
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 03 September 2015
Sec. Pathology
Volume 2 - 2015 | https://doi.org/10.3389/fmed.2015.00059
This article is part of the Research TopicOrgan Fibrosis: Triggers, Pathways and Cellular PlasticityView all 7 articles
Chronic organ injury leads to fibrosis and eventually organ failure. Fibrosis is characterized by excessive synthesis, remodeling, and contraction of extracellular matrix produced by myofibroblasts. Myofibroblasts are the key cells in the pathophysiology of fibrotic disorders and their differentiation can be triggered by multiple stimuli. To develop anti-fibrotic therapies, it is of paramount importance to understand the molecular basis of the signaling pathways contributing to the activation and maintenance of myofibroblasts. Several signal transduction pathways, such as transforming growth factor (TGF)-β, Wingless/Int (WNT), and more recently yes-associated protein 1 (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) signaling, have been linked to the pathophysiology of fibrosis. Activation of the TGF-β1-induced SMAD complex results in the upregulation of genes important for myofibroblast function. Similarly, WNT-stabilized β-catenin translocates to the nucleus and initiates transcription of its target genes. YAP and TAZ are two transcriptional co-activators from the Hippo signaling pathway that also rely on nuclear translocation for their functioning. These three signal transduction pathways have little molecular similarity but do share one principle: the cytosolic/nuclear regulation of its transcriptional activators. Past research on these pathways often focused on the isolated cascades without taking other signaling pathways into account. Recent developments show that parts of these pathways converge into an intricate network that governs the activation and maintenance of the myofibroblast phenotype. In this review, we discuss the current understanding on the signal integration between the TGF-β, WNT, and YAP/TAZ pathways in the development of organ fibrosis. Taking a network-wide view on signal transduction will provide a better understanding on the complex and versatile processes that underlie the pathophysiology of fibrotic disorders.
Regardless of the initial trigger, chronic organ injury disturbs the cellular and molecular processes of normal wound healing, resulting in organ fibrosis and eventually organ failure (1). Chronic injury causes prolonged activation of effector cells, such as fibroblasts (2), pericytes (3–5), bone marrow-derived cells (6–8), and possibly cells from epithelial (9) or endothelial origin (10, 11), which differentiate toward myofibroblasts. In normal granulation tissue, myofibroblasts are essential for the deposition, contraction, and remodeling of the extracellular matrix (ECM) and thereby promote wound healing (12). However, aberrant wound healing results in increased proliferation and attenuated apoptosis of myofibroblasts. The well-developed cytoskeletal apparatus of myofibroblasts contains actin and myosin, which are linked to the so-called supermature focal adhesions that connect the cells actin filaments to the ECM (13). This allows myofibroblasts to contract the ECM around them and create contractures that impede organ function (14). The de novo expression of smooth muscle α-actin (αSMA), an isoform usually expressed in smooth muscle cells, further enhances their contractile capabilities (15, 16). Moreover, myofibroblasts are notorious producers of ECM components, such as collagens, glycoproteins, and proteoglycans, resulting in the formation of fibrous scar tissue. Cross-linking of collagen in fibrous scar tissue makes it highly resistant to protease degradation and results in irreversible scarring and destruction of the tissue architecture (17). Although a large body of knowledge exists on myofibroblast biology, as of to date, no approved therapies are available that can reverse fibrosis (18, 19). Thus, understanding the molecular mechanisms that govern the differentiation and maintenance of myofibroblasts in fibrotic diseases is of paramount importance.
The differentiation of myofibroblasts is governed by an interplay between different mechanisms. Under increased tissue stiffness and mechanical strain, fibroblasts become activated and show increased β- and γ-actin and αSMA-containing stress fibers, linked to focal adhesions (15). They also start to express the ED-A splice variant of cellular fibronectin – crucial for myofibroblast differentiation – at the plasma membrane (20, 21). Membrane protruding integrin molecules connect the ECM components to the actin fibers, which allows for the conversion of mechanical into biochemical cues that are relayed to the nucleus.
Alternatively, myofibroblast differentiation is driven by biochemical signaling of extracellular growth factors. Many growth factor families have been studied extensively in the context of organ fibrosis, with an emphasis on the transforming growth factor (TGF)-β and Wingless/Int (WNT) signaling pathways as key mediators [reviewed in Ref. (22, 23)]. Their mode of action describes the production of soluble growth factor ligands by a variety of cell types. The growth factors are stored in the ECM, until they are activated and released by mechanical tension or proteolytic cleavage, which enables these ligands to engage their membrane-bound receptors. The receptors relay the biochemical signal inwards, via kinase complexes, to the nucleus. Nuclear transcriptional modulators then act on the chromatin complex in order to change the transcriptional landscape, and thereby promote or repress transcription of target genes. Recently, in fibrosis research the attention shifted toward a relatively new signaling cascade: yes-associated protein 1 (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) signaling. Interestingly, the three mentioned signal transduction pathways have but little molecular similarity but do share one principle: the cytosolic/nuclear regulation of their transcriptional modulators.
In the past, signaling cascades were often studied in isolation, i.e., a ligand signals through its receptor and mediates the nuclear accumulation of one or several transcription factors to modulate target gene expression. This view changed since recent advances suggest that these cascades are in fact organized into complex signaling networks which, dependent on the cellular and environmental context, govern cell function and fate in fibrotic disorders. This inter-pathway communication allows for increased versatility and fine tuning of cellular responses, which may explain the variety of phenotypes found in fibrotic disorders. The aim of this review is to discuss the current understanding on the signal integration between the TGF-β, WNT, and YAP/TAZ pathways in the development of fibrosis. We will start with a short overview of the three pathways, and extend our discussion with a detailed view on how these pathways connect at multiple levels of signal transduction in the context of myofibroblast function and fibrosis. Finally, we touch upon the challenges and considerations in the design of anti-fibrotic therapies, with focus on the cross-talk between the three signal transduction cascades.
The TGF-β superfamily of growth factors consists of multiple proteins that govern a wide range of physiological processes, such as stem cell pluripotency, cell fate determination, proliferation, and differentiation. In humans, over 30 members of the TGF-β superfamily have been documented, including TGF-βs, activins, inhibins, nodal, growth/differentiation factors (GDFs), and bone morphogenetic proteins (BMPs). In this review, we focus mainly on the canonical signaling through TGF-β1, since considerable evidence exists for its role in fibrosis (22) (Figure 1).
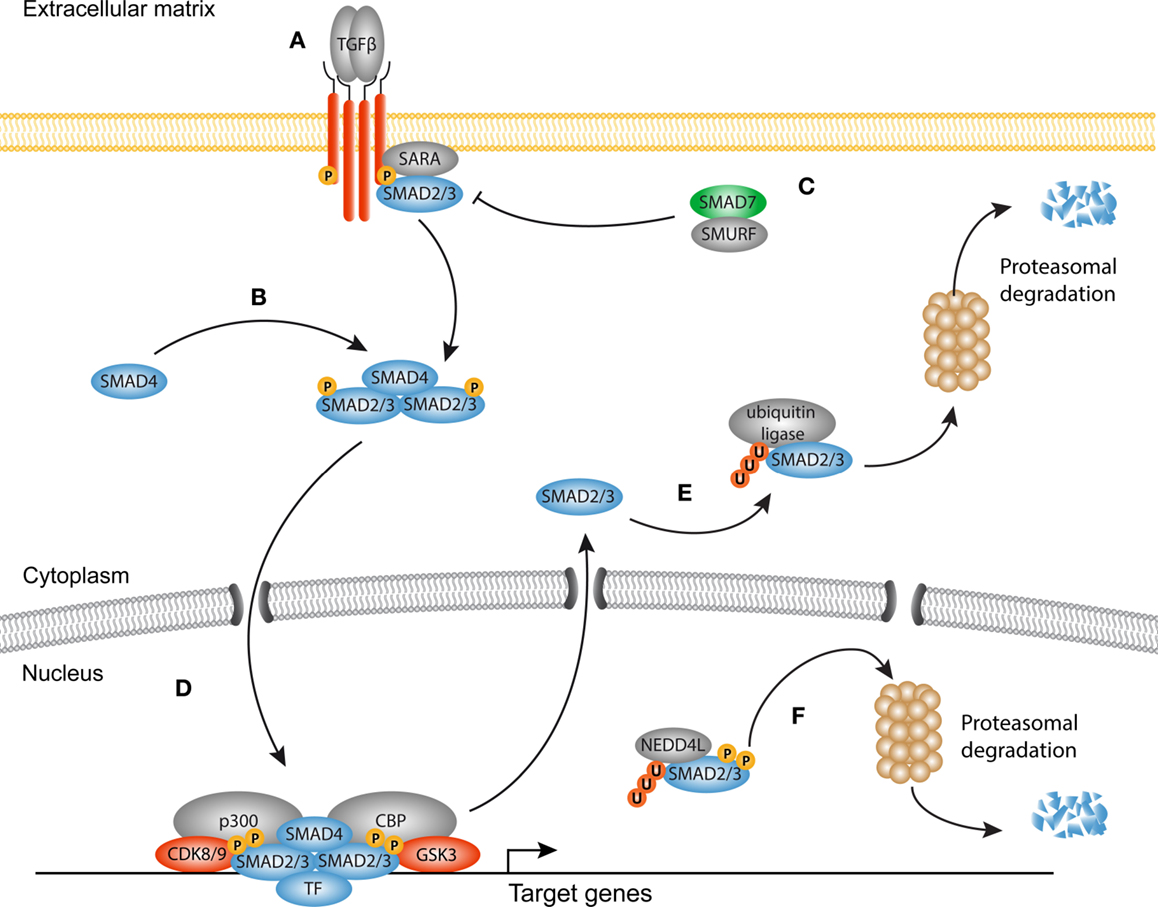
Figure 1. Canonical TGF-β signaling in fibrosis. (A) Transforming growth factor-β (TGF-β) homodimers engage the type II receptors, which phosphorylate and form a heterotetrameric complex with two type I receptors and additional Smad-binding proteins such as SARA. The signaling domain of the type I receptor mediates phosphorylation and activation of Smad proteins. (B) Smad4 associates with phosphorylated Smads to form an active heterotrimeric complex. (C) The inhibitory Smad7 together with Smad-specific E3 ubiquitin protein ligases (Smurf) inhibits the receptor complex by targeting it for ubiquitination and proteasomal degradation. (D) The activated Smad complex forms a transcriptional module with several transcription factors, co-factors such as p300 and Creb binding protein (CBP) to promote transcription of target genes (e.g., PAI1, COL1A1, CCN2). (E) Dephosphorylated Smad proteins continuously shuttle between the nucleus and the cytoplasm. In the cytoplasm, they can be targeted for degradation by ubiquitin ligases. (F) Consecutive phosphorylation by CDK8/9 and GSK3 in the nucleus recruits the ubiquitin ligase Nedd4L that target Smad proteins for proteasomal degradation in the cytoplasm, and possibly the nucleus.
In homeostatic conditions, TGF-β is trapped in the ECM together with latency-associated peptides (LAPs) and latent TGF-β-binding proteins (LTBPs) in the so-called large latent complex (LLC) (24). Upon injury, proteolytic cleavage of the LAP (25), or binding of integrins together with increased mechanical forces (26–28), cause release of TGF-β from the LLC allowing it to engage its receptors. Signaling propagation occurs when a TGF-β homodimer interacts with two type I and two type II receptors. Ligand binding initiates the phosphorylation of the SGSGSG domain on the type I receptor by the type II receptor (29, 30). Subsequently, the activated type I receptor is now able to bind and phosphorylate Smad proteins, the central modulators of canonical TGF-β signaling. There are three classes of Smad proteins: regulatory (R)-Smads, co-activator (Co)-Smads, and inhibitory (I)-Smads. R-Smads (Smad2 and Smad3) are phosphorylated by the type I receptor and form heteromeric complexes with the Co-Smad, Smad4. Both R-Smads and Smad4 consist of a N-terminal MH1 and a C-terminal MH2 domain connected by a linker region. Upon phosphorylation of the MH2 domain, Smad complexes shuttle to the nucleus and together with DNA-binding proteins (31–33) localize to specific CAGAC motifs, the so-called Smad-binding elements (SBE), to regulate transcription of target genes (34).
There is ample evidence that TGF-β signaling is a key regulator of myofibroblast biology in the heart, lungs, liver, kidneys, and skin (35–49). TGF-β levels are elevated in fibrotic tissues and myofibroblasts display nuclear accumulation of Smads in vivo accompanied with an increased expression of TGF-β target genes and decreased levels of the inhibitory Smad6 and Smad7. Despite a tremendous body of experimental work, the mechanisms underlying Smad-induced fibroblast activation are incompletely understood, as both activation and inhibition of Smads can promote fibrogenesis, dependent on the context (37, 47, 50–55). Furthermore, inhibition of the Smad signaling cascade does not completely attenuate the fibrotic response, which suggests that several other signaling cascades are involved in activating the transcriptional program of myofibroblasts. It has become evident that transcriptional output of Smad signaling is tightly controlled by the interplay with a variety of master transcription factors, DNA-binding (co)factors, repressors, and chromatin readers, and writers (33, 56).
Discovered in Sophophora (Drosophila) as Wingless and in the mouse as Int1, together termed WNT in mammals, canonical WNT signaling comprises the molecular interactions leading to the nuclear translocation of β-catenin [reviewed in Ref. (23, 57)] (Figure 2). WNTs have primarily been studied in fetal development as they are responsible for the formation and polarity of the primary body axis (58), but it has become evident that they are versatile growth factors in both homeostasis and disease. Soluble WNT ligands bind to a family of seven transmembrane receptors named Frizzled (Fz). A single WNT ligand can interact with several Fz receptors, and vice versa (59). In a WNT-off state, the concentration of endogenous WNT antagonists outweighs that of WNT ligands, which results in the phosphorylation of cytoplasmic β-catenin by two subunits from the β-catenin destruction complex, glycogen synthase kinase (GSK)3, and casein kinase (CK)1. These phosphorylation events trigger subsequent ubiquitination and proteasomal degradation of β-catenin (60). In a WNT-on state, ligands engage the Fz receptors which function together with the low-density-lipoprotein-receptor-related proteins (LRP)5 and LRP6 co-receptors to activate the downstream signaling cascade. LRP is phosphorylated in its cytoplasmic tail by GSK3 and CK1 proteins (61–63). The activated Fz/LRP complex interacts with Disheveled (DVL), Axin, and GSK3 through Pro-Pro-Pro-(Ser/Tyr)-Pro repeats (62, 63). Axin functions as a scaffold for the destruction complex, as it directly interacts with β-catenin, GSK3, CK1, the tumor suppressor protein adenomatous polyposis coli (APC), and the ubiquitin ligase β-TrCP. As Axin and GSK3 are sequestered to the plasma membrane by the Fz/LRP complex, β-TrCP is excluded from the destruction complex, limiting β-catenin ubiquitination and degradation (64). An alternative route of WNT activation describes the formation of LRP5/6 aggregates that are internalized together with the destruction complex in the so-called multivesicular bodies. Once inside the multivesicular bodies, a large portion of β-catenin cannot interact with the destruction complex and thus escapes ubiquitination (65, 66). Stabilized β-catenin now accumulates in the nucleus where it associates with T-cell factor/lymphoid enhancer-binding factor-1 (TCF/Lef-1) transcription factors and several co-factors like p300 and CREB binding protein (CBP) to regulate transcription of target genes (67, 68).
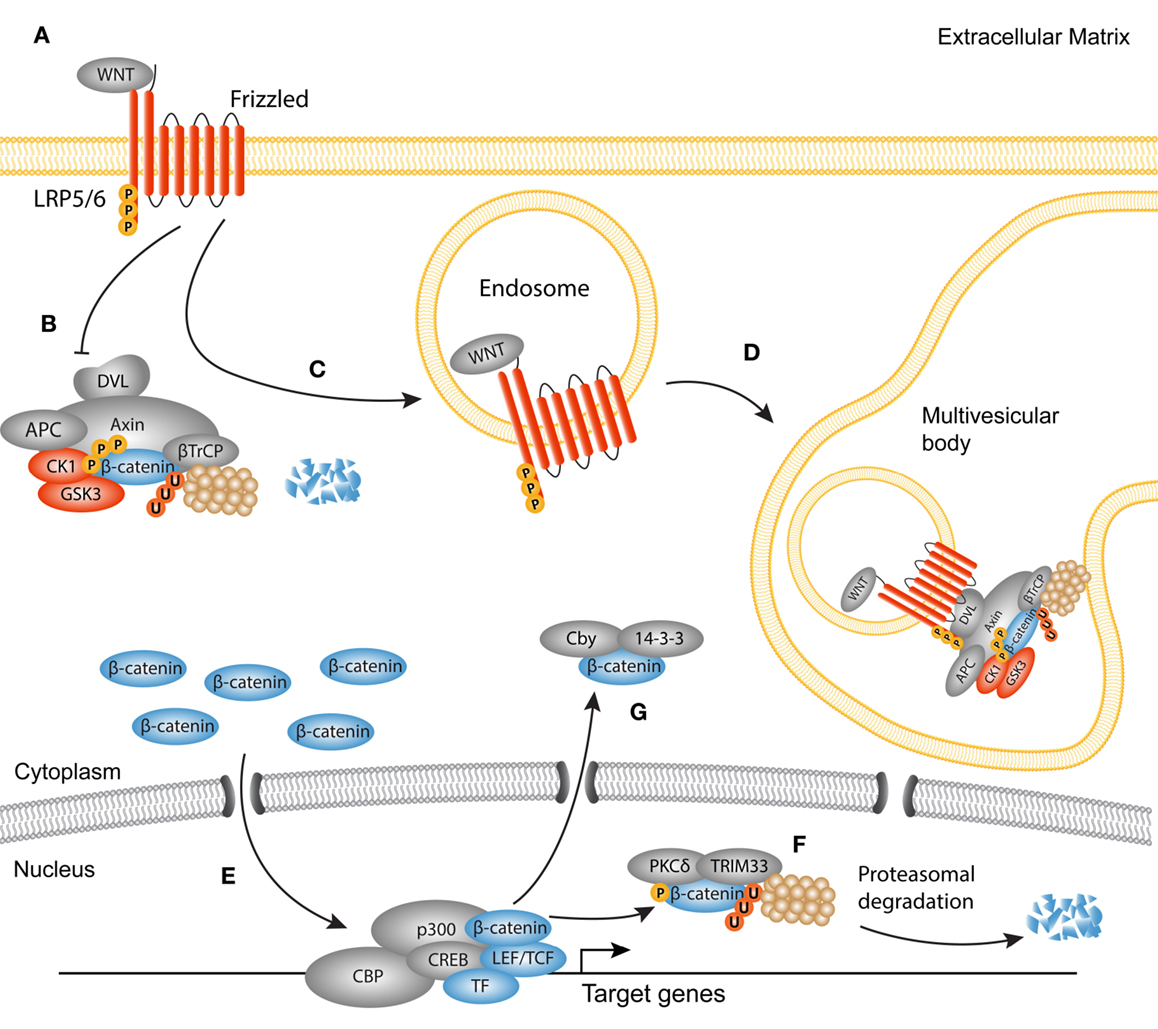
Figure 2. Active canonical WNT signaling in fibrosis. Simplified scheme showing the activated canonical WNT cascade and translocation of β-catenin. (A) WNT ligands bind to a frizzled receptor and form a complex with the co-receptor Lipoprotein-related-receptor protein (LRP). (B) The ubiquitination activity of the destruction complex [comprised of disheveled (DVL) Axin, adenomatous polyposis coli (APC), glycogen synthase kinase 3 (GSK3), and casein kinase 1(CK1)] is inhibited causing β-catenin to accumulate in the cytoplasm. (C) As later event, clusters of receptor complexes are internalized into endosomes, which triggers the sequestering of the destruction complex, and subsequent inhibition of GSK3. (D) GSK3 is then transported to multivesicular bodies where it cannot interact with cytoplasmic β-catenin, and thus protects β-catenin from proteasomal degradation. (E) Newly synthesized β-catenin translocates to the nucleus to interact with LEF/TCF transcription factors and other co-factors, such as p300 and CBP. (F) Termination of the WNT/β-catenin signaling cycle may occur through phosphorylation of β-catenin by protein kinase C (PKC)δ and subsequent ubiquitination by tripartite motif (TRIM)33. These steps target β-catenin for proteasomal degradation in the nucleus. (G) Another possible route for the termination of β-catenin activation is the cytoplasmic sequestering by 14-3-3ζ and Chibby (Cby).
About 10 years ago, the first evidence of WNT involvement in myofibroblast biology was found (69). Since then, many studies have emphasized a key role for canonical WNT signaling in fibrogenesis of the heart, lungs, kidneys, and several fibrotic disorders of the skin (70–86). Aberrant activation of WNT signaling can be caused by increased expression of WNT agonists (87), or by silencing of endogenous WNT antagonists, such as proteins from the Dikkopf (DKK) and secreted frizzled-related protein (sFRP) families (77, 86, 88, 89). Experimental models that use exogenous overexpression of WNT ligands or sustained nuclear accumulation of β-catenin suggest that canonical WNT signaling is enough to trigger the expression of a fibrogenic program in fibroblasts (85, 86). However, depletion of β-catenin in the same model could not completely prevent the development of fibrosis, suggesting that β-catenin functions in concert with other pro-fibrotic signals (86). Similar findings come from pulmonary fibroblasts, in which β-catenin stabilization was not sufficient for the upregulation of myofibroblasts-specific genes (74). The discrepancy between the different studies may be due to differences in the constructs used to stabilize β-catenin. Taken together, it has become evident that regulation of β-catenin cytoplasmic/nuclear shuttling is an intricate process, evidenced by the complex expression pattern of WNT ligands in the course of fibrogenesis (71, 77, 82).
YAP and TAZ are regarded as the main output of the Hippo pathway (Figure 3). YAP and TAZ have been extensively studied in relation to the Hippo core kinase complex, and its role in organ size control, stem cell fate, and cancer (90–92). Both YAP and TAZ contain a WW domain that binds to Pro–Pro–X–Tyr motifs of associated proteins (93–95). The Hippo signaling cascade consists of the Ser/Thr kinases MST1 and MST2, which are orthologs of the Drosophila Hippo kinase (96). MST1/2 binds to Salvador (SAV)/WW45 to form an active enzyme complex that phosphorylates the MOB1A/B subunits of LATS1/2 (97). The activated LATS1/2–MOB1A/B complex in turn phosphorylates YAP and TAZ. The primary phosphorylation of YAP and TAZ triggers subsequent phosphorylation by CK1 kinases. This generates a “phosphodegron” recognized by β-TrCP, leading to YAP and TAZ polyubiquitination and subsequent proteasomal degradation (98). The Serine residues relevant for the inactivation of YAP and TAZ are Ser127 (Ser89 in TAZ) and S381 (S311 in TAZ) (99, 100). When the Hippo pathway is inactive, YAP and TAZ are dephosphorylated and translocate to the nucleus, where they associate with transcription factors and other DNA-binding proteins to modulate target gene transcription. Despite a similar mechanism of activation, YAP and TAZ can bind different transcription factors, but also display overlap as seen with the association with TEA DNA-binding domain (TEAD) transcription factors (101–104). This suggests that their functions only partially overlap, but do share redundancy in some biological contexts.
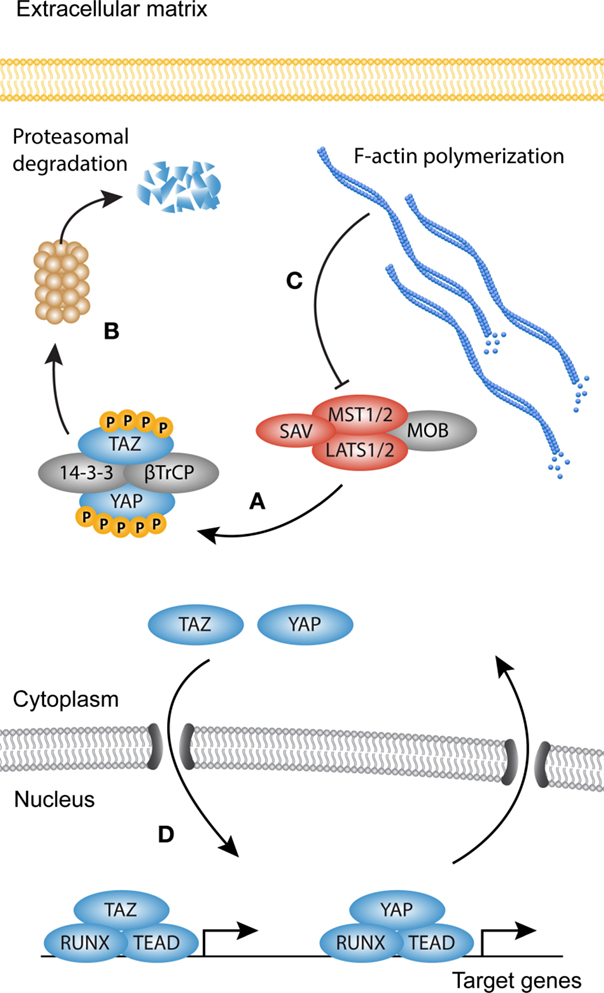
Figure 3. YAP and TAZ signaling. Simplified scheme showing activation of YAP and TAZ. (A) When the Hippo kinase complex [comprised of Serine/threonine-protein kinases (MST1/2), MOB kinase activator 1 (MOB1), Salvador (SAV), and serine/threonine-protein kinases (LATS1/2)] is active, YAP and TAZ become phosphorylated on multiple sites, creating a so-called phosphodegron. (B) Both YAP and TAZ are then sequestered in the cytoplasm by 14-3-3 proteins or targeted for degradation by β-TrCP. (C) Polymerization of the F-actin cytoskeleton inhibits the activity of MST1/2, rendering the core kinase complex inactive (several other upstream activators of the core kinase complex are not shown). (D) YAP and TAZ now translocate to the nucleus where they associate with transcription factors such as Runt-related transcription factor (RUNX) and TEA domain family member (TEAD) to modulate transcription.
The capabilities of YAP and TAZ to regulate organ growth and size are striking, but at the same time incompletely understood. It is however clear that they perform these functions, at least in part, as mechanical rheostats independent of the core kinase complex. This becomes evident in cells cultured in vitro, which show strong nuclear localization of YAP/TAZ in semi-confluent cultures, but when reaching confluence YAP and TAZ translocate to the cytoplasm (97). A similar biomechanical program can be observed in cells grown on pathologically stiff substrates or substrates that allow cell spreading, as they display predominantly nuclear accumulation of YAP and TAZ and increased transcription of their target genes. By contrast, cells grown on compliant substrates or space limiting substrates display cytoplasmic localization of YAP and TAZ (105–107). F-actin polymerization determines cell morphology and increases in cells cultured in sub-confluence or on stiff substrates. Indeed, F-actin polymerization proves to be the link between cell spreading and YAP and TAZ nuclear translocation (106). The mechanical properties of YAP and TAZ were recently translated to myofibroblast activation and the induction of fibrosis. In biopsies from idiopathic pulmonary fibrosis, both YAP and TAZ levels are elevated, and display a predominantly nuclear localization, which suggests increased transcriptional activity (108). Moreover, YAP and TAZ knockdown in mouse lung and liver fibroblasts cultured on stiff substrates reduces the levels of proteins associated with myofibroblast differentiation such as pro-collagen, αSMA, and plasminogen activator inhibitor (PAI)1 (108, 109). Adding to this, mice heterozygous for TAZ show a remarkable resilience against bleomycin-induced pulmonary fibrosis, possibly due to reduced levels of CCN2 (CTGF), one of the YAP and TAZ target genes (110). Also in cardiac fibrosis, YAP and TAZ have been a topic of investigation, but whether YAP and TAZ promote fibrogenesis remains elusive, and is probably dependent on the context of injury (111, 112).
Taken together, these findings suggest that the nuclear translocation of YAP and TAZ is the sum of multiple tiers of regulation acting in concert. Because YAP and TAZ activity is not only controlled by Hippo signaling but also by mechanical signals and other signaling cascades, we refer to YAP and TAZ signaling in its broader context.
In fibrosis, it is becoming clear that the TGF-β, WNT, and YAP and TAZ signaling pathways work in concert, instead of being isolated entities. Several studies have hinted at the inter-pathway cross-talk in the differentiation of myofibroblasts. For instance, in lung fibrosis, protein levels of both YAP and TAZ are increased and have increased nuclear localization (108). This corresponds to the increased levels of nuclear β-catenin and phosphorylated R-Smads found in fibrotic tissues. During skin wound healing in mice, both YAP and TAZ are increased upon injury and translocated to the nucleus. Moreover, TGF-β1 levels are also increased in the dermis, suggesting a link between activation of YAP and TAZ and the production of TGF-β1 (113). Adding to this, YAP- and TAZ-deficient fibroblasts are less reactive to TGF-β stimulation in vitro, produced less ECM, have lower expression of myofibroblast markers PAI1 and αSMA, and show lower contractile capabilities (108).
The mechanisms through which these pathways communicate are diverse and range from modulating the availability of growth factors and the availability of membrane bound receptors to nuclear entry and activation of transcription factors (Figure 4).
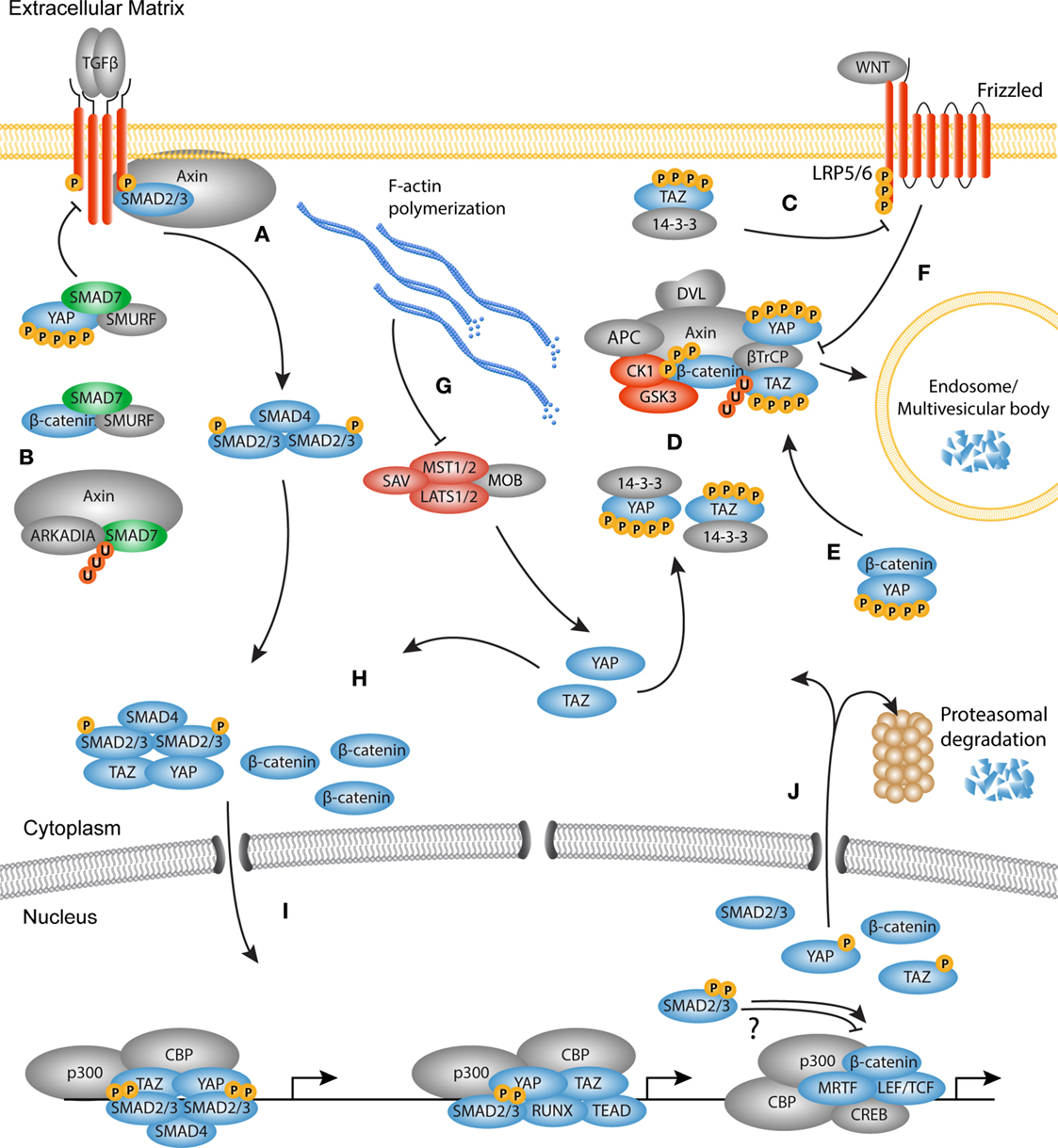
Figure 4. The TGF-β, WNT, and YAP/TAZ signaling pathways converge. Schematic overview of the molecular cross-talk between components of the TGFβ, WNT, and YAP/TAZ pathways. (A) Upon TGF-β stimulation, Axin promotes the tail-phosphorylation of Smad3. (B) Axin also promotes the degradation of inhibitory Smad7, thereby further enhancing the TGF-β signal. Smad7 can associate with both YAP and β-catenin. Binding of YAP to Smad7 increases the affinity for the type I receptor and increases the repressive effects on TGF-β signaling. Smad7 binding with β-catenin can mediate both degradation and stabilization of β-catenin. (C) TAZ inhibits the phosphorylation of disheveled (DVL) by casein kinase (CK)1, providing either positive or negative feedback depending on the WNT ligand present. (D) The active Hippo core kinase complex phosphorylates both YAP and TAZ creating a phosphodegron. Phosphorylated YAP and TAZ are either sequestered by 14-3-3 proteins or associate with the β-catenin destruction complex. In the destruction complex, YAP and TAZ are necessary for docking of β-TrCP to the complex. (E) Phosphorylated YAP also associates with β-catenin to inhibit its nuclear translocation and promote its degradation. (F) Upon WNT activation, the destruction complex is inhibited because YAP and TAZ dissociate from the complex. As later event, the destruction complex is sequestered by the LRP/Frizzled/DVL receptor complex and targeted for degradation in the microvascular bodies. (G) F-actin polymerization inactivates the core kinase complex, causing YAP and TAZ to be dephosphorylated. Concurrently, β-catenin is not degraded by the inactive destruction complex, so that newly synthesized β-catenin accumulates in the cytoplasm. (H) The activated Smad complex associates with YAP or TAZ and translocates to the nucleus. Free and stabilized β-catenin also translocates to the nucleus. (I) In the nucleus, the transcription factors may co-localize at the chromatin depending on the context to govern transcription of myofibroblast related genes. (J) At the end of the transcription cycle, transcription factors are degraded in the nucleus, or translocate back to the cytoplasm for either degradation or a new round of activation.
The most straightforward form of cross-talk between signaling cascades occurs when activity of one pathway enhances the production of agonists or antagonists of the second. This type of cross-talk can create a feed-forward or feedback loop to enhance or attenuate, respectively, the transcriptional activity of another signaling cascade. For instance, stimulation of fibroblasts with WNT3a enhances the expression of TGF-β1 and subsequent phosphorylation of the MH2 domain of Smad2 (114). Consistently, absence of WNT signaling through LRP5 in bleomycin-induced lung fibrosis decreases the expression of TGF-β1 and attenuates the fibrotic response (115). Reconstitution of active TGF-β1 signaling in LRP5-deficient mice overrides the protective effects of abrogated WNT signaling. Moreover, it was found that TGF-β also enhances WNT signaling through the inhibition of DKK1 (116). Reduced expression of DKK1 enhances the stability and nuclear accumulation of β-catenin in both epithelial cells and fibroblasts, whereas reconstitution of DDK1 in vivo attenuates TGF-β-induced fibrosis. This allows cells to communicate over a certain distance and influence the microenvironment of its neighboring cells. Despite the strong effects of altered growth factor signaling, this is often not enough to modulate complex fibrogenic responses. To achieve this kind of complexity, direct interaction between signaling components is required.
TGF-β, WNT, and YAP/TAZ output activity all rely on a general mechanism: the nuclear translocation of its transcriptional modulators. To prevent continuous activation, the cell has several means to prevent spontaneous nuclear entry or binding to the DNA. For instance, Smad proteins continuously shuttle between the cytoplasm and the nucleus (33). Without tail-phosphorylation by the TGF-β type I receptor, Smads cannot interact with Smad4 and are unable to engage the DNA. Instead, they are phosphorylated in the linker region by CDK8/9 and GSK3 which renders them susceptible for polyubiquitination and degradation (31). By contrast, β-catenin and YAP and TAZ are sequestered in protein complexes with E-cadherin or 14-3-3 proteins, respectively, or directly targeted for proteasomal degradation by associating with β-TrCP (60, 94, 97).
Recent studies highlighted that extensive cross-talk occurs on the level of cytoplasmic retention and degradation. One example of cross-talk between the YAP and TAZ, and TGF-β pathways is through the interaction with Smad7. As mentioned above, activated Smad proteins need to form a complex with Smad4 in order to become transcriptionally active modules. To regulate the Smad activation cycle, the TGF-β pathway uses Smad7 to form a negative feedback loop via various mechanisms. First, Smad7 can associate with the TGF-β type I receptor. Consequently, R-Smad phosphorylation and complex formation between R-Smads and Smad4 are inhibited (117). Smad7 also recruits E3 ubiquitin ligases, such as Smurf1 and Smurf2, to initiate receptor ubiquitination and degradation of the receptor complex. This self-regulating layer of the TGF-β signaling cascade can be linked to YAP and TAZ signaling, as YAP was found to associate with Smad7 at the type I receptor (118). By binding to YAP, Smad7 has a higher affinity for the type I receptor and increases its repressive effects on TGF-β signaling. Another line of evidence revealed that Smad7 interacts with β-catenin to promote Smurf2-induced mediated ubiquitination and degradation, attenuating WNT activity in the skin (119). By contrast, in cancer epithelial cells it was found that Smad7 promotes the stability of β-catenin by enhancing its association with E-cadherin at the plasma membrane (120). Moreover, upon TGF-β stimulation, the WNT scaffold protein Axin can form a complex with Smad7 and the E3 ubiquitin ligase Arkadia to promote Smad7 degradation (121). These conflicting reports underline that Smad7 may act as repressor or enhancer of cellular signaling depending on the cell type and environmental context.
As mentioned above, the type I receptor initiates phosphorylation of R-Smads, a process that is regulated through the interaction with several adaptor proteins. Interestingly, in unstimulated fibroblasts, Axin facilitates the binding of Smad3 with the type I receptor, independent from the adapter protein SARA (122). Upon TGF-β simulation, Axin promotes the tail-phosphorylation of Smad3 and subsequently dissociates from the type I receptor. Depletion of Axin results in decreased expression of TGF-β responsive genes such as PAI1, suggesting that Axin mediates cytoplasmic cross-talk between the TGF-β and WNT pathways which promotes the transcription of pro-fibrotic genes.
Recently it was found that TAZ also communicates with WNT signaling through the interaction of TAZ and β-catenin in the cytoplasm (123). In a WNT-off state, both β-catenin and TAZ associate with β-TrCP and are ubiquitinated and degraded in the proteasome. This process requires active GSK3 phosphorylation of the β-catenin phosphodegron. Upon WNT stimulation, GSK3 dissociates from the destruction complex, β-catenin is dephosphorylated and unable to bind to TAZ. Thus, WNT stimulation induces the stability and nuclear localization of its own transcriptional modulator, β-catenin, as well as TAZ.
Furthermore, inactive YAP and TAZ form a complex together with β-catenin, GSK3, and Axin1 (124). Cytoplasmic YAP/TAZ specifically bind Axin in absence of WNT signals. In this case, Axin facilitates the function of cytoplasmic anchor, as Axin depletion results in a rapid nuclear accumulation of YAP/TAZ. Indeed, stimulation by WNT3a causes dissociation of YAP/TAZ from the destruction complex after which they translocate to the nucleus, and modulate transcription of TEAD target genes. Vice versa, as part of the destruction complex, YAP/TAZ are needed for the docking of β-TrCP to the destruction complex. By releasing YAP/TAZ upon WNT stimulation, β-TrCP cannot bind to the destruction complex and ubiquitinate β-catenin. Furthermore, it was found that protein kinase C zeta (PKCζ) associates with the destruction complex and can phosphorylate YAP and β-catenin on several residues, adding to quick proteasomal degradation (125).
Next to its function in the destruction complex, it was found that TAZ binds to the PY and PDZ domains of DVL2 upon stimulation with WNT3a (126). TAZ binding inhibits the phosphorylation of DVL2 by CK1, which results in reduced β-catenin-mediated activity of LEF/TCF transcription. Interestingly, WNT3a and WNT5a – which in part have opposite functions in β-catenin stabilization – both induce the phosphorylation of CK1, suggesting that TAZ binding to DVL2 may have different outcomes depending on the WNT isoform and receptor pair present (127).
Furthermore, it was found that YAP too fulfills multiple roles in YAP/WNT cross-talk. By directly binding to β-catenin, phosphorylated YAP prevents nuclear translocation of β-catenin and subsequent transcription of LEF/TCF target genes (128). This process is dependent on the activity of the Hippo core kinase complex, as increased Hippo activity induces phosphorylation of YAP and concomitantly reduces levels of β-catenin in the nucleus. Evidence thus shows that the transcriptional modulators of the TGF-β, WNT, and YAP/TAZ pathways are integral factors in the cross regulation between these pathways. Cytoplasmic retention of transcription factors and transcriptional activators proves to be an ingenious system through which the three different pathways tightly regulate their own and each other’s activity.
The original view on growth factor signaling described the nuclear accumulation of transcriptional modulators solely as a consequence of ligand-mediated activation. In the absence of a growth factor ligand, Smads and β-catenin were thought to reside exclusively in the cytoplasm and translocate only to the nucleus upon receptor activation. We now know that transcription factor shuttling is not as black and white as once proposed. Without stimulation, R-Smad proteins continuously shuttle between the nucleus and cytoplasm, but display a significant higher concentration in the cytoplasm (129). It is thought that R-Smads reside in the cytoplasm, until TGF-β stimulation releases them for nuclear translocation, enhances their affinity for nuclear importin proteins, and induces nuclear anchoring. In the case of canonical WNT signaling, β-catenin levels are maintained low due to degradation in absence of WNT signals, although β-catenin can be observed in both the cytoplasm and the nucleus. Recent developments reveal that the fold-change in β-catenin levels after WNT stimulation is more important for transcriptional modulation than the absolute levels of β-catenin (130). This finding suggests that even in cells with low basal β-catenin levels, slight changes in nuclear β-catenin are sufficient to initiate transcriptional changes. Nuclear accumulation of YAP and TAZ is governed by the activity of the Hippo signaling cascade as well as biomechanical signals that are relayed from outside the cell. It is becoming clear that fine tuning of nucleocytoplasmic shuttling is not just mediated by a single signaling pathway, but rather by the cross-talk of several components of the TGF-β, WNT, and YAP/TAZ cascades.
In fibrosis, epithelial to mesenchymal transition (EMT) describes the process of epithelial cells that undergo transdifferentiating toward a myofibroblast-like phenotype, a phenomenon observed in both cancer metastasis and fibrosis (9, 131, 132). Upon injury, epithelial cells lose their characteristic cellular junctions and acquire a spindle-like morphology. Cells undergoing EMT often show increased motility, de novo expression of αSMA, and elevated expression of ECM components, such as collagens and fibronectin. Also during EMT, several studies have provided evidence that TGF-β, WNT, and YAP/TAZ interact with each other to drive the transformation toward a mesenchymal-like cell type.
One of the first studies, describing the integration of YAP/TAZ and TGF-β signaling in nucleocytoplasmic shuttling, found that TAZ interacts with Smad2/4 and Smad3/4 complexes in epithelial cells (133). The coiled-coil domain in the C-terminal region of TAZ binds to the MH1 domain of Smad2/3 and thereby promotes the nuclear accumulation of Smad2/3 and increases their transcriptional activity on target genes such as PAI1 and SMAD7. Interestingly, low levels of TAZ promote nuclear accumulation, but when the concentration of TAZ increased, it is predominantly located in the cytoplasm and nuclear localization of Smad2/3 is blocked. This suggests that Smad accumulation is strongly dependent on the expression levels and activation status of TAZ.
Similar findings were obtained for YAP, which forms a complex with Smad3, TEAD, and p300 on the CCN2 promoter in mesothelioma cells (134). Knock down of YAP results in attenuated expression of endothelin1 (ET1) and CCN2, whereas no immediate differences are seen in the expression of fibronectin and collagens, suggesting that YAP controls the expression of a subset of TGF-β responsive genes. Moreover, levels of metalloproteinase 2 (MMP2) are increased upon YAP knock down, strengthening the hypothesis that YAP, together with Smads, governs a pro-fibrotic phenotype. These findings were corroborated in mammary epithelial cells, as YAP/TAZ associates with Smad2/3 and TEADs (135).
A question that then arises is whether transcriptional modulators only need each other for nuclear entry, or also associate with each other at specific promoter or enhancer regions to modulate transcription. Interestingly, during EMT in alveolar epithelial cells, simultaneous stimulation by TGF-β and WNT ligands has synergistic effects on the expression of αSMA as well as the activity of LEF/TCF responsive elements (136). TGF-β alone induces nuclear translocation of β-catenin by inactivating GSK3-mediated degradation, which is further enhanced by WNT stimulation. Delicate ChIP-re-ChIP experiments revealed that β-catenin and Smad3 co-localize at the SBE1 containing region of the αSMA promoter, in a CBP-dependent fashion. These findings are supported by co-localization of β-catenin, Smad3, and CBP in nuclei of epithelial cells in idiopathic pulmonary fibrosis biopsies. Other reports contradict these findings and propose that β-catenin induces the expression of αSMA through interaction with myocardin-related transcription factor (MRTF), a process inhibited by Smad3 (52, 137). One explanation for these conflicting results may be the differences in experimental setup and the different species studied.
Smads and β-catenin were also found to interact on other genes involved in fibrogenesis. As proof of principle, TGF-β and WNT3a synergistically enhanced the promoter activity of sequences containing both SBE and LEF/TCF responsive elements (138). Co-stimulation resulted in a unique expression profile distinct from that seen after stimulation with single growth factors. Interestingly, recent developments describe how YAP can compete with Smad2/3 for promoter occupancy in the transcription of genes involved in mesendoderm differentiation. Gene transcription strongly depends on the phosphorylation status of RNA polymerase II (RNAPII) [reviewed in Ref. (139)]. Briefly, phosphorylation of RNAPII on Ser5 is important for the initiation of transcription, whereas subsequent phosphorylation on Ser2 and Ser7 are crucial for the elongation steps of transcription. β-catenin and LEF-1 associate with enhancer regions of mesendodermal genes such as MIXL1 and EOMES and recruit Ser5 phosphorylated RNAPII to initiate transcription (140). Upon activin stimulation, Smad2/3 localize to the promoter region of these genes to enhance the phosphorylation of Ser2 and Ser7 on RNAPII and thereby promote elongation of transcription. YAP was found to actively inhibit this process by recruitment of the negative elongation factor NELF. Knockdown of YAP reduces the occupancy of NELF and enhances the phosphorylation on Ser2 and Ser7 at target genes, which promotes transcription. Although these results do not directly link to myofibroblast function, they have significant implications on the mechanism by which YAP regulates gene transcription. Future research will reveal if similar mechanisms apply to the regulation of myofibroblast-related genes.
Taken together, TGF-β, WNT, and YAP/TAZ signals converge by modulating the nuclear accumulation and transcriptional activity of their transcription factors. Furthermore, the outcome of this type of cross-talk is not only dependent on the concentration of transcription factors but also on the availability of co-activators and co-repressors, chromatin conformation, and the phosphorylation status of RNAPII, which may vary from one cell type to another (31, 140).
The final stage of the signal transduction cascades involves the process of transcription factor recycling. In the case of Smad proteins, tail-phosphorylation of the MH2 domain induces nuclear accumulation. Whether nuclear R-Smads engage in transcription or are targeted for nuclear exit and proteasomal degradation relies on a series of phosphorylation and dephosphorylation events by multiple kinases and phosphatases [reviewed in Ref. (141)]. The variety of kinases and phosphatases introduces another level of complexity in the regulation of Smad action, and is greatly dependent on signaling through other pathways at a specific place and time.
One of the examples, through which other pathways interact with Smad recycling describes the temporal regulation by CDK8/9 and GSK3. First CDK8/9 phosphorylate Smad1 on Ser206 and Ser214, which allows binding with YAP and simultaneously triggers phosphorylation by GSK3 on Thr202 and Ser210. The latter phosphorylation events cause YAP to dissociate and Smurf2 to bind with Smad1, and initiate ubiquitination (32). Although Smad1 is not activated by TGF-β but rather by BMPs, one can envision identical mechanisms in the recycling of canonical R-Smads by Nedd4L (31, 142). Whether the association of TAZ with Smad2/3 has similar effects on their recycling remains to be determined. As mentioned above, YAP is able to enhance the repressive functions of Smad7 at the type I receptor. Interestingly, Smad7 has also been found to inhibit TGF-β signaling in the nucleus, where it can use its MH2 domain to bind to DNA sequences containing SBEs (143). DNA bound Smad7 competes with Smad2/Smad4 complexes, thus, directly impairing the transcription of TGF-β responsive genes such as PAI1. Whether the interactions between YAP and Smad7 are of importance in this process remain to be elucidated.
Remarkable progress in both biology and pharmacology has led to advances in the development of anti-fibrotic therapies. Many of these therapies aim to target the usual suspects such as ligands and receptors of the TGF-β and WNT signaling cascades using antagonistic antibodies or small-molecule inhibitors. Although the therapeutic efficacy in animal models proves promising (78), trials often fail to achieve significance in clinical endpoints or suffer from severe adverse effects (73) with the exception of one recent study in systemic sclerosis (144). The discrepancy in efficacy between rodents and humans suggests that animal models poorly mimic the pathophysiology of human fibrotic disorders. As we gain insight in the molecular mechanisms that link the TGF-β, WNT, and YAP/TAZ cascades, we come to understand the challenges and pitfalls of targeting one specific signaling pathway [thoroughly reviewed in Ref. (19, 145–147)]. We have seen that signaling cascades are complex and that many pathway components fulfill multiple functions. Because TGF-β, WNT, and YAP/TAZ signals have distinct functions in different cell types and tissues, specific targeting of the fibrotic lesion is crucial. The temporal properties of signal transduction in different phases of disease and homeostasis pose another difficulty in the administration of pathway-wide modulating agents. It is therefore not just a matter of up- or downregulation. For example, targeting of TGF-β or WNT signaling with neutralizing antibodies may have widespread effects on the functioning of several components of the TGF-β, WNT, and YAP and TAZ signaling pathways, as well as numerous other growth factor cascades. To circumvent the wide-spread effects of growth factor inhibition and limit adverse effects, we increasingly depend on the development of small-molecule intracellular inhibitors.
The small-molecule intracellular inhibitor LY2157299 specifically targets the kinase pocket of the type I receptor of the TGF-β cascade without inhibiting the type II receptor and thereby attenuates Smad2/3-dependent transcription of target genes (145). However, LY2157299 has not been studied in clinical trials to halt or reverse fibrosis. Examples of small-molecule intracellular inhibitors for the WNT pathway, such as PKF115–584 and CGP049090 (148), act on the association between β-catenin and LEF/TCF transcriptions factors. Other strategies focus on inhibition of the PDZ domain of DVL or the transcriptional co-activators CBP and p300 (145). By inducing a shift from β-catenin association with p300 to CBP, genes such as COL1A1 may be negatively regulated (149). However, other studies report that inhibition of β-catenin–CBP also ameliorates fibrosis, suggesting that there is no such thing as pure “good” and “bad” β-catenin signaling (73, 78). As YAP and TAZ have but recently been linked to fibrogenesis, no clinical trials have been performed. Thus, whether targeting of YAP and TAZ is a fruitful strategy against fibrosis progression remains to be elucidated. One of the challenges in the targeting of YAP and TAZ is that they do not possess catalytic domains, but rather depend on specific protein binding domains for the interaction with their binding partners such as LATS1/2, Src family kinases, and TEADs. Nonetheless, a recent study described a potent inhibitor of YAP–TEAD: the benzoporphyrin derivative verteporfin (150). Verteporfin is currently used in the clinic for the treatment of macular degeneration, which makes it appealing for the use in clinical trials for fibrosis. Although inhibition of the YAP–TEAD complex seems a promising anti-fibrotic strategy, as several pro-fibrotic genes are not under control of TEADs, this may prove not to be the best approach.
The disadvantages of pathway-wide molecular inhibitors challenge the scientific community to develop specific targeting strategies against intracellular processes and protein–protein interactions. The increasing insight in the molecular cross-talk between signaling cascades adds new possibilities in drug development. Additionally, by focusing on the elucidation of the crystal structures of protein complexes, we can pursue the rational design of novel small molecular inhibitors to interfere at the cross-roads of signal transduction cascades.
Recent advancements in the field of TGF-β, WNT, and YAP/TAZ signaling have revealed that these signaling entities do not act alone. The notion that pathway components can have multiple and even opposed functions within one cell partly explains how the inhibition of a single molecular target often does not result in the desired therapeutic effect. This does not only add to the mere understanding of fibrotic processes, but also promotes the necessity to develop highly specific small-molecule intracellular inhibitors that act on protein–protein interactions at the cross-roads of signaling cascades. It should be noted that several of the studies described in this review used artificial ectopic expression of the proteins investigated. This may introduce artifacts that can influence the activity and functionality of the signaling cascades involved. Thus, more detailed studies in representative models for fibrosis focusing on endogenous proteins are required to completely understand the molecular cross-talk in vivo. Broadening our view on signal transduction will provide a better understanding of how a limited set of growth factors is able to govern the complex processes that underlie the physiology and pathology of fibrotic disorders.
BP, RB, and MB designed the manuscript. BP and MB collected literature and BP drafted the manuscript. All authors critically discussed and revised the content of the manuscript and had final approval of the manuscript in its present form.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by a grant from the Dutch government to the Netherlands Institute for Regenerative Medicine (NIRM, grant No. FES0908) and the Dutch Kidney Foundation.
1. Rockey DC, Bell P, Hill JA. Fibrosis – a common pathway to organ injury and failure. N Engl J Med (2015) 372:1138–49. doi:10.1056/NEJMra1300575
2. Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest (2014) 124:2921–34. doi:10.1172/JCI74783
3. Humphreys BD, Lin S, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol (2010) 176:85–97. doi:10.2353/ajpath.2010.090517
4. Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, et al. Perivascular Gli1(+) progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell (2014) 16:51–66. doi:10.1016/j.stem.2014.11.004
5. Hung C, Linn G, Chow YH, Kobayashi A, Mittelsteadt K, Altemeier WA, et al. Role of lung pericytes and resident fibroblasts in the pathogenesis of pulmonary fibrosis. Am J Respir Crit Care Med (2013) 188:820–30. doi:10.1164/rccm.201212-2297OC
6. Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest (2004) 113:243–52. doi:10.1172/JCI18847
7. Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol (2006) 45:429–38. doi:10.1016/j.jhep.2006.04.014
8. Wada T, Sakai N, Matsushima K, Kaneko S. Fibrocytes: a new insight into kidney fibrosis. Kidney Int (2007) 72:269–73. doi:10.1038/sj.ki.5002325
9. Quaggin SE, Kapus A. Scar wars: mapping the fate of epithelial-mesenchymal-myofibroblast transition. Kidney Int (2011) 80:41–50. doi:10.1038/ki.2011.77
10. Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol (2008) 19:2282–7. doi:10.1681/asn.2008050513
11. Xavier S, Vasko R, Matsumoto K, Zullo JA, Chen R, Maizel J, et al. Curtailing endothelial TGF-beta signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol (2015) 26:817–29. doi:10.1681/ASN.2013101137
12. Desmoulière A, Tuchweber B, Gabbiani G. Role of the myofibroblast differentiation during liver fibrosis. J Hepatol (1995) 22:61–4.
13. Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol (2006) 85:175–81. doi:10.1016/j.ejcb.2005.09.004
14. Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia (1971) 27:549–50. doi:10.1007/BF02147594
15. Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell (2001) 12:2730–41. doi:10.1091/mbc.12.9.2730
16. Strauch AR, Hariharan S. Dynamic interplay of smooth muscle α-actin gene-regulatory proteins reflects the biological complexity of myofibroblast differentiation. Biology (Basel) (2013) 2:555–86. doi:10.3390/biology2020555
17. van der Slot AJ, Zuurmond AM, van den Bogaerdt AJ, Ulrich MM, Middelkoop E, Boers W, et al. Increased formation of pyridinoline cross-links due to higher telopeptide lysyl hydroxylase levels is a general fibrotic phenomenon. Matrix Biol (2004) 23:251–7. doi:10.1016/j.matbio.2004.06.001
18. Ho YY, Lagares D, Tager AM, Kapoor M. Fibrosis – a lethal component of systemic sclerosis. Nat Rev Rheumatol (2014) 10:390–402. doi:10.1038/nrrheum.2014.53
19. Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med (2013) 5:167sr1. doi:10.1126/scitranslmed.3004700
20. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol (2002) 3:349–63. doi:10.1038/nrm809
21. Kohan M, Muro AF, White ES, Berkman N. EDA-containing cellular fibronectin induces fibroblast differentiation through binding to alpha4beta7 integrin receptor and MAPK/Erk 1/2-dependent signaling. FASEB J (2010) 24:4503–12. doi:10.1096/fj.10-154435
22. Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J (2004) 18:816–27. doi:10.1096/fj.03-1273rev
23. Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell (2012) 149:1192–205. doi:10.1016/j.cell.2012.05.012
24. Zilberberg L, Todorovic V, Dabovic B, Horiguchi M, Couroussé T, Sakai LY, et al. Specificity of latent TGF-β binding protein (LTBP) incorporation into matrix: role of fibrillins and fibronectin. J Cell Physiol (2012) 227:3828–36. doi:10.1002/jcp.24094
25. Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev (2000) 14:163–76. doi:10.1101/gad.14.2.163
26. Wipff PJ, Hinz B. Integrins and the activation of latent transforming growth factor beta1 – an intimate relationship. Eur J Cell Biol (2008) 87:601–15. doi:10.1016/j.ejcb.2008.01.012
27. Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, et al. Latent TGF-β structure and activation. Nature (2011) 474:343–9. doi:10.1038/nature10152
28. Worthington JJ, Klementowicz JE, Travis MATGF. β: a sleeping giant awoken by integrins. Trends Biochem Sci (2010) 36:47–54. doi:10.1016/j.tibs.2010.08.002
29. Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-beta receptor. Nature (1994) 370:341–7. doi:10.1038/370341a0
30. Massagué J. TGF-beta signal transduction. Annu Rev Biochem (1998) 67:753–91. doi:10.1146/annurev.biochem.67.1.753
31. Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell (2009) 139:757–69. doi:10.1016/j.cell.2009.09.035
32. Aragón E, Goerner N, Zaromytidou AI, Xi Q, Escobedo A, Massagué J, et al. A Smad action turnover switch operated by WW domain readers of a phosphoserine code. Genes Dev (2011) 25:1275–88. doi:10.1101/gad.2060811
33. Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol (2012) 13:616–30. doi:10.1038/nrm3434
34. Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell (2003) 113:685–700. doi:10.1016/S0092-8674(03)00432-X
35. Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, et al. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation (2007) 116:2127–38. doi:10.1161/CIRCULATIONAHA.107.704197
36. Wang B, Omar A, Angelovska T, Drobic V, Rattan SG, Jones SC, et al. Regulation of collagen synthesis by inhibitory Smad7 in cardiac myofibroblasts. Am J Physiol Heart Circ Physiol (2007) 293:H1282–90. doi:10.1152/ajpheart.00910.2006
37. Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res (2010) 107:418–28. doi:10.1161/CIRCRESAHA.109.216101
38. Takizawa H, Tanaka M, Takami K, Ohtoshi T, Ito K, Satoh M, et al. Increased expression of transforming growth factor-β1 in small airway epithelium from tobacco smokers and patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med (2001) 163:1476–83. doi:10.1164/ajrccm.163.6.9908135
39. Springer J, Scholz FR, Peiser C, Groneberg DA, Fischer A. SMAD-signaling in chronic obstructive pulmonary disease: transcriptional down-regulation of inhibitory SMAD 6 and 7 by cigarette smoke. Biol Chem (2004) 385:649–53. doi:10.1515/BC.2004.080
40. Pulichino A, Wang I, Caron A, Mortimer J, Auger A, Boie Y, et al. Identification of transforming growth factor β1-driven genetic programs of acute lung fibrosis. Am J Respir Cell Mol Biol (2008) 39:324–36. doi:10.1165/rcmb.2007-0186OC
41. Sandbo N, Lau A, Kach J, Ngam C, Yau D, Dulin NO. Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-β. Am J Physiol Lung Cell Mol Physiol (2011) 301:656–66. doi:10.1152/ajplung.00166.2011
42. Liu C, Gaça MD, Swenson E, Vellucci VF, Reiss M, Wells RG. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J Biol Chem (2003) 278:11721–8. doi:10.1074/jbc.M207728200
43. Uemura M, Swenson ES, Gaça MD, Giordano FJ, Reiss M, Wells RG. Smad2 and Smad3 play different roles in rat hepatic stellate cell function and α-smooth muscle actin organization. Mol Biol Cell (2005) 16:4214–24. doi:10.1091/mbc.E05-02-0149
44. Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. Transforming growth factor-beta1 induces an epithelial-to-mesenchymal transition state in mouse hepatocytes in vitro. J Biol Chem (2007) 282:22089–101. doi:10.1074/jbc.M700998200
45. Fukasawa H, Yamamoto T, Togawa A, Ohashi N, Fujigaki Y, Oda T, et al. Down-regulation of Smad7 expression by ubiquitin-dependent degradation contributes to renal fibrosis in obstructive nephropathy in mice. Proc Natl Acad Sci U S A (2004) 101:8687–92. doi:10.1073/pnas.0400035101
46. Fukasawa H, Yamamoto T, Suzuki H, Togawa A, Ohashi N, Fujigaki Y, et al. Treatment with anti-TGF-beta antibody ameliorates chronic progressive nephritis by inhibiting Smad/TGF-beta signaling. Kidney Int (2004) 65:63–74. doi:10.1111/j.1523-1755.2004.00393.x
47. Poncelet AC, Schnaper H, Tan R, Liu Y, Runyan CE. Cell phenotype-specific down-regulation of Smad3 involves decreased gene activation as well as protein degradation. J Biol Chem (2007) 282:15534–40. doi:10.1074/jbc.M701991200
48. Ishida W, Mori Y, Lakos G, Sun L, Shan F, Bowes S, et al. Intracellular TGF-beta receptor blockade abrogates Smad-dependent fibroblast activation in vitro and in vivo. J Invest Dermatol (2006) 126:1733–44. doi:10.1038/sj.jid.5700303
49. Meng X, Huang X, Chung AC, Qin W, Shao X, Igarashi P, et al. Smad2 protects against TGF-β/Smad3-mediated renal fibrosis. J Am Soc Nephrol (2010) 21:1477–87. doi:10.1681/ASN.2009121244
50. Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JJ, Mizel DE, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol (1999) 1:260–6. doi:10.1038/12971
51. Arany PR, Flanders KC, Kobayashi T, Kuo CK, Stuelten C, Desai KV, et al. Smad3 deficiency alters key structural elements of the extracellular matrix and mechanotransduction of wound closure. Proc Natl Acad Sci U S A (2006) 103:9250–5. doi:10.1073/pnas.0602473103
52. Masszi A, Speight P, Charbonney E, Lodyga M, Nakano H, Szászi K, et al. Fate-determining mechanisms in epithelial–myofibroblast transition: major inhibitory role for Smad3. J Cell Biol (2010) 188:383–99. doi:10.1083/jcb.200906155
53. Burch ML, Zheng W, Little PJ. Smad linker region phosphorylation in the regulation of extracellular matrix synthesis. Cell Mol Life Sci (2011) 68:97–107. doi:10.1007/s00018-010-0514-4
54. Duan WJ, Yu X, Huang XR, Yu JW, Lan HY. Opposing roles for Smad2 and Smad3 in peritoneal fibrosis in vivo and in vitro. Am J Pathol (2014) 184:2275–84. doi:10.1016/j.ajpath.2014.04.014
55. Neelisetty S, Alford C, Reynolds K, Woodbury L, Nlandu-Khodo S, Yang H, et al. Renal fibrosis is not reduced by blocking transforming growth factor-β signaling in matrix-producing interstitial cells. Kidney Int (2015). doi:10.1038/ki.2015.51
56. Mullen AC, Orlando DA, Newman JJ, Lovén J, Kumar RM, Bilodeau S, et al. Master transcription factors determine cell-type-specific responses to TGF-β signaling. Cell (2011) 147:565–76. doi:10.1016/j.cell.2011.08.050
57. Niehrs C. The complex world of WNT receptor signalling. Nat Rev Mol Cell Biol (2012) 13:767–79. doi:10.1038/nrm3470
58. Petersen CP, Reddien PW. Wnt signaling and the polarity of the primary body axis. Cell (2009) 139:1056–68. doi:10.1016/j.cell.2009.11.035
59. van Amerongen R, Mikels A, Nusse R. Alternative wnt signaling is initiated by distinct receptors. Sci Signal (2008) 1:re9. doi:10.1126/scisignal.135re9
60. Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J (1997) 16:3797–804. doi:10.1093/emboj/16.13.3797
61. Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell (2002) 108:837–47. doi:10.1016/S0092-8674(02)00685-2
62. Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, et al. Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature (2005) 438:867–72. doi:10.1038/nature04170
63. Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, et al. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature (2005) 438:873–7. doi:10.1038/nature04185
64. Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell (2012) 149:1245–56. doi:10.1016/j.cell.2012.05.002
65. Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, et al. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science (2007) 316:1619–22. doi:10.1126/science.1137065
66. Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell (2010) 143:1136–48. doi:10.1016/j.cell.2010.11.034
67. Behrens J, von Kries J, Kühl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature (1996) 382:638–42. doi:10.1038/382638a0
68. Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell (1996) 86:391–9. doi:10.1016/S0092-8674(00)80112-9
69. Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol (2002) 282:F431–41. doi:10.1152/ajprenal.0009.2001
70. Kobayashi K, Luo M, Zhang Y, Wilkes DC, Ge G, Grieskamp T, et al. Secreted frizzled-related protein 2 is a procollagen C proteinase enhancer with a role in fibrosis associated with myocardial infarction. Nat Cell Biol (2008) 11:46–55. doi:10.1038/ncb1811
71. Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, et al. Wnt1/β-catenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J (2012) 31:429–42. doi:10.1038/emboj.2011.418
72. Ye B, Ge Y, Perens G, Hong L, Xu H, Fishbein MC, et al. Canonical Wnt/β-catenin signaling in epicardial fibrosis of failed pediatric heart allografts with diastolic dysfunction. Cardiovasc Pathol (2013) 22:54–7. doi:10.1016/j.carpath.2012.03.004
73. Henderson WR, Chi EY, Ye X, Nguyen C, Tien YT, Zhou B, et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A (2010) 107:14309–14. doi:10.1073/pnas.1001520107
74. Lam AP, Flozak AS, Russell S, Wei J, Jain M, Mutlu GM, et al. Nuclear β-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol (2011) 45:915–22. doi:10.1165/rcmb.2010-0113OC
75. Ulsamer A, Wei Y, Kim KK, Tan K, Wheeler S, Xi Y, et al. Axin pathway activity regulates in vivo pY654-β-catenin accumulation and pulmonary fibrosis. J Biol Chem (2012) 297:5164–72. doi:10.1074/jbc.M111.322123
76. Surendran K, Schiavi S, Hruska KA. Wnt-dependent β-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol (2005) 16:2373–84. doi:10.1681/ASN.2004110949
77. He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol (2009) 20:765–76. doi:10.1681/ASN.2008060566
78. Hao S, He W, Li Y, Ding H, Hou Y, Nie J, et al. Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol (2011) 22:1642–53. doi:10.1681/ASN.2010101079
79. Ren S, Johnson BG, Kida Y, Ip C, Davidson KC, Lin SL, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci U S A (2013) 110:1440–5. doi:10.1073/pnas.1211179110
80. DiRocco DP, Kobayashi A, Taketo MM, McMahon AP, Humphreys BD. Wnt4/β-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol (2013) 24:1399–412. doi:10.1681/ASN.2012050512
81. Sato M. Upregulation of the Wnt/β-catenin pathway induced by transforming growth factor-β in hypertrophic scars and keloids. Acta Derm Venereol (2006) 86:300–7. doi:10.2340/00015555-0101
82. Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, et al. Wnt/β-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum (2012) 64:2734–45. doi:10.1002/art.34424
83. Kapoor M, Liu S, Shi-wen X, Huh K, McCann M, Denton CP, et al. GSK-3beta in mouse fibroblasts controls wound healing and fibrosis through an endothelin-1-dependent mechanism. J Clin Invest (2008) 118:3279–90. doi:10.1172/JCI35381
84. Bergmann C, Akhmetshina A, Dees C, Palumbo K, Zerr P, Beyer C, et al. Inhibition of glycogen synthase kinase 3β induces dermal fibrosis by activation of the canonical Wnt pathway. Ann Rheum Dis (2011) 70:2191–8. doi:10.1136/ard.2010.147140
85. Hamburg EJ, Atit RP. Sustained β-catenin activity in dermal fibroblasts is sufficient for skin fibrosis. J Invest Dermatol (2012) 235:686–97. doi:10.1038/jid.2012.155
86. Beyer C, Schramm A, Akhmetshina A, Dees C, Kireva T, Gelse K, et al. β-catenin is a central mediator of pro-fibrotic Wnt signaling in systemic sclerosis. Ann Rheum Dis (2012) 71:761–7. doi:10.1136/annrheumdis-2011-200568
87. Distler A, Ziemer C, Beyer C, Lin NY, Chen CW, Palumbo-Zerr K, et al. Inactivation of evenness interrupted (EVI) reduces experimental fibrosis by combined inhibition of canonical and non-canonical Wnt signalling. Ann Rheum Dis (2014) 73:624–7. doi:10.1136/annrheumdis-2013-203995
88. Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci (2003) 116:2627–34. doi:10.1242/jcs.00623
89. Dees C, Schlottmann I, Funke R, Distler A, Palumbo-Zerr K, Zerr P, et al. The Wnt antagonists DKK1 and SFRP1 are downregulated by promoter hypermethylation in systemic sclerosis. Ann Rheum Dis (2014) 73:1232–9. doi:10.1136/annrheumdis-2012-203194
90. Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell (2005) 122:421–34. doi:10.1016/j.cell.2005.06.007
91. Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol (2007) 17:2054–60. doi:10.1016/j.cub.2007.10.039
92. Piccolo S, Dupont S, Cordenonsi M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev (2014) 94:1287–312. doi:10.1152/physrev.00005.2014
93. Sudol M, Bork P, Einbond A, Kastury K, Druck T, Negrini M, et al. Characterization of the mammalian YAP (Yes-associated Protein) gene and its role in defining a novel protein module, the WW domain. J Biol Chem (1995) 270:14733–41. doi:10.1074/jbc.270.24.14733
94. Kanai F, Marignani P, Sarbassova D, Yagi R, Hall R, Donowitz M, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J (2000) 19:6778–91. doi:10.1093/emboj/19.24.6778
95. Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, Kalmukova R, et al. TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science (2005) 309:1074–8. doi:10.1126/science.1110955
96. Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell (2003) 114:445–56. doi:10.1016/S0092-8674(03)00549-X
97. Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev (2007) 21:2747–61. doi:10.1101/gad.1602907
98. Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFbeta-TrCP E3 ligase. J Biol Chem (2010) 285:37159–69. doi:10.1074/jbc.M110.152942
99. Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J Biol Chem (2008) 283:27534–46. doi:10.1074/jbc.M804380200
100. Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev (2010) 24:72–85. doi:10.1101/gad.1843810
101. Vassilev A, Kaneko K, Shu H, Zhao Y, DePamphilis MTEAD. /TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev (2001) 15:1229–41. doi:10.1101/gad.888601
102. Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev (2008) 22:1962–71. doi:10.1101/gad.1664408
103. Mahoney WM, Hong JH, Yaffe MB, Farrance IK. The transcriptional co-activator TAZ interacts differentially with transcriptional enhancer factor-1 (TEF-1) family members. Biochem J (2005) 388:217–25. doi:10.1042/BJ20041434
104. Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem (2009) 284:13355–62. doi:10.1074/jbc.M900843200
105. Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, et al. Role of YAP/TAZ in mechanotransduction. Nature (2011) 474:179–83. doi:10.1038/nature10137
106. Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development (2011) 138:3907–14. doi:10.1242/dev.070987
107. Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J (2011) 30:2325–35. doi:10.1038/emboj.2011.157
108. Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol (2015) 308:L344–57. doi:10.1152/ajplung.00300.2014
109. Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol (2015) 63:679–88. doi:10.1016/j.jhep.2015.04.011
110. Mitani A, Nagase T, Fukuchi K, Aburatani H, Makita R, Kurihara H. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am J Respir Crit Care Med (2009) 180:326–38. doi:10.1164/rccm.200812-1827OC
111. Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A (2013) 110:13839–44. doi:10.1073/pnas.1313192110
112. Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res (2014) 115:354–63. doi:10.1161/CIRCRESAHA.115.303632
113. Lee MJ, Ran Byun M, Furutani-Seiki M, Hong JH, Jung HS. YAP and TAZ regulate skin wound healing. J Invest Dermatol (2014) 134:518–25. doi:10.1038/jid.2013.339
114. Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-β signaling through SMAD2 in a β-catenin-dependent manner. PLoS One (2011) 6:e19809. doi:10.1371/journal.pone.0019809
115. Lam AP, Herazo-Maya JD, Sennello JA, Flozak AS, Russell S, Mutlu GM, et al. Wnt coreceptor Lrp5 is a driver of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med (2014) 190:185–95. doi:10.1164/rccm.201401-0079OC
116. Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, et al. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun (2012) 3:735. doi:10.1038/ncomms1734
117. Hayashi H, Abdollah S, Qiu Y, Cai J, Xu Y, Grinnell B, et al. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell (1997) 89:1165–73. doi:10.1016/S0092-8674(00)80303-7
118. Ferrigno O, Lallemand F, Verrecchia F, L’Hoste S, Camonis J, Atfi A, et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene (2002) 21:4879–84. doi:10.1038/sj.onc.1205623
119. Han G, Li AG, Liang YY, Owens P, He W, Lu S, et al. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell (2006) 11:301–12. doi:10.1016/j.devcel.2006.06.014
120. Tang Y, Liu Z, Zhao L, Clemens TL, Cao X. Smad7 stabilizes beta-catenin binding to E-cadherin complex and promotes cell-cell adhesion. J Biol Chem (2008) 283:23956–63. doi:10.1074/jbc.M800351200
121. Liu W, Rui H, Wang J, Lin S, He Y, Chen M, et al. Axin is a scaffold protein in TGF-beta signaling that promotes degradation of Smad7 by Arkadia. EMBO J (2006) 25:1646–58. doi:10.1038/sj.emboj.7601057
122. Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, et al. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol (2001) 21:5132–41. doi:10.1128/MCB.21.15.5132-5141.2001
123. Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, et al. Role of TAZ as mediator of Wnt signaling. Cell (2012) 151:1443–56. doi:10.1016/j.cell.2012.11.027
124. Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, et al. YAP/TAZ incorporation in the β-catenin destruction complex orchestrates the Wnt response. Cell (2014) 158:157–70. doi:10.1016/j.cell.2014.06.013
125. Llado V, Nakanishi Y, Duran A, Reina-Campos M, Shelton PM, Linares JF, et al. Repression of intestinal stem cell function and tumorigenesis through direct phosphorylation of beta-catenin and Yap by PKCzeta. Cell Rep (2015) 10:740–54. doi:10.1016/j.celrep.2015.01.007
126. Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, Cockburn K, Larsen BG, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-β-SMAD pathway. Dev Cell (2010) 19:831–44. doi:10.1016/j.devcel.2010.11.012
127. Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol (2006) 4:e115. doi:10.1371/journal.pbio.0040115
128. Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J (2012) 31:1109–22. doi:10.1038/emboj.2011.487
129. Hill CS. Nucleocytoplasmic shuttling of Smad proteins. Cell Res (2009) 19:36–46. doi:10.1038/cr.2008.325
130. Goentoro L, Kirschner MW. Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol Cell (2009) 36:872–84. doi:10.1016/j.molcel.2009.11.017
131. De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer (2013) 13:97–110. doi:10.1038/nrc3447
132. Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol (2011) 73:413–35. doi:10.1146/annurev-physiol-012110-142225
133. Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol (2008) 10:837–48. doi:10.1038/ncb1748
134. Fujii M, Nakanishi H, Toyoda T, Tanaka I, Kondo Y, Osada H, et al. Convergent signaling in the regulation of connective tissue growth factor in malignant mesothelioma: TGFbeta signaling and defects in the Hippo signaling cascade. Cell Cycle (2012) 11:3373–9. doi:10.4161/cc.21397
135. Hiemer SE, Szymaniak AD, Varelas X. The transcriptional regulators TAZ and YAP direct transforming growth factor β-induced tumorigenic phenotypes in breast cancer cells. J Biol Chem (2014) 289:13461–74. doi:10.1074/jbc.M113.529115
136. Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang H, et al. Interactions between β-catenin and transforming growth factor-β signaling pathways mediate epithelial-mesenchymal transition and are dependent on the transcriptional co-activator cAMP-response element-binding protein (CREB)-binding protein (CBP). J Biol Chem (2012) 287:7026–38. doi:10.1074/jbc.M111.276311
137. Charbonney E, Speight P, Masszi A, Nakano H, Kapus A. beta-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition. Mol Biol Cell (2011) 22:4472–85. doi:10.1091/mbc.E11-04-0335
138. Labbé E, Lock L, Letamendia A, Gorska AE, Gryfe R, Gallinger S, et al. Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res (2007) 67:75–84. doi:10.1158/0008-5472.CAN-06-2559
139. Heidemann M, Hintermair C, Voss K, Eick D. Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription. Biochim Biophys Acta (2013) 1829:55–62. doi:10.1016/j.bbagrm.2012.08.013
140. Estarás C, Benner C, Jones KA. SMADs and YAP compete to control elongation of beta-catenin:LEF-1-recruited RNAPII during hESC differentiation. Mol Cell (2015) 58:780–93. doi:10.1016/j.molcel.2015.04.001
141. Wrighton KH, Lin X, Feng XH. Phospho-control of TGF-beta superfamily signaling. Cell Res (2009) 19:8–20. doi:10.1038/cr.2008.327
142. Gao S, Alarcon C, Sapkota G, Rahman S, Chen PY, Goerner N, et al. Ubiquitin ligase Nedd4L targets activated Smad2/3 to limit TGF-beta signaling. Mol Cell (2009) 36:457–68. doi:10.1016/j.molcel.2009.09.043
143. Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, et al. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol (2007) 27:4488–99. doi:10.1128/MCB.01636-06
144. Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest (2015) 125:2795–807. doi:10.1172/JCI77958
145. Akhurst RJ, Hata A. Targeting the TGFβ signalling pathway in disease. Nat Rev Drug Discov (2012) 11:790–811. doi:10.1038/nrd3810
146. Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov (2014) 13:513–32. doi:10.1038/nrd4233
147. Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov (2013) 13:63–79. doi:10.1038/nrd4161
148. Lepourcelet M, Chen YPN, France DS, Wang H, Crews P, Petersen F, et al. Small-molecule antagonists of the oncogenic Tcf/beta-catenin protein complex. Cancer Cell (2014) 5:91–102. doi:10.1016/S1535-6108(03)00334-9
149. Ghosh AK, Vaughan DE. Fibrosis: is it a coactivator disease. Front Biosci (Elite Ed) (2012) 4:1556–70. doi:10.2741/E480
Keywords: fibrosis, myofibroblast, TGF-β, WNT, YAP/TAZ, Hippo, signaling
Citation: Piersma B, Bank RA and Boersema M (2015) Signaling in fibrosis: TGF-β, WNT, and YAP/TAZ converge. Front. Med. 2:59. doi: 10.3389/fmed.2015.00059
Received: 26 May 2015; Accepted: 13 August 2015;
Published: 03 September 2015
Edited by:
Achim Weber, University of Zurich, SwitzerlandReviewed by:
Francesco Trapasso, University “Magna Græcia” of Catanzaro, ItalyCopyright: © 2015 Piersma, Bank and Boersema. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bram Piersma, Matrix Research Group, Department of Pathology and Medical Biology, University Medical Center Groningen, University of Groningen, Hanzeplein 1, Groningen 9728HR, Netherlands,Yi5waWVyc21hQHVtY2cubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.