-y
26. EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. (2017). Available online at: http://www.eucast.org
Keywords: Yersinia enterocolitica, septicemia, aortic aneurysm, serogroup O:9, virulence factors
Citation: Rodio DM, Bressan A, Ambrosi C, Scribano D, Tolli R, Mansour W, Speziale F, Antonelli G, Trancassini M and Pietropaolo V (2018) Yersinia enterocolitica in Italy: A Case of Septicemia and Abdominal Aortic Aneurysm Infection. Front. Med. 5:156. doi: 10.3389/fmed.2018.00156
Received: 14 February 2018; Accepted: 04 May 2018;
Published: 24 May 2018.
Edited by:
Nicola Petrosillo, Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani (IRCCS), ItalyReviewed by:
Marco Falcone, Sapienza Università di Roma, ItalyGiovanni Di Bonaventura, Università degli Studi G. d'Annunzio Chieti e Pescara, Italy
Copyright © 2018 Rodio, Bressan, Ambrosi, Scribano, Tolli, Mansour, Speziale, Antonelli, Trancassini and Pietropaolo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Pietropaolo, dmFsZXJpYS5waWV0cm9wYW9sb0B1bmlyb21hMS5pdA==
†These authors have contributed equally to this work.
 Donatella M. Rodio1†
Donatella M. Rodio1† Alessia Bressan
Alessia Bressan Cecilia Ambrosi
Cecilia Ambrosi Daniela Scribano
Daniela Scribano Rita Tolli
Rita Tolli Maria Trancassini
Maria Trancassini Valeria Pietropaolo
Valeria Pietropaolo