- 1Division of Haematology and Central Haematology Laboratory, CHUV, Lausanne University Hospital, University of Lausanne, Lausanne, Switzerland
- 2Faculté de Biologie et Médecine, UNIL, University of Lausanne, Lausanne, Switzerland
Massive hemorrhage is a leading cause of death worldwide. During the last decade several retrospective and some prospective clinical studies have suggested a beneficial effect of early plasma-based resuscitation on survival in trauma patients. The underlying mechanisms are unknown but appear to involve the ability of plasma to preserve the endothelial glycocalyx. In this mini-review, we summarize current knowledge on glycocalyx structure and function, and present data describing the impact of hemorrhagic shock and resuscitation fluids on glycocalyx. Animal studies show that hemorrhagic shock leads to glycocalyx shedding, endothelial inflammatory changes, and vascular hyper-permeability. In these animal models, plasma administration preserves glycocalyx integrity and functions better than resuscitation with crystalloids or colloids. In addition, we briefly present data on the possible plasma components responsible for these effects. The endothelial glycocalyx is increasingly recognized as a critical component for the physiological vasculo-endothelial function, which is destroyed in hemorrhagic shock. Interventions for preserving an intact glycocalyx shall improve survival of trauma patients.
The aim of this mini-review is to give an overview on plasma treatment in massive bleeding. We will briefly describe current pathophysiological concepts of vascular damage in hemorrhagic shock, summarize data on the use of plasma as a resuscitation fluid, and report experimental data suggesting a protective role of plasma on endothelial integrity.
Trauma, Massive Hemorrhage, and Trauma-Induced Coagulopathy (TIC)
Epidemiology and Definition of Massive Hemorrhage
The World Health Organization estimates that in the year 2000, 5 million people died of injuries, accounting for 9% of global annual mortality (1). After central nervous injury, massive hemorrhage represents the second-leading cause of death, being responsible for 30–40% of trauma-related mortality (1). Death can occur within 3–6 h by exsanguination from uncontrolled hemorrhage and one-third to half of the deaths occur before reaching the hospital (1, 2). Modern transfusion practices and blood supply make massive hemorrhage a potentially preventable cause of death in different settings (e.g., civilian or military trauma, surgery, post-partum). The benefit of blood component transfusion in the context of trauma has been discussed for many years but it is only since the retrospective study of Borgmann published in 2007 (3) that plasma transfusion has been recognized as a probable positive factor for survival. However, “survival bias” remains an unsolved pitfall of retrospective studies, not only for interpreting potentially causative factors related to survival (i.e., did the patient “survive because she received plasma transfusion” or did she “get plasma transfusion because survived long enough to receive it”?) but also for defining massive hemorrhage. In fact, the classical definition of massive hemorrhage is based on the number of packed red blood cells (PRBC) units transfused during the first 24 h after admission. High mortality rates during the first 24 h and rapid time course of massive hemorrhage make transfusion rate (e.g., ≥3 PRBC units/60 min) a more appropriate definition (4). In addition, data analysis from the PROMMT study enabled Rahbar et al. to identify those patients most likely to develop massive hemorrhage based on emergency admission variables, such as systolic blood pressure, heart rate, pH, and hemoglobin (5). This prospective observational study showed that transfusion with higher ratio of plasma to PRBC early in resuscitation is associated with an improved survival at 24 h (6). Specifically, adult trauma patients surviving beyond 30 min from admission and transfused with ≥1 unit of PRBC in the first 6 h and ≥3 units of PRBC during the first 24 h showed a significantly higher survival at 6 and 24 h, and 30 days when receiving plasma units and PRBC at a ratio of at least 1:1 (6, 7). Of note, such high plasma to PRBC ratios beyond the first 24 h was not associated with survival by day 30.
Pathophysiological Concepts
The so-called acute trauma coagulopathy (ATC) (8) and TIC (9) have been conceptualized through different models, all converging to the key concept of «endothelial stress» (10–20), also named «endotheliopathy of trauma» (21, 22) or «shock-induced endotheliopathy» (23). The endothelium covering an area of about 5,000 m2 is one of the frailest and initial victims of massive hemorrhage (24). For instance, severe hypo-perfusion is associated with increased levels of circulating heparan sulfate, a component of the endothelial surface with anticoagulatory properties similar to heparin (25). Moreover, the co-existence of severe tissue injury, leading to high in vivo thrombin generation, and severe hypo-perfusion, leading to endothelial sufferance and thrombomodulin shedding, is complicated by circulating thrombin–thrombomodulin complexes culminating in systemic protein C activation and fibrinolysis (8, 9).
Several factors drive the system into a vicious circle: (1) on the one side, endothelial injury with enhanced vascular permeability leads to further loss of intravascular volume, hypovolemia, tissue hypoxia, and exacerbated shock and (2) on the other side, resuscitation-related blood dilution with acidosis and hypothermia (the classical iatrogenic triad) further impair vasculo-endothelial functions. In sum, massive hemorrhage means perfusion, oxygenation, coagulation, and metabolic failures.
Plasma as a Resuscitation Fluid
Plasma Type, Delivery, and Supply
Plasma sources and plasma processing have been developed during these last decades (18). Each preparation addresses and mitigates particular risks related to transfusion hazards: single donor fresh frozen plasma (FFP) vs pooled plasma or quarantine FFP vs pathogen-inactivated FFP to diminish the risk of transfusion-transmitted infections; FP24 (frozen within 24 h after donation) instead of standard FFP (frozen within 8 h after donation) to enable HLA testing and remove high risk units for TRALI; frozen plasma vs liquid or thawed plasma to extend storage duration; lyophilization formulas for rapid reconstitution. Study of the variability of coagulation factors and natural anticoagulants levels in different plasma preparations are summarized elsewhere (26). Of note, the factor V and factor VIII, known to be «labile» and critical in the evaluation of manufacture practice, show heterogeneous decrease during storage, depending on formulas. Several studies reveal how processing conditions (whole blood hold-time, storage duration/temperature before freezing, freezing mode, leucodepletion, pathogen inactivation, lyophilization) specifically influence coagulation factor levels, microparticles content, clot generation capacity and protein composition in plasma (27–30) and clotting factor stability after thawing (31). In massive hemorrhage management, logistical concerns, besides biological aspects such as type of plasma, FFP to PRBC ratio or functional monitoring of clot generation, matters as well. Time between trauma and transfusion, transport of plasma from blood bank to the clinical unit, mode of checking plasma unit before transfusion and provision of thawed/liquid plasma are most critical aspects in massive transfusion protocols (32–34).
Hence, one logistical challenge for blood bankers is plasma supply. Benefit from plasma transfusion in massive hemorrhage lead to growing use of «universal» but scarce AB plasma (35). At the same time, implementation of the «male policy» (plasma donation from male donors only) to improve the transfusion safety regarding risk of TRALI significantly restricts plasma availability. This demand/supply imbalance led the American Red Cross to consider the use of group A plasma to adult trauma patients (36). Novak et al. reported the experience of 12 trauma centers participating in the PROPPR study in managing plasma inventory to meet new guidelines issued by the American College of Surgeons in 2013 for trauma resuscitation (37). Rapid delivery is made possible by the selection of group A plasma with low titer anti-B, in addition to plasma formulations and thawing systems with short turnaround time.
Benefit of Plasma Transfusion: Do Coagulation Factors Tell the Whole Story?
Since the time Borgman et al. demonstrated in 2007 that FFP transfusion in massive hemorrhage resulted in increased survival (3), researcher started to wonder which mechanisms may be responsible for this effect. The first hypothesis at hand would have been the correction of coagulopthy. However, plasma transfusion in the form of FFP cannot replace coagulation factor loss (38). Therefore, several publications aimed to investigate the benefit of plasma resuscitation on other pathophysiological variables, such as endothelial restoration (39–45).
Endothelial Glycocalyx
The Endothelial Glycocalyx Structure and Function
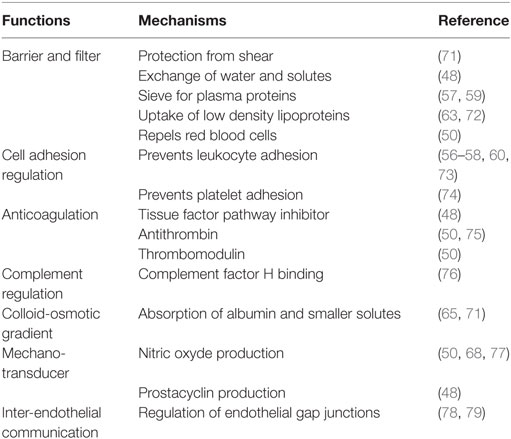
The endothelial glycocalyx is a thick (about 0.2–3.0 µm in vivo) (46, 47), negatively charged carbohydrate-rich layer coating the vascular endothelium (48–52). The glycocalyx sensu stricto is formed by cell membrane-bound sulfated proteoglycans, consisting of a core protein (e.g., transmembrane syndecan, membrane-bound glypican, or basement matrix-associated perlecan) with glycosaminoglycans side chains (e.g., heparan sulfate, hyaluronic acid, and chondroitin sulfate) (53), and cell membrane glycoproteins bearing sialoproteins (50, 53). Syndecan-1 (CD 138), a heparan sulfate containing proteoglycan, is one of the major constituents ensuring endothelial integrity (51). Under physiological conditions, positively charged soluble components (such as plasma proteins, enzymes, growth factors, cytokines, amino acids, and cations) and water are trapped in the glycocalyx forming an extended endothelial surface layer. The mesh formed by the gylcocalyx contains ~1 to 1.5 l plasma, which are in dynamic equilibrium with the flowing blood (48, 54, 55).
The glycocalyx has several recognized functions (Table 1) (49–52). In particular, it forms a physical barrier between blood and vessel wall (48, 56–60); it maintains blood fluidity by modulating the interactions of the endothelium with blood cells and proteins (50, 61–63); it regulates cell adhesion and vascular permeability (64); it creates a high intravascular colloid-osmotic gradient (65, 66); and it acts as a mechano-transducer, e.g., by sensing shear stress and inducing endothelial release of nitric oxide (60, 63, 67, 68). As it may be expected from its many functions, the disruption of the glycocalyx leads to several clinically relevant pathologies (48, 52, 61). In the following paragraph, we will discuss the effect of hemorrhagic shock and type of resuscitation fluid on the glycocalyx.
Hemorrhagic Shock, Endothelial Glycocalyx, and Resuscitation Fluid
Shedding of endothelial glycocalyx components has been shown to occur in response to, e.g., ischemia and hypoxia (80), reactive oxygen species (81), inflammation and sepsis (82), and trauma-related sympatho-adrenal activation (83). As recently reviewed by Becker et al., loss of glycocalyx appears to be mediated by “sheddases,” such as matrix metalloproteases, heparanases, hyaluronidases, and proteases (60) and to be responsible for endothelial inflammatory changes and vascular hyperpermeability (51, 64). In hemorrhagic shock, loss of the endothelial glycocalyx correlates with a dismal outcome. For instance, human studies indicate that in trauma patients with severe bleeding, high levels of syndecan-1 on admission (≥40 ng/ml) correlate with the extent of tissue damage, laboratory indicators of ATC and, in particular, mortality (84–86). Rahbar et al. showed that high circulating syndecan-1 levels correlate with increased vascular permeability (87). Since plasma-based resuscitation appears to exert a beneficial effect on survival (6, 7, 88), the question is whether plasma as a resuscitation fluid may have an impact on the endothelial glycocalyx and, therefore, potentially on vascular integrity and function.
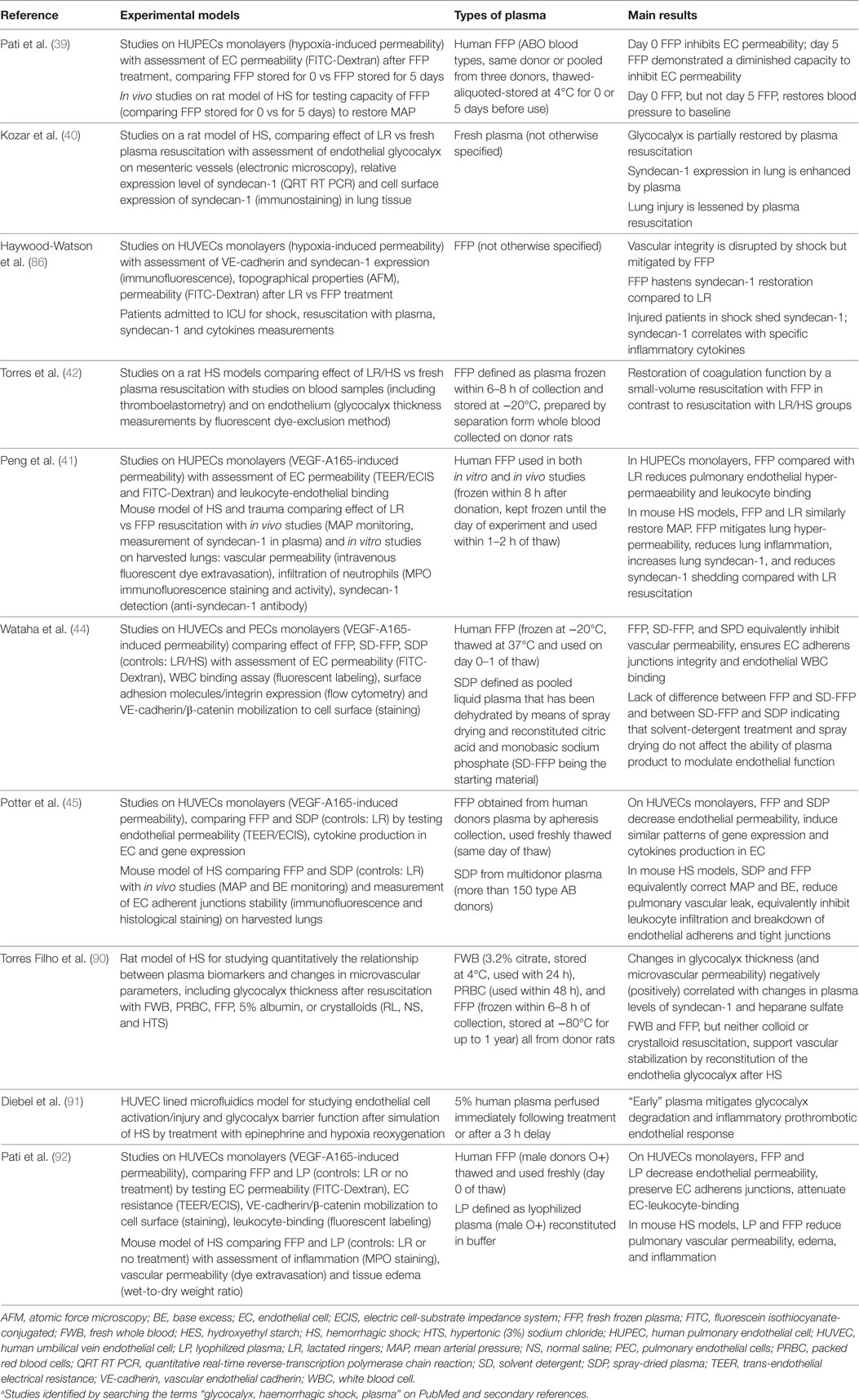
Investigations in animal models may help framing a working concept (Table 2). Kozar et al. (40) employed a pressure-controlled model of hemorrhagic shock. Rats were bled to a mean arterial pressure of 30 mmHg for 90 min then resuscitated with either lactated Ringer’s solution (LR) or fresh plasma to a mean arterial pressure of 80 mmHg. These animals were compared to shams (all procedures without bleeding) and positive controls (hemorrhagic shock without resuscitation). The authors found that (1) hemorrhagic shock is associated with a significant shedding of the endothelial glycocalyx, as indicated by circulating syndecan-1 levels, cell surface expression of syndecan-1, and electron microscopy imaging; (2) loss of the endothelial syndecan-1 correlates with the extent of lung injury, as assessed by alveolar wall thickness, capillary congestion, and cellularity; (3) resuscitation with plasma partially restores the endothelial glycocalyx while LR cannot, as assessed by electron microscopy on post-capillary venules obtained from the small bowel mesentery and by syndecan-1 expression in the lung; (4) the endothelial glycocalyx appears to be restored within 3 h after plasma resuscitation; (5) a clinically potential beneficial effect of plasma is suggested by the observations that plasma resuscitation required significantly less volume to maintain the mean arterial pressure at 80 mmHg compared to LR, and by the fact that plasma reduced lung injury while LR resuscitation increased it (40). These observations were expanded by the work of Torres et al. (42). In their model, a 40% blood volume hemorrhage was induced in rats. After 30 min of shock, animals were resuscitated with LR, hydroxyethyl starch (HES) or FFP, and compared to sham and hemorrhage without resuscitation. First, the authors confirmed that the endothelial glycocalyx is significantly damaged by the hemorrhagic shock and can be restored only with FFP, as assessed by circulating syndecan-1 levels and glycocalyx thickness. Second, a clinically beneficial effect of plasma-based resuscitation was indicated by the fact that FFP corrected metabolic acidosis significantly better than LR and HES, as assessed by pH, base excess, and lactate. This was associated with an improved microcirculation and a lesser degree of hemodilution by FFP compared to LR and HES (42). This latter point was also observed by a recent publication of Nelson et al. (89), who demonstrated that resuscitation with FFP resulted in a circulating volume expansion equaling the volume of blood loss, while circulating volume expansion by Ringer’s acetate was less effective.
The pulmonary effects of hemorrhagic shock and resuscitation fluids were addressed by Peng et al. (41). They investigated pulmonary endothelial inflammation and hyper-permeability employing a coagulopathic mouse model of hemorrhagic shock and trauma. Mice were bled to a mean arterial pressure of 35 ± 5 mmHg for 90 min (93) and subsequently resuscitated over 15 min with either LR (at 3× shed blood volume) or FFP (at 1× shed blood volume). Resuscitated animals were compared to shams (all procedures without shock) and positive controls (hemorrhagic shock without resuscitation). Major findings were as follows: (1) lung permeability, assessed in vivo by the extravasation of a fluorescent dextrane or Evan’s blue, was significantly increased after hemorrhagic shock compared to shams, and FFP resuscitation was significantly more effective than LR in preventing/correcting shock-induced pulmonary hyper-permeability; (2) similarly, lung inflammation, assessed by detecting myeloperoxidase which reflects neutrophils infiltration, significantly increased after hemorrhagic shock and was lessened by FFP resuscitation; (3) shock-induced loss of pulmonary syndecan-1 was most efficiently prevented by resuscitation with FFP. Of note, similar results on pulmonary inflammation and permeability were reported by Potter et al. employing FFP and spray-dried plasma (SDP) (45).
A recent publication by Torres Filho et al. (90) employing a rat model of hemorrhagic shock showed that (1) syndecan-1 and heparan sulfate represent valuable biomarkers of glycocalyx shedding and (2) fresh whole blood and FFP support vascular stabilization by reconstitution of the endothelial glycocalyx (see Table 2).
Syndecan-1 as a Key Mediator of Plasma’s Effect
A key question is which plasma component may exert a beneficial effect on the glycocalyx. In vitro experiments have shown that FFP enhances pulmonary endothelial syndecan-1 expression in a time- and dose-dependent manner (94). A key role for syndecan-1 is supported by in vivo experiments as well. Utilizing the model of trauma-hemorragic shock described by Peng (41), Wu et al. investigated the pulmonary response to the type of resuscitation fluid (FFP vs LR) in wild-type and Syndecan gene knock-out (Sdc1−/−) mice (94). They found that the inability to synthesize syndecan-1 abrogated the protective effect observed with plasma. In particular, they demonstrated that in absence of syndecan-1 synthesis: (1) the ability of FFP to mitigate the increase in lung permeability induced by hemorrhagic shock was abrogated; (2) FFP lost its ability to dampen the shock-induced increase of pulmonary neutrophil infiltration; and (3) FFP lost its protective effect on histopathologic signs of lung injury. Similar results have been reported by Ban et al. with an animal model of gut injury and inflammation after hemorrhagic shock (95).
Plasma: Coagulation Factors or Other Components?
Intriguingly, a major plasma component that may play a role in preserving endothelial integrity appears to be albumin. While loss of circulating albumin correlated with loss of the glycocalyx and increased fluid extravasation (96), albumin supplementation attenuated glycocalyx shedding and reduced interstitial edema in a guinea pig heart model of cold ischemia (97). Kheirabadi et al. (98) studied the role of albumin in a model of uncontrolled hemorrhage. Rabbits were subjected to a splenic injury. Ten minutes after injury, at a mean arterial pressure less than 40 mmHg, the rabbits received equal volumes (15 ml/kg) of rabbit plasma, HES, or 5% human albumin, targeting a mean arterial pressure of 65 mmHg. The authors observed that: (1) onset of resuscitation initiated additional bleeding and total blood loss did not differ among the three groups; (2) thromboelastography revealed a faster and stronger clot formation in the plasma and albumin groups compared to HES; (3) shock indices were increased in all three groups but less in the albumin one; (4) the albumin group had the highest survival rate (8 out of 9 rabbits) compared to plasma and HES (both 4/10), and positive controls (1/9). This apparent beneficial role of albumin, if confirmed in further studies, may be related to its ability to attenuate neutrophil adhesion to the endothelium and other anti-inflammatory properties, its scavenging and buffering capacity, its potential to enhance nitric oxide production and stabilize glycocalyx (50, 60, 99). However, a recent publication showed that a four-factor prothrombin complex concentrate (containing vitamin K-dependent coagulation factors and several other plasma proteins) and FFP but not albumin inhibit vascular permeability in an in vivo mice model (100). Thus far, it is not known which soluble factor present in the factor concentrate might be responsible for its beneficial effect (100).
As of coagulation factors, despite a current of thought supporting the use of fibrinogen in massive bleeding, we are not aware of publications investigating its impact on glycocalyx and endothelial functions. A recent work observed a U-shaped association between initial fibrinogen concentration in major bleeding and in-hospital mortality, with similar rates of increased mortality for fibrinogen levels <1 g/l and >4 g/l (101). A possible explanation for the negative effect of higher fibrinogen levels is offered by in vitro data, suggesting that fibrin promotes endothelial transmigration of neutrophils and inflammation (102).
As of other plasma proteins, adiponectin is an interesting candidate (103). Adiponectin is produced in adipocytes and has been shown to have anti-inflammatory properties and to prevent cytokine-induced endothelial cell hyper-permeability (104–106). Employing a mouse model, Deng et al. demonstrated that (1) hemorrhagic shock leads to a significant decrease of adiponectin levels and a disruption of the lung vascular barrier function; (2) plasma resuscitation improves adiponectin levels and reverses lung injury; (3) the beneficial effect of plasma-based resuscitation is abolished by immunodepletion of adiponectin; and (4) it is restored when plasma was replenished with adiponectin (103). These findings suggest that adiponectin may be an important component contributing to a vasoprotective effect of plasma-based resuscitation.
In sum, several animal studies suggest that early use of plasma in hemorrhagic shock may exert a clinically significant beneficial effect by preserving or even restoring the glycocalyx layer and, therefore, maintaining critical endothelial functions. This appears to be due to the ability of a plasma component to lessen endothelial inflammatory response, possibly by limiting neutrophil adhesion. As of today, it is not known which plasma components are responsible for these effects, which impact plasma processing may exert on them, and which might be the dose–effect relationship.
Human Studies
From a clinical point of view, the key question is whether early resuscitation of hemorrhagic shock with plasma is truly able to improve vasculo-endothelial function and survival. As a proof of principle, a small study in non-bleeding critically ill patients demonstrated that plasma transfusion decreased syndecan-1 and factor VIII levels, suggesting an endothelial stabilizing effect (107). To our knowledge, the only human study prospectively investigating the effect of early plasma-based resuscitation in humans is the COMBAT study (108). In this prospective randomized trial, casualties are treated with 2 units of FFP (thawed in the ambulance) vs conventional crystalloids as initial pre-hospital resuscitation. The study aims to verify whether a “plasma first” resuscitation strategy might be able to (1) attenuate acute traumatic coagulopathy; (2) improve metabolic recovery; (3) decrease blood component transfusion; (4) reduce the incidence of acute lung injury and multiple organ failure; (5) decrease mortality at 24 h or 28 days. According to www.clinicaltrials.gov, the study has been closed after having enrolled 144 patients as per protocol. Results are eagerly awaited.
In conclusion, plasma as early resuscitation fluid for massive hemorrhage appears to exert beneficial effects improving patient survival. Experimental data suggest that this may be related to its ability to preserve endothelial glycocalyx structure and function. We think that these fascinating data shall be confirmed in prospective randomized clinical trials and the mechanisms underlying these effects shall be revealed in order to develop more targeted treatments.
Author Contributions
Both authors discussed the literature, wrote the manuscript, and approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma (2006) 60(6 Suppl):S3–11. doi:10.1097/01.ta.0000199961.02677.19
2. Seghatchian J, Samama MM. Massive transfusion: an overview of the main characteristics and potential risks associated with substances used for correction of a coagulopathy. Transfus Apher Sci (2012) 47(2):235–43. doi:10.1016/j.transci.2012.06.001
3. Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma (2007) 63(4):805–13. doi:10.1097/TA.0b013e3181271ba3
4. Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. J Trauma Acute Care Surg (2013) 74(2):396–400. doi:10.1097/TA.0b013e31827a3639
5. Rahbar MH, del Junco DJ, Huang H, Ning J, Fox EE, Zhang X, et al. A latent class model for defining severe hemorrhage: experience from the PROMMTT study. J Trauma Acute Care Surg (2013) 75(1 Suppl 1):S82–8. doi:10.1097/TA.0b013e31828fa3d3
6. Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg (2013) 148(2):127–36. doi:10.1001/2013.jamasurg.387
7. del Junco DJ, Holcomb JB, Fox EE, Brasel KJ, Phelan HA, Bulger EM, et al. Resuscitate early with plasma and platelets or balance blood products gradually: findings from the PROMMTT study. J Trauma Acute Care Surg (2013) 75(1 Suppl 1):S24–30. doi:10.1097/TA.0b013e31828fa3b9
8. Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care (2007) 13(6):680–5. doi:10.1097/MCC.0b013e3282f1e78f
9. Giordano S, Spiezia L, Campello E, Simioni P. The current understanding of trauma-induced coagulopathy (TIC): a focused review on pathophysiology. Intern Emerg Med (2017) 12(7):981–91. doi:10.1007/s11739-017-1674-0
10. Maegele M, Spinella PC, Schochl H. The acute coagulopathy of trauma: mechanisms and tools for risk stratification. Shock (2012) 38(5):450–8. doi:10.1097/SHK.0b013e31826dbd23
11. Hess JR, Lawson JH. The coagulopathy of trauma versus disseminated intravascular coagulation. J Trauma (2006) 60(6 Suppl):S12–9. doi:10.1097/01.ta.0000199545.06536.22
12. Cap A, Hunt BJ. The pathogenesis of traumatic coagulopathy. Anaesthesia (2015) 70(Suppl 1):e32–4. doi:10.1111/anae.12914
13. Bjerkvig CK, Strandenes G, Eliassen HS, Spinella PC, Fosse TK, Cap AP, et al. “Blood failure” time to view blood as an organ: how oxygen debt contributes to blood failure and its implications for remote damage control resuscitation. Transfusion (2016) 56(Suppl 2):S182–9. doi:10.1111/trf.13500
14. Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood (2016) 128(8):1043–9. doi:10.1182/blood-2016-01-636423
15. Gando S, Hayakawa M. Pathophysiology of trauma-induced coagulopathy and management of critical bleeding requiring massive transfusion. Semin Thromb Hemost (2016) 42(2):155–65. doi:10.1055/s-0035-1564831
16. Poole D, Cortegiani A, Chieregato A, Russo E, Pellegrini C, De Blasio E, et al. Blood component therapy and coagulopathy in trauma: a systematic review of the literature from the trauma update group. PLoS One (2016) 11(10):e0164090. doi:10.1371/journal.pone.0164090
17. Stensballe J, Ostrowski SR, Johansson PI. Haemostatic resuscitation in trauma: the next generation. Curr Opin Crit Care (2016) 22(6):591–7. doi:10.1097/MCC.0000000000000359
18. Watson JJ, Pati S, Schreiber MA. Plasma transfusion: history, current realities, and novel improvements. Shock (2016) 46(5):468–79. doi:10.1097/SHK.0000000000000663
19. Wong H, Curry N, Stanworth SJ. Blood products and procoagulants in traumatic bleeding: use and evidence. Curr Opin Crit Care (2016) 22(6):598–606. doi:10.1097/MCC.0000000000000354
20. Jenkins DH, Rappold JF, Badloe JF, Berseus O, Blackbourne L, Brohi KH, et al. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation: definitions, current practice, and knowledge gaps. Shock (2014) 41(Suppl 1):3–12. doi:10.1097/SHK.0000000000000140
21. Naumann DN, Hazeldine J, Dinsdale RJ, Bishop JR, Midwinter MJ, Harrison P, et al. Endotheliopathy is associated with higher levels of cell-free DNA following major trauma: a prospective observational study. PLoS One (2017) 12(12):e0189870. doi:10.1371/journal.pone.0189870
22. Naumann DN, Hazeldine J, Davies DJ, Bishop J, Midwinter MJ, Belli A, et al. Endotheliopathy of trauma is an on-scene phenomenon, and is associated with multiple organ dysfunction syndrome: a prospective observational study. Shock (2018) 49(4):420–8. doi:10.1097/SHK.0000000000000999
23. Johansson P, Stensballe J, Ostrowski S. Shock induced endotheliopathy (SHINE) in acute critical illness – a unifying pathophysiologic mechanism. Crit Care (2017) 21(1):25. doi:10.1186/s13054-017-1605-5
25. Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg (2012) 73(1):60–6. doi:10.1097/TA.0b013e31825b5c10
26. Boyd TM, Lockhart E, Welsby I. Split blood products. In: Marcucci CE, Schoettker P, editors. Perioperative Hemostasis. Berlin Heidelberg: Springer (2015). p. 151–75.
27. Seltsam A, Muller TH. Update on the use of pathogen-reduced human plasma and platelet concentrates. Br J Haematol (2013) 162(4):442–54. doi:10.1111/bjh.12403
28. Chan KS, Sparrow RL. Microparticle profile and procoagulant activity of fresh-frozen plasma is affected by whole blood leukoreduction rather than 24-hour room temperature hold. Transfusion (2014) 54(8):1935–44. doi:10.1111/trf.12602
29. Runkel S, Hitzler WE, Hellstern P. The impact of whole blood processing and freezing conditions on the quality of therapeutic plasma prepared from whole blood. Transfusion (2015) 55(4):796–804. doi:10.1111/trf.12914
30. Steil L, Thiele T, Hammer E, Bux J, Kalus M, Volker U, et al. Proteomic characterization of freeze-dried human plasma: providing treatment of bleeding disorders without the need for a cold chain. Transfusion (2008) 48(11):2356–63. doi:10.1111/j.1537-2995.2008.01856.x
31. Thiele T, Kellner S, Hron G, Wasner C, Nauck M, Zimmermann K, et al. Storage of thawed plasma for a liquid plasma bank: impact of temperature and methylene blue pathogen inactivation. Transfusion (2012) 52(3):529–36. doi:10.1111/j.1537-2995.2011.03317.x
32. Holcomb JB, Pati S. Optimal trauma resuscitation with plasma as the primary resuscitative fluid: the surgeon’s perspective. Hematology Am Soc Hematol Educ Program (2013) 2013:656–9. doi:10.1182/asheducation-2013.1.656
33. Etchill E, Sperry J, Zuckerbraun B, Alarcon L, Brown J, Schuster K, et al. The confusion continues: results from an American Association for the Surgery of Trauma survey on massive transfusion practices among United States trauma centers. Transfusion (2016) 56(10):2478–86. doi:10.1111/trf.13755
34. Treml AB, Gorlin JB, Dutton RP, Scavone BM. Massive transfusion protocols: a survey of academic medical centers in the United States. Anesth Analg (2017) 124(1):277–81. doi:10.1213/ANE.0000000000001610
35. Yazer M, Eder AF, Land KJ. How we manage AB plasma inventory in the blood center and transfusion service. Transfusion (2013) 53(8):1627–33. doi:10.1111/trf.12223
36. Dunbar NM, Yazer MH; Biomedical Excellence for Safer Transfusion Collabrative. A possible new paradigm? A survey-based assessment of the use of thawed group A plasma for trauma resuscitation in the United States. Transfusion (2016) 56(1):125–9. doi:10.1111/trf.13266
37. Novak DJ, Bai Y, Cooke RK, Marques MB, Fontaine MJ, Gottschall JL, et al. Making thawed universal donor plasma available rapidly for massively bleeding trauma patients: experience from the Pragmatic, Randomized Optimal Platelets and Plasma Ratios (PROPPR) trial. Transfusion (2015) 55(6):1331–9. doi:10.1111/trf.13098
38. Garrigue D, Godier A, Glacet A, Labreuche J, Kipnis E, Paris C, et al. French lyophilized plasma versus fresh frozen plasma for the initial management of trauma-induced coagulopathy: a randomized open-label trial. J Thromb Haemost (2018) 16(3):481–9. doi:10.1111/jth.13929
39. Pati S, Matijevic N, Doursout MF, Ko T, Cao Y, Deng X, et al. Protective effects of fresh frozen plasma on vascular endothelial permeability, coagulation, and resuscitation after hemorrhagic shock are time dependent and diminish between days 0 and 5 after thaw. J Trauma (2010) 69(Suppl 1):S55–63. doi:10.1097/TA.0b013e3181e453d4
40. Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg (2011) 112(6):1289–95. doi:10.1213/ANE.0b013e318210385c
41. Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, et al. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan 1. Shock (2013) 40(3):195–202. doi:10.1097/SHK.0b013e31829f91fc
42. Torres LN, Sondeen JL, Ji L, Dubick MA, Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma Acute Care Surg (2013) 75(5):759–66. doi:10.1097/TA.0b013e3182a92514
43. Matijevic N, Wang YW, Cotton BA, Hartwell E, Barbeau JM, Wade CE, et al. Better hemostatic profiles of never-frozen liquid plasma compared with thawed fresh frozen plasma. J Trauma Acute Care Surg (2013) 74(1):84–90. doi:10.1097/TA.0b013e3182788e32
44. Wataha K, Menge T, Deng X, Shah A, Bode A, Holcomb JB, et al. Spray-dried plasma and fresh frozen plasma modulate permeability and inflammation in vitro in vascular endothelial cells. Transfusion (2013) 53(Suppl 1):80S–90S. doi:10.1111/trf.12040
45. Potter DR, Baimukanova G, Keating SM, Deng X, Chu JA, Gibb SL, et al. Fresh frozen plasma and spray-dried plasma mitigate pulmonary vascular permeability and inflammation in hemorrhagic shock. J Trauma Acute Care Surg (2015) 78(6 Suppl 1):S7–17. doi:10.1097/TA.0000000000000630
46. Betteridge KB, Arkill KP, Neal CR, Harper SJ, Foster RR, Satchell SC, et al. Sialic acids regulate microvessel permeability, revealed by novel in vivo studies of endothelial glycocalyx structure and function. J Physiol (2017) 595(15):5015–35. doi:10.1113/JP274167
47. Ebong EE, Macaluso FP, Spray DC, Tarbell JM. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler Thromb Vasc Biol (2011) 31(8):1908–15. doi:10.1161/ATVBAHA.111.225268
48. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch (2007) 454(3):345–59. doi:10.1007/s00424-007-0212-8
49. Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng (2007) 9:121–67. doi:10.1146/annurev.bioeng.9.060906.151959
50. Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia (2014) 69(7):777–84. doi:10.1111/anae.12661
51. Ushiyama A, Kataoka H, Iijima T. Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care (2016) 4(1):59. doi:10.1186/s40560-016-0182-z
52. Schott U, Solomon C, Fries D, Bentzer P. The endothelial glycocalyx and its disruption, protection and regeneration: a narrative review. Scand J Trauma Resusc Emerg Med (2016) 24:48. doi:10.1186/s13049-016-0239-y
53. Li L, Ly M, Linhardt RJ. Proteoglycan sequence. Mol Biosyst (2012) 8(6):1613–25. doi:10.1039/c2mb25021g
54. Rehm M, Zahler S, Lotsch M, Welsch U, Conzen P, Jacob M, et al. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology (2004) 100(5):1211–23. doi:10.1097/00000542-200405000-00025
55. Nieuwdorp M, van Haeften TW, Gouverneur MC, Mooij HL, van Lieshout MH, Levi M, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes (2006) 55(2):480–6. doi:10.2337/diabetes.55.02.06.db05-1103
56. Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol (1999) 277(2 Pt 2):H508–14.
57. Lipowsky HH, Gao L, Lescanic A. Shedding of the endothelial glycocalyx in arterioles, capillaries, and venules and its effect on capillary hemodynamics during inflammation. Am J Physiol Heart Circ Physiol (2011) 301(6):H2235–45. doi:10.1152/ajpheart.00803.2011
58. Constantinescu AA, Vink H, Spaan JA. Endothelial cell glycocalyx modulates immobilization of leukocytes at the endothelial surface. Arterioscler Thromb Vasc Biol (2003) 23(9):1541–7. doi:10.1161/01.ATV.0000085630.24353.3D
59. Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am J Physiol Heart Circ Physiol (2000) 278(1):H285–9. doi:10.1152/ajpheart.2000.278.1.H285
60. Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol (2015) 80(3):389–402. doi:10.1111/bcp.12629
61. Chelazzi C, Villa G, Mancinelli P, De Gaudio AR, Adembri C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit Care (2015) 19:26. doi:10.1186/s13054-015-0741-z
62. Bansch P, Nelson A, Ohlsson T, Bentzer P. Effect of charge on microvascular permeability in early experimental sepsis in the rat. Microvasc Res (2011) 82(3):339–45. doi:10.1016/j.mvr.2011.08.008
63. Kolarova H, Ambruzova B, Svihalkova Sindlerova L, Klinke A, Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm (2014) 2014:694312. doi:10.1155/2014/694312
64. Schmidt EP, Lee WL, Zemans RL, Yamashita C, Downey GP. On, around, and through: neutrophil-endothelial interactions in innate immunity. Physiology (Bethesda) (2011) 26(5):334–47. doi:10.1152/physiol.00011.2011
65. Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation (2007) 116(17):1896–906. doi:10.1161/CIRCULATIONAHA.106.684852
66. Biddle C. Like a slippery fish, a little slime is a good thing: the glycocalyx revealed. AANA J (2013) 81(6):473–80.
67. Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res (2003) 93(10):e136–42. doi:10.1161/01.RES.0000101744.47866.D5
68. Yen W, Cai B, Yang J, Zhang L, Zeng M, Tarbell JM, et al. Endothelial surface glycocalyx can regulate flow-induced nitric oxide production in microvessels in vivo. PLoS One (2015) 10(1):e0117133. doi:10.1371/journal.pone.0117133
69. Mitra R, O’Neil GL, Harding IC, Cheng MJ, Mensah SA, Ebong EE. Glycocalyx in atherosclerosis-relevant endothelium function and as a therapeutic target. Curr Atheroscler Rep (2017) 19(12):63. doi:10.1007/s11883-017-0691-9
70. Sieve I, Munster-Kuhnel AK, Hilfiker-Kleiner D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vascul Pharmacol (2018) 100:26–33. doi:10.1016/j.vph.2017.09.002
71. Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch (2000) 440(5):653–66. doi:10.1007/s004240000307
72. Segrest JP, Jones MK, De Loof H, Dashti N. Structure of apolipoprotein B-100 in low density lipoproteins. J Lipid Res (2001) 42(9):1346–67.
73. Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am J Physiol Heart Circ Physiol (2002) 283(4):H1282–91. doi:10.1152/ajpheart.00117.2002
74. Chappell D, Brettner F, Doerfler N, Jacob M, Rehm M, Bruegger D, et al. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: an animal study. Eur J Anaesthesiol (2014) 31(9):474–81. doi:10.1097/EJA.0000000000000085
75. Dimitrievska S, Gui L, Weyers A, Lin T, Cai C, Wu W, et al. New functional tools for antithrombogenic activity assessment of live surface glycocalyx. Arterioscler Thromb Vasc Biol (2016) 36(9):1847–53. doi:10.1161/ATVBAHA.116.308023
76. Boels MG, Lee DH, van den Berg BM, Dane MJ, van der Vlag J, Rabelink TJ. The endothelial glycocalyx as a potential modifier of the hemolytic uremic syndrome. Eur J Intern Med (2013) 24(6):503–9. doi:10.1016/j.ejim.2012.12.016
77. Jacob M, Rehm M, Loetsch M, Paul JO, Bruegger D, Welsch U, et al. The endothelial glycocalyx prefers albumin for evoking shear stress-induced, nitric oxide-mediated coronary dilatation. J Vasc Res (2007) 44(6):435–43. doi:10.1159/000104871
78. Ampey BC, Morschauser TJ, Lampe PD, Magness RR. Gap junction regulation of vascular tone: implications of modulatory intercellular communication during gestation. Adv Exp Med Biol (2014) 814:117–32. doi:10.1007/978-1-4939-1031-1_11
79. Radeva MY, Waschke J. Mind the gap: mechanisms regulating the endothelial barrier. Acta Physiol (Oxf) (2018) 222(1). doi:10.1111/apha.12860
80. Annecke T, Fischer J, Hartmann H, Tschoep J, Rehm M, Conzen P, et al. Shedding of the coronary endothelial glycocalyx: effects of hypoxia/reoxygenation vs ischaemia/reperfusion. Br J Anaesth (2011) 107(5):679–86. doi:10.1093/bja/aer269
81. van Golen RF, Reiniers MJ, Vrisekoop N, Zuurbier CJ, Olthof PB, van Rheenen J, et al. The mechanisms and physiological relevance of glycocalyx degradation in hepatic ischemia/reperfusion injury. Antioxid Redox Signal (2014) 21(7):1098–118. doi:10.1089/ars.2013.5751
82. Nieuwdorp M, Meuwese MC, Mooij HL, van Lieshout MH, Hayden A, Levi M, et al. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis (2009) 202(1):296–303. doi:10.1016/j.atherosclerosis.2008.03.024
83. Ostrowski SR, Henriksen HH, Stensballe J, Gybel-Brask M, Cardenas JC, Baer LA, et al. Sympathoadrenal activation and endotheliopathy are drivers of hypocoagulability and hyperfibrinolysis in trauma: a prospective observational study of 404 severely injured patients. J Trauma Acute Care Surg (2017) 82(2):293–301. doi:10.1097/TA.0000000000001304
84. Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg (2011) 254(2):194–200. doi:10.1097/SLA.0b013e318226113d
85. Gonzalez Rodriguez E, Ostrowski SR, Cardenas JC, Baer LA, Tomasek JS, Henriksen HH, et al. Syndecan-1: a quantitative marker for the endotheliopathy of trauma. J Am Coll Surg (2017) 225(3):419–27. doi:10.1016/j.jamcollsurg.2017.05.012
86. Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, et al. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS One (2011) 6(8):e23530. doi:10.1371/journal.pone.0023530
87. Rahbar E, Cardenas JC, Baimukanova G, Usadi B, Bruhn R, Pati S, et al. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J Transl Med (2015) 13:117. doi:10.1186/s12967-015-0481-5
88. Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg (2008) 248(3):447–58. doi:10.1097/SLA.0b013e318185a9ad
89. Nelson A, Statkevicius S, Schott U, Johansson PI, Bentzer P. Effects of fresh frozen plasma, Ringer’s acetate and albumin on plasma volume and on circulating glycocalyx components following haemorrhagic shock in rats. Intensive Care Med Exp (2016) 4(1):6. doi:10.1186/s40635-016-0080-7
90. Torres Filho IP, Torres LN, Salgado C, Dubick MA. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am J Physiol Heart Circ Physiol (2016) 310(11):H1468–78. doi:10.1152/ajpheart.00006.2016
91. Diebel LN, Martin JV, Liberati DM. Microfluidics: a high throughput system for the assessment of the endotheliopathy of trauma and the effect of timing of plasma administration on ameliorating shock associated endothelial dysfunction. J Trauma Acute Care Surg (2017) 84(4):575–82. doi:10.1097/TA.0000000000001791
92. Pati S, Peng Z, Wataha K, Miyazawa B, Potter DR, Kozar RA. Lyophilized plasma attenuates vascular permeability, inflammation and lung injury in hemorrhagic shock. PLoS One (2018) 13(2):e0192363. doi:10.1371/journal.pone.0192363
93. Chesebro BB, Rahn P, Carles M, Esmon CT, Xu J, Brohi K, et al. Increase in activated protein C mediates acute traumatic coagulopathy in mice. Shock (2009) 32(6):659–65. doi:10.1097/SHK.0b013e3181a5a632
94. Wu F, Peng Z, Park PW, Kozar RA. Loss of syndecan-1 abrogates the pulmonary protective phenotype induced by plasma after hemorrhagic shock. Shock (2017) 48(3):340–5. doi:10.1097/SHK.0000000000000832
95. Ban K, Peng Z, Pati S, Witkov RB, Park PW, Kozar RA. Plasma-mediated gut protection after hemorrhagic shock is lessened in syndecan-1-/- mice. Shock (2015) 44(5):452–7. doi:10.1097/SHK.0000000000000452
96. Jacob M, Bruegger D, Rehm M, Welsch U, Conzen P, Becker BF. Contrasting effects of colloid and crystalloid resuscitation fluids on cardiac vascular permeability. Anesthesiology (2006) 104(6):1223–31. doi:10.1097/00000542-200606000-00018
97. Jacob M, Paul O, Mehringer L, Chappell D, Rehm M, Welsch U, et al. Albumin augmentation improves condition of guinea pig hearts after 4 hr of cold ischemia. Transplantation (2009) 87(7):956–65. doi:10.1097/TP.0b013e31819c83b5
98. Kheirabadi BS, Valdez-Delgado KK, Terrazas IB, Miranda N, Dubick MA. Is limited prehospital resuscitation with plasma more beneficial than using a synthetic colloid? An experimental study in rabbits with parenchymal bleeding. J Trauma Acute Care Surg (2015) 78(4):752–9. doi:10.1097/TA.0000000000000591
99. Zazzeron L, Gattinoni L, Caironi P. Role of albumin, starches and gelatins versus crystalloids in volume resuscitation of critically ill patients. Curr Opin Crit Care (2016) 22(5):428–36. doi:10.1097/MCC.0000000000000341
100. Pati S, Potter DR, Baimukanova G, Farrel DH, Holcomb JB, Schreiber MA. Modulating the endotheliopathy of trauma: factor concentrate versus fresh frozen plasma. J Trauma Acute Care Surg (2016) 80(4):576–84. doi:10.1097/TA.0000000000000961
101. McQuilten ZK, Bailey M, Cameron PA, Stanworth SJ, Venardos K, Wood EM, et al. Fibrinogen concentration and use of fibrinogen supplementation with cryoprecipitate in patients with critical bleeding receiving massive transfusion: a bi-national cohort study. Br J Haematol (2017) 179(1):131–41. doi:10.1111/bjh.14804
102. Yakovlev S, Mikhailenko I, Cao C, Zhang L, Strickland DK, Medved L. Identification of VLDLR as a novel endothelial cell receptor for fibrin that modulates fibrin-dependent transendothelial migration of leukocytes. Blood (2012) 119(2):637–44. doi:10.1182/blood-2011-09-382580
103. Deng X, Cao Y, Huby MP, Duan C, Baer L, Peng Z, et al. Adiponectin in fresh frozen plasma contributes to restoration of vascular barrier function after hemorrhagic shock. Shock (2016) 45(1):50–4. doi:10.1097/SHK.0000000000000458
104. Bluher M, Mantzoros CS. From leptin to other adipokines in health and disease: facts and expectations at the beginning of the 21st century. Metabolism (2015) 64(1):131–45. doi:10.1016/j.metabol.2014.10.016
105. van Meurs M, Castro P, Shapiro NI, Lu S, Yano M, Maeda N, et al. Adiponectin diminishes organ-specific microvascular endothelial cell activation associated with sepsis. Shock (2012) 37(4):392–8. doi:10.1097/SHK.0b013e318248225e
106. Xu SQ, Mahadev K, Wu X, Fuchsel L, Donnelly S, Scalia RG, et al. Adiponectin protects against angiotensin II or tumor necrosis factor alpha-induced endothelial cell monolayer hyperpermeability: role of cAMP/PKA signaling. Arterioscler Thromb Vasc Biol (2008) 28(5):899–905. doi:10.1161/ATVBAHA.108.163634
107. Straat M, Muller MC, Meijers JC, Arbous MS, Spoelstra-de Man AM, Beurskens CJ, et al. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: a prospective substudy of a randomized trial. Crit Care (2015) 19:163. doi:10.1186/s13054-015-0828-6
Keywords: massive hemorrhage, shock, resuscitation, fresh frozen plasma, endothelium, glycocalyx
Citation: Barelli S and Alberio L (2018) The Role of Plasma Transfusion in Massive Bleeding: Protecting the Endothelial Glycocalyx? Front. Med. 5:91. doi: 10.3389/fmed.2018.00091
Received: 14 January 2018; Accepted: 22 March 2018;
Published: 18 April 2018
Edited by:
Stefano Fontana, Transfusion Interrégionale CRS SA, SwitzerlandReviewed by:
Thomas Thiele, Universitätsmedizin Greifswald, GermanyThomas Pierre Lecompte, Université de Genève, Switzerland
Copyright: © 2018 Barelli and Alberio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorenzo Alberio, bG9yZW56by5hbGJlcmlvQGNodXYuY2g=
ORCID ID: orcid.org/0000-0001-9686-9920
 Stefano Barelli
Stefano Barelli Lorenzo Alberio
Lorenzo Alberio