- Department of Clinical Chemistry and Hematology, University Medical Center Utrecht, Utrecht, Netherlands
The contact system was originally identified as an obsolete part of the coagulation system, but it has been repeatedly implicated in inflammatory states, such as infection, as well as in allergic- and chronic inflammatory disease. Under these conditions, there is surprisingly little evidence that factor XII (FXII) acts as a coagulation factor, and its activity appears to be mainly directed toward activation of the kallikrein–kinin system. The contact system factors interact with pathogens as well as cells of the (innate) immune system on several levels. Among others, these cells may provide negatively charged surfaces that contribute to contact activation as well as release enzymes that feed into this system. Furthermore, cellular receptors have been identified that bind contact factors at sites of inflammation. Based on the accumulated evidence, we propose a model for enzymatic crosstalk between inflammatory cells and the plasma contact system. During these reactions, FXII is enzymatically cleaved by non-contact system enzymes. This generates unactivated FXII fragments that can subsequently be rapidly activated in the fluid phase. The resulting enzyme lacks procoagulant properties, but retains its pro-inflammatory characteristic as a prekallikrein activator.
Introduction
Continuous maintenance of vascular integrity is essential for effective blood circulation and overall survival. When tissues are infected or injured, local increases in vascular permeability and controlled inflammation are needed for protection and repair. The short-lived peptide bradykinin is a well-known mediator of inflammation and vascular leakage. To this day, the plasma contact system is considered to be the most important source of intravascular bradykinin production. However, the physiological mechanisms that drive this system to generate bradykinin remain elusive.
The plasma contact system consists of the two proteases, factor XII (FXII) and plasma prekallikrein (pro-pKal), and their non-enzymatic cofactor high molecular weight kininogen (HK). These coagulation factors spontaneously activate in the presence of negatively charged surfaces, which [as previously reviewed in Ref. (1, 2)] can be non-natural (e.g., kaolin) or cell-derived (e.g., polyphosphate). Surface-binding of FXII is accompanied by a conformational shift (3). This may generate the first spark of enzymatic activity (4). In chorus, HK (in complex with pro-pKal) also binds to the negatively charged surface, thereby presenting pro-pKal for activating cleavage by active FXII (FXIIa). In turn, activated plasma kallikrein (pKal) can reciprocally cleave and activate more FXII, forming a powerful activation feedback loop that can avoid inhibition by C1-esterase inhibitor (C1inh). In part, this can be attributed to the negative charge of the activating surfaces, which electrostatically repel C1inh (which is also negatively charged). When sufficient amounts of FXII activate on the surface, FXIIa activates factor XI (FXI) in a mechanism that closely resembles pro-pKal activation. Deficiencies in contact factors were originally identified as in vitro defects in surface-mediated clotting reactions (5, 6). As a direct result, it is generally thought that contact activation will inherently lead to blood coagulation. Mysteriously, deficiencies in the contact factors are without bleeding diatheses, providing reasons to believe that the contact system has become redundant for physiological hemostasis. But is activation of blood coagulation by the contact system truly its first and foremost important function?
At this point, it is noteworthy that only a subset of negatively charged activators of the contact system support activation of FXI by FXIIa. Generally, these surfaces are insoluble particles (7–9). However, a second type of contact system activator (generally negatively charged soluble polymers) is unable to support FXII-driven blood coagulation in vitro or activate FXI in vivo (8, 10). Surprisingly, this class of activators still powerfully promotes pKal activity and bradykinin production. The fundamental principles that make the contact system “decide” whether or not to trigger coagulation in response to specific activators are still unknown, but we propose that this is related to alternative conformational changes that FXII undergoes when it binds to activating surfaces (7). Furthermore, earlier biochemical investigations have pointed out that surface-bound FXII becomes activated in a step-wise mechanism (Figure 1, “Classic” contact activation). A first pKal-mediated cleavage activates FXII into a full-length two-chain molecule with surface-binding and procoagulant characteristics. Further cleavage by pKal fragments the molecule, allowing it to dissociate into solution. This enzymatic fragment has lost the ability to activate FXI, but can still act as a powerful pro-pKal activator (11).
The Contact System in Inflammatory Pathology
The contact system has attracted strong scientific attention as a result of its contribution to pathological thrombus formation and the potential it holds for developing safe antithrombotic strategies without an associated bleeding risk (12). However, this system has also been repeatedly implicated in acute inflammatory and allergic reactions, as well as chronic inflammatory disease, often without a clear link to the coagulation system.
Sepsis
Patients with sepsis undergo a systemic inflammatory response and can experience fever, hypotension, tachycardia, and organ failure (13). Sepsis can be caused by various pathogens, although bacterial infection is most common. When primates are challenged in an Escherichia coli-induced sepsis model, contact system activity is observed in the systemic circulation (14–16). Inhibition of contact system activity attenuates complement activation and diminishes neutrophil degranulation. Resultantly, overall survival rates increase. However, when FXII is inactivated by a blocking antibody, disseminated intravascular coagulation still occurs, indicating that the contact system is not responsible for the thrombotic aspect of this pathology. Presumably, expression of tissue factor by circulating cells and diffuse vascular damage, leading to subendothelial exposure, are the driving factor behind this prothrombotic aspect of sepsis. When guinea pigs are experimentally infected with Pseudomonas aeruginosa (P. aeruginosa), it triggers development of peritonitis (17). Activation of the contact system is observed during these infections and plays a role in this pathology: antibody-mediated depletion of FXII prevents the onset of septic shock. In contrast, depletion of α2-macroglobulin, which has the ability to inhibit pKal during these infections, exacerbated the outcome. While these two studies highlight a prominent role for bradykinin production in septic shock models, it is important to note that not all sepsis models show identical results. In a porcine sepsis model with P. aeruginosa, the onset of symptoms was not influenced by a kinin B2 receptor antagonist. However animals did recover faster as a result of this treatment (18), suggesting that the contact system does not play a lead role throughout the entire pathological process. In a similar porcine infection model with Neisseria meningitides, administration of a kinin B2 receptor antagonist had no effect (19). This raises the question whether all forms of sepsis are accompanied by contact system activation.
In a human study of systemic inflammatory response syndrome, continuous infusion of a bradykinin antagonist had no overall effect on the 28-day survival (20). However, a subset of patients with a Gram-negative bacterial infection did show improvements in recovery. Another study reports that levels FXIIa–C1inh and PK–C1inh complexes were transiently increased in 40% of patients during the course of their sepsis (21). These observations in human patients parallel observations in animal studies and suggest that not all types of infection that lead to sepsis act on the contact system in the same manner. Some pathogens trigger contact system activity via outer surface components: Curli-expressing E. coli have been shown to directly bind and activate the contact system on their surface (22). Furthermore, E. coli, Bacteroides fragilis, Bacteroides vulgatus, and Fusobacterium mortiferum LPS have been postulated to be able to directly activate FXII (23, 24). However, other pathogens appear to trigger contact system activity in an enzymatic manner. For example, P. aeruginosa expresses a form of elastase that, after administration in guinea pigs, provokes massive consumption of FXII, PPK, and HK and triggers bradykinin formation, recapitulating key features of pseudomonal sepsis (25). Several other microbial enzymes with similar functions have been identified (26). Three main groups of proteinases can be distinguished: (I) those that activate FXII, but not pro-pKal; (II) those that can activate both FXII and pro-pKal; and (III) those that directly liberate bradykinin from HK. Finally, recent studies have shown that bacterial strains that carry direct plasminogen activators (e.g., streptokinase) can trigger plasmin-triggered bradykinin production via the contact system (27), which is highly reminiscent of earlier studies that identified plasmin as an activating enzyme of FXII (28) as well as recent findings that implicate plasmin as FXII-activating enzyme in hereditary angioedema (HAE) (29). This may help to explain the changes in blood pressure that take place during sepsis but also possibly points toward a bradykinin-dependent mechanism of pathogen host invasion.
Anaphylaxis
Anaphylaxis is a severe allergic reaction with a possible deadly outcome. Attacks can be triggered in reaction to food, insect bites and/or stings, and medication. As a result, patients can experience gastrointestinal and skin manifestations, as well as arrhythmias, bronchial constriction, and vascular leakage, which causes hypotension. These effects are generally thought to be mainly due to extensive degranulation of mast cells and basophils. However, several lines of evidence point to a role for bradykinin in the exacerbation of allergic reactions.
Patients who undergo attacks of anaphylaxis show strong consumption of contact system factors (30, 31). Interestingly, plasminogen activation is simultaneously seen in these same patients (32). Consumption of contact factors has also been reported in IgE-mediated mouse models for anaphylaxis (10). This raises the questions of how (and why) the contact system activates during anaphylaxis. Upon mast cell and basophil degranulation, a wide variety of vasoactive mediators and proinflammotory effectors are secreted which belong to a wide variety of cytokines, chemokines, or lipid mediators (33). Alongside these substances, the highly sulfated glycosaminoglycan heparin is also secreted. While heparin is mostly known for its anti-coagulant properties in the clinic, for mast cells, heparin acts as scaffold and carrier for several proteases and is essential for the proper morphology of the secretory granules (34, 35). To execute this function, heparin shares many properties with negatively charged polymers that can active the contact system. Indeed, when heparin is isolated from peritoneal mast cells and subsequently added to plasma, or administered intravenously to mice, contact system activation and bradykinin formation ensues (10). It should be remarked that therapeutic heparin preparations do not trigger significant or dangerous systemic contact system activation, unless highly charged impurities are present (36). However, when mast cells release heparin in vivo, significant contact activation follows. In IgE-dependent mouse models for anaphylaxis, genetic and pharmacological targeting of the contact pathway attenuates symptomatic hypotension and cutaneous swelling (10). As such, it is proposed that mast cell heparin is an important endogenous contact system activator in anaphylaxis in mice and humans. Furthermore, these experiments indicate that mast cell/histamine driven allergic reactions are mechanistically coupled to bradykinin production and do not present exclusively operating mechanisms for vascular leakage.
Multiple Sclerosis
Multiple sclerosis (MS) is a severe neuroinflammatory disease, which affects local function of the central nervous system (CNS). Patients can experience loss of their vision, muscle coordination, and sensation. While the precise cause of MS is enigmatic, the general consensus is that MS is caused by the local destruction of the optic nerve, brain stem, basal ganglia, and spinal cord, and certain areas of white matter in the brain. Triggers for this pathology are linked to immune system activity or failure of the myelin producing cells. MS can be classified as an inflammatory disease, but in addition, a dysregulation of the extrinsic pathways of coagulation has been repeatedly indicated (37–40). Excitingly, a recent publication by Göbel et al. suggests that the contact system and FXII in particular, might be a directly involved in the onset of MS (41). It was demonstrated that when an inflammatory response against the CNS is evoked in a mouse model for MS, FXII-knockout mice show a delayed disease onset and reduced disease severity compared to their wild-type counter parts (WT). The role of FXII in this context is critically dependent on the expression of the cell-surface receptor CD87 by dendritic cells, which is crucial for T-cell differentiation. Interestingly, CD87 is also known as uPAR, a key receptor in the urokinase plasminogen activation system, that mediates plasminogen activation on endothelial cells but also has cell signaling properties during FXII binding that influence angiogenesis (42). These recent studies indicate a novel role for FXII in MS, which is independent of intrinsic coagulation or the kallikrein–kinin system. However, a contribution of bradykinin or its metabolites to MS is still probable: blockade of the kinin B1 receptor, which is mainly expressed at sites of inflammation, reduces pathology in mouse models for MS by preventing T-cell migration into the nervous tissue by restoring excessive permeability of the blood–brain barrier (43). These combined studies point out that the role of FXII in immune modulation and inflammation in extravascular tissues may so far have been underappreciated.
Two External Enzymes That Feed into the Contact System During Inflammation
It can be assumed that the mechanisms that drive physiological contact system activation are restricted to the same factors that are required for surface-triggered FXII-dependent coagulation (or pro-pKal activation) in vitro. In this context, pKal is the main enzyme that cleaves and activates FXII. However, extensive studies on endothelial cells have identified new external players that feed into the contact system, both by acting as pro-pKal activators (44, 45). In this next section, we will review examples of two enzymes from the serine-protease family that can feed into the contact system.
Plasmin
Plasmin is foremost known for its thrombolytic function through fibrin breakdown and has strong therapeutic value in the treatment of thrombotic pathology (e.g., stroke). However, plasminogen activation can take place in the complete absence of a thrombus on the surface of endothelial cells in a receptor-mediated manner by the urokinase plasminogen activation system. Hypoxia is one of the triggers for expression of the required receptors (46). This among others helps to explain the generation of fibrinolytic activity during attacks of (fibrin-poor) thrombotic microangiopathy (47).
Hereditary angioedema is characterized by swelling attacks of the extremities, face, trunk, airway, or viscera of the abdomen. The onset can be spontaneous or secondary to trauma. The contact system and bradykinin production are heavily implicated, as HAE is often related to C1inh deficiency (48), as well as by mutations in FXII (49), and can be treated with kinin B2 receptor antagonists, C1inh reconstitution therapy, and antagonists of pKal (50). The mechanisms that underlie the attacks are currently unknown, but strikingly similar symptoms are seen in a subset of stroke patients (~5%) who undergo thrombolytic therapy (51). Furthermore, thrombolytic therapy after stroke exacerbates brain edema (52) with kallikrein–kinin system as known mediator (53). This connects well to the finding that the contact system is systemically activated after administration of plasminogen activators and the capacity of plasmin to cleave and activate FXII in a mechanism that closely resembles the function of pKal (28, 54). Another noteworthy influence of plasmin on the contact system is that it has the potential to “prime” HK for kallikrein-mediated liberation of bradykinin (55). We recently reported that three types of FXII mutations that cause HAE enhance the capacity of plasmin to cleave and activate FXII through introduction of novel enzymatic cleavage sites, leading to uncontrolled bradykinin production despite the presence of normal C1inh levels (29). Interestingly, the plasminogen activation system is linked in many of the inflammatory conditions described above, ranging from bacterial plasminogen activators to concurrent activation of plasminogen and the contact system in anaphylaxis (27, 30, 32). Based on these combined findings, we propose that plasmin is of importance for FXII-mediated bradykinin production in a context that expands beyond HAE.
Elastase
Several cell types of the (innate) immune system, such as mast cells, basophils, and neutrophils contain and release elastase. This enzyme has been extensively studied as an inflammatory mediator in lung injury. Elastase has the capacity to cleave FXII into 28 kDa and 52 kDa fragments in peripheral blood. This destroys its procoagulant activity through removal of the surface-binding domains (56, 57), but does not directly activate the FXII molecule. As a result, elastase is currently seen as a negative regulator of the plasma contact system, but is this really the case? The cleavage pattern that FXII generates in response to elastase is strikingly similar to that of pKal, indicating that the cleavage sites for elastase and pKal in FXII are in close vicinity to each other. At this point, it is noteworthy to remember that pKal exerts a similar function during “classic” contact activation, removing the surface-binding domains of FXII to yield a fragmented active form of FXII with selective pro-pKal activating properties. In case of elastase cleavage, the soluble fragment has not (yet) been activated. In a mirroring mechanism, elastase is able cleave the light chain of HK. This eliminates its procoagulant properties, but leaves the kinin sequence untouched (56). It is attractive to hypothesize that this “primes” FXII for activation by pKal (or plasmin) and HK for liberation of bradykinin by pKal in the fluid phase. Finally, elastase can proteolytically inactivate α2-antiplasmin and C1inh (58). Taken together, the combined properties seem to point at the potential of elastase as a positive regulator of contact system activation, while shifting its actions toward a pro-inflammatory focus.
Cleavage Does Not Equal Destruction: A Conceptual Model for Two-Stage Fluid Phase Activation of Factor XII
“Classic” Contact Activation
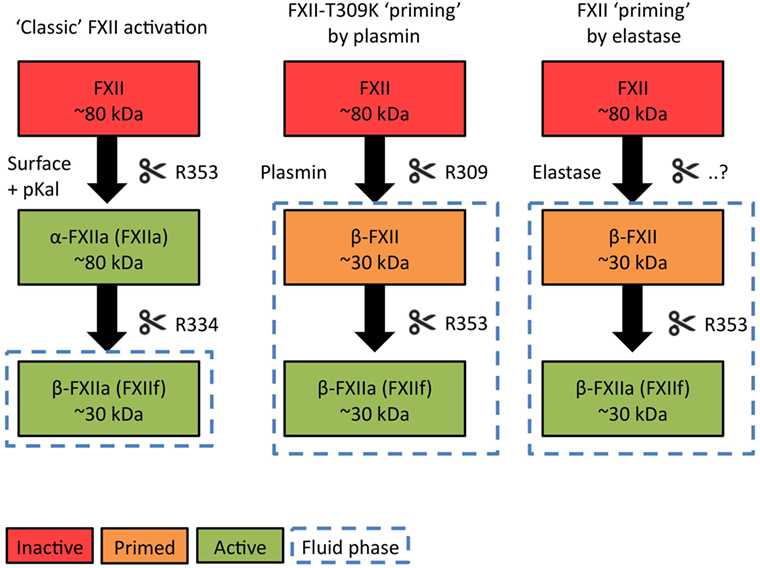
As discussed earlier, the pattern by which FXII is cleaved by pKal is decisive in determining whether it acts as a procoagulant or pro-inflammatory enzyme (11) (Figure 1, left column). Three pKal-sensitive cleavage sites on FXII have been identified: R334, R343, and R353 [mature protein numbering (59)]. In the “classic” model of contact activation, α-FXIIA is formed first by cleavage at R353, which “locks” this molecule into a two-chain active conformation. In short, R353 cleavage is critical for FXII activation (59). Next, R334 cleavage generates β-FXIIA, a fluid phase pro-pKal activator. The functional consequences of R343 cleavage are still unknown. However, since pKal is not the only serine protease that is able to cleave FXII, the sequence of events that occur during physiological FXII activation may be different than were originally discovered during surface-triggered contact system activation in vitro.
A “Priming” Model for FXII-HAE
We recently reported that mutations in FXII that cause HAE (FXII-HAE) introduce new cleavage sites in the unstructured proline-rich region, which accelerate fluid phase activation by plasmin (29) (Figure 1, middle column). These sites are near to the R334 site, which pKal usually cleaves to generate β-FXIIA after initial activation. Our findings in FXII-HAE have led us to hypothesize that during activation of FXII-HAE mutants, the unactivated FXII protein is fragmented in solution first, rather than after initial activation. This step not only eliminates its procoagulant properties but also removes a functional sequence that shields site R353. This “primes” the FXII molecule and lowers the threshold for fluid phase activation by pKal or plasmin.
Factor XII “Priming” by Elastase
Based on available biochemical data, the currently unidentified cleavage site for elastase is in the very same region as the FXII-HAE mutations, as well as near R334 (Figure 1, right column). In analogy to the mechanism described above, we propose that when elastase cleaves FXII, it converts the molecule into a fragment that is unable to generate clotting activity, but is has a significantly increased propensity for activation by pKal or plasmin in the fluid phase.
Summary
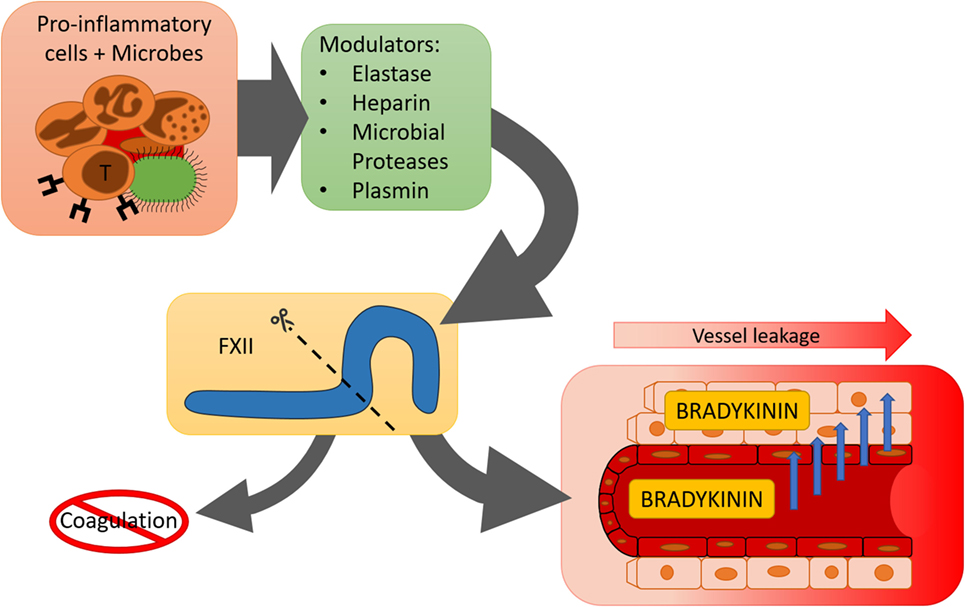
Accumulating evidence shows that the contact system is involved in inflammatory mechanisms that are not directly linked to blood coagulation (Figure 2). Alternative enzymatic processing of FXII by non-contact system enzymes may help to explain how the contact system “chooses” its direction.
Author Contributions
BNJ, SM, and CM performed literature searches and wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
CM acknowledges financial support from the International Patient Organization for C1-Inhibitor Deficiencies (HAEi), Stichting Vrienden van Het UMC Utrecht, and Landsteiner Foundation for Blood Transfusion Research (LSBR).
References
1. Maas C, Renné T. Regulatory mechanisms of the plasma contact system. Thromb Res (2012) 129(Suppl 2):S73–6. doi:10.1016/j.thromres.2012.02.039
2. Maas C, Oschatz C, Renné T. The plasma contact system 2.0. Semin Thromb Hemost (2011) 37:375–81. doi:10.1055/s-0031-1276586
3. Samuel M, Pixley RA, Villanueva MA, Colman RW, Villanueva GB. Human factor XII (Hageman factor) autoactivation by dextran sulfate. Circular dichroism, fluorescence, and ultraviolet difference spectroscopic studies. J Biol Chem (1992) 267:19691–7.
4. Nuijens JH, Huijbregts CC, Eerenberg-Belmer AJ, Meijers JC, Bouma BN, Hack CE. Activation of the contact system of coagulation by a monoclonal antibody directed against a neodeterminant in the heavy chain region of human coagulation factor XII (Hageman factor). J Biol Chem (1989) 264:12941–9.
5. Ratnoff OD, Colopy JE. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest (1955) 34:602–13. doi:10.1172/JCI103109
6. Saito H. Studies on Fletcher trait and Fitzgerald trait. A rare chance to disclose body’s defense reactions against injury. Thromb Haemost (2010) 104:867–74. doi:10.1160/TH10-01-0058
7. de Maat S, Maas C. Factor XII: form determines function. J Thromb Haemost (2016) 14:1498–506. doi:10.1111/jth.13383
8. de Maat S, van Dooremalen S, de Groot PG, Maas C. A nanobody-based method for tracking factor XII activation in plasma. Thromb Haemost (2013) 110:458–68. doi:10.1160/TH12-11-0792
9. Bock PE, Srinivasan KR, Shore JD. Activation of intrinsic blood coagulation by ellagic acid: insoluble ellagic acid-metal ion complexes are the activating species. Biochemistry (Mosc) (1981) 20:7258–66. doi:10.1021/bi00528a032
10. Oschatz C, Maas C, Lecher B, Jansen T, Björkqvist J, Tradler T, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity (2011) 34:258–68. doi:10.1016/j.immuni.2011.02.008
11. Revak SD, Cochrane CG, Bouma BN, Griffin JH. Surface and fluid phase activities of two forms of activated Hageman factor produced during contact activation of plasma. J Exp Med (1978) 147:719–29. doi:10.1084/jem.147.3.719
12. Labberton L, Kenne E, Long AT, Nickel KF, Di Gennaro A, Rigg RA, et al. Neutralizing blood-borne polyphosphate in vivo provides safe thromboprotection. Nat Commun (2016) 7:12616. doi:10.1038/ncomms12616
13. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016) 315:801–10. doi:10.1001/jama.2016.0287
14. Pixley RA, De La Cadena R, Page JD, Kaufman N, Wyshock EG, Chang A, et al. The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. In vivo use of a monoclonal anti-factor XII antibody to block contact activation in baboons. J Clin Invest (1993) 91:61–8. doi:10.1172/JCI116201
15. Jansen PM, Pixley RA, Brouwer M, de Jong IW, Chang AC, Hack CE, et al. Inhibition of factor XII in septic baboons attenuates the activation of complement and fibrinolytic systems and reduces the release of interleukin-6 and neutrophil elastase. Blood (1996) 87:2337–44.
16. Pixley RA, DeLa Cadena RA, Page JD, Kaufman N, Wyshock EG, Colman RW, et al. Activation of the contact system in lethal hypotensive bacteremia in a baboon model. Am J Pathol (1992) 140:897–906.
17. Khan MM, Shibuya Y, Kambara T, Yamamoto T. Role of alpha-2-macroglobulin and bacterial elastase in guinea-pig pseudomonal septic shock. Int J Exp Pathol (1995) 76:21–8.
18. Ridings PC, Sugerman HJ, Blocher CR, Fisher BJ, Fowler AA. Hemodynamic effects of bradykinin antagonism in porcine gram-negative sepsis. J Investig Surg (1995) 8:115–22. doi:10.3109/08941939509016514
19. Barratt-Due A, Johansen HT, Sokolov A, Thorgersen EB, Hellerud BC, Reubsaet JL, et al. The role of bradykinin and the effect of the bradykinin receptor antagonist icatibant in porcine sepsis. Shock Augusta Ga (2011) 36:517–23. doi:10.1097/SHK.0b013e3182336a34
20. Fein AM, Bernard GR, Criner GJ, Fletcher EC, Good JT, Knaus WA, et al. Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, deltibant (CP-0127). Results of a randomized, double-blind, placebo-controlled trial. CP-0127 SIRS and Sepsis Study Group. JAMA (1997) 277:482–7. doi:10.1001/jama.1997.03540300050033
21. Nuijens JH, Huijbregts CC, Eerenberg-Belmer AJ, Abbink JJ, Strack van Schijndel RJ, Felt-Bersma RJ, et al. Quantification of plasma factor XIIa-Cl(-)-inhibitor and kallikrein-Cl(-)-inhibitor complexes in sepsis. Blood (1988) 72:1841–8.
22. Ben Nasr A, Olsén A, Sjöbring U, Müller-Esterl W, Björck L. Assembly of human contact phase proteins and release of bradykinin at the surface of Curli-expressing Escherichia coli. Mol Microbiol (1996) 20:927–35. doi:10.1111/j.1365-2958.1996.tb02534.x
23. Morrison DC, Cochrane CG. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J Exp Med (1974) 140:797–811. doi:10.1084/jem.140.3.797
24. Bjornson HS. Activation of Hageman factor by lipopolysaccharides of Bacteroides fragilis, Bacteroides vulgatus, and Fusobacterium mortiferum. Rev Infect Dis (1984) 6(Suppl 1):S30–3. doi:10.1093/clinids/6.Supplement_1.S30
25. Khan MM, Yamamoto T, Araki H, Shibuya Y, Kambara T. Role of Hageman factor/kallikrein-kinin system in pseudomonal elastase-induced shock model. Biochim Biophys Acta (1993) 1157:119–26. doi:10.1016/0304-4165(93)90055-D
26. Molla A, Yamamoto T, Akaike T, Miyoshi S, Maeda H. Activation of hageman factor and prekallikrein and generation of kinin by various microbial proteinases. J Biol Chem (1989) 264:10589–94.
27. Nitzsche R, Rosenheinrich M, Kreikemeyer B, Oehmcke-Hecht S. Streptococcus pyogenes triggers activation of the human contact system by streptokinase. Infect Immun (2015) 83:3035–42. doi:10.1128/IAI.00180-15
28. Kaplan AP, Austen KF. A prealbumin activator of prekallikrein. II. Derivation of activators of prekallikrein from active Hageman factor by digestion with plasmin. J Exp Med (1971) 133:696–712. doi:10.1084/jem.133.4.696
29. de Maat S, Björkqvist J, Suffritti C, Wiesenekker CP, Nagtegaal W, Koekman A, et al. Plasmin is a natural trigger for bradykinin production in patients with hereditary angioedema with factor XII mutations. J Allergy Clin Immunol (2016). doi:10.1016/j.jaci.2016.02.021
30. van der Linden PW, Hack CE, Eerenberg AJ, Struyvenberg A, van der Zwan JK. Activation of the contact system in insect-sting anaphylaxis: association with the development of angioedema and shock. Blood (1993) 82:1732–9.
31. Sala-Cunill A, Björkqvist J, Senter R, Guilarte M, Cardona V, Labrador M, et al. Plasma contact system activation drives anaphylaxis in severe mast cell–mediated allergic reactions. J Allergy Clin Immunol (2015) 135:1031.e–43.e. doi:10.1016/j.jaci.2014.07.057
32. van der Linden PW, Hack CE, Struyvenberg A, Roem D, Brouwer MC, de Boer JP, et al. Controlled insect-sting challenge in 55 patients: correlation between activation of plasminogen and the development of anaphylactic shock. Blood (1993) 82:1740–8.
33. Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol (2008) 9:1215–23. doi:10.1038/ni.f.216
34. Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature (1999) 400:773–6. doi:10.1038/23488
35. Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, et al. Heparin is essential for the storage of specific granule proteases in mast cells. Nature (1999) 400:769–72. doi:10.1038/23481
36. Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med (2008) 358:2457–67. doi:10.1056/NEJMoa0803200
37. Gveric D, Herrera B, Petzold A, Lawrence DA, Cuzner ML. Impaired fibrinolysis in multiple sclerosis: a role for tissue plasminogen activator inhibitors. Brain J Neurol (2003) 126:1590–8. doi:10.1093/brain/awg167
38. Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, et al. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med (2007) 204:571–82. doi:10.1084/jem.20061931
39. Ryu JK, Petersen MA, Murray SG, Baeten KM, Meyer-Franke A, Chan JP, et al. Blood coagulation protein fibrinogen promotes autoimmunity and demyelination via chemokine release and antigen presentation. Nat Commun (2015) 6:8164. doi:10.1038/ncomms9164
40. Han MH, Hwang S-I, Roy DB, Lundgren DH, Price JV, Ousman SS, et al. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature (2008) 451:1076–81. doi:10.1038/nature06559
41. Göbel K, Pankratz S, Asaridou C-M, Herrmann AM, Bittner S, Merker M, et al. Blood coagulation factor XII drives adaptive immunity during neuroinflammation via CD87-mediated modulation of dendritic cells. Nat Commun (2016) 7:11626. doi:10.1038/ncomms11626
42. LaRusch GA, Mahdi F, Shariat-Madar Z, Adams G, Sitrin RG, Zhang WM, et al. Factor XII stimulates ERK1/2 and Akt through uPAR, integrins, and the EGFR to initiate angiogenesis. Blood (2010) 115:5111–20. doi:10.1182/blood-2009-08-236430
43. Göbel K, Pankratz S, Schneider-Hohendorf T, Bittner S, Schuhmann MK, Langer HF, et al. Blockade of the kinin receptor B1 protects from autoimmune CNS disease by reducing leukocyte trafficking. J Autoimmun (2011) 36:106–14. doi:10.1016/j.jaut.2010.11.004
44. Joseph K, Tholanikunnel BG, Kaplan AP. Heat shock protein 90 catalyzes activation of the prekallikrein-kininogen complex in the absence of factor XII. Proc Natl Acad Sci U S A (2002) 99:896–900. doi:10.1073/pnas.022626899
45. Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem (2002) 277:17962–9. doi:10.1074/jbc.M106101200
46. Graham CH, Fitzpatrick TE, McCrae KR. Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood (1998) 91:3300–7.
47. Tersteeg C, de Maat S, De Meyer SF, Smeets MWJ, Barendrecht AD, Roest M, et al. Plasmin cleavage of von Willebrand factor as an emergency bypass for ADAMTS13 deficiency in thrombotic microangiopathy. Circulation (2014) 129:1320–31. doi:10.1161/CIRCULATIONAHA.113.006727
48. Nussberger J, Cugno M, Cicardi M. Bradykinin-mediated angioedema. N Engl J Med (2002) 347:621–2. doi:10.1056/NEJM200208223470820
49. Dewald G, Bork K. Missense mutations in the coagulation factor XII (Hageman factor) gene in hereditary angioedema with normal C1 inhibitor. Biochem Biophys Res Commun (2006) 343:1286–9. doi:10.1016/j.bbrc.2006.03.092
50. Hofman Z, de Maat S, Hack CE, Maas C. Bradykinin: inflammatory product of the coagulation system. Clin Rev Allergy Immunol (2016) 51:152–61. doi:10.1007/s12016-016-8540-0
51. Pinho J, Alves JN, Oliveira L, Pereira S, Barros J, Machado C, et al. Orolingual angioedema after thrombolysis is not associated with insular cortex ischemia on pre-thrombolysis CT. J Neurol Sci (2016) 369:48–50. doi:10.1016/j.jns.2016.07.043
52. Rudolf J, Grond M, Stenzel C, Neveling M, Heiss WD. Incidence of space-occupying brain edema following systemic thrombolysis of acute supratentorial ischemia. Cerebrovasc Dis (1998) 8:166–71. doi:10.1159/000015843
53. Unterberg A, Dautermann C, Baethmann A, Müller-Esterl W. The kallikrein-kinin system as mediator in vasogenic brain edema. Part 3: inhibition of the kallikrein-kinin system in traumatic brain swelling. J Neurosurg (1986) 64:269–76. doi:10.3171/jns.1986.64.2.0269
54. Ewald GA, Eisenberg PR. Plasmin-mediated activation of contact system in response to pharmacological thrombolysis. Circulation (1995) 91:28–36. doi:10.1161/01.CIR.91.1.28
55. Kleniewski J, Blankenship DT, Cardin AD, Donaldson V. Mechanism of enhanced kinin release from high molecular weight kininogen by plasma kallikrein after its exposure to plasmin. J Lab Clin Med (1992) 120:129–39.
56. Kleniewski J, Donaldson V. Granulocyte elastase cleaves human high molecular weight kininogen and destroys its clot-promoting activity. J Exp Med (1988) 167:1895–907. doi:10.1084/jem.167.6.1895
57. Meier HL, Schulman ES, Heck LW, MacGlashan D, Newball HH, Kaplan AP. Release of elastase from purified human lung mast cells and basophils. Identification as a Hageman factor cleaving enzyme. Inflammation (1989) 13:295–308. doi:10.1007/BF00914396
58. Brower MS, Harpel PC. Proteolytic cleavage and inactivation of alpha 2-plasmin inhibitor and C1 inactivator by human polymorphonuclear leukocyte elastase. J Biol Chem (1982) 257:9849–54.
Keywords: factor XII, inflammation, bradykinin, plasmin, elastase
Citation: Jukema BN, de Maat S and Maas C (2016) Processing of Factor XII during Inflammatory Reactions. Front. Med. 3:52. doi: 10.3389/fmed.2016.00052
Received: 01 October 2016; Accepted: 21 October 2016;
Published: 04 November 2016
Edited by:
Joost Meijers, University of Amsterdam, NetherlandsReviewed by:
Owen McCarty, Oregon Health & Science University, USAHeiko Herwald, Lund University, Sweden
Copyright: © 2016 Jukema, de Maat and Maas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Coen Maas, Y21hYXM0QHVtY3V0cmVjaHQubmw=
 Bernard Nico Jukema
Bernard Nico Jukema Steven de Maat
Steven de Maat Coen Maas
Coen Maas