- The Ohio State University College of Medicine, Columbus, OH, United States
A sinus of Valsalva aneurysm is a rare malformation of the aortic root that can fistulize to another cardiac structure such as the right atrium. Although transthoracic echocardiography and computed tomography angiography have demonstrated utility for the diagnosis of a sinus of Valsalva-to-right atrial fistula, there are few cases where a misdiagnosis may occur. Intraoperative transesophageal echocardiography may be an essential imaging tool for the diagnosis and management of incidental findings such as a sinus of Valsalva-to-right atrial fistula during cardiac surgery and should be used routinely.
Introduction
A sinus of Valsalva aneurysm (SOVA) is a rare cardiac anomaly where dilation of the wall of the aortic root area between the aortic valve annulus and the sinotubular ridge develops and is often associated with numerous cardiac complications that dictate the acuity and severity of its clinical presentation (1–8). Surgical intervention has shown promising outcomes with 10-year survival ranging between 63 and 100%, but given the often-urgent nature of the presentation of a SOVA, accurate diagnosis and anticipation of associated complications is imperative to guide appropriate, and potentially life-saving, therapy (9, 10).
The authors present the case of a patient undergoing a SOVA repair in which an aorto-right atrial fistula was incidentally identified on intraoperative transesophageal echocardiography (TEE) examination; the presence of the fistula prompted a change in the intraoperative management of the patient.
Case Presentation
A 57-year-old male with a past medical history of hypertension, myocardial infarction, hyperlipidemia, and sepsis was found by his physician to have a murmur during a visit for severe exertional dyspnea. A transthoracic echocardiogram (TTE) showed a large SOVA located at the non-coronary cusp, moderate enlargement of the right atrium (RA) and the right ventricle (RV), mild systolic dysfunction of the left ventricle, and no echocardiographic evidence of valvular pathology or intracardiac shunts. A computed tomography angiogram (CTA) was obtained to further characterize the SOVA and revealed a 2.2 cm × 1.6 cm × 2.3 cm saccular aneurysm herniating from the non-coronary cusp into the RA, in addition to a dilated RA and a dilated RV. The CTA further demonstrated the following: complete opacification of the RA, the RV, and the aortic root by the intravenous contrast agent, and extensive reflux of contrast into the inferior vena cava and the hepatic veins during systole that was suggestive of significant tricuspid valve insufficiency or a large left-to-right shunt. The likelihood of an intracardiac shunt was high considering the absence of tricuspid insufficiency on the TTE examination; however, further cardiac imaging was not considered since TEE monitoring was being employed intraoperatively. A percutaneous coronary angiogram was also performed, which revealed mild non-obstructive atherosclerotic disease.
The patient was subsequently scheduled for repair of the SOVA. A left radial intra-arterial catheter was inserted for hemodynamic monitoring prior to the induction of general anesthesia and endotracheal intubation. Post-intubation, a TEE probe was inserted to guide central venous catheter placement and hemodynamic monitoring. The TEE demonstrated a 1.9 cm × 2.6 cm saccular aneurysm at the level of the non-coronary sinus, which communicated with the RA upon color flow Doppler analysis (Figures 1 and 2; Videos S1 and S2 in Supplementary Material), a finding not apparent on the pre-operative CTA or TTE. Substantial communication between the ascending aorta and the RA was identified and the decision was made to avoid placing a pulmonary arterial catheter, which potentially could access the SOVA–RA fistula (Figures 3 and 4) and cause aortic injury. Cardiopulmonary bypass was then instituted after adequate systemic anticoagulation with heparin. The orifice of the SOVA was repaired with a bovine pericardial patch successfully. Afterward, the fistula tract was accessed using a right atriotomy and was excised and oversewn. The patient tolerated the procedure well and had an uneventful recovery.
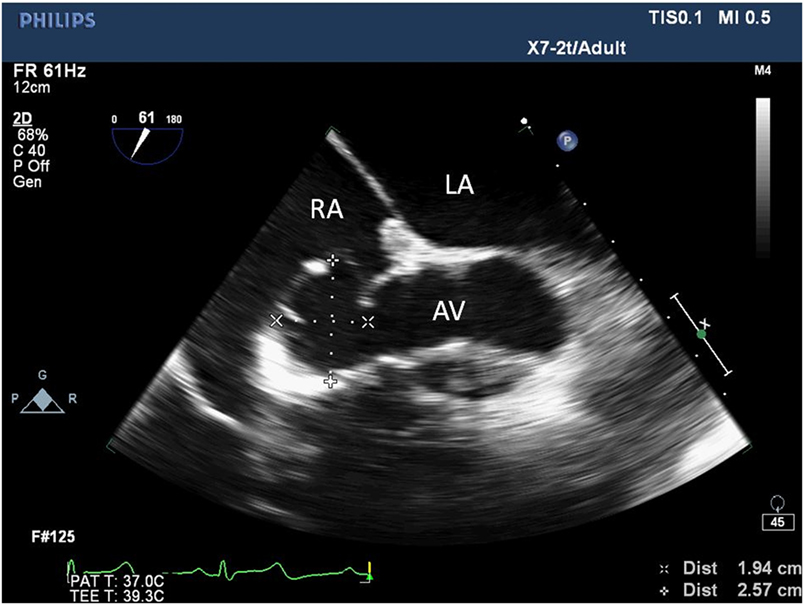
Figure 1. Two-dimensional echocardiogram short-axis view of the aortic valve showing an aneurysm at the non-coronary sinus of Valsalva measuring 1.94 cm × 2.57 cm. LA, left atrium; RA, right atrium; AV, aortic valve.
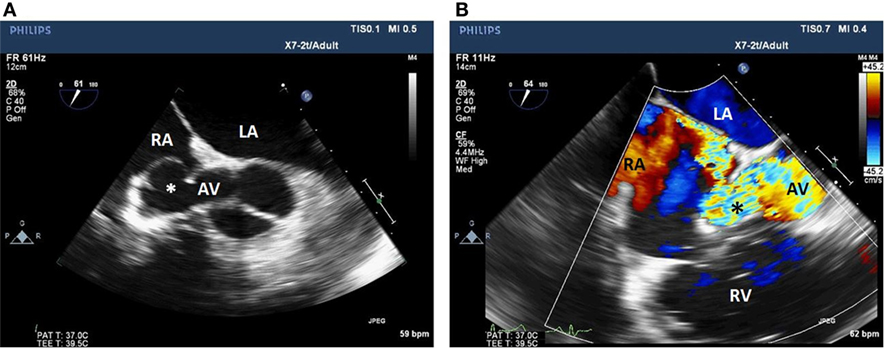
Figure 2. Two-dimensional echocardiogram short-axis view of the aortic valve (A) with color flow Doppler (B) showing a fistula between the non-coronary sinus of Valsalva aneurysm and the RA. LA, left atrium; RA, right atrium; AV, aortic valve; Asterix, sinus of Valsalva aneurysm; RV, right ventricle.
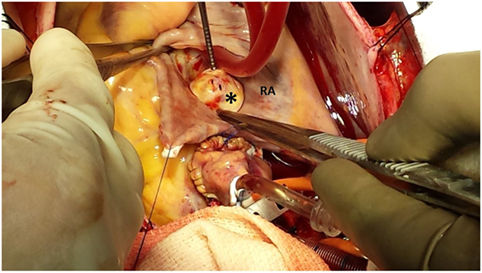
Figure 3. Image demonstrating the non-coronary sinus of Valsalva aneurysm communicating with the RA. RA, right atrium; Asterix, sinus of Valsalva aneurysm.
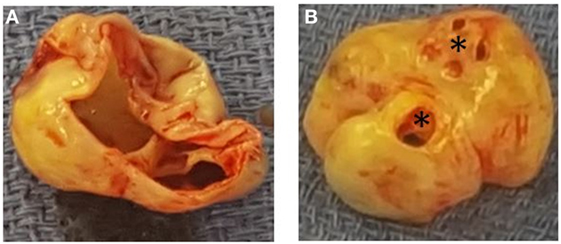
Figure 4. Panel demonstrating the non-coronary sinus of Valsalva aneurysm form the perspective of the aortic root (A) and the perspective of the right atrium (B). Asterix, sinus of Valsalva aneurysm-to-right atrial fistulas.
Discussion
A SOVA is a rare cardiac malformation involving the aortic root. It predominantly originates from the right coronary cusp (39–89%), followed by the non-coronary cusp, and rarely from the left coronary cusp (3–6, 9, 11–20). Diagnosis of a SOVA often warrants further evaluation, as its presence is often associated with various cardiac sequelae such as aneurysmal rupture, aneurysmal fistulization, ventricular septal defect, RV outflow tract obstruction, and aortic valve dysfunction (3).
Transthoracic echocardiography, the initial diagnostic study performed in the presented case, often provides sufficient information regarding the presence, location, and complicating cardiac lesions associated with a SOVA. Although TTE appropriately identified the presence and origin of a SOVA, it failed to discover the communicating tract between the SOVA and the RA. Furthermore, the TTE did not indicate any evidence of aortic insufficiency or any other valvular pathology, as initially anticipated following the discovery of the murmur, which in retrospect was due to blood flow from the aorta root to the RA. In a recent report by Cheng et al., TTE provided a diagnostic sensitivity, specificity, and accuracy in the identification of SOVA of 93.9, 99.9, and 99.8%, respectively (3). Moreover, the authors reported that TTE could be relied upon to accurately diagnose the presence of aneurysmal rupture in most cases (97.5%).
Multimodal cardiac imaging, in the form of CTA and cardiac magnetic resonance imaging (CMRI), can complement echocardiography. Both CTA and CMRI offer excellent anatomical depiction of a SOVA and may help identify fistulous tracts (21, 22). The CTA in the case presented, however, did not identify the SOVA to RA fistula due to concomitant opacification of the aorta and the RA by the radiographic contrast. The reflux of contrast material into the inferior vena cava and hepatic venous system was due to the fistula (23). Retrograde aortography previously served as an invaluable diagnostic tool for patients with suspected SOVA and was relied upon particularly prior to the advancements in echocardiography (24). Considering that contrast is administered in the aorta immediately before imaging, the presence of a fistula to the RA can be rapidly identified with a retrograde aortogram. In the presented case, direct ostial access of the right coronary and left coronary circulation during the cardiac catheterization precluded any assessment of the non-coronary sinus, thus resulting in the failure to identify the fistulous tract.
Finding an aorto-RA fistula early during the operative course holds major implications for both the cardiac anesthesiologist and the cardiothoracic surgeon. During pulmonary artery catheter placement, inadvertent passage of the catheter through the fistulous tract may cause aneurysmal rupture and hemodynamic compromise. Furthermore, identification of such findings may alter surgical management and should be communicated as quickly as possible to accommodate additional procedures as needed.
In the case presented, despite undergoing a comprehensive cardiac work-up prior to surgery inclusive of a TTE, a CTA, and a coronary angiogram, the fistulous tract between the non-coronary cusp and the RA was clearly not identified, and highlights the utility of, and further advocates for early intraoperative application of TEE for the elucidation of overall cardiac pathophysiology in patients undergoing SOVA repair.
Summary
Transesophageal echocardiography is a useful imaging modality that can provide clarity and insight into often challenging cardiac presentations. It is incumbent upon the cardiac anesthesiologist to display medical acumen and effectively utilize this imaging modality. Although standard practice does not dictate early application of TEE during the operative course, we recommend that TEE be utilized immediately post-induction to detect any previously unforeseen cardiac pathology and better guide both anesthetic and surgical care in patients undergoing SOVA repair.
Ethics Statement
Consent was obtained for publication of this case report.
Author Contributions
All authors contributed equally to the creation of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fmed.2017.00095/full#supplementary-material.
Video S1. Two-dimensional echocardiogram right ventricular inflow–outflow view demonstrating a non-coronary sinus of Valsalva aneurysm.
Video S2. Two-dimensional echocardiogram right ventricular inflow–outflow view with color flow Doppler demonstrating a non-coronary sinus of Valsalva aneurysm-to-right atrial fistula.
References
2. Smith WA. Aneurysm of the sinus of Valsalva with report of two cases. J Am Med Assoc (1914) 62:1878. doi:10.1001/jama.1914.02560490024006
3. Cheng T, Yang Y, Xie M, Wang XF, Dong NG, Su W, et al. Echocardiographic diagnosis of sinus of Valsalva aneurysm: a 17-year (1995-2012) experience of 212 surgically treated patients from one single medical center in China. Int J Cardiol (2014) 173:33–9. doi:10.1016/j.ijcard.2014.02.003
4. Chu S, Hung C, How S, Chang H, Wang SS, Tsai CH, et al. Ruptured aneurysms of sinus of Valsalva in oriental patients. J Thorac Cardiovasc Surg (1990) 99:228–98.
5. Dong C, Wu Q, Tang Y. Ruptured sinus of Valsalva aneurysm: a Beijing experience. Ann Thorac Surg (2002) 74:1621–4. doi:10.1016/S0003-4975(02)03987-5
6. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of Valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann (2008) 16:361–5. doi:10.1177/021849230801600504
7. Weinreich M, Yu PJ, Trost B. Sinus of Valsalva aneurysms: review of the literature and an update on management. Clin Cardiol (2015) 38(3):185–9. doi:10.1002/clc.22359
8. Feldman D, Roman M. Aneurysms of the sinus of Valsalva. Cardiology (2006) 106:73–81. doi:10.1159/000092635
9. Moustafa S, Mookadam F, Cooper L, Adam G, Zehr K, Stulak J, et al. Sinus of Valsalva aneurysms – 47 years of a single center experience and systematic overview of published reports. Am J Cardiol (2007) 99:1159–64. doi:10.1016/j.amjcard.2006.11.047
10. Zhao G, Seng J, Yan B, Wei H, Qiao C, Zhao S, et al. Diagnosis and surgical treatment of ruptured aneurysm in sinus of Valsalva. Chin Med J (Engl) (2003) 116:1047–50.
11. Takach TJ, Reul GJ, Duncan JM, Cooley DA, Livesay JJ, Ott DA, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg (1999) 68:1573–7. doi:10.1016/S0003-4975(99)01045-0
12. Nowicki E, Aberdeen E, Friedman S, Rashkind WJ. Congenital left aortic sinus-left ventricle fistula and review of aorto cardiac fistulas. Ann Thorac Surg (1977) 23(4):323–78. doi:10.1016/S0003-4975(10)64149-5
13. van Son JA, Danielson GK, Schaff HV, Orszulak TA, Edwards WD, Seward JB. Long-term outcome of surgical repair of ruptured sinus of Valsalva aneurysm. Circulation (1994) 90:II20–9.
14. Choudhary S, Bhan A, Shara R, Airan B, Kumar AS, Venugopal P. Sinus of Valsalva aneurysms: 20 years experience. J Card Surg (1997) 12:300–8. doi:10.1111/j.1540-8191.1997.tb00143.x
15. Au WK, Chiu SW, Mok CK, Lee WT, Cheung D, He GW. Repair of ruptured sinus of Valsalva aneurysm: determinants of long-term survival. Ann Thorac Surg (1998) 66:1604–10. doi:10.1016/S0003-4975(98)00782-6
16. Azakie A, David T, Peniston C. Ruptured sinus of Valsalva aneurysm: early recurrence and fate of the aortic valve. Ann Thorac Surg (2000) 70:1466–70. doi:10.1016/S0003-4975(00)01734-3
17. Shah RP, Ding ZP, Ng AS, Quek SS. A ten-year review of ruptured sinus of Valsalva: clinico-pathological and echo-Doppler features. Singapore Med J (2001) 42:473–6.
18. Murashita T, Kubota T, Kamikubo Y, Shiiya N, Yasuda K. Long-term results of aortic valve regurgitation and repair of ruptured sinus of Valsalva aneurysms. Ann Thorac Surg (2002) 73:1466–71. doi:10.1016/S0003-4975(02)03493-8
19. Harkness JR, Fitton TP, Barreiro CJ, Alejo D, Gott VL, Baumgartner WA, et al. A 32-year experience with surgical repair of sinus of Valsalva aneurysms. J Card Surg (2005) 20:198–204. doi:10.1111/j.0886-0440.2005.200430.x
20. Sarikaya S, Adademir T, Elibol A, Büyükbayrak F, Onk A, Kirali K. Surgery for ruptured sinus of Valsalva aneuryms: 25-year experience with 55 patients. Eur J Cardiothorac Surg (2013) 43:591–6. doi:10.1093/ejcts/ezs450
21. Bricker AO, Avutu B, Mohammed TL, Williamson EE, Syed IS, Julsrud PR, et al. Valsalva sinus aneurysms: findings at CT and MR imaging. Radiographics (2010) 30:99–110. doi:10.1148/rg.301095719
22. Hoey ET, Gulati GS, Singh S, Watkin RW, Nazir S, Ganeshan A, et al. The role of multi-modality imaging for sinus of Valsalva aneurysms. Int J Cardiovasc Imaging (2012) 28(7):1725–38. doi:10.1007/s10554-011-0001-5
23. Yeh B, Kurzman P, Foster E, Qayyum A, Joe B, Coakley F. Clinical relevance of retrograde inferior vena cava or hepatic vein opacification during contrast-enhanced CT. AJR Am J Roentgenol (2004) 183:1227–32. doi:10.2214/ajr.183.5.1831227
Keywords: sinus of Valsalva aneurysm, right atrium, transesophageal echocardiography, sinus of Valsalva-to-right atrial fistula, computed tomography angiography
Citation: Rosero-Britton BR, Nguyen A, Warsame I, Shabsigh M, Dong L, Wolfe J, Whitson B and Essandoh M (2017) Incidental Finding of an Aorto-Right Atrial Fistula in a Patient Undergoing Repair of a Sinus of Valsalva Aneurysm. Front. Med. 4:95. doi: 10.3389/fmed.2017.00095
Received: 19 May 2017; Accepted: 20 June 2017;
Published: 30 June 2017
Edited by:
Samir G. Sakka, Witten/Herdecke University, GermanyReviewed by:
Marina Soro, Hospital Clinico Universitario de Valencia, SpainMassimiliano Sorbello, Policlinico Universitario di Catania, Italy
Copyright: © 2017 Rosero-Britton, Nguyen, Warsame, Shabsigh, Dong, Wolfe, Whitson and Essandoh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Essandoh, bWljaGFlbC5lc3NhbmRvaEBvc3VtYy5lZHU=
 Byron R. Rosero-Britton
Byron R. Rosero-Britton Anthony Nguyen
Anthony Nguyen Muhammad Shabsigh
Muhammad Shabsigh Michael Essandoh
Michael Essandoh