
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 04 December 2017
Sec. Hematology
Volume 4 - 2017 | https://doi.org/10.3389/fmed.2017.00219
This article is part of the Research Topic Transfusion Medicine and Blood View all 23 articles
In line with current microbial risk reduction efforts, pathogen inactivation (PI) technologies for blood components promise to reduce the residual risk of known and emerging infectious agents. The implementation of PI of labile blood components is slowly but steadily increasing. This review discusses the relevance of PI for the field of transfusion medicine and describes the available and emerging PI technologies that can be used to treat cellular blood products such as platelet and red blood cell units. In collaboration with the French medical device manufacturer Macopharma, the German Red Cross Blood Services developed a new UVC light-based PI method for platelet units, which is currently being investigated in clinical trials.
From the late 1970s to the mid-1980s, contaminated hemophilia blood products were a serious public health problem, resulting in the infection of large numbers of hemophiliacs with the human immunodeficiency virus (HIV). If safety measures had been implemented in a timely and consistent manner after identification of the acquired immune deficiency syndrome (AIDS) epidemic in 1981 and isolation of the HIV in 1983, the transmission of HIV infection by these blood products could have been prevented in most cases. This contaminated blood scandal made the community aware that new pathogens may emerge and threaten blood safety at any time. However, there was a significant delay in the introduction of HIV detection systems in some countries and in some cases, the detection tests that were implemented proved to be unreliable. In addition, the plasma products used for therapy were not even treated by heat inactivation—a pathogen inactivation (PI) method that was readily available and approved at that time. Consequently, blood and blood components became subject to drug law in some countries (1, 2).
Increasingly stringent donor eligibility criteria and more sensitive virus detection methods have reduced the risk of transfusion-transmitted infection (TTI) by blood products significantly, but a residual risk of TTI with viruses, bacteria, protozoa, and prions remains. False-negative test results due to test failures, very low-pathogen concentrations in the peripheral blood or escaped mutants can result in TTI in spite of negative screening tests (e.g., for Treponema pallidum, hepatitis B, hepatitis C, and HIV). In addition, transfusion recipients may be infected by pathogens not targeted in regular blood donor screening programs (e.g., hepatitis A and bacteria). Transfusion safety is particularly susceptible to pathogens that enter regions in which they are not yet endemic. The fact that viruses that are usually endemic in tropical regions have recently caused outbreaks in Western countries demonstrates that these pathogens can arise and threaten transfusion safety at any time (3, 4).
Blood safety is still mainly based on the reactive principle of introducing new test systems or new donor election criteria after a threat to transfusion recipients has been identified. In other words, infections by contaminated blood products must first occur before appropriate counter-measures are established. At the beginning of the last decade, a number of cases of West Nile virus occurred in the USA through the transmission of blood components before the first detection system for donor testing was implemented (3). The recent Zika virus outbreak on the American continent has heightened concerns over this reactive approach to blood supply safety (5, 6).
During an international consensus conference, transfusion experts and other stakeholders in the field of transfusion medicine recommended a change from the hitherto reactive strategy toward a proactive, preventive approach to blood safety (7). Recently, developed and approved PI technologies for cellular blood products, such as red blood cell (RBC) and platelet units, are considered key measures for closing or at least reducing the safety gap caused by emerging pathogens. While virus reduction procedures are an integral part of the process of manufacturing plasma derivatives from plasma pools, and although the methylene blue system has been used for PI of single donor plasma units for nearly two decades (8), a new generation of PI methods for platelet units have recently become available (9, 10). PI technologies for the treatment of RBC units are still in development and have not received market authorization yet.
The use of PI technologies for blood products has a number of advantages. Because they inactivate most clinically relevant viruses, bacteria, and protozoa, they can help to eliminate the residual risk of infection during the “window period” when transfusion-relevant pathogens (e.g., HIV) cannot be detected by donor screening tests. Their broad activity against pathogens also helps to reduce the risk of recognizable infectious agents (e.g., bacteria), which still cannot be prevented completely. In contrast to screening tests for transfusion-borne pathogens, PI proactively protects against emerging infectious agents entering the blood supply in a given community.
All PI methods used to treat cellular blood products work by impairing the target pathogen’s ability to replicate. When used alone or in combination, ultraviolet (UV) light and alkylating agents cause irreversible damage to the nucleic acids of pathogens. Therefore, they effectively eliminate classical pathogens such as viruses, bacteria, fungi, and protozoa, but are ineffective against prions. The latter protein-based pathogenic agents can cause sporadic and variant Creutzfeldt–Jakob disease in humans.
The following PI technologies for cellular products are currently available or in the pipeline.
The INTERCEPT Blood System for platelets and plasma is manufactured by Cerus Corporation (Concord, CA, USA). The mechanism of action of this PI technology is based on the properties of amotosalen HCl (S-59), a photoactive compound which penetrates cellular and nuclear membranes and binds to the double-stranded regions of DNA and RNA. When activated by low-energy UVA light (320–400 nm), amatosalen cross-links nucleic acids and thus irreversibly blocks the replication of DNA and RNA (11). After illumination, residual amotosalen and its photoproducts must be removed during an incubation step lasting up to 16 h. The amatosalen/UVA procedure is not suitable for RBCs because of UVA light absorption by hemoglobin.
The MIRASOL system was developed by TerumoBCT (Lakewood, CO, USA). This photodynamic procedure employs riboflavin (vitamin B2) and broad spectrum UV light (mainly UVA und UVB, 285–365 nm). On exposure to UVA and UVB light, riboflavin associates with nucleic acids and mediates oxygen-independent electron transfer, causing irreversible damage to the nucleic acids (12). Because naturally occurring vitamin B2 and its photodegradation products are non-toxic and non-mutagenic, they do not need to be removed prior to transfusion. In addition to plasma and platelets, protocols for extension of the MIRASOL system to whole blood are now in development.
THERAFLEX UV-Platelets is a novel UVC-based PI technology that works without photoactive substances. It is the product of a joint venture between Macopharma (Mouvaux, France) and the German Red Cross Blood Service NSTOB in Springe, Germany. Shortwave UVC light (254 nm) directly interacts with nucleic acids to form pyrimidine dimers that block the elongation of nucleic acid transcripts (13). UVC irradiation mainly affects the nucleic acids of pathogens and leukocytes and does not impair plasma and platelet quality. As no photoactive substances are involved, UVC treatment is just as simple but faster (takes less than 1 min) than gamma irradiation, and can easily be integrated into the manufacturing processes at blood banks (Figure 1). The THERAFLEX system was originally developed for platelets but is also suitable for plasma and RBC units.
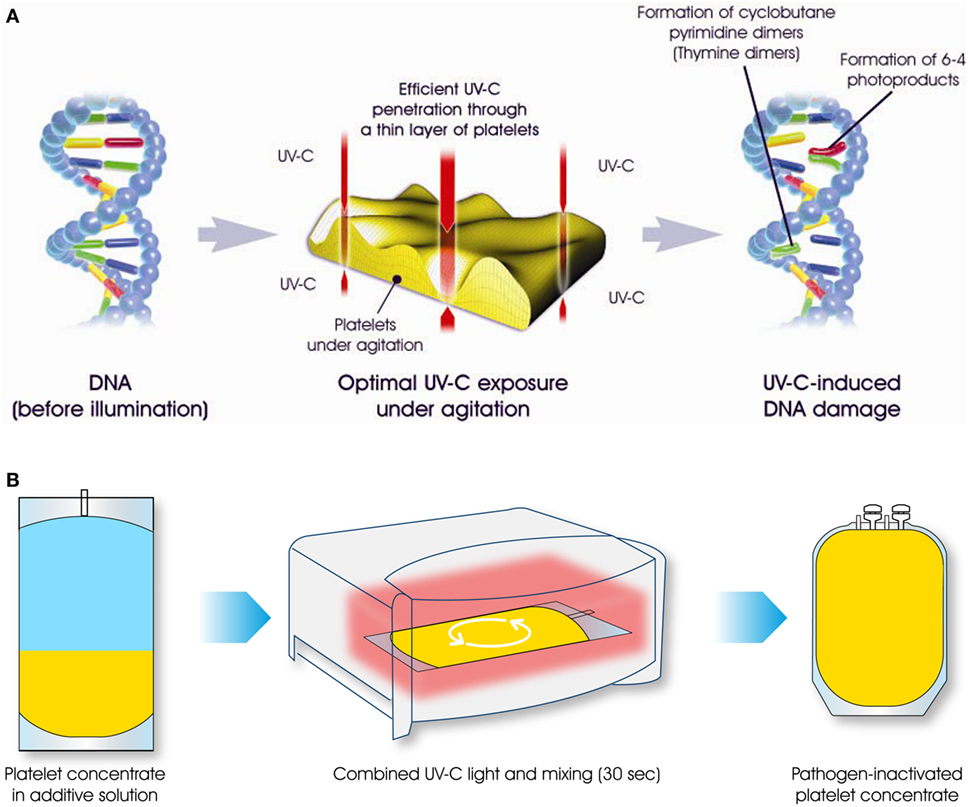
Figure 1. The THERAFLEX ultraviolet (UV)-Platelets pathogen inactivation system uses UVC light to induce irreversible damage to the nucleic acids of viruses, bacteria, fungi, protozoa, and leukocytes. Intense agitation of the platelet bag during UVC illumination results in efficient mixing, ensuring the uniform treatment of all blood compartments (A). For the illumination step of this simple and rapid procedure, platelet units are placed in the irradiation device for a period of less than 1 min (B). Afterward, the pathogen-reduced platelet product can be used for transfusion.
The S-303 PI system (INTERCEPT RBC system, Cerus Corporation, Concord, CA, USA) was specifically developed for RBC units. S-303 is a modular compound that prevents nucleic acid replication by targeting and cross-linking nucleic acids. Once added to the RBC unit, this amphipathic compound rapidly passes through cell and viral envelope membranes and intercalates into the helical regions of nucleic acids. S-300, the non-reactive byproduct of this reaction, is subsequently removed by incubation and centrifugation, which can take up to 20 h (14). In contrast to the other PI technologies described here, the S-303 system does not require UV light. However, glutathione (GSH), a naturally occurring antioxidant, must be used to prevent non-specific reactions between S-303 and other nucleophiles present in the RBC unit. These may include small molecules, such as phosphate and water, and macromolecules, such as proteins.
The INTERCEPT and MIRASOL systems for platelets and plasma have already been approved in the USA and some European and Asian countries, while both the THERAFLEX system and the S-303 system are still in clinical development. The UVC-based THERAFLEX system is expected to receive marketing authorization within the next few years (Table 1).
Clinical studies show that platelets retain their hemostatic efficacy after PI treatment. Following prophylactic transfusion, there was no difference in the ability of pathogen-reduced and untreated platelet units to prevent severe bleeding (15). However, almost all clinical trials demonstrated that post-transfusion survival and recovery rates were consistently lower in patients receiving platelets treated with PI technology than in those transfused with untreated platelets (16–19). Accordingly, the transfusion of pathogen-reduced platelets resulted in lower platelet count increments (CIs), lower corrected count increments, shorter intervals between platelet transfusions, and a higher number of platelet transfusions per patient. However, observational studies showed no evidence of increased product consumption rates when pathogen-reduced platelet units were used in a routine setting (20).
Interestingly, the rate of acute transfusion reactions may be lower after the transfusion of pathogen-reduced versus untreated platelets. However, there have been concerns over acute respiratory distress associated with amatosalen/UVA-treated platelets (15). While the results of animal studies suggest that UV light-treated platelets mediated a higher risk of pulmonary toxicity (21), an analysis of clinical data by an expert panel does not confirm significant differences in the rates of acute lung disorders between PI-treated and untreated platelets (22). The results of ongoing large-scale phase III and hemovigilance studies will help to further clarify open questions with respect to therapeutic efficacy and potential side effects of pathogen-reduced platelets (23).
The S-303 system, which is in clinical development, is the only PI technology available for RBCs. Current studies are investigating the second-generation S-303 PI process. The first-generation S-303 procedure only marginally affected RBC quality and function, but after reports of immunization against pathogen-inactivated RBCs in transfused patients emerged, a new generation of the S-303 system had to be developed. In the second-generation S-303 system, the quencher concentration of GSH was increased from 2 to 20 mmol/l in order to decrease the affinity of S-303 for proteins and thus to avoid the formation of neoantigens on the surface of erythrocytes (24). However, recent studies show that immunization against S-303-coated RBCs still occurs after modification of the S-303 system (25). In particular, the fact that antibodies against S-303-treated cells were also detected in healthy blood donors who had never been transfused with pathogen-reduced RBCs suggests that some individuals may be immunized by S-303-like substances in the environment (e.g., food or air) or may have naturally occurring antibodies against epitopes on the S-303 molecule. These data clearly show that the use of chemical agents for PI of cellular products increases the risk of immune responses against blood components in transfusion recipients. Various phase III clinical trials to test the second-generation S-303 PI system for RBCs in acute and chronic anemia patients are currently ongoing or planned.
The INTERCEPT and MIRASOL PI systems for platelets and plasma are used in some parts of Asia, Europe, and the USA. In Europe, the willingness to use pathogen-reduced platelet units varies between countries and regions. PI technologies are implemented nationwide in some countries (e.g., Switzerland and Belgium), but only regionally in others (e.g., Poland). Evaluation of PI technologies for platelets is under way at some blood centers in Germany. In 2011, the Swiss national authority (Swissmedic) ordered the nationwide implementation of PI of platelet units. This measure was mainly aimed at preventing or at least minimizing the risk of fatal transfusion reactions caused by bacterially contaminated platelet units. Analysis of Swiss hemovigilance data revealed that without PI, one fatal case of transfusion-transmitted sepsis by contaminated platelet units would occur in Switzerland every 2 years. The US Food and Drug Administration (FDA) recently recommended the use of approved PI technologies as an alternative to bacterial detection methods in order to adequately control the risk of bacterial contamination of platelets (26, 27).
The preventive potential of PI of cellular blood components first became apparent during a chikungunya virus epidemic on the French island of La Reunion in the Indian Ocean in 2006 (28). Because more than 30% of the inhabitants were infected, local blood donation was suspended to prevent TTI. To sustain the availability of platelet components, the French national blood service (Etablissement Français du Sang) implemented universal PI of platelet components on the island. The success of this measure demonstrated that PI can effectively support the availability of safe labile blood components during an epidemic.
The West Nile virus epidemic in the USA was the first example of a large-scale arboviral threat to the blood supply of a Western country that required an urgent response across government agencies and non-governmental organizations. The dramatic spread of Zika virus in the Americas since 2015 has generated a sense of public health urgency akin to AIDS, along with immediate concerns over blood safety. In areas of active transmission, “FDA guidance recommends that blood be outsourced from unaffected areas, unless there are measures to screen donations using a laboratory test, or unless the blood components are subjected to PI technology” with an approved method (29). The INTERCEPT system was approved by the FDA in 2014 and has already been implemented at a number of US blood centers.
Despite the increasing and profound safety and efficacy record of pathogen-reduced blood cellular products, there are still concerns that impede the introduction of PI technology in hemotherapy. The INTERCEPT protocol includes incubation and adsorption steps that result in a significant loss of platelets (up to 15%) during preparation and PI treatment. However, this loss could be offset by performing PI with higher platelet counts in the starting products. The platelet yields could be increased by using more buffy coats (e.g., five instead of four) to manufacture pooled platelets, or by collecting higher numbers of platelets during the apheresis process. Moreover, this measure could compensate for reduced platelet CIs in transfusion recipients and thus lower the possible need for increased platelet unit utilization.
All PI technologies mentioned in this review exhibit gaps in their PI efficacy. The amatosalen/UVA-based system (INTERCEPT) is ineffective for non-enveloped viruses such as hepatitis A, hepatitis E, and parvovirus B19 (30). The riboflavin/UV-based system (MIRASOL) has only weakly effects against bacteria and some viruses (31). The UVC light-based system (THERAFLEX) is highly effective against bacteria and most transfusion-relevant viruses, but only moderately effective against HIV (32). However, when highly sensitive screening tests for HIV are performed, UVC-based PI could further reduce the risk of virus transmission during the “window period” in which the pre-nucleic acid testing can be negative and in patients with occult infections. Despite these weaknesses, PI systems generally have the potential to significantly add an additional layer of safety to blood transfusion.
Major concerns surrounding the implementation of PI have to do with its impact on the integrity of blood components and the toxicity of the chemicals used in these systems. In particular, acute and chronic toxicities may be caused by PI technologies that use active chemicals. Although only small quantities of photochemical compounds are used in PI technologies and they appear to provide sufficient safety margins, it cannot be excluded that alkylating agents such as amatosalen may be carcinogenic in the long term in a subset of transfused patients. A major advantage of the THERAFLEX system is that it works without photoactive substances, thus eliminating the risk of photoreagent-related adverse events (10, 13).
According to various stakeholders in the field of transfusion medicine, it is crucial to inactivate pathogens in all blood components in order to increase the safety margin of the entire blood supply. As long as PI is not routinely implemented in the production of RBC units (the most commonly used blood components), PI cannot achieve its full potential to enhance blood safety. Experts and health authorities are increasingly recommending the implementation of PI systems for platelets and plasma as an important step toward improving blood safety. A Canadian risk-benefit analysis suggests that if a new pathogen entered the blood supply, the use of pathogen-reduced plasma and platelets would reduce the risk of TTI by 40% (33).
The additional costs of PI implementation may be responsible for the hesitant acceptance of this technology by hospitals and funding agencies. Although based on assumptions and simplifications, the available cost-effectiveness analyses suggest that PI implementation, like other measures for the improvement of blood safety, has an acceptable cost–benefit ratio in this specific application (34, 35). The potential cost savings from PI implementation could offset some costs associated with the technology (e.g., production costs); however, the amount of potential offsetting cost reductions may vary considerably between different countries and regions and must be evaluated on an individual basis for blood centers and hospitals (36). Finally, the available resources influence how politicians and health authorities decide on how to meet public concerns for safety in transfusion medicine. If emerging evidence continues to demonstrate the efficacy of PI, it will be difficult to explain to individuals with severe transfusion-associated infections why this readily available risk mitigation and safety measure was not implemented.
The author confirms being the sole contributor of this work and approved it for publication.
The author works for German Red Cross Blood Service NSTOB, a blood donation center that collaborates with Macopharma on the development of a pathogen inactivation system for platelets. The author did not receive any financial support relevant to this manuscript.
1. Engelfriet CP, Reesink HW, Snyder EL, Dzik WH, Masse M, Naegelen C, et al. The official requirements for platelet concentrates. Vox Sang (1998) 75(4):308–17. doi:10.1046/j.1423-0410.1998.75403081.x
2. Devine DV, Bradley AJ, Maurer E, Levin E, Chahal S, Serrano K, et al. Effects of prestorage white cell reduction on platelet aggregate formation and the activation state of platelets and plasma enzyme systems. Transfusion (1999) 39(7):724–34. doi:10.1046/j.1537-2995.1999.39070724.x
3. Harrington T, Kuehnert MJ, Kamel H, Lanciotti RS, Hand S, Currier M, et al. West Nile virus infection transmitted by blood transfusion. Transfusion (2003) 43(8):1018–22. doi:10.1046/j.1537-2995.2003.00481.x
4. Bianco C. Dengue and chikungunya viruses in blood donations: risks to the blood supply? Transfusion (2008) 48(7):1279–81. doi:10.1111/j.1537-2995.2008.01806.x
5. Lanteri MC, Kleinman SH, Glynn SA, Musso D, Keith Hoots W, Custer BS, et al. Zika virus: a new threat to the safety of the blood supply with worldwide impact and implications. Transfusion (2016) 56(7):1907–14. doi:10.1111/trf.13677
6. Motta IJ, Spencer BR, Cordeiro da Silva SG, Arruda MB, Dobbin JA, Gonzaga YB, et al. Evidence for transmission of Zika virus by platelet transfusion. N Engl J Med (2016) 375(11):1101–3. doi:10.1056/NEJMc1607262
7. Klein HG, Anderson D, Bernardi MJ, Cable R, Carey W, Hoch JS, et al. Pathogen inactivation: making decisions about new technologies. Report of a consensus conference. Transfusion (2007) 47(12):2338–47. doi:10.1111/j.1537-2995.2007.01512.x
8. Pereira A. Methylene-blue-photoinactivated plasma and its contribution to blood safety. Transfusion (2004) 44(6):948–50; author reply 950. doi:10.1111/j.0041-1132.2004.359_6.x
9. Pelletier JP, Transue S, Snyder EL. Pathogen inactivation techniques. Best Pract Res Clin Haematol (2006) 19(1):205–42. doi:10.1016/j.beha.2005.04.001
10. Seltsam A, Muller TH. Update on the use of pathogen-reduced human plasma and platelet concentrates. Br J Haematol (2013) 162(4):442–54. doi:10.1111/bjh.12403
11. Irsch J, Lin L. Pathogen inactivation of platelet and plasma blood components for transfusion using the INTERCEPT blood system. Transfus Med Hemother (2011) 38(1):19–31. doi:10.1159/000323937
12. Marschner S, Goodrich R. Pathogen reduction technology treatment of platelets, plasma and whole blood using riboflavin and UV light. Transfus Med Hemother (2011) 38(1):8–18. doi:10.1159/000324160
13. Seltsam A, Muller TH. UVC irradiation for pathogen reduction of platelet concentrates and plasma. Transfus Med Hemother (2011) 38(1):43–54. doi:10.1159/000323845
14. Henschler R, Seifried E, Mufti N. Development of the S-303 pathogen inactivation technology for red blood cell concentrates. Transfus Med Hemother (2011) 38(1):33–42. doi:10.1159/000324458
15. McCullough J, Vesole DH, Benjamin RJ, Slichter SJ, Pineda A, Snyder E, et al. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT trial. Blood (2004) 104(5):1534–41. doi:10.1182/blood-2003-12-4443
16. van Rhenen D, Gulliksson H, Cazenave JP, Pamphilon D, Ljungman P, Kluter H, et al. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood (2003) 101(6):2426–33. doi:10.1182/blood-2002-03-0932
17. Kerkhoffs JL, van Putten WL, Novotny VM, Te Boekhorst PA, Schipperus MR, Zwaginga JJ, et al. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. Br J Haematol (2010) 150(2):209–17. doi:10.1111/j.1365-2141.2010.08227.x
18. Mirasol Clinical Evaluation Study Group. A randomized controlled clinical trial evaluating the performance and safety of platelets treated with MIRASOL pathogen reduction technology. Transfusion (2010) 50(11):2362–75. doi:10.1111/j.1537-2995.2010.02694.x
19. Rebulla P, Vaglio S, Beccaria F, Bonfichi M, Carella A, Chiurazzi F, et al. Clinical effectiveness of platelets in additive solution treated with two commercial pathogen-reduction technologies. Transfusion (2017) 57(5):1171–83. doi:10.1111/trf.14042
20. Cazenave JP, Isola H, Waller C, Mendel I, Kientz D, Laforet M, et al. Use of additive solutions and pathogen inactivation treatment of platelet components in a regional blood center: impact on patient outcomes and component utilization during a 3-year period. Transfusion (2011) 51(3):622–9. doi:10.1111/j.1537-2995.2010.02873.x
21. Gelderman MP, Chi X, Zhi L, Vostal JG. Ultraviolet B light-exposed human platelets mediate acute lung injury in a two-event mouse model of transfusion. Transfusion (2011) 51(11):2343–57. doi:10.1111/j.1537-2995.2011.03135.x
22. Corash L, Lin JS, Sherman CD, Eiden J. Determination of acute lung injury after repeated platelet transfusions. Blood (2011) 117(3):1014–20. doi:10.1182/blood-2010-06-293399
23. Ypma PF, van der Meer PF, Heddle NM, van Hilten JA, Stijnen T, Middelburg RA, et al. A study protocol for a randomised controlled trial evaluating clinical effects of platelet transfusion products: the pathogen reduction evaluation and predictive analytical rating score (PREPAReS) trial. BMJ Open (2016) 6(1):e010156. doi:10.1136/bmjopen-2015-010156
24. Benjamin RJ, McCullough J, Mintz PD, Snyder E, Spotnitz WD, Rizzo RJ, et al. Therapeutic efficacy and safety of red blood cells treated with a chemical process (S-303) for pathogen inactivation: a phase III clinical trial in cardiac surgery patients. Transfusion (2005) 45(11):1739–49. doi:10.1111/j.1537-2995.2005.00583.x
25. North AK, Henschler R, Geisen C, Garratty G, Arndt PA, Kattamis A, et al. Evaluation of naturally occurring antibodies to pathogen inactivated red blood cells. Transfusion (2010) 50(Suppl):38A.
26. Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion. Draft Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research (2016).
27. Sachais BS, Paradiso S, Strauss D, Shaz BH. Implications of the US Food and Drug Administration draft guidance for mitigating septic reactions from platelet transfusions. Blood Adv (2017) 1(15):1142–7. doi:10.1182/bloodadvances.2017008334
28. Rasongles P, Angelini-Tibert MF, Simon P, Currie C, Isola H, Kientz D, et al. Transfusion of platelet components prepared with photochemical pathogen inactivation treatment during a chikungunya virus epidemic in Ile de La Reunion. Transfusion (2009) 49(6):1083–91. doi:10.1111/j.1537-2995.2009.02111.x
29. Kuehnert MJ, Epstein JS. Assuring blood safety and availability: Zika virus, the latest emerging infectious disease battlefront. Transfusion (2016) 56(7):1669–72. doi:10.1111/trf.13673
30. Hauser L, Roque-Afonso AM, Beyloune A, Simonet M, Deau Fischer B, Burin des Roziers N, et al. Hepatitis E transmission by transfusion of Intercept blood system-treated plasma. Blood (2014) 123(5):796–7. doi:10.1182/blood-2013-09-524348
31. Goodrich RP, Gilmour D, Hovenga N, Keil SD. A laboratory comparison of pathogen reduction technology treatment and culture of platelet products for addressing bacterial contamination concerns. Transfusion (2009) 49(6):1205–16. doi:10.1111/j.1537-2995.2009.02126.x
32. Mohr H, Steil L, Gravemann U, Thiele T, Hammer E, Greinacher A, et al. A novel approach to pathogen reduction in platelet concentrates using short-wave ultraviolet light. Transfusion (2009) 49(12):2612–24. doi:10.1111/j.1537-2995.2009.02334.x
33. Kleinman S, Cameron C, Custer B, Busch M, Katz L, Kralj B, et al. Modeling the risk of an emerging pathogen entering the Canadian blood supply. Transfusion (2010) 50(12):2592–606. doi:10.1111/j.1537-2995.2010.02724.x
34. Postma MJ, van Hulst M, De Wolf JT, Botteman M, Staginnus U. Cost-effectiveness of pathogen inactivation for platelet transfusions in the Netherlands. Transfus Med (2005) 15(5):379–87. doi:10.1111/j.1365-3148.2005.00609.x
35. Custer B, Agapova M, Martinez RH. The cost-effectiveness of pathogen reduction technology as assessed using a multiple risk reduction model. Transfusion (2010) 50(11):2461–73. doi:10.1111/j.1537-2995.2010.02704.x
Keywords: transfusion, platelets, pathogen inactivation, ultraviolet light, red blood cells
Citation: Seltsam A (2017) Pathogen Inactivation of Cellular Blood Products—An Additional Safety Layer in Transfusion Medicine. Front. Med. 4:219. doi: 10.3389/fmed.2017.00219
Received: 26 September 2017; Accepted: 20 November 2017;
Published: 04 December 2017
Edited by:
Christoph Niederhauser, Transfusion Interrégionale CRS SA, SwitzerlandReviewed by:
Owen McCarty, Oregon Health & Science University, United StatesCopyright: © 2017 Seltsam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Axel Seltsam, YXhlbC5zZWx0c2FtQGJzZC1uc3RvYi5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.