- 1Department of Internal Medicine, St. Barnabas Hospital Bronx, Bronx, NY, USA
- 2Department of Anesthesiology, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Immune thrombocytopenic purpura (ITP) is defined as an acquired thrombocytopenia with antibodies detected against platelet surface antigens, and it is the most common form of thrombocytopenia in otherwise asymptomatic adults. ITP secondary to an underlying condition is a diagnosis of exclusion that is essential to establish for treatment efficacy. Secondary thrombocytopenia caused by cytomegalovirus (CMV) is common; however, case reports associated with diagnosis in immunocompetent adults are rare, and to the best of our knowledge only 20 publications have been associated with this diagnosis. Our report is based on a clinical presentation of a 37-year-old female complaining of petechiae, heavy menses, shortness of breath, and a platelet count of 1 × 109/L. Treatment with IVIG and steroids failed to improve platelet count. Subsequently, an infectious laboratory workup was performed, detecting CMV infection, and treatment with antiviral agents was initiated, causing platelet count to increase as viral load decreased.
Introduction
Immune thrombocytopenic purpura (ITP) is a form of acquired thrombocytopenia triggered by anti-platelet antibodies that destroy platelets peripherally, damage megakaryocytes, and inhibits platelet production in the marrow (1). Agents such as CMV, hepatitis C, Epstein–Barr virus, and Parvovirus B19, among others, have been identified as the culprits behind this form of ITP (2). Steroids and IVIG are the treatment of choice for ITP. The primary infection should be treated in order to allow ITP standard therapies to be effective. We present a case of a 37-year-old female patient with a history of petechia, heavy menstrual bleed, and low platelets (1 × 109/L) after a CMV infection. Cytomegalovirus (CMV) is a known cause of morbidity and mortality in patients with immunosuppressed states, whereas in the immunocompetent patients, the virus commonly manifests as an asymptomatic infection or as mononucleosis-like syndrome. CMV can cause disease in immunocompromised patients either via a primary CMV infection or reactivation of a latent CMV infection. It is characterized by malaise, myalgia, headache, sore throat, and fever. Associated clinical syndromes include encephalitis, pneumonitis, hepatitis, uveitis, retinitis, colitis, and graft rejection. Treatment of the primary infection is imperative because standard therapies for ITP regain their efficacy once the infection is resolved (3).
Case Presentation
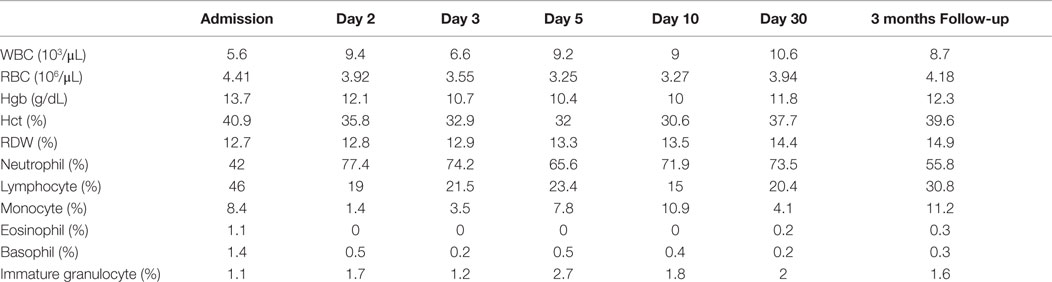
A 37-year-old female presented to the emergency department complaining of shortness of breath, pruritic rash, epistaxis, heavy menses, and night sweats. A physical examination revealed a petechial rash on her torso, lower extremities, and oral mucosa. On admission, the patient was found to have platelet count of 1.0 × 109/L (Figure 1).The normal range for platelets in healthy Caucasians is 150,000–400,000/mm3 (a cubic millimeter equals a microliter), or 150–400 × 109/L (4). The patient’s vitals were stable, including the absence of fever, and on physical examination no splenomegaly was found. White blood cells (WBC) and red blood cells (RBC) were unremarkable (Table 1); nonetheless, a slight elevation in AST and ALT was found. The patient’s symptoms aroused suspicion of immune thrombocytopenia. Initial administration of high-dose steroids (dexamethasone 40 mg PO ×4 days and prednisone 60 mg PO ×2 days) showed improvement in the patient’s platelet count; however a subsequent course of high-dose methylprednisolone (solumedrol 60 mg IV ×3 days) did not show any further clinical benefit. Intravenous Immunoglobulin (IVIG 1 g/kg ×2 days) was administered, and far from alleviating existing symptoms, caused right pleural effusion, prompting its discontinuation and the possibility of secondary ITP was suspected. An infectious work up was performed, and results were all negative except by PCR for CMV–DNA that showed CMV IgM more than fourfold, a viral load of 46,881 copies/MI (Figure 2). A bone marrow biopsy revealed a combined pattern of normocellular and hypercellular areas along with the presence of megakaryocytes. Per Infectious Disease and Hematology-Oncology medical team recommendations, a combination of valgancyclovir (900 mg PO BID) and romiplostin (Nplate® 1 μg/kg SC/week) was started, causing a significant drop in viral load (Figure 2). Resolution of CMV infection and subsequent promotion of platelet growth contributed to a significant improvement in patient’s symptoms. The patient was discharged with a platelet count of 49 × 109/L (Figure 1). One month later, platelet count improved to 228 × 109/L, and CMV viral load dropped to <200 copies/MI and asymptomatic (Figure 2).
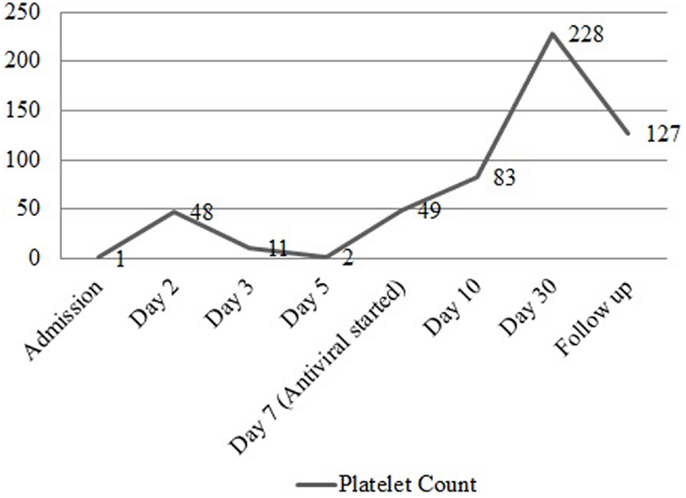
Figure 1. Platelet count before and after treatment. Graph representing the trend of platelet count from the time of admission to the post treatment period with valgancyclovir, romiplostim, and IVIG.
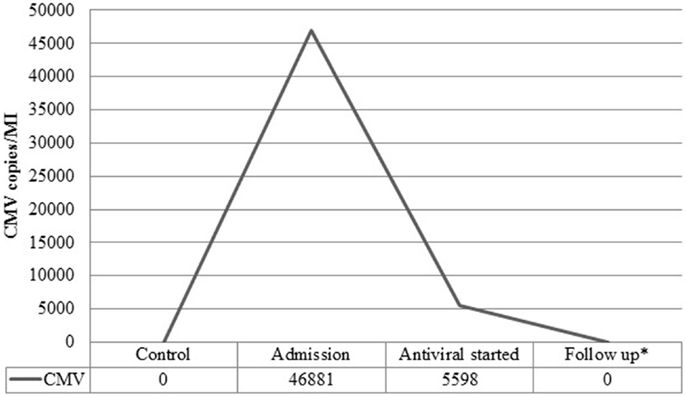
Figure 2. Viral load before and after treatment. Viral load in copies/MI for Cytomegalovirus (CMV) measured at admission, post treatment with valgancyclovir and during follow-up.
Informed Consent Statement
The subject described in this report has given verbal informed consent. Written informed consent is not required if data is drawn from observed behavior or if data does not contain identifying information, as it is in the present report.
Discussion
Secondary ITP is an acquired thrombocytopenia caused by autoantibodies against platelets (5). Initial presentations of ITP are petechiae and purpura, with a more severe progression to intracranial hemorrhage or gastrointestinal bleeding, leading to a fatal outcome, if treatment is not started on a promptly manner (3, 5). Chronic conditions, such as systemic lupus erythematosus (SLE), chronic lymphocytic leukemia (CLL), and infectious agents such as immunodeficiency virus (HIV), hepatitis C virus (HCV), and CMV, can trigger this form of ITP (5–7). The various theories of CMV infection-induced thrombocytopenia were described by Crapnell et al., as CMV-induced direct cytotoxicity to hematopoietic cells with immune-mediated destruction of infected cells or impairment of bone marrow stromal function (7).
Immune thrombocytopenic purpura is frequently an exclusionary diagnosis, characterized by the absence of any other clinical condition accountable for the low platelet count and isolated thrombocytopenia. Generally, risk of bleeding in ITP is low, but it increases when platelet count is <10 × 109/L. Initial treatment should focus on preventing clinically significant bleeding and treating the primary infection, rather than to normalize platelet count, as the risk of bleeding is low even in patients with severely low platelet counts (5). Some physicians believe treatment with steroids should be avoided in patients with CMV-induced thrombocytopenia, as immunosuppressive treatment may be responsible for the primary CMV infection exacerbation and trigger further decrease in platelet count (3, 8). It has been demonstrated that standard therapies for ITP, including IVIG and splenectomy, regain their efficacy once the primary CMV infection is controlled with antiviral agents such as ganciclovir or valganciclovir (3, 9). Treatment with ganciclovir or valganciclovir is currently recommended as first-line treatment for immunecompromised adults with severe CMV disease, and few studies have evaluated the use of these antiviral drugs for treatment in immunocompetent adults due to the major side effects of these agents such as myelosupression (10). Nevertheless, untreated CMV disease in immunocompetent adults is associated with increased morbidity and mortality. There are some studies that provide evidence of significant clinical improvement after treatment with antiviral agents is started (10–13). On the other hand, the use of thrombopoeitin receptor (TPO-R) agonists such as romiplostin (Nplate®) has been studied, and literature has shown that these agents are effective in refractory ITP compared to placebo (14–16).
Conclusion
Our case report will add to the existing body of research and will increase awareness of this form of thrombocytopenia. Further research is needed in order to establish general treatment guidelines for CMV-induced thrombocytopenia in immunocompetent patients while steroids administration is still debatable.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med (2006) 355(16):1672–81. doi: 10.1056/NEJMoa054626
2. Wei SH, Ho MC, Ni YH, Lin DT, Lee PH. Cytomegalovirus-associated immune thrombocytopenic purpura after liver transplantation. J Formos Med Assoc (2007) 106(4):327–9. doi:10.1016/S0929-6646(09)60261-8
3. Shrestha R, Rondelli D, Sherpa MT. Cytomegalovirus: a possible cause of persistent refractory immune thrombocytopenic purpura. J Adv Intern Med (2014) 3(1):42–5. doi:10.3126/jaim.v3i1.10704
4. Ross DW, Ayscue LH, Watson J, Bentley SA. Stability of hematologic parameters in healthy subjects. Intraindividual versus interindividual variation. Am J Clin Pathol (1988) 90(3):262–7.
5. James NG, Donald MA. Immune Thrombocytopenia (ITP) in Adults: Clinical Manifestations and Diagnosis (2015). Available from: http://www.uptodate.com/contents/immune-thrombocytopenia-itp-in-adults-clinical-manifestations-and-diagnosis
6. Sheng YZ, Tang LF, Zou CC, Yan Zheng J, Zhao ZY. Cytomegalovirus-associated idiopathic thrombocytopenic purpura in Chinese children. Scand J Infect Dis (2008) 40(11–12):922–7. doi:10.1080/00365540802238471
7. Yenicesu I, Yetgin S, Ozyurek E, Aslan D. Virus-associated immune thrombocytopenic purpura in childhood. Pediatr Hematol Oncol (2002) 19(6):433–7. doi:10.1080/08880010290097233
8. Liebman HA. Viral-associated immune thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program (2008) 2008(1):212–8. doi:10.1182/asheducation-2008.1.212
9. DiMaggio D, Anderson A, Bussel JB. Cytomegalovirus can make immune thrombocytopenic purpura refractory. Br J Haematol (2009) 146(1):104–12. doi:10.1111/j.1365-2141.2009.07714.x
10. Lancini D, Faddy HM, Flower R, Hogan C. Cytomegalovirus disease in immunocompetent adults. Med J Aust (2014) 201(10):578–80. doi:10.5694/mja14.00183
11. Kocak ED, Sherwin JC, Hall AJ. Cytomegalovirus disease in immunocompetent adults. Med J Aust (2015) 202(8):419. doi:10.5694/mja14.01704
12. Serna-Higuera C, Gonzalez-Garcia M, Milicua JM, Munoz V. Acute cholestatic hepatitis by cytomegalovirus in an immunocompetent patient resolved with ganciclovir. J Clin Gastroenterol (1999) 29(3):276–7. doi:10.1097/00004836-199910000-00010
13. Rafailidis PI, Mourtzoukou EG, Varbobitis IC, Falagas ME. Severe cytomegalovirus infection in apparently immunocompetent patients: a systematic review. Virol J (2008) 5:47. doi:10.1186/1743-422X-5-47
14. Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet (2008) 371(9610):395–403. doi:10.1016/S0140-6736(08)60203-2
15. Newland A, Caulier MT, Kappers-Klunne M, Schipperus MR, Lefrere F, Zwaginga JJ, et al. An open-label, unit dose-finding study of AMG 531, a novel thrombopoiesis-stimulating peptibody, in patients with immune thrombocytopenic purpura. Br J Haematol (2006) 135(4):547–53. doi:10.1111/j.1365-2141.2006.06339.x
Keywords: immune thrombocytopenic purpura, cytomegalovirus, steroids, valgancyclovir, bleeding
Citation: Flores-Chang BS, Arias-Morales CE, Wadskier FG, Gupta S and Stoicea N (2015) Immune thrombocytopenic purpura secondary to cytomegalovirus infection: a case report. Front. Med. 2:79. doi: 10.3389/fmed.2015.00079
Received: 14 August 2015; Accepted: 20 October 2015;
Published: 03 November 2015
Edited by:
César Aldecoa, Hospital Universitario Río Hortega, SpainReviewed by:
Ohta Yoshinori, Hyogo College of Medicine, JapanJesus Rico-Feijoo, Hospital Universitario Río Hortega, Spain
Copyright: © 2015 Flores-Chang, Arias-Morales, Wadskier, Gupta and Stoicea. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bessy S. Flores-Chang, YmVzc3lzdXlpbkBnbWFpbC5jb20=
 Bessy S. Flores-Chang
Bessy S. Flores-Chang Carlos E. Arias-Morales
Carlos E. Arias-Morales Francis G. Wadskier
Francis G. Wadskier Sorab Gupta
Sorab Gupta Nicoleta Stoicea
Nicoleta Stoicea