
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. Technol. , 03 April 2025
Sec. Diagnostic and Therapeutic Devices
Volume 7 - 2025 | https://doi.org/10.3389/fmedt.2025.1505184
Background: Preventing needlestick injuries caused by hypodermic needles is crucial for healthcare personnel. In this context, port access needles play an important role. However, systematic comparisons of different safety-engineered port access needles have not been conducted. Therefore, we evaluated differences in product characteristics and user preferences of safety-engineered protection mechanisms of port access needles.
Methods: Port puncture was performed using port access needles with four different safety mechanisms: (a) EZ Huber™ PFM Medical, (b) Gripstick® Safety OMT, (c) Gripper Micro® Smiths Medical and (d) pps ct® Vygon. Each needle type was used in three consecutive tries: an uninstructed first handling, after which instructions were given according to operating manual. Subsequently, a first and second trial were conducted. Study endpoints included successful activation, activation time, way of activation (one hand or two hands), correct activation, possible risk of needlestick injury, possibility of deactivation and preferred safety mechanism.
Results: Overall, successful activation rate during the second trial was equal for all four devices (100%). Median activation time was (a) 6 s, (b) 3 s, (c) 11 s and (d) 6 s. Single-handed activation during the second trial was (a) 0%, (b) 75%, (c) 1% and (d) 1%. Single-handed activation after further preparation with two hands during the second trial was (a) 0%, (b) 0%, (c) 0% and (d) 50%. Correct activation during the second trial was (a) 97%, (b) 66%, (c) 19% and (d) 44%. Possible risk of needlestick injury during the second trial was highest with (b). Possibility of deactivation was (a) 75%, (b) 94%, (c) 97% and (d) 22%. Individual preferences for each system were (a) n = 5, (b) n = 2, (c) n = 1 and (d) n = 24. The main written reasons given for preference were the safety protection mechanism and handling of the port needle.
Conclusion: We have shown significant differences regarding product characteristics of safety mechanisms of port access needles. Our evaluation approach provides specific data for both, technical (e.g., single-handed activation) and personal device selection criteria (e.g., preference of the safety mechanism).
Port access needles, used for accessing implanted ports, play a crucial role in providing reliable and convenient access to the vascular system (1). Despite their significance, issues related to safety and the potential for needlestick injuries remain a concern (2–4). In this context, various safety-engineered protection mechanisms have been introduced in the clinical setting to solve the general problem of needlestick injuries (5). Nevertheless, needlestick injuries still occur, even after education and training with devices containing safety-engineered protection mechanisms (6). Looking at factors affecting the occurrence of needlestick injuries on the level of tool and technology factors, the use of personal protective equipment had the highest relative weight followed by the safety design of devices (7).
For designing safety-engineered protection mechanisms, detailed specifications such as the ability to activate the device with one hand are described by current regulations (8–10). To date, a wide range of devices with safety-engineered protection mechanisms have been introduced, including blood collection needles, winged blood collection needles, peripheral intravenous catheters and port access needles. Several studies have been conducted to evaluate different types of safety-engineered protection mechanisms (2, 11–14). These investigations repeatedly found that most injuries occur before or even during activation of the safety-engineered protection mechanism, highlighting the impact of the mechanism itself on the prevention of needle-stick injuries and the need for ongoing optimization of safety-engineered protection mechanisms (15–19).
In the context of available frameworks for implementation of sharp injury preventing programs (8, 10, 20), we proposed a systematic model-based user evaluation of devices with safety-engineered protection mechanism prior to clinical implementation (21). To date, only few user-acceptability studies prior to introduction of safety-engineered port access needles into the clinical area have been published (22–24). New promising approaches focus on virtual reality and corresponding haptic simulation methods, enabling training, evaluation and design optimizations (25–28); however, virtual reality technology is still challenging to simulate fine motor interactions (29). In a previous study, the Polyperf® Safe (PPS) Huber needle was evaluated in cancer patients (22). Compared to the standard Gripper® needle in this study, most nurses were convinced that the PPS needle was safer than the Gripper® needle. However, this study was solely based on questionnaire evaluations with no further information regarding safety aspects. Hence, a systematic comparison of different safety-engineered port access needles and their underlying fundamental mechanisms has not been conducted. Therefore, we expanded our model-based user evaluation of devices with safety-engineered protection mechanism using a lifelike simulation model for port access needles.
In this randomized lifelike model-based study, we hypothesized that significant differences in product characteristics and inexperienced healthcare personnel would reveal user preferences of safety-engineered protection mechanisms of port access needles.
The study was approved by the local Medical Research Ethics Committee Freiburg (Research Ethics Committee Reference Number: 44/14). Third-year medical students from the University Medical School Freiburg (Germany) were selected randomly using a standard random generator (Microsoft Excel) and invited to participate in the study. Exclusion criteria included prior routine experience with port puncture and safety-engineered port needles (e.g., prior employment as a nurse or physician assistant). The participants had to give their informed written consent to be tested and analysed.
Four different port needles representing four different safety-engineered protection mechanisms were tested (Figure 1): i.e., (a) EZ Huber™ PFM Medical (pfm medical gmbh, Köln, Germany) = protector slipped over the needle while pulling out, (b) Gripstick® Safety OMT (OMT GmbH & Co. KG, Frittlingen, Germany) = needle protector closed over the needle via spring mechanism after pressing the release button, (c) Gripper Micro® Smiths Medical (Smiths Medical Deutschland GmbH, Grasbrunn, Germany) = two parted mechanism which removes the safety part and leaves a blunt cannula: while removing the safety part, a protection snaps into place, and (d) pps ct® Vygon (Vygon, Aachen, Germany) = protective cover pushed over the needle while withdrawing.
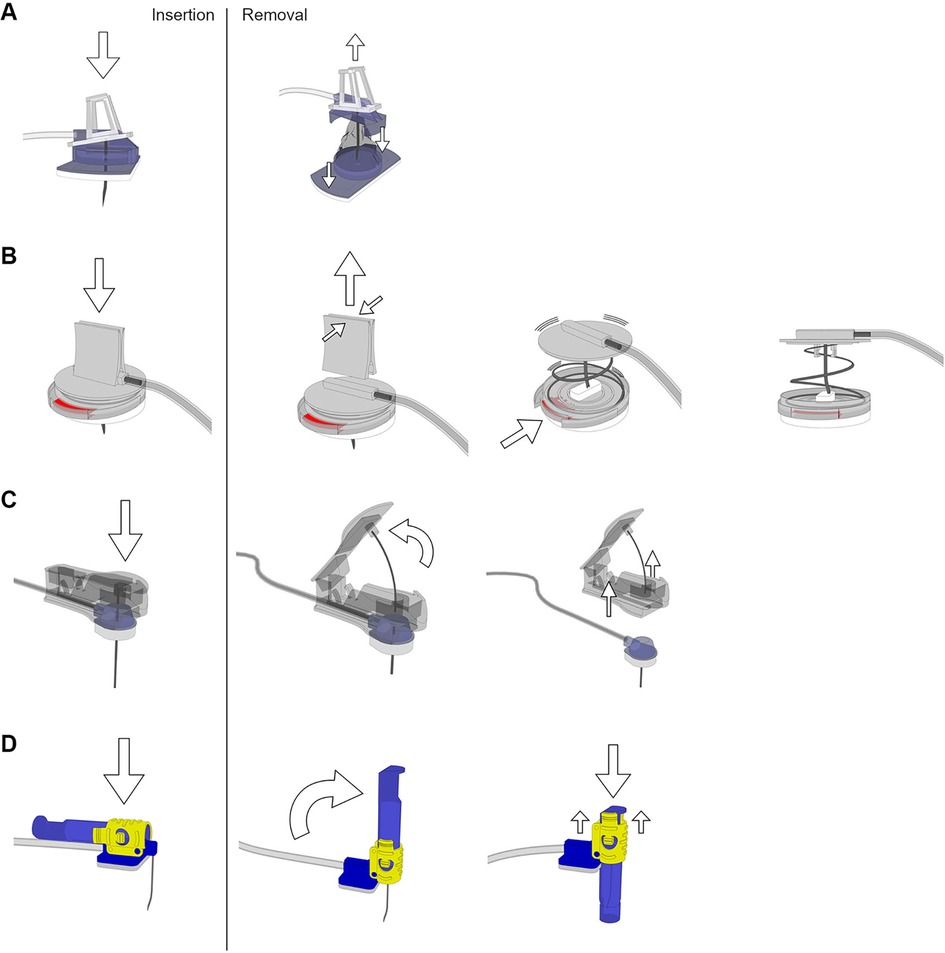
Figure 1. Port needles with different safety-engineered protection mechanisms. Handling while insertion and removal of the needles: (A) EZ Huber™ PFM Medical (needle pulled into a protective cover on removal), (B) Gripstick® Safety OMT (spring retraction of the needle via the release button), (C) Gripper Micro® Smiths Medical (removal of the needle in conjunction with the safety component after insertion) and (D) pps ct® Vygon (protective cover pushed over the needle during removal). Images have been created by 3D modeling and rendering using Autodesk Maya 2012 (Autodesk Inc., San Rafael, USA) and Adobe Photoshop (Adobe Inc., San José, USA).
The experimental set-up for port puncture included a simulation model (“Chester Chest”, Laerdal Medical GmbH, Puchheim, Germany) and a commercial video documentation camera (Sony Alpha 6400, Sony Europe B.V., Berlin) for post-hoc analysis of each puncture attempt (Figure 2). At first, all participants were instructed to perform each port puncture as follows: wearing gloves, skin disinfection, preparation of the port needle, port puncture, position control, decannulation and subsequent activation of the specific safety-engineered protection mechanism. Three consecutive attempts were recorded: an uninstructed first handling, followed by instruction according to the manufacturer's operating manual, followed by a first and a second trial. The order in which the four different port needles were used was randomized for all participants. For the uninstructed first handling, participants were asked to perform a port puncture immediately after randomization. This shows whether the safety-engineered protection mechanism is self-explanatory or not. After the second trial, participants were asked to deactivate the safety-engineered protection mechanism. Subsequently, the participants had to answer a questionnaire on their experience with each safety-engineered protection mechanism of the four port needles using a Likert score (1 = strongly agree; 2 = agree; 3 = neutral; 4 = disagree; 5 = strongly disagree).
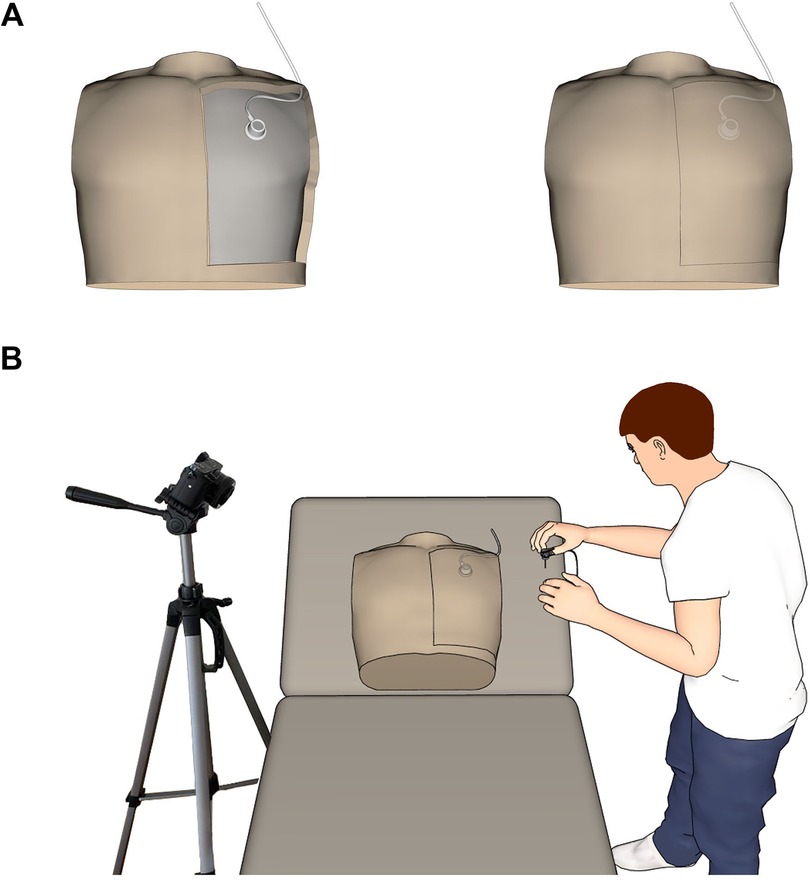
Figure 2. Experimental set-up for port needle evaluation using the Chester Chest™ lifelike model of common long-term vascular access routes. (A) Placement of the intravascular access device within the torso. (B) Positioning of the model and the video camera during the puncture trial. Images have been created by 3D modeling and rendering using Autodesk Maya 2012 (Autodesk Inc., San Rafael, USA) and Adobe Photoshop (Adobe Inc., San José, USA).
Endpoints analyzed by video included successful activation of the safety-engineered protection mechanism, the time required for activation, ability to execute one- or two-handed activation, correct activation of the safety mechanism, the risk of needlestick injury, troubles with handing before starting the puncture, the possibility of deactivation and the preferred safety-engineered protection mechanism. The time required for activation was defined as the time required from decannulation of the needle tip outside the skin (EZ Huber™ PFM Medical, Gripper Micro® Smiths Medical, and pps ct® Vygon) or contact of the fingers with the release slide (Gripstick® Safety OMT) until complete activation of the safety-engineered protection mechanism. Correct activation was defined according to the manufacturer's instruction. Risk of possible needlestick injury was defined as one finger coming within a distance of less than 1 cm next to the tip of the needle prior to activation of the safety-engineered protection mechanism. Troubles with handling before starting the puncture were defined for taking more than 2 s or even failing to remove the needle protection cap. Possible deactivation was defined as a free needle tip after maximum manipulation of the activated safety-engineered protection mechanism.
Nonparametric data were tested for differences using Cochrane's Q test followed by McNemars's exact test and Friedman's test followed by Wilcoxon's signed rank sum test if indicated. Data from “not possible activation of safety-engineered protection mechanism trials” were excluded from calculation of activation times. GraphPad Prism® 9.2.0 for Microsoft Windows (GraphPad Software Inc., La Jolla, California, USA) and MedCalc® 20.014 for Microsoft Windows (MedCalc Software bvba, Ostend, Belgium) were used for statistical analysis. A P-value of <0.05 was chosen as the level of significance. In cases of multiple comparisons, P was corrected using Bonferroni's approach for posthoc tests resulting in a P-value of <0.0083 being considered significant.
From 300 third-year medical students, 32 were randomly selected and all of them consented to participate in this study. Of the enclosed participants, 22 were women. The average age was 26 years.
Results from the port puncture simulations were summarized in Table 1. Overall successful activation rate for uninstructed first handling was best for EZ Huber™ PFM Medical (84%), followed by pps ct® Vygon (56%), Gripper Micro® Smiths Medical (41%) and Gripstick® Safety OMT (31%). Overall, successful activation rate improved during the second trial for all the devices (100%). Median time required for safety mechanism activation was shorter for Gripstick® Safety OMT compared to the other three devices (P < 0.0083). Compared to the other devices, activation with one hand during the second trial was significantly higher with Gripstick® Safety OMT (75%) (P < 0.0083) and higher for pps ct® Vygon (50%) for activation with one hand after further preparation with two hands (P < 0.0083). Correct activation of the safety mechanism during second trial was higher for EZ Huber™ PFM Medical (97%) compared to Gripper Micro® Smiths Medical (19%) and pps ct® Vygon (44%) and Gripstick® Safety OMT (66%) compared to Gripper Micro® Smiths Medical (19%) (P < 0.0083) and increased for all four devices after instruction compared to first handling (P < 0.05). All four devices could protect from risk of possible needlestick injury during the second trial. Trouble with handling before starting the puncture for uninstructed first handling (91%) and the first trial (19%) occurred while using the Gripstick® Safety OMT due to difficulties in removing the needle protection cap (P < 0.0083). Deactivation of safety mechanism was possible with Gripper Micro® Smiths Medical (97%), Gripstick® Safety OMT (94%), EZ Huber™ PFM Medical (75%) and pps ct® Vygon (22%). Premature activation occurred only with Gripstick® Safety OMT during first handling (34%) and first trial (3%).
Results from the questionnaire are summarized in Table 2. Compared to the other devices the pps ct® Vygon was rated best for ease of determining activation, effectiveness in reducing needlestick injuries, impossibility of deactivation and operator safety (P < 0.0083). Gripstick® Safety OMT and pps ct® Vygon were rated best for activation using one hand (P < 0.0083). Gripper Micro® Smiths Medical was rated worst for no training needed for use and ease of handling after being briefed on the user's manual (P < 0.0083). No differences were found between the devices regarding the visualization of the needle tip. Twenty-four medical students preferred the pps ct® Vygon needle, five the EZ Huber™ PFM Medical, two the Gripstick® Safety OMT and one the Gripper Micro® Smiths Medical (Table 2).
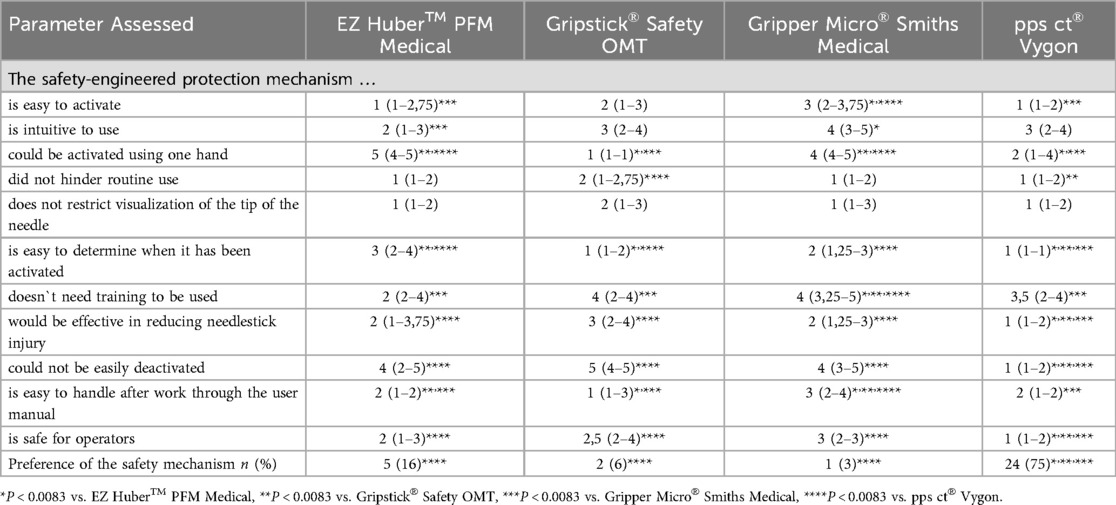
Table 2. Summary of the results from the questionary. Results are taken as Likert score (1 = strongly agree; 2 = agree; 3 = neutral; 4 = disagree; 5 = strongly disagree). Values are reported as median (IQR).
Overall text comments for preference of one of the four port needles included: (i) safest protection mechanism (n = 13), (ii) the port access needle is best to handle (n = 13), and (iii) the mechanism cannot be deactivated (n = 11).
The main findings of this study can be summarized as follows: (i) the overall successful activation rate during the second trial was equal for all devices, (ii) the median time required for safety mechanism activation was shortest for Gripstick® Safety OMT, (iii) single-handed activation during the second trial was best for the Gripstick® Safety OMT, (iv) the risk of possible needlestick injury during second trial was equal for all devices, (v) trouble with handling before starting the puncture including premature activation was highest for Gripstick® Safety OMT, (vi) deactivation of the safety mechanism was lowest for pps ct® Vygon, and (vii) users preferred the most comprehensive safety-engineered protection mechanism and a mechanism that cannot be deactivated.
As shown for other devices (21), the overall successful activation rate was high and equal for all port access needles. However, the median time required for safety mechanism activation was shortest for Gripstick® Safety OMT with 3 s vs. 6–11 s for the other needles. Single-handed activation during the second trial was also best for Gripstick® Safety OMT with 75% vs. 0%–3% for the other needles. These characteristics may provide an additional level of safety in day-to-day clinical settings. The notable proficiency in activating the safety mechanism, particularly with EZ Huber™ PFM Medical and Gripstick® Safety OMT, implies that the other two safety mechanisms may need more comprehensive instructions for correct use. We have identified a potential issue with the Gripstick® Safety OMT concerning inadvertent premature activation when used without proper guidance. Consequently, none of the assessed safety mechanisms was fully self-explanatory.
Several studies repeatedly found that most injuries occur before or even during activation of the safety-engineered protection mechanism, highlighting the impact of the mechanism itself on the prevention of needle-stick injuries and the need for ongoing optimization of safety-engineered protection mechanisms (15–19). Our findings show that all four devices could protect from risk of possible needlestick injury during the second trial with almost no identifiable risk of possible needlestick injury. One safety-engineered protection mechanism (Gripstick® Safety OMT) showed significant trouble with the handling before starting the puncture during first handling and first trial due to premature activation of the spring mechanism by unintentionally pressing the release button during removal of the needle protection cap. Therefore, our findings could lead to an improvement of the current mechanisms as well as for the future designs of safety-engineered protection mechanisms.
All healthcare personnel, including students and trainees, should be educated and trained in locally available safety-engineered protection devices with a priority on educational interventions in high-risk settings (6, 10). However, if education and training have not been carried out, safety-engineered protection mechanisms should be as self-explanatory as possible (21). We have identified several problems during uninstructed first handling: the activation with two hands in most cases, a possible risk of needlestick injury with almost all devices, several premature activations and troubles with handling before the puncture with one device. This information may aid in optimizing designs and identifying safety-engineered protection devices that are self-explanatory.
As concluded in an analysis of safety-engineered protection mechanisms of winged blood collection needles (21), Jagger and Perry highlighted the crucial involvement of healthcare workers in selecting safety-engineered devices (9). Adams and Elliott suggested evaluating safety-engineered needle devices before introduction, recognizing that no single device can satisfy all requirements or preferences of healthcare workers (13). Our findings indicate that healthcare personnel are not only capable of assessing various devices with safety-engineered protection mechanisms, as demonstrated previously (21), but also capable of offering detailed insights into their preferred devices and the reasons behind their choices. Moreover, our study suggests a preference for needle retraction devices (such as pps ct® Vygon) over needle shielding devices (such as EZ Huber™ PFM Medical and Gripstick® Safety OMT), as previously shown for winged blood collection needles (21). These results are in line with a prior study where most nurses believed the PPS needle was safer than the traditional Deltec™ Gripper® needle (22).
In practice, there are sometimes disagreements about which criteria are decisive for selecting a certain device. To address this issue, relevant findings can further be presented using comprehensive visualization methods (see Supplemental Materials: Graphs S1 and S2). Furthermore, our approach could be integrated into a comprehensive procurement framework within various healthcare facilities. We recommend a prioritization of the following fundamental criteria: successful activation rate, risk of potential needlestick injury and preference. This may ensure a high user compliance rate and add additional safety during the disposal process of hypodermic needles.
In addition, the specific design characteristics and differences between the four evaluated products may explain some of the observed differences in handling and efficacy. The EZ Huber™ PFM Medical is a modified version of a traditional needle, incorporating the safety-engineered protection mechanism into an established product. The Gripstick® Safety OMT represents a push button approach with a focus on ease of activation. In contrast, the Gripper Micro® Smiths Medical was designed to minimize the size of the port needle itself while in place on the patient, in combination with a safety-engineered protection mechanism. The pps ct® Vygon can be interpreted as a partially compromise between size and a sophisticated safety-engineered protection mechanism. In this context, virtual reality and corresponding haptic simulation methods (25–28) may enable the study of other testing parameters, such as patient movement or other difficulties during insertion/removal, as well as simulation of new developed safety-engineered protection mechanism principles during prototyping and prior to application in patients.
One limitation of the study is the lifelike model of vascular access routes, as in other simulation studies (21), complicating factors like different nature of skin quality, bleeding, patient behaviour such as movement of the chest during port cannulation or stress of the unexperienced user during the procedure cannot be simulated reliably. We did not investigate potential additional training effects beyond three attempts with each port access needle, nor did we assess the impact of an accompanying education and training program on preferences, as suggested (6, 10). Our study also did not analyse the effect on needlestick injury rates; thus, it does not establish the actual safety performance of the various devices. Instead, our focus was on the features of the safety-engineered protection mechanism itself and its usability, particularly when utilized by inexperienced and minimally trained healthcare personnel.
In summary, our research has revealed substantial variations among inexperienced healthcare workers in their perception of safety-engineered port access needle features. Specifically, we found that the preference for the most comprehensive safety mechanism was a key factor, with devices incorporating needle retraction being favoured over those employing needle shielding. Furthermore, our evaluation approach provides specific data for technical device selection criteria (e.g., single-handed activation), which are crucial for hypodermic needles. Consequently, we propose our lifelike model-based study as a potential tool for evaluating new healthcare devices before clinical deployment, aiding in the integration of safer needle designs into healthcare settings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethik-Kommission der Albert-Ludwigs-Universität Freiburg, Engelberger Straße 21, 79106 Freiburg—GERMANY. Local ethics committee approval number for this study: 44/14. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FG: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing, Visualization, Software. PH: Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. PD: Formal analysis, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. DS: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing, Software.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmedt.2025.1505184/full#supplementary-material
Graph 1 | Visualization of the results from the port puncture simulation for comparison of the four port needles with different safety-engineered protection mechanisms using enhanced circular layout generation (RS1).
Graph 2 | Visualization of the results from the questionnaire for comparison of the four port needles with different safety-engineered protection mechanisms using enhanced circular layout generation (RS1).
1. York N. Best practice for using ports and non-coring needles. Br J Nurs. (2019) 28(Sup14a):S11–5. doi: 10.12968/bjon.2019.28.Sup14a.S11
2. Tosini W, Ciotti C, Goyer F, Lolom I, L’Hériteau F, Abiteboul D, et al. Needlestick injury rates according to different types of safety-engineered devices: results of a French multicenter study. Infect Control Hosp Epidemiol. (2010) 31(4):402–7. doi: 10.1086/651301
3. Hoffmann C, Buchholz L, Schnitzler P. Reduction of needlestick injuries in healthcare personnel at a university hospital using safety devices. J Occup Med Toxicol. (2013) 8(1):20. doi: 10.1186/1745-6673-8-20
4. Civetta G, Lombardi L, Lanotte A, Delvecchio AM, Colonnata M, Todisco A, et al. Needle insertion difficulty algorithm (NIDA): a novel pilot study to predict Huber needle insertion difficulty in totally implanted devices. J Vasc Access. (2023) 24(3):492–6. doi: 10.1177/11297298211040343
5. Reddy VK, Lavoie MC, Verbeek JH, Pahwa M. Devices for preventing percutaneous exposure injuries caused by needles in healthcare personnel. Cochrane Database Syst Rev. (2017) 11(11):CD009740. doi: 10.1002/14651858.CD009740.pub3
6. Cheetham S, Ngo HT, Liira J, Liira H. Education and training for preventing sharps injuries and splash exposures in healthcare workers. Cochrane Database Syst Rev. (2021) 14(4):CD012060. doi: 10.1002/14651858.CD012060.pub2
7. Mousavi SM, Yazdanirad S, Althubiti S, Majdabadi MA, Najarian F, Sepehr P. Determination and prioritization of factors affecting the occurrence of needle stick injuries among healthcare workers using techniques of delphi and fuzzy analytical hierarchy process (FAHP). BMC Public Health. (2023) 23(1):2009. doi: 10.1186/s12889-023-16969-x
9. Jagger J, Perry J, Gomaa A, Phillips EK. The impact of U.S. policies to protect healthcare workers from bloodborne pathogens: the critical role of safety-engineered devices. J Infect Public Health. (2008) 1(2):62–71. doi: 10.1016/j.jiph.2008.10.002
10. Council directive 2010/32/EU of 10 May 2010, implementing the Framework Agreement on prevention from sharp injuries in the hospital and healthcare sector concluded by HOSPEEM and EPSU. Pub. L. No. 134, 67–74. (2010).
11. Mendelson MH, Lin-Chen BY, Solomon R, Bailey E, Kogan G, Goldbold J. Evaluation of a safety resheathable winged steel needle for prevention of percutaneous injuries associated with intravascular-access procedures among healthcare workers. Infect Control Hosp Epidemiol. (2003) 24(2):105–12. doi: 10.1086/502174
12. Asai T, Hidaka I, Kawashima A, Miki T, Inada K, Kawachi S. Efficacy of catheter needles with safeguard mechanisms. Anaesthesia. (2002) 57(6):572–7. doi: 10.1046/j.1365-2044.2002.02571.x
13. Adams D, Elliott TSJ. A comparative user evaluation of three needle-protective devices. Br J Nurs. (2003) 12(8):470–4. doi: 10.12968/bjon.2003.12.8.11273
14. Menezes JA, Bandeira CS, Quintana M, de Lima E, Silva JCA, Calvet GA, et al. Impact of a single safety-engineered device on the occurrence of percutaneous injuries in a general hospital in Brazil. Am J Infect Control. (2014) 42(2):174–7. doi: 10.1016/j.ajic.2013.07.017
15. Black L, Parker G, Jagger J. Chinks in the armor: activation patterns of hollow-bore safety-engineered sharp devices. Infect Control Hosp Epidemiol. (2012) 33(8):842–4. doi: 10.1086/666630
16. Black L. Chinks in the armor: percutaneous injuries from hollow bore safety-engineered sharps devices. Am J Infect Control. (2013) 41(5):427–32. doi: 10.1016/j.ajic.2012.05.025
17. Lu Y, Senthilselvan A, Joffe AM, Beach J. Effectiveness of safety-engineered devices in reducing sharp object injuries. Occup Med. (2015) 65(1):39–44. doi: 10.1093/occmed/kqu152
18. Malinowski M, Serafin A, Prazmowska-Wilanowska A. Dropsafe safety pen needle helps to prevent accidental needlesticks after injections: results of a simulated clinical study. J Infect Prev. (2021) 22(1):19–27. doi: 10.1177/1757177420948580
19. Serafin A, Ryk A, Fendler W. Safe and effective use of a passive safety needle by healthcare professionals in a simulated environment, including perceptions and preferences. Expert Rev Med Devices. (2023) 20(11):963–71. doi: 10.1080/17434440.2023.2254680
20. Workbook for Designing, Implementing, and Evaluating a Sharp Injury Preventing Program. (2008). Centers for Disease Control and Prevention website. Available online at: http://www.cdc.gov/sharpssafety/pdf/sharpsworkbook_2008.pdf (accessed March 14, 2024).
21. Haupt C, Spaeth J, Ahne T, Goebel U, Steinmann D. A model-based product evaluation protocol for comparison of safety-engineered protection mechanisms of winged blood collection needles. Infect Control Hosp Epidemiol. (2016) 37(5):505–11. doi: 10.1017/ice.2016.14
22. Goossens GA, Moons P, Jérôme M, Stas M. Prospective clinical evaluation of the Polyperf® safe, a safety huber needle, in cancer patients. J Vasc Access. (2011) 12(3):200–6. doi: 10.5301/JVA.2010.6075
23. Shimono C, Tanaka A, Fujita A, Ishimoto M, Oura S, Yamaue H, et al. (Comparison of port needle with safety device between Huber plus (HP) and poly PERF safe (PPS)). Gan To Kagaku Ryoho. (2010) 37(5):947–51.20495336
24. Meade E, Munoz-Mozas G, Moodley N, Williams J. Clinical experiences of using ports and non-coring needles. Br J Nurs. (2019) 28(Sup14a):S16–9. doi: 10.12968/bjon.2019.28.Sup14a.S16
25. Tsai S, Sin-Kuo C, Li-Feng H, Shirling L, Fang-Meei T, Wen-Hsu S, et al. The use of virtual reality computer simulation in learning port-A cath injection. Adv Health Sci Educ Theory Pract. (2008) 14(1):71–87. doi: 10.1007/s10459-006-9025-3
26. Shu-Feng S, Li-Ling H, Suh-Ing H. Effects of digital learning and virtual reality in port-A catheter training course for oncology nurses: a mixed-methods study. Healthcare. (2023) 11(7):1017. doi: 10.3390/healthcare11071017
27. Boutin J, Kamoonpuri J, Faieghi R, Chung J, de Ribaupierre S, Eagleson R. Smart haptic gloves for virtual reality surgery simulation: a pilot study on external ventricular drain training. Front Robot AI. (2024) 10:1273631. doi: 10.3389/frobt.2023.1273631
28. Gutiérrez-Fernández A, Fernández-Llamas C, Vázquez-Casares AM, Mauriz E, Riego-del-Castillo V, John NW. Immersive haptic simulation for training nurses in emergency medical procedures. Vis Comput. (2024) 40:7527–37. doi: 10.1007/s00371-023-03227-9
Keywords: port access needles, needlestick injury, safety-engineered protection mechanism, lifelike model, medical students
Citation: Gabler F, Heiden P, Deibert P and Steinmann D (2025) Evaluation of different safety-engineered protection mechanisms of port access needles using a lifelike model of vascular access routes. Front. Med. Technol. 7:1505184. doi: 10.3389/fmedt.2025.1505184
Received: 2 October 2024; Accepted: 6 March 2025;
Published: 3 April 2025.
Edited by:
Dietlind Tittelbach-Helmrich, Duale Hochschule Baden-Württemberg, Karlsruhe, GermanyReviewed by:
Konstantinos Kapnisis, Cyprus University of Technology, CyprusCopyright: © 2025 Gabler, Heiden, Deibert and Steinmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Steinmann, ZGFuaWVsLnN0ZWlubWFubkB1bmlrbGluaWstZnJlaWJ1cmcuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.