- 1Scripps Translational Science Institute, The Scripps Research Institute, La Jolla, CA, United States
- 2Division of Gastroenterology and Hepatology, Department of Medicine, Scripps Clinic – Scripps Green Hospital, La Jolla, CA, United States
- 3Division of Allergy and Immunology, Department of Medicine, Scripps Clinic—Scripps Green Hospital, La Jolla, CA, United States
- 4Division of Allergy and Immunology, Department of Pediatrics, University of California, San Diego, La Jolla, CA, United States
- 5Division of Allergy and Immunology, Department of Medicine, University of California, San Diego, La Jolla, CA, United States
- 6Rady Children’s Hospital - San Diego, San Diego, CA, United States
Chronic eosinophilic inflammation is associated with tissue remodeling and fibrosis in a number of chronic T-helper 2 (Th2)-mediated diseases including eosinophilic esophagitis (EoE) and asthma. Chronic inflammation results in dysregulated tissue healing, leading to fibrosis and end organ dysfunction, manifesting clinically as irreversible airway obstruction in asthma and as esophageal rigidity, strictures, narrowing, dysmotility, dysphagia, and food impactions in EoE. Current therapies for EoE and asthma center on reducing inflammation-driven tissue remodeling and fibrosis with corticosteroids, coupled with symptomatic control and allergen avoidance. Additional control of Th2 inflammation can be achieved in select asthma patients with biologic therapies such as anti-IL-5 and anti-IL-13 antibodies, which have also been trialed in EoE. Recent molecular analysis suggests an emerging role for structural cell dysfunction, either inherited or acquired, in the pathogenesis and progression of EoE and asthma tissue remodeling. In addition, new data suggest that inflammation-independent end organ rigidity can alter structural cell function. Herein, we review emerging data and concepts for the pathogenesis of tissue remodeling and fibrosis primarily in EoE and relevant pathogenetic parallels in asthma, focusing additionally on emerging disease-specific therapies and the ability of these therapies to reduce tissue remodeling in subsets of patients.
Introduction
Allergic inflammation has the capacity to recruit eosinophils to the site of inciting stimulus. Prolonged eosinophil infiltration can contribute to significant tissue injury, leading to maladaptive tissue remodeling and fibrosis. We will focus primarily on eosinophilic disorders associated with robust tissue remodeling, specifically eosinophilic esophagitis (EoE) and its relevant pathogenetic parallels in asthma.
Clinical Features of Tissue Remodeling
The hypereosinophilic syndrome (HES)-associated tissue remodeling is arguably the most severe with cardiac damage leading to potential morbidity due to endomyocardial fibrosis. Asthma-associated airway remodeling occurs with epithelial denudation and goblet cell metaplasia, subepithelial fibrosis, angiogenesis, and smooth muscle hypertrophy (1). Remodeling is believed to be the mechanism to irreversible airway obstruction (2). EoE is an emerging chronic allergen-driven immune-mediated inflammatory disease that has been gaining recognition, with an increasing prevalence reaching 1 case per 1,000 persons (3–5). Chronic, unbridled inflammation in EoE leads to progressive esophageal fibrostenosis with rigidity and dysmotility with food impactions (6–9). Adult studies clearly demonstrate a natural history to stricture formation (6, 7). In both asthma and EoE, remodeling begins early in life, before the age of 6 years, and children with EoE can have histologic remodeling at as young as 2 years of age (2, 10).
Eosinophilic esophagitis is defined as a marked esophageal eosinophilic inflammation (≥15 eosinophils per high power field) that includes other inflammatory cells that likely contribute to remodeling such as mast cells, basophils, and adaptive as well as innate lymphoid cells (11–15). In the face of chronic antigen exposure and tissue damage, a progressive maladaptive esophageal tissue remodeling response causes clinical manifestations of dysphagia, food impactions, and, sometimes, spontaneous esophageal perforation (6, 16–20). In children, EoE often presents clinically as abdominal pain, nausea, vomiting, regurgitation, feeding difficulty, food aversion, weight loss, and failure to thrive; in adults, dysphagia and food impactions become more clinically prominent due to progression of esophageal dysfunction and fibrosis (13, 21). EoE severity has been associated with a lower body mass index, likely secondary to chronic nutritional deficit from recurrent dysphagia, food impaction, and food aversion (22). Although most EoE patients are well appearing, they often require a multimodal management approach that includes chronic medical treatment, dietary restriction, lifestyle changes, and repeated endoscopic diagnostic and therapeutic evaluations, creating a significant healthcare burden and impaired quality of life (18, 23–28).
In EoE, endoscopic features of remodeling vary between age groups. In children, features of esophageal pallor and furrows associate with histologic fibrosis and clinical dysphagia (29). In contrast, adult features of remodeling include concentric rings, narrowing, strictures, and the esophageal “pull” sign (30, 31). The narrowed and fibrostenotic esophagi are often the endoscopic features of adult EoE and can be intermittently observed in a subset of children (32). Functional readouts of esophageal rigidity include esophageal manometry and the novel application of the functional luminal imaging probe to assess esophageal rigidity and motility (33, 34). Indeed, esophageal rigidity predicts the risk of food impactions. Ultrasound studies in both adults and children show transmural esophageal thickening (35, 36). Similarly, CT scans of asthmatic airways demonstrate airway wall thickening even in children, while the HES heart can show increased cardiac muscle fibrosis with decreased chamber space (2).
Histologic Features of Remodeling
Asthmatic airways demonstrate subepithelial fibrosis, with increased trichrome staining. The asthmatic epithelium demonstrates defective epithelial barrier function and loss of junctional proteins, with goblet cell metaplasia (2, 37). Airway epithelial barrier function is thought to regulate asthma pathogenesis (38). Subepithelial angiogenesis accounts for airway wall edema, while thickened airway smooth muscle causes airway hyperreactivity. On the basis of the findings in remodeled asthmatic airways, our lab sought to understand whether esophageal biopsies from children with severe EoE had histologic findings akin to the remodeled asthmatic airway. Indeed, histopathologic analysis has shown extensive cellular and extracellular remodeling changes in EoE (13, 21, 39, 40). Remodeling is manifested in the epithelium as basal cell hyperplasia, dilated intercellular spaces, and desquamation; and in the subepithelium as fibrosis, angiogenesis, and smooth muscle hyperplasia (16, 21, 41). The loss of barrier function is a cardinal feature of the EoE esophagus with decreased expression of desmoglein-1 and filaggrin in addition to decreased E-cadherin and claudin-1 (42–44).
Molecular Mechanisms of Tissue Remodeling
Interleukins and Cytokines Involved in Remodeling
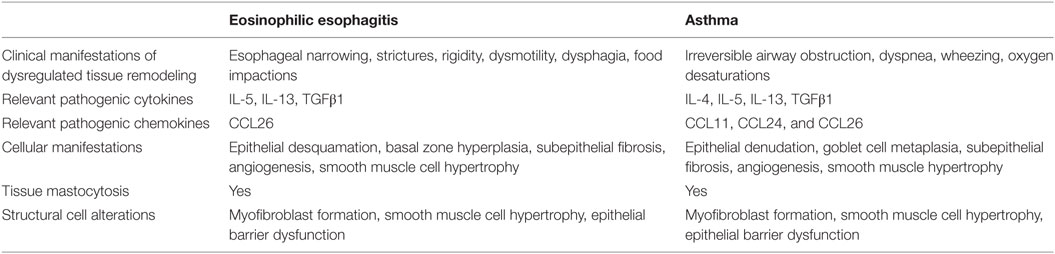
Current concepts of tissue remodeling have centralized on cellular and extracellular matrix responses to repetitive tissue injury and ineffective tissue regeneration in the context of chronic inflammation (1, 13, 21, 45). It appears that the mechanisms of remodeling are similar in asthma and EoE (Table 1). IL-4 and IL-13 play pivotal roles in asthma pathogenesis (1, 46). Progress in EoE pathogenesis to date has focused mainly on IL-13 (42, 47). Allergen-mediated induction of IL-4, IL-5, and IL-13 promotes a T-helper 2 (Th2) immune response, resulting in eosinophil recruitment and activation. In addition, profibrotic factors such as TGFβ1 appear to play an important role in the remodeling associated with these allergic diatheses (21).
IL-13 has emerged as a master regulator in EoE and drives the recruitment and activation of eosinophils via eotaxin-3/CCL26 and IL-5, further augmenting Th2 inflammation in the esophagus that can result in irreversible stricture formation (42, 47, 48). IL-13 contributes to the disruption of the epithelial barrier function, in part, via induction of calpain-14 that cleaves desmoglein-1 (49). Esophageal epithelial cells respond to IL-13 stimulation with STAT6-dependent expression of eotaxin-3/CCL26 that amplifies the chemotactic signals for further eosinophilic recruitment (47). IL-13 either alone or in combination with TGFβ1 can induce tissue fibroblasts to express periostin, further promoting eosinophil adhesion to fibronectin (50). IL-13 overexpression in an inducible transgenic murine model causes esophageal eosinophilia and stricture formation; turning off IL-13 overexpression to remove allergic inflammation reduces tissue eosinophilia but is unable to reverse the established esophageal stricture (48). In addition, GATA-1-null eosinophil-deficient IL-13 transgenic mice are able to develop esophageal tissue remodeling as evidenced by esophageal epithelial thickness, collagen deposition, and cellular hyperplasia (51). In contrast to IL-5, IL-13-mediated esophageal dysmotility and dysfunction via collagen deposition, angiogenesis, and epithelial hyperplasia can occur independently of eosinophilic inflammation (48, 51). In murine models of asthma, airway structural remodeling has been shown to persist even after complete resolution of allergic inflammation (52, 53).
IL-5 is a major cytokine that regulates eosinophilopoeisis and the trafficking, survival, and activation of eosinophils (54). Arguably, the best evidence for the role of IL-5 in human asthma is the success of humanized, monoclonal anti-IL-5 antibodies in treating eosinophilic asthma although their ability to decrease remodeling in human tissues is not clear. Peripheral blood from patients with active EoE have increased frequency of circulating activated eosinophils and IL-5-expressing CD4+ T cells, and peripheral blood mononuclear cells from EoE patients produce significantly more IL-5 compared to healthy controls when stimulated with house dust mites, ragweed, milk, Aspergillus fumigatus, or soy (55–59). Upregulated local expression of IL-5 promotes eosinophilic trafficking to the esophagus (60–62). Mice deficient in either IL-5 or eosinophils have diminished lamina propria collagen and fibronectin deposition in experimental EoE (62, 63). Esophageal strictures develop in IL-5-overexpressing transgenic mice, but not if these mice are also genetically deficient in eosinophils (48), demonstrating that the pro-remodeling effects of IL-5 are not intrinsic to this interleukin but, rather, through its capacity to recruit and activate inflammatory cells.
Eosinophils and Other Immune Cells in Tissue Remodeling
Tissue inflammation in EoE is patchy and can be transmural, with immune cell infiltration and structural changes extending from the epithelium to the underlying muscle layers allowing multiple tissue layers to be directly exposed to the damage induced by inflammatory cells (39, 64–66). Epithelial barrier disruption activates a program of IL-33, TSLP, and eotaxin-3/CCL26 expression in EoE that promotes Th2 immune activation and eosinophil infiltration (12, 67–71). Eotaxin-3/CCL26 is a potent chemoattractant for eosinophils that is highly upregulated in esophageal biopsies and sera of EoE patients (72, 73); plasma levels of eotaxin-1/CCL11 and eotaxin-2/CCL24 are not increased in active EoE (73). In comparison, epithelial levels of CCL24 and CCL26, but not CCL11, are elevated in severe asthma (1, 74). Asthmatic eosinophils migrate better in response to ex vivo stimulation with CCL26 than CCL11 or CCL24 (75); in addition, CCL26 stimulation of asthmatic eosinophils demonstrates a biphasic migration pattern that potentially contributes to eosinophil-dependent pathogenesis of persistent asthma. IL-33 and TSLP can activate the recently discovered Th2-promoting group 2 innate lymphocytes (ILC2), which are enriched in active EoE and may promote remodeling via the expression of IL-5 and IL-13 (14). Infiltrating eosinophils further drive EoE inflammation via a multitude of mechanisms including degranulation, inflammatory, and profibrotic cytokine secretion such as IL-4, IL-5, IL-13, GM-CSF, and TGFβ1, and eosinophil extracellular trap formation, which correlates with inflammatory features such as white exudates in active EoE (40, 69). GM-CSF blockade reduces basal cell hyperplasia and epithelial remodeling in experimental EoE (76). Other eosinophil blocking strategies are also successful in EoE animal models including antibody blockade with anti-Siglec-F (63, 77). Although eosinophils infiltrate densely in EoE, their complex interactions with non-immune cells such as epithelial cells, fibroblasts, and smooth muscle cells and other immune cells such as mast cells, ILC2, basophils, T cells, and invariant natural killer T cells likely dictate the histologic and clinical remodeling outcomes of the disease (5, 13, 14, 40, 78).
Eosinophilic esophagitis and asthma are also characterized by tissue mastocytosis, which contributes to esophageal and airway dysfunction. Murine models of EoE, which are deficient in mast cells, show that mast cells contribute to smooth muscle cell mass (11). Mast cells are also reservoirs for profibrotic factors such as TGFβ1, and decreases in mucosal mast cell numbers are likely one mechanism by which fibrosis improves following therapy (79). Similarly, other tryptase-positive cells such as basophils have been implicated in EoE, and blocking the TSLP receptor diminishes basophil-induced complications such as food impactions in experimental EoE (12).
Profibrotic Cytokines
Symptomatic EoE presents clinically as dysphagia, stemming from maladaptive esophageal tissue remodeling that results in fibrosis causing esophageal dysfunction and dysmotility. Eosinophils and mast cells are significant sources of TGFβ1, as previously identified in the esophagus of EoE patients and in the lungs of asthmatic patients (16, 79, 80). Eosinophils and eosinophil-derived products increase extracellular matrix production of fibronectin and collagen I in primary human esophageal fibroblasts and muscle cells in a process dependent on TGFβ1 and p38 signaling (81). TGFβ1 expression is elevated in the epithelium and subepithelium of adult and pediatric EoE patients (16, 82). TGFβ1 signaling induces collagen deposition and production of fibronectin and other extracellular matrix proteins; and blockade of the canonical TGFβ1 signaling pathway, Smad2/3, decreases remodeling in an oral ova murine EoE model (83). Also invoking the canonical TGFβ1 pathway, there is an increased epithelial and subepithelial expression of nuclear Smad2/3 in pediatric EoE patients. In addition, eosinophil-derived products, secreted products from eosinophil-fibroblast/muscle cell co-cultures, TGFβ1, or IL-13 altered esophageal muscle contraction in a feline EoE model (81). In a cohort of pediatric EoE patients, fibrosis was associated with eosinophilic degranulation in the epithelium as measured by staining for eosinophilic major basic protein, whereas fibrosis was not associated with the degree of esophageal eosinophilia, the number of mast cells, or mast cell degranulation (67). Kita and colleagues proposed that detection of eosinophil degranulation might be a more accurate assessment of EoE severity, based on their observations of marked deposition of eosinophil-derived neurotoxin in adult EoE biopsies (84).
In addition to its profibrotic effects, TGFβ1 can alter tissue contractility. TGFβ1 activates tissue fibroblasts, resulting in myofibroblast differentiation that further contributes to extracellular matrix deposition and collagen contraction (85). In addition, TGFβ1 induces primary esophageal smooth muscle cell contraction, a mechanism dependent on the canonical Smad2/3 pathway and phospholamban, a sarcoendoplasmic reticulum protein that regulates calcium flux, which is upregulated in EoE biopsies (79, 85). It is interesting to speculate if esophageal phospholamban plays a role akin to asthmatic orosomucoid like 3, which is clearly implicated in the pathogenesis of asthma.
TGFβ1 also has significant effects on the epithelium. It breaks down epithelial barriers in asthma by decreasing the expression of adhesion molecules. In EoE, remodeling has been associated with epithelial–mesenchymal transition, a TGFβ1-regulated process (86, 87). TGFβ1 significantly induces plasminogen activator inhibitor 1 (PAI-1)/serpinE1 in esophageal epithelial cells. Epithelial PAI-1 reflects the severity of histologic fibrosis and is also required for TGFβ1-induced expression of phospholamban and α-smooth muscle actin (αSMA) in esophageal fibroblasts, suggesting that it is part of the pathway to esophageal myofibroblast accumulation (88). Children with genotype TT at the TGFβ1 promoter have significantly elevated numbers of TGFβ1-positive cells, increased mast cells (but not eosinophils), more severe epithelial remodeling, and, when food sensitized, worse fibrosis than children of non-TT genotype (89).
Fibrosis may also occur independently of TGFβ1, as other profibrotic molecules such as CCL18 and fibroblast growth factor-9 (FGF9) are elevated in EoE tissue biopsies (90, 91) and not all adult subjects have elevated TGFβ1 (82, 91). CCL18 is similarly elevated in the bronchoalveolar lavage and sera of asthmatic patients and preferentially attracts Th2 cells and basophils (92). Eosinophil-derived major basic protein induces FGF9 production that can contribute to the fibroproliferative response in EoE (90). To our knowledge, the role of FGF9 in asthma has not yet been described.
Mechanotransduction and Remodeling
There is accumulating evidence that mechanical signals (“mechanosignaling”) alter the function of structural cells in the airway and esophagus in a manner that can be independent of, dependent on, or synergistic with, inflammation (93–95). Our recently published data demonstrate that rigid matrix alters the gene expression profile of primary human esophageal smooth muscle cells toward a pathogenic profile similar to that induced by TGFβ1 (95). EoE fibroblasts from children and adults had increased αSMA and traction force when cultured on a rigid matrix (94). Airway epithelial cells respond to physical parameters such as compressive forces mimicking those seen in an edematous airway with increased production of disease relevant inflammatory markers such as endothelin and TGFβ2 and decreasing expression of barrier proteins (93). In addition, compression forces increase fibroblast expression of collagens. Asthmatic bronchial fibroblasts exhibit higher elastic modulus than control cells; TGFβ1-induced differentiation of bronchial fibroblasts into myofibroblasts is enhanced by increasing matrix stiffness (96, 97). Airway smooth muscle cell contraction induces the release of more active TGFβ1 (98). Methacholine-induced bronchoconstriction in the absence of inflammation is sufficient to induce airway remodeling in asthmatic patients (99). Taken together, these compelling data invoke a shift in the thought paradigm from focus almost exclusively on inflammation to one with an integrated focus on the mechanosignaling coupled to inflammation. Indeed normalization of mechanosignaling is likely required to effectively reduce the propagation of inflammation and dysregulated structural cell gene expression. Currently, it is not clear what direct or indirect effects there are on inflammatory cells cultured either in an environment that is rigid or compressed. However, it is well accepted that cells such as mast cells respond to physical insults such as scratching.
Current and Emerging Therapeutics for Allergic Remodeling
Currently, there are no FDA-approved drugs indicated for the treatment of EoE. Some of the current treatment strategies and their effects on airway and esophageal remodeling are summarized below (5, 18, 28, 100, 101).
Topical Corticosteroids
Topical esophageal corticosteroids constitute the most commonly utilized EoE therapy in children and adults. Similarly, inhaled corticosteroids are the most common agent used for persistent asthma. There has been relatively rapid accumulation of data for EoE since biopsies are procured regularly as part of disease monitoring. In contrast, airway biopsy is done in the context of clinical trials. Short-term studies in children have demonstrated that topical corticosteroids decrease fibrosis, VCAM-1, epithelial remodeling, subepithelial TGFβ1, and nuclear Smad2/3-positive cells in the subset of patients who have resolution of epithelial eosinophils following therapy (102). As such, it appears that in “responder” children, remodeling is in flux and can be reversed or improved with short-term therapy. Such treatment-responsive remodeling likely constitutes a physiologic rather than a pathologic process. In contrast, children who are “non-responders” to therapy, as defined by persistent esophageal eosinophilia despite therapy, have continued subepithelial fibrosis, vascular activation, and TGFβ1-expressing cells. Topical fluticasone treatment downregulates mRNA expression of eotaxin-3 and decreases the degree of eosinophilic and lymphocytic tissue infiltration in EoE esophagi (47, 103, 104). In addition, topical fluticasone treatment of EoE patients reduces IL-13 mRNA expression and reverses expression of 98% of IL-13-induced EoE transcriptome to the levels of healthy controls (47). EoE esophageal mucosal integrity is improved with topical fluticasone, as seen with normalization of expression of desmoglein-1 and filaggrin (105, 106). Peripheral blood eosinophils isolated from adult corticosteroid-treated EoE patients sustain their activated phenotype (107), but exhibit decreased CD18 surface expression, with resultant diminished adherence of eosinophils to ICAM-1, ICAM-2, and endothelial cells (108). Budesonide treatment of adult EoE patients results in a statistically significant reduction in absolute blood eosinophil count and serum levels of CCL17, CCL18, CCL26, eosinophil-cationic protein, and mast cell tryptase. In addition, the absolute blood eosinophil count changes correlate with esophageal eosinophil density (109, 110).
Since EoE is a chronic disease, chronic therapy seems warranted. Studying a group of 32 children over a mean of 5 years (maximum of 10 years) treated with corticosteroids, Rajan and colleagues showed that children with EoE who persistently respond well to therapy have significantly less fibrosis and lower endoscopic scores than children who respond suboptimally to therapy (10). The clinical reasons for differences in response to therapy are not clear. However, it is possible that a “remodeling first-inflammation second” EoE phenotype is less responsive to steroid therapy. It is also possible that mechanical alterations in the esophagus, such as rigidity, change the structural and/or inflammatory cell response to interventions. This concept is echoed in the adult literature where the fibrostenotic, dysmotile esophagus is substantially more resistant to topical therapy with corticosteroids (9, 111). In terms of endoscopic and symptoms severity, topical corticosteroids can improve the diameter of the strictured adult EoE esophagus and decrease the rate of food impactions (8, 112).
In asthma, the effects of inhaled corticosteroids on remodeling and the best remodeling endpoint to follow are not entirely clear (2). This is likely due to the paucity of repeated human airway tissue for study and the complexity of the pulmonary structure as branching occurs. In a murine model of allergen-induced asthma, corticosteroids prevent myofibroblast accumulation and peribronchial collagen deposition and fibrosis (113). In addition, corticosteroids can improve a subset of gene transcripts in asthmatic airway fibroblasts (2). Combination treatments with inhaled corticosteroids and long-acting β2-adrenergic receptor agonists together have demonstrated superior prevention of asthma exacerbations (114). Systemic corticosteroids used during severe asthmatic exacerbations exhibit variable responsiveness, thought related to the underlying asthma heterogeneity, for example, corticosteroid-responsive type 2-high airway inflammation-driven “concordant disease” versus corticosteroid-resistant type 2-low “discordant disease” (115–119). Although inhaled steroids can improve epithelial shedding, this is not a consistent finding. Studies of the reticular basement membrane thickening demonstrate improvements, but whether improvement in basement membrane thickening corresponds to improvements in asthma complications such as difficult-to-treat airway hyperreactivity or irreversible airflow obstruction is not clear. Although there is not a paucity of human tissue for study in EoE, the most clinically meaningful endpoint of remodeling is still unclear, although the best targets are likely to be fibrosis and early-onset esophageal rigidity.
Efficacy of topical corticosteroid therapy is dependent on mucosal drug delivery and esophageal mucosal contact time (120). Swallowed aerosolized corticosteroid has variable delivery, with oral viscous corticosteroid preparation achieving superior esophageal mucosal delivery and treatment efficacy (120). Emerging non-proprietary and proprietary formulations of corticosteroid are expected to improve treatment options, drug bioavailability, and treatment efficacy (120–124). While corticosteroid treatment for EoE is effective, there exists a significant number of EoE patients who do not respond to topical corticosteroid treatments (122, 125). It has been proposed that topical corticosteroids are unable to penetrate the deeper esophageal layers where significant eosinophilic inflammation and tissue remodeling and fibrosis are likely to take place. Oftentimes, biopsies are limited to the superficial layers and may offer an incomplete picture of the histologic response. Targeting the fibrotic tissue may offer enhanced corticosteroid uptake. Currently, a clinical trial for EoE is examining the effect of losartan, an angiotensin II receptor blocker used clinically for hypertension that also exerts anti-fibrotic effect through suppression of active TGFβ1 levels (126). Losartan has been shown to inhibit collagen I synthesis, resulting in improved distribution and efficacy of antitumoral agents (126). Another potential beneficial effect of anti-fibrotic therapy might involve an indirect improvement of structural cell dysfunction by reducing tissue rigidity. This is based on the novel observation by Aceves and colleagues that a rigid matrix induces morphologic and transcriptional changes in esophageal smooth muscle cells with increased collagen deposition and cellular hypertrophy (95). Similar subsequent work by Muir et al. demonstrated the role of matrix stiffness in modifying TGFβ1signaling and contractility of primary esophageal fibroblasts (94). Taken together, targeting inflammation-dependent and inflammation-independent, rigidity-dependent pathways may represent novel strategies to modulate tissue remodeling and fibrosis in EoE and beyond.
Elimination Diets in EoE
In children, dietary modification to remove allergen-derived antigenic stimulation has been shown to reverse subepithelial fibrosis in EoE (127, 128). In addition, the combination of elimination diet and topical corticosteroids can decrease fibrosis in children (127). The effect of elimination diet on adult remodeling is not as clear.
Biologic Therapy
Anti-IL-5 blockade with mepolizumab is safe and achieves significant reduction in circulating peripheral eosinophils and inflamed tissue eosinophilia (129–131). Even though IL-5 is a key regulatory cytokine of eosinophils that is upregulated in EoE, anti-IL-5 therapy using two different humanized monoclonal antibodies partially reduces tissue eosinophilia but does not alter esophageal fibrosis (132, 133). Although histologic or radiographic endpoints have not been systematically assessed in asthma, anti-IL-5 is effective in patients with severe, steroid refractory asthma and can be steroid sparing in patients with HES (134–136). In children with EoE, mepolizumab treatment decreases the numbers of tryptase-positive cells, IL-9-positive cells, and esophageal eosinophil–mast cell couplets (137).
Anti-IL-13 monoclonal antibody QAX576 significantly reduces esophageal eosinophilia and expression of EoE-related genes up to 6 months after treatment, but demonstrates only a trend for improved clinical symptoms (138). IL-13 blockade with a humanized monoclonal antibody RPC4046 significantly reduces esophageal eosinophilia and endoscopic features in EoE patients and also improves dysphagia; however, the effect is more prominent in steroid refractory EoE patients, suggesting that severe subjects may do well with anti-IL-13 therapy (139). This is consistent with the decrease in transcription of some remodeling genes including periostin for up to 6 months following treatment (138). Anti-IL-13 therapy also decreases markers of remodeling such as periostin and osteopontin in asthmatics, and subjects with higher serum periostin levels are more responsive to anti-IL-13 therapy (136, 140). Dupilumab, a blocker of both IL-4 and IL-13, may be of utility in asthma and EoE-associated remodeling (140–142).
Conclusion
Both EoE and asthma are diseases that involve robust tissue remodeling as part of the disease processes with resultant end organ dysfunction in a subset of subjects. In asthma, the clinical complication is irreversible airway obstruction. In EoE, it is stricture formation. One mechanism to this complication is prolonged, unbridled inflammation that can occur due to lack of therapeutic intervention or the failure of therapies to adequately control disease progression. The presumed inflammatory signals are from infiltrating cells that respond to alterations in structural cell physiology such as decreased barrier function and the onset of chemokine production. However, other signals such as mechanical changes in the airways due to airway rigidity and epithelial contraction during repeated rounds of bronchoconstriction drive structural cells such as epithelium to generate inflammatory signals that could propagate inflammation and be unresponsive to standard anti-inflammatory therapies such as corticosteroids.
In addition to these issues, a number of additional considerations should be made when assessing the Th2-associated remodeling. The first issue is what parameters reflect remodeling most reliably? The second is the issue of pathogenic versus physiologic remodeling. The use of physiologic markers such as esophageal strictures or fixed airway obstruction likely represents an endgame of chronic disease and will likely be difficult to control. For this reason, one goal should be to find early markers of remodeling and control them. Of course, remodeling is also a normal process of wound healing that is necessary and required. What is not clear is how the shift from physiologic to pathogenic remodeling occurs. Possible explanations include disease duration, chronic inflammation, and/or mechanical signals such as tissue rigidity. Understanding the molecular mechanisms and the clinical phenotypes of these processes will be essential to better control allergic tissue remodeling and its consequences.
Author Contributions
QN and SA reviewed the literature and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported by NIH/NIAID AI 092135 (SA) and NIH/NCATS CTSA 5 UL1 TR001114 and 5KL2 TR001112 (QN).
References
1. Trejo Bittar HE, Yousem SA, Wenzel SE. Pathobiology of severe asthma. Annu Rev Pathol (2015) 10:511–45. doi:10.1146/annurev-pathol-012414-040343
2. Fehrenbach H, Wagner C, Wegmann M. Airway remodeling in asthma: what really matters. Cell Tissue Res (2017) 367(3):551–69. doi:10.1007/s00441-016-2566-8
3. Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am (2014) 43(2):201–18. doi:10.1016/j.gtc.2014.02.002
4. Furuta GT, Katzka DA. Eosinophilic esophagitis. N Engl J Med (2015) 373(17):1640–8. doi:10.1056/NEJMra1502863
5. Cianferoni A, Spergel J. Eosinophilic esophagitis: a comprehensive review. Clin Rev Allergy Immunol (2016) 50(2):159–74. doi:10.1007/s12016-015-8501-z
6. Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology (2013) 145(6):1230–6.e1–2. doi:10.1053/j.gastro.2013.08.015
7. Dellon ES, Kim HP, Sperry SL, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc (2014) 79(4):577–85.e4. doi:10.1016/j.gie.2013.10.027
8. Kuchen T, Straumann A, Safroneeva E, Romero Y, Bussmann C, Vavricka S, et al. Swallowed topical corticosteroids reduce the risk for long-lasting bolus impactions in eosinophilic esophagitis. Allergy (2014) 69(9):1248–54. doi:10.1111/all.12455
9. Colizzo JM, Clayton SB, Richter JE. Intrabolus pressure on high-resolution manometry distinguishes fibrostenotic and inflammatory phenotypes of eosinophilic esophagitis. Dis Esophagus (2016) 29(6):551–7. doi:10.1111/dote.12360
10. Rajan J, Newbury RO, Anilkumar A, Dohil R, Broide DH, Aceves SS.Long-term assessment of esophageal remodeling in patients with pediatric eosinophilic esophagitis treated with topical corticosteroids. J Allergy Clin Immunol (2016) 137(1):147–56.e8. doi:10.1016/j.jaci.2015.05.045
11. Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A. Pathogenic role of mast cells in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol (2013) 304(12):G1087–94. doi:10.1152/ajpgi.00070.2013
12. Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med (2013) 19(8):1005–13. doi:10.1038/nm.3281
13. Aceves SS. Eosinophilic esophagitis. Immunol Allergy Clin North Am (2015) 35(1):145–59. doi:10.1016/j.iac.2014.09.007
14. Doherty TA, Baum R, Newbury RO, Yang T, Dohil R, Aquino M, et al. Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol (2015) 136(3):792–4.e3. doi:10.1016/j.jaci.2015.05.048
15. Singla MB, Chehade M, Brizuela D, Maydonovitch CL, Chen YJ, Riffle ME, et al. Early comparison of inflammatory vs. fibrostenotic phenotype in eosinophilic esophagitis in a multicenter longitudinal study. Clin Transl Gastroenterol (2015) 6:e132. doi:10.1038/ctg.2015.62
16. Aceves SS, Newbury RO, Dohil R, Bastian JF, Broide DH. Esophageal remodeling in pediatric eosinophilic esophagitis. J Allergy Clin Immunol (2007) 119(1):206–12. doi:10.1016/j.jaci.2006.10.016
17. Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, et al. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol (2011) 128(1):3–20.e6; quiz 1–2. doi:10.1016/j.jaci.2011.02.040
18. Straumann A, Schoepfer AM. Therapeutic concepts in adult and paediatric eosinophilic oesophagitis. Nat Rev Gastroenterol Hepatol (2012) 9(12):697–704. doi:10.1038/nrgastro.2012.182
19. Nicodeme F, Hirano I, Chen J, Robinson K, Lin Z, Xiao Y, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol (2013) 11(9):1101–7.e1. doi:10.1016/j.cgh.2013.03.020
20. Falk GW. Clinical presentation of eosinophilic esophagitis in adults. Gastroenterol Clin North Am (2014) 43(2):231–42. doi:10.1016/j.gtc.2014.02.009
21. Aceves SS, Ackerman SJ. Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunol Allergy Clin North Am (2009) 29(1):197–211, xiii–xiv. doi:10.1016/j.iac.2008.10.003
22. Wolf WA, Piazza NA, Gebhart JH, Rusin S, Covey S, Higgins LL, et al. Association between body mass index and clinical and endoscopic features of eosinophilic esophagitis. Dig Dis Sci (2017) 62(1):143–9. doi:10.1007/s10620-016-4357-1
23. Hirano I. Dilation in eosinophilic esophagitis: to do or not to do? Gastrointest Endosc (2010) 71(4):713–4. doi:10.1016/j.gie.2009.12.026
24. Taft TH, Kern E, Keefer L, Burstein D, Hirano I. Qualitative assessment of patient-reported outcomes in adults with eosinophilic esophagitis. J Clin Gastroenterol (2011) 45(9):769–74. doi:10.1097/MCG.0b013e3182166a5a
25. Klinnert MD, Silveira L, Harris R, Moore W, Atkins D, Fleischer DM, et al. Health-related quality of life over time in children with eosinophilic esophagitis and their families. J Pediatr Gastroenterol Nutr (2014) 59(3):308–16. doi:10.1097/MPG.0000000000000451
26. Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol (2015) 110(5):626–32. doi:10.1038/ajg.2014.316
27. Safroneeva E, Coslovsky M, Kuehni CE, Zwahlen M, Haas NA, Panczak R, et al. Eosinophilic oesophagitis: relationship of quality of life with clinical, endoscopic and histological activity. Aliment Pharmacol Ther (2015) 42(8):1000–10. doi:10.1111/apt.13370
28. Singla MB, Moawad FJ. An overview of the diagnosis and management of eosinophilic esophagitis. Clin Transl Gastroenterol (2016) 7:e155. doi:10.1038/ctg.2016.4
29. Aceves SS, Newbury RO, Dohil MA, Bastian JF, Dohil R. A symptom scoring tool for identifying pediatric patients with eosinophilic esophagitis and correlating symptoms with inflammation. Ann Allergy Asthma Immunol (2009) 103(5):401–6. doi:10.1016/S1081-1206(10)60359-6
30. Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR.Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut (2013) 62(4):489–95. doi:10.1136/gutjnl-2011-301817
31. Dellon ES, Gebhart JH, Higgins LL, Hathorn KE, Woosley JT, Shaheen NJ. The esophageal biopsy “pull” sign: a highly specific and treatment-responsive endoscopic finding in eosinophilic esophagitis (with video). Gastrointest Endosc (2016) 83(1):92–100. doi:10.1016/j.gie.2015.05.046
32. Menard-Katcher C, Swerdlow MP, Mehta P, Furuta GT, Fenton LZ. Contribution of esophagram to the evaluation of complicated pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr (2015) 61(5):541–6. doi:10.1097/MPG.0000000000000849
33. Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology (2011) 140(1):82–90. doi:10.1053/j.gastro.2010.09.037
34. Carlson DA, Lin Z, Kahrilas PJ, Sternbach J, Donnan EN, Friesen L, et al. The functional lumen imaging probe detects esophageal contractility not observed with manometry in patients with achalasia. Gastroenterology (2015) 149(7):1742–51. doi:10.1053/j.gastro.2015.08.005
35. Fox VL, Nurko S, Teitelbaum JE, Badizadegan K, Furuta GT. High-resolution EUS in children with eosinophilic “allergic” esophagitis. Gastrointest Endosc (2003) 57(1):30–6. doi:10.1067/mge.2003.33
36. Straumann A, Conus S, Degen L, Frei C, Bussmann C, Beglinger C, et al. Long-term budesonide maintenance treatment is partially effective for patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol (2011) 9(5):400–9.e1. doi:10.1016/j.cgh.2011.01.017
37. Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol (2011) 128(3):549–56.e1–12. doi:10.1016/j.jaci.2011.05.038
38. Heijink IH, Nawijn MC, Hackett TL. Airway epithelial barrier function regulates the pathogenesis of allergic asthma. Clin Exp Allergy (2014) 44(5):620–30. doi:10.1111/cea.12296
39. Wechsler JB, Bryce PJ. Allergic mechanisms in eosinophilic esophagitis. Gastroenterol Clin North Am (2014) 43(2):281–96. doi:10.1016/j.gtc.2014.02.006
40. Davis BP, Rothenberg ME. Mechanisms of disease of eosinophilic esophagitis. Annu Rev Pathol (2016) 11:365–93. doi:10.1146/annurev-pathol-012615-044241
41. Cheng E, Souza RF, Spechler SJ. Tissue remodeling in eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol (2012) 303(11):G1175–87. doi:10.1152/ajpgi.00313.2012
42. Blanchard C, Stucke EM, Burwinkel K, Caldwell JM, Collins MH, Ahrens A, et al. Coordinate interaction between IL-13 and epithelial differentiation cluster genes in eosinophilic esophagitis. J Immunol (2010) 184(7):4033–41. doi:10.4049/jimmunol.0903069
43. Abdulnour-Nakhoul SM, Al-Tawil Y, Gyftopoulos AA, Brown KL, Hansen M, Butcher KF, et al. Alterations in junctional proteins, inflammatory mediators and extracellular matrix molecules in eosinophilic esophagitis. Clin Immunol (2013) 148(2):265–78. doi:10.1016/j.clim.2013.05.004
44. Sherrill JD, Kc K, Wu D, Djukic Z, Caldwell JM, Stucke EM, et al. Desmoglein-1 regulates esophageal epithelial barrier function and immune responses in eosinophilic esophagitis. Mucosal Immunol (2014) 7(3):718–29. doi:10.1038/mi.2013.90
45. Broide DH. Immunologic and inflammatory mechanisms that drive asthma progression to remodeling. J Allergy Clin Immunol (2008) 121(3):560–70; quiz 71–2. doi:10.1016/j.jaci.2008.01.031
46. Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine (2015) 75(1):68–78. doi:10.1016/j.cyto.2015.05.014
47. Blanchard C, Mingler MK, Vicario M, Abonia JP, Wu YY, Lu TX, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol (2007) 120(6):1292–300. doi:10.1016/j.jaci.2007.10.024
48. Mavi P, Rajavelu P, Rayapudi M, Paul RJ, Mishra A. Esophageal functional impairments in experimental eosinophilic esophagitis. Am J Physiol Gastrointest Liver Physiol (2012) 302(11):G1347–55. doi:10.1152/ajpgi.00013.2012
49. Davis BP, Stucke EM, Khorki ME, Litosh VA, Rymer JK, Rochman M, et al. Eosinophilic esophagitis-linked calpain 14 is an IL-13-induced protease that mediates esophageal epithelial barrier impairment. JCI Insight (2016) 1(4):e86355. doi:10.1172/jci.insight.86355
50. Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, et al. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol (2008) 1(4):289–96. doi:10.1038/mi.2008.15
51. Zuo L, Fulkerson PC, Finkelman FD, Mingler M, Fischetti CA, Blanchard C, et al. IL-13 induces esophageal remodeling and gene expression by an eosinophil-independent, IL-13R alpha 2-inhibited pathway. J Immunol (2010) 185(1):660–9. doi:10.4049/jimmunol.1000471
52. Johnson JR, Wiley RE, Fattouh R, Swirski FK, Gajewska BU, Coyle AJ, et al. Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med (2004) 169(3):378–85. doi:10.1164/rccm.200308-1094OC
53. Leigh R, Ellis R, Wattie J, Southam DS, De Hoogh M, Gauldie J, et al. Dysfunction and remodeling of the mouse airway persist after resolution of acute allergen-induced airway inflammation. Am J Respir Cell Mol Biol (2002) 27(5):526–35. doi:10.1165/rcmb.2002-0048OC
54. Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol (2009) 21(12):1303–9. doi:10.1093/intimm/dxp102
55. Yamazaki K, Murray JA, Arora AS, Alexander JA, Smyrk TC, Butterfield JH, et al. Allergen-specific in vitro cytokine production in adult patients with eosinophilic esophagitis. Dig Dis Sci (2006) 51(11):1934–41. doi:10.1007/s10620-005-9048-2
56. Bullock JZ, Villanueva JM, Blanchard C, Filipovich AH, Putnam PE, Collins MH, et al. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr (2007) 45(1):22–31. doi:10.1097/MPG.0b013e318043c097
57. Marlais M, Francis ND, Fell JM, Rawat DJ. Blood tests and histological correlates in children with eosinophilic oesophagitis. Acta Paediatr (2011) 100(8):e75–9. doi:10.1111/j.1651-2227.2011.02210.x
58. Nguyen T, Gernez Y, Fuentebella J, Patel A, Tirouvanziam R, Reshamwala N, et al. Immunophenotyping of peripheral eosinophils demonstrates activation in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr (2011) 53(1):40–7. doi:10.1097/MPG.0b013e318212647a
59. Botan V, dos Santos Borges TK, Rocha Alves EA, Claudino Pereira Couto S, Bender Kohnert Seidler H, Muniz-Junqueira MI. Enhanced activation of eosinophils in peripheral blood and implications for eosinophilic esophagitis diagnosis. J Gastroenterol Hepatol (2017) 32(7):1318–27. doi:10.1111/jgh.13710
60. Straumann A, Bauer M, Fischer B, Blaser K, Simon HU. Idiopathic eosinophilic esophagitis is associated with a T(H)2-type allergic inflammatory response. J Allergy Clin Immunol (2001) 108(6):954–61. doi:10.1067/mai.2001.119917
61. Mishra A, Hogan SP, Brandt EB, Rothenberg ME. IL-5 promotes eosinophil trafficking to the esophagus. J Immunol (2002) 168(5):2464–9. doi:10.4049/jimmunol.168.5.2464
62. Mishra A, Wang M, Pemmaraju VR, Collins MH, Fulkerson PC, Abonia JP, et al. Esophageal remodeling develops as a consequence of tissue specific IL-5-induced eosinophilia. Gastroenterology (2008) 134(1):204–14. doi:10.1053/j.gastro.2007.10.002
63. Rubinstein E, Cho JY, Rosenthal P, Chao J, Miller M, Pham A, et al. Siglec-F inhibition reduces esophageal eosinophilia and angiogenesis in a mouse model of eosinophilic esophagitis. J Pediatr Gastroenterol Nutr (2011) 53(4):409–16. doi:10.1097/MPG.0b013e3182182ff8
64. Fontillon M, Lucendo AJ. Transmural eosinophilic infiltration and fibrosis in a patient with non-traumatic Boerhaave’s syndrome due to eosinophilic esophagitis. Am J Gastroenterol (2012) 107(11):1762. doi:10.1038/ajg.2012.226
65. Saffari H, Peterson KA, Fang JC, Teman C, Gleich GJ, Pease LF III. Patchy eosinophil distributions in an esophagectomy specimen from a patient with eosinophilic esophagitis: implications for endoscopic biopsy. J Allergy Clin Immunol (2012) 130(3):798–800. doi:10.1016/j.jaci.2012.03.009
66. Cianferoni A, Spergel JM, Muir A. Recent advances in the pathological understanding of eosinophilic esophagitis. Expert Rev Gastroenterol Hepatol (2015) 9(12):1501–10. doi:10.1586/17474124.2015.1094372
67. Chehade M, Sampson HA, Morotti RA, Magid MS. Esophageal subepithelial fibrosis in children with eosinophilic esophagitis. J Pediatr Gastroenterol Nutr (2007) 45(3):319–28. doi:10.1097/MPG.0b013e31806ab384
68. Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet (2010) 42(4):289–91. doi:10.1038/ng.547
69. Simon D, Radonjic-Hosli S, Straumann A, Yousefi S, Simon HU. Active eosinophilic esophagitis is characterized by epithelial barrier defects and eosinophil extracellular trap formation. Allergy (2015) 70(4):443–52. doi:10.1111/all.12570
70. Chandramouleeswaran PM, Shen D, Lee AJ, Benitez A, Dods K, Gambanga F, et al. Preferential secretion of thymic stromal lymphopoietin (TSLP) by terminally differentiated esophageal epithelial cells: relevance to eosinophilic esophagitis (EoE). PLoS One (2016) 11(3):e0150968. doi:10.1371/journal.pone.0150968
71. Judd LM, Heine RG, Menheniott TR, Buzzelli J, O’Brien-Simpson N, Pavlic D, et al. Elevated IL-33 expression is associated with pediatric eosinophilic esophagitis, and exogenous IL-33 promotes eosinophilic esophagitis development in mice. Am J Physiol Gastrointest Liver Physiol (2016) 310(1):G13–25. doi:10.1152/ajpgi.00290.2015
72. Blanchard C, Wang N, Stringer KF, Mishra A, Fulkerson PC, Abonia JP, et al. Eotaxin-3 and a uniquely conserved gene-expression profile in eosinophilic esophagitis. J Clin Invest (2006) 116(2):536–47. doi:10.1172/JCI26679
73. Konikoff MR, Blanchard C, Kirby C, Buckmeier BK, Cohen MB, Heubi JE, et al. Potential of blood eosinophils, eosinophil-derived neurotoxin, and eotaxin-3 as biomarkers of eosinophilic esophagitis. Clin Gastroenterol Hepatol (2006) 4(11):1328–36. doi:10.1016/j.cgh.2006.08.013
74. Coleman JM, Naik C, Holguin F, Ray A, Ray P, Trudeau JB, et al. Epithelial eotaxin-2 and eotaxin-3 expression: relation to asthma severity, luminal eosinophilia and age at onset. Thorax (2012) 67(12):1061–6. doi:10.1136/thoraxjnl-2012-201634
75. Provost V, Larose MC, Langlois A, Rola-Pleszczynski M, Flamand N, Laviolette M. CCL26/eotaxin-3 is more effective to induce the migration of eosinophils of asthmatics than CCL11/eotaxin-1 and CCL24/eotaxin-2. J Leukoc Biol (2013) 94(2):213–22. doi:10.1189/jlb.0212074
76. McNamee EN, Biette KA, Hammer J, Harris R, Miyazawa H, Lee JJ, et al. Targeting granulocyte-macrophage colony-stimulating factor in epithelial and vascular remodeling in experimental eosinophilic esophagitis. Allergy (2017) 72(8):1232–42. doi:10.1111/all.13105
77. Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy (2008) 63(9):1156–63. doi:10.1111/j.1398-9995.2008.01709.x
78. Rothenberg ME. Molecular, genetic, and cellular bases for treating eosinophilic esophagitis. Gastroenterology (2015) 148(6):1143–57. doi:10.1053/j.gastro.2015.02.002
79. Aceves SS, Chen D, Newbury RO, Dohil R, Bastian JF, Broide DH. Mast cells infiltrate the esophageal smooth muscle in patients with eosinophilic esophagitis, express TGF-beta1, and increase esophageal smooth muscle contraction. J Allergy Clin Immunol (2010) 126(6):1198–204.e4. doi:10.1016/j.jaci.2010.08.050
80. Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, et al. Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol (1997) 17(3):326–33. doi:10.1165/ajrcmb.17.3.2733
81. Rieder F, Nonevski I, Ma J, Ouyang Z, West G, Protheroe C, et al. T-helper 2 cytokines, transforming growth factor beta1, and eosinophil products induce fibrogenesis and alter muscle motility in patients with eosinophilic esophagitis. Gastroenterology (2014) 146(5):1266–77.e1–9. doi:10.1053/j.gastro.2014.01.051
82. Straumann A, Conus S, Degen L, Felder S, Kummer M, Engel H, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology (2010) 139(5):1526–37, 37.e1. doi:10.1053/j.gastro.2010.07.048
83. Cho JY, Doshi A, Rosenthal P, Beppu A, Miller M, Aceves S, et al. Smad3-deficient mice have reduced esophageal fibrosis and angiogenesis in a model of egg-induced eosinophilic esophagitis. J Pediatr Gastroenterol Nutr (2014) 59(1):10–6. doi:10.1097/MPG.0000000000000343
84. Kephart GM, Alexander JA, Arora AS, Romero Y, Smyrk TC, Talley NJ, et al. Marked deposition of eosinophil-derived neurotoxin in adult patients with eosinophilic esophagitis. Am J Gastroenterol (2010) 105(2):298–307. doi:10.1038/ajg.2009.584
85. Beppu LY, Anilkumar AA, Newbury RO, Dohil R, Broide DH, Aceves SS. TGF-beta1-induced phospholamban expression alters esophageal smooth muscle cell contraction in patients with eosinophilic esophagitis. J Allergy Clin Immunol (2014) 134(5):1100–7.e4. doi:10.1016/j.jaci.2014.04.004
86. Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest (2003) 112(12):1776–84. doi:10.1172/JCI20530
87. Kagalwalla AF, Akhtar N, Woodruff SA, Rea BA, Masterson JC, Mukkada V, et al. Eosinophilic esophagitis: epithelial mesenchymal transition contributes to esophageal remodeling and reverses with treatment. J Allergy Clin Immunol (2012) 129(5):1387–96.e7. doi:10.1016/j.jaci.2012.03.005
88. Rawson R, Yang T, Newbury RO, Aquino M, Doshi A, Bell B, et al. TGF-beta1-induced PAI-1 contributes to a profibrotic network in patients with eosinophilic esophagitis. J Allergy Clin Immunol (2016) 138(3):791–800.e4. doi:10.1016/j.jaci.2016.02.028
89. Rawson R, Anilkumar A, Newbury RO, Bafna V, Aquino M, Palmquist J, et al. The TGFbeta1 promoter SNP C-509T and food sensitization promote esophageal remodeling in pediatric eosinophilic esophagitis. PLoS One (2015) 10(12):e0144651. doi:10.1371/journal.pone.0144651
90. Mulder DJ, Pacheco I, Hurlbut DJ, Mak N, Furuta GT, MacLeod RJ, et al. FGF9-induced proliferative response to eosinophilic inflammation in oesophagitis. Gut (2009) 58(2):166–73. doi:10.1136/gut.2008.157628
91. Lucendo AJ, Arias A, De Rezende LC, Yague-Compadre JL, Mota-Huertas T, Gonzalez-Castillo S, et al. Subepithelial collagen deposition, profibrogenic cytokine gene expression, and changes after prolonged fluticasone propionate treatment in adult eosinophilic esophagitis: a prospective study. J Allergy Clin Immunol (2011) 128(5):1037–46. doi:10.1016/j.jaci.2011.08.007
92. de Nadai P, Charbonnier AS, Chenivesse C, Senechal S, Fournier C, Gilet J, et al. Involvement of CCL18 in allergic asthma. J Immunol (2006) 176(10):6286–93. doi:10.4049/jimmunol.176.10.6286
93. Gosens R, Grainge C. Bronchoconstriction and airway biology: potential impact and therapeutic opportunities. Chest (2015) 147(3):798–803. doi:10.1378/chest.14-1142
94. Muir AB, Dods K, Henry SJ, Benitez AJ, Lee D, Whelan KA, et al. Eosinophilic esophagitis-associated chemical and mechanical microenvironment shapes esophageal fibroblast behavior. J Pediatr Gastroenterol Nutr (2016) 63(2):200–9. doi:10.1097/MPG.0000000000001100
95. Tkachenko E, Rawson R, La E, Doherty TA, Baum R, Cavagnero K, et al. Rigid substrate induces esophageal smooth muscle hypertrophy and eosinophilic esophagitis fibrotic gene expression. J Allergy Clin Immunol (2016) 137(4):1270–2.e1. doi:10.1016/j.jaci.2015.09.020
96. Shi Y, Dong Y, Duan Y, Jiang X, Chen C, Deng L. Substrate stiffness influences TGF-beta1-induced differentiation of bronchial fibroblasts into myofibroblasts in airway remodeling. Mol Med Rep (2013) 7(2):419–24. doi:10.3892/mmr.2012.1213
97. Sarna M, Wojcik KA, Hermanowicz P, Wnuk D, Burda K, Sanak M, et al. Undifferentiated bronchial fibroblasts derived from asthmatic patients display higher elastic modulus than their non-asthmatic counterparts. PLoS One (2015) 10(2):e0116840. doi:10.1371/journal.pone.0116840
98. Oenema TA, Maarsingh H, Smit M, Groothuis GM, Meurs H, Gosens R. Bronchoconstriction induces TGF-beta release and airway remodelling in guinea pig lung slices. PLoS One (2013) 8(6):e65580. doi:10.1371/journal.pone.0065580
99. Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, et al. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med (2011) 364(21):2006–15. doi:10.1056/NEJMoa1014350
100. Cianferoni A, Spergel JM. Immunotherapeutic approaches for the treatment of eosinophilic esophagitis. Immunotherapy (2014) 6(3):321–31. doi:10.2217/imt.14.3
101. Goyal A, Cheng E. Recent discoveries and emerging therapeutics in eosinophilic esophagitis. World J Gastrointest Pharmacol Ther (2016) 7(1):21–32. doi:10.4292/wjgpt.v7.i1.21
102. Aceves SS, Newbury RO, Chen D, Mueller J, Dohil R, Hoffman H, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy (2010) 65(1):109–16. doi:10.1111/j.1398-9995.2009.02142.x
103. Noel RJ, Putnam PE, Collins MH, Assa’ad AH, Guajardo JR, Jameson SC, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol (2004) 2(7):568–75. doi:10.1016/S1542-3565(04)00240-X
104. Lucendo AJ, De Rezende L, Comas C, Caballero T, Bellon T. Treatment with topical steroids downregulates IL-5, eotaxin-1/CCL11, and eotaxin-3/CCL26 gene expression in eosinophilic esophagitis. Am J Gastroenterol (2008) 103(9):2184–93. doi:10.1111/j.1572-0241.2008.01937.x
105. Katzka DA, Tadi R, Smyrk TC, Katarya E, Sharma A, Geno DM, et al. Effects of topical steroids on tight junction proteins and spongiosis in esophageal epithelia of patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol (2014) 12(11):1824–9.e1. doi:10.1016/j.cgh.2014.02.039
106. van Rhijn BD, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RM, de Jonge WJ, et al. Histological response to fluticasone propionate in patients with eosinophilic esophagitis is associated with improved functional esophageal mucosal integrity. Am J Gastroenterol (2015) 110(9):1289–97. doi:10.1038/ajg.2015.247
107. Johnsson M, Bove M, Bergquist H, Olsson M, Fornwall S, Hassel K, et al. Distinctive blood eosinophilic phenotypes and cytokine patterns in eosinophilic esophagitis, inflammatory bowel disease and airway allergy. J Innate Immun (2011) 3(6):594–604. doi:10.1159/000331326
108. Lingblom C, Bergquist H, Johnsson M, Sundstrom P, Quiding-Jarbrink M, Bove M, et al. Topical corticosteroids do not revert the activated phenotype of eosinophils in eosinophilic esophagitis but decrease surface levels of CD18 resulting in diminished adherence to ICAM-1, ICAM-2, and endothelial cells. Inflammation (2014) 37(6):1932–44. doi:10.1007/s10753-014-9926-x
109. Schlag C, Miehlke S, Heiseke A, Brockow K, Krug A, von Arnim U, et al. Peripheral blood eosinophils and other non-invasive biomarkers can monitor treatment response in eosinophilic oesophagitis. Aliment Pharmacol Ther (2015) 42(9):1122–30. doi:10.1111/apt.13386
110. Min SB, Nylund CM, Baker TP, Ally M, Reinhardt B, Chen YJ, et al. Longitudinal evaluation of noninvasive biomarkers for eosinophilic esophagitis. J Clin Gastroenterol (2017) 51(2):127–35. doi:10.1097/MCG.0000000000000621
111. Eluri S, Runge TM, Cotton CC, Burk CM, Wolf WA, Woosley JT, et al. The extremely narrow-caliber esophagus is a treatment-resistant subphenotype of eosinophilic esophagitis. Gastrointest Endosc (2016) 83(6):1142–8. doi:10.1016/j.gie.2015.11.019
112. Lee J, Huprich J, Kujath C, Ravi K, Enders F, Smyrk TC, et al. Esophageal diameter is decreased in some patients with eosinophilic esophagitis and might increase with topical corticosteroid therapy. Clin Gastroenterol Hepatol (2012) 10(5):481–6. doi:10.1016/j.cgh.2011.12.042
113. Miller M, Cho JY, McElwain K, McElwain S, Shim JY, Manni M, et al. Corticosteroids prevent myofibroblast accumulation and airway remodeling in mice. Am J Physiol Lung Cell Mol Physiol (2006) 290(1):L162–9. doi:10.1152/ajplung.00252.2005
114. Loymans RJ, Gemperli A, Cohen J, Rubinstein SM, Sterk PJ, Reddel HK, et al. Comparative effectiveness of long term drug treatment strategies to prevent asthma exacerbations: network meta-analysis. BMJ (2014) 348:g3009. doi:10.1136/bmj.g3009
115. Fitzpatrick AM, Stephenson ST, Brown MR, Nguyen K, Douglas S, Brown LA. Systemic corticosteroid responses in children with severe asthma: phenotypic and endotypic features. J Allergy Clin Immunol Pract (2017) 5(2):410–9.e4. doi:10.1016/j.jaip.2016.08.001
116. Bossley CJ, Fleming L, Ullmann N, Gupta A, Adams A, Nagakumar P, et al. Assessment of corticosteroid response in pediatric patients with severe asthma by using a multidomain approach. J Allergy Clin Immunol (2016) 138(2):413–20e6. doi:10.1016/j.jaci.2015.12.1347
117. Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Effects of age and disease severity on systemic corticosteroid responses in asthma. Am J Respir Crit Care Med (2017) 195(11):1439–48. doi:10.1164/rccm.201607-1453OC
118. Brisk R, Heaney LG. Asthma control and exacerbations: two different sides of the same coin. Curr Opin Pulm Med (2016) 22(1):32–7. doi:10.1097/MCP.0000000000000222
119. Robinson D, Humbert M, Buhl R, Cruz AA, Inoue H, Korom S, et al. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy (2017) 47(2):161–75. doi:10.1111/cea.12880
120. Dellon ES, Sheikh A, Speck O, Woodward K, Whitlow AB, Hores JM, et al. Viscous topical is more effective than nebulized steroid therapy for patients with eosinophilic esophagitis. Gastroenterology (2012) 143(2):321–4.e1. doi:10.1053/j.gastro.2012.04.049
121. Rubinstein E, Lee JJ, Fried A, Logvinenko T, Ngo P, McDonald D, et al. Comparison of 2 delivery vehicles for viscous budesonide to treat eosinophilic esophagitis in children. J Pediatr Gastroenterol Nutr (2014) 59(3):317–20. doi:10.1097/MPG.0000000000000436
122. Gupta SK, Vitanza JM, Collins MH. Efficacy and safety of oral budesonide suspension in pediatric patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol (2015) 13(1):66–76.e3. doi:10.1016/j.cgh.2014.05.021
123. Hefner JN, Howard RS, Massey R, Valencia M, Stocker DJ, Philla KQ, et al. A randomized controlled comparison of esophageal clearance times of oral budesonide preparations. Dig Dis Sci (2016) 61(6):1582–90. doi:10.1007/s10620-015-3990-4
124. Miehlke S, Hruz P, Vieth M, Bussmann C, von Arnim U, Bajbouj M, et al. A randomised, double-blind trial comparing budesonide formulations and dosages for short-term treatment of eosinophilic oesophagitis. Gut (2016) 65(3):390–9. doi:10.1136/gutjnl-2014-308815
125. Albert D, Heifert TA, Min SB, Maydonovitch CL, Baker TP, Chen YJ, et al. Comparisons of fluticasone to budesonide in the treatment of eosinophilic esophagitis. Dig Dis Sci (2016) 61(7):1996–2001. doi:10.1007/s10620-016-4110-9
126. Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A (2011) 108(7):2909–14. doi:10.1073/pnas.1018892108
127. Abu-Sultaneh SM, Durst P, Maynard V, Elitsur Y. Fluticasone and food allergen elimination reverse sub-epithelial fibrosis in children with eosinophilic esophagitis. Dig Dis Sci (2011) 56(1):97–102. doi:10.1007/s10620-010-1259-5
128. Lieberman JA, Morotti RA, Konstantinou GN, Yershov O, Chehade M. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy (2012) 67(10):1299–307. doi:10.1111/j.1398-9995.2012.02881.x
129. Garrett JK, Jameson SC, Thomson B, Collins MH, Wagoner LE, Freese DK, et al. Anti-interleukin-5 (mepolizumab) therapy for hypereosinophilic syndromes. J Allergy Clin Immunol (2004) 113(1):115–9. doi:10.1016/j.jaci.2003.10.049
130. Stein ML, Villanueva JM, Buckmeier BK, Yamada Y, Filipovich AH, Assa’ad AH, et al. Anti-IL-5 (mepolizumab) therapy reduces eosinophil activation ex vivo and increases IL-5 and IL-5 receptor levels. J Allergy Clin Immunol (2008) 121(6):1473–83, 1483.e1–4. doi:10.1016/j.jaci.2008.02.033
131. Conus S, Straumann A, Bettler E, Simon HU. Mepolizumab does not alter levels of eosinophils, T cells, and mast cells in the duodenal mucosa in eosinophilic esophagitis. J Allergy Clin Immunol (2010) 126(1):175–7. doi:10.1016/j.jaci.2010.04.029
132. Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology (2011) 141(5):1593–604. doi:10.1053/j.gastro.2011.07.044
133. Spergel JM, Rothenberg ME, Collins MH, Furuta GT, Markowitz JE, Fuchs G III, et al. Reslizumab in children and adolescents with eosinophilic esophagitis: results of a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol (2012) 129(2):456–63, 463.e1–3. doi:10.1016/j.jaci.2011.11.044
134. Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov (2012) 11(12):958–72. doi:10.1038/nrd3792
135. Pelaia G, Vatrella A, Busceti MT, Gallelli L, Preiano M, Lombardo N, et al. Role of biologics in severe eosinophilic asthma – focus on reslizumab. Ther Clin Risk Manag (2016) 12:1075–82. doi:10.2147/TCRM.S111862
136. Bel EH, Ten Brinke A. New anti-eosinophil drugs for asthma and COPD: targeting the trait! Chest (2017). doi:10.1016/j.chest.2017.05.019
137. Otani IM, Anilkumar AA, Newbury RO, Bhagat M, Beppu LY, Dohil R, et al. Anti-IL-5 therapy reduces mast cell and IL-9 cell numbers in pediatric patients with eosinophilic esophagitis. J Allergy Clin Immunol (2013) 131(6):1576–82. doi:10.1016/j.jaci.2013.02.042
138. Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti-IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. J Allergy Clin Immunol (2015) 135(2):500–7. doi:10.1016/j.jaci.2014.07.049
139. Dellon ES, Collins M, Assouline-Dayan Y, Evans L, Gupta SK, Schoepfer A, et al. A randomized, double-blind, placebo-controlled trial of a novel recombinant, humanized, anti-interleukin-13 monoclonal antibody (RPC4046) in patients with active eosinophilic esophagitis: results of the HEROES study. American College of Gastroenterology (ACG) 2016 Annual Scientific Meeting; Abstract #19; 2016 October 18; Las Vegas, NV (2016).
140. Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW.A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol (2016) 170(2):122–31. doi:10.1159/000447692
141. Kau AL, Korenblat PE. Anti-interleukin 4 and 13 for asthma treatment in the era of endotypes. Curr Opin Allergy Clin Immunol (2014) 14(6):570–5. doi:10.1097/ACI.0000000000000108
Keywords: eosinophilic esophagitis, asthma, inflammation, tissue remodeling, fibrosis, structural cell dysfunction, corticosteroid, biologic therapy
Citation: Nhu QM and Aceves SS (2017) Tissue Remodeling in Chronic Eosinophilic Esophageal Inflammation: Parallels in Asthma and Therapeutic Perspectives. Front. Med. 4:128. doi: 10.3389/fmed.2017.00128
Received: 10 May 2017; Accepted: 21 July 2017;
Published: 07 August 2017
Edited by:
Mats W. Johansson, University of Wisconsin-Madison, United StatesReviewed by:
Joanne Carol Masterson, University of Colorado Denver, United StatesAdam Collison, University of Newcastle, Australia
Copyright: © 2017 Nhu and Aceves. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Quan M. Nhu, bmh1LnF1YW5Ac2NyaXBwc2hlYWx0aC5vcmc=, cW5odUB1Y3NkLmVkdQ==;
Seema S. Aceves, c2FjZXZlc0B1Y3NkLmVkdQ==
 Quan M. Nhu
Quan M. Nhu Seema S. Aceves4,5,6*
Seema S. Aceves4,5,6*