- 1Health Technology Graduate Program, Pontifícia Universidade Católica do Paraná, Curitiba, Brazil
- 2Physiotherapy Under-Graduate Program, Pontifícia Universidade Católica do Paraná, Curitiba, Brazil
Introduction: The radio electric asymmetric conveyer (REAC) is a technology that has the purpose of restoring the cellular polarity triggering the rebalancing of the endogenous bioelectric field, which considering the neurological dysfunctions, affects the neural communication mechanisms. The studies published so far show that the REAC neuromodulation technology has positive effects in treating these dysfunctions, with the principles of endogenous bioelectricity as a basis to achieve these effects.
Objectives: This study aims to review the literature that explored the effects of REAC protocols on motor control and to identify which mechanisms would be involved.
Materials and methods: This integrative review considered studies that used REAC as a therapeutic intervention directed at human motor control and experimental research with animals that applied REAC to obtain effects related to motor behavior.
Results: Ten articles were included, eight clinical and two experimental studies. The clinical studies used the neuro postural optimization (NPO) protocol in 473 patients, of which 53 were healthy subjects, 91 were Alzheimer's disease patients, 128 were patients with atypical swallowing, 12 subjects with neurological diseases, and 189 were without the specification of disease. The experimental studies used the antalgic neuromodulation and neurodegeneration protocols in animal models.
Conclusion: The information integrated in this review made it possible to consider REAC technology a promising resource for treating motor control dysfunctions. It is possible to infer that the technology promotes functional optimization of neuronal circuits that may be related to more efficient strategies to perform motor tasks.
Introduction
The radio electric asymmetric conveyer (REAC) technology aims to elicit cellular reprogramming activity and modulate adaptive responses in the body by restoring the balance of cellular endogenous bioelectric fields (1). It has been the target of studies in diverse areas of health sciences such as neurology (2, 3), orthopedics (4, 5), gerontology (6), cardiology (7), rheumatology (8) and histopathology (9–11). The basis for achieving the desired effects of REAC technology is the endogenous bioelectricity which affects neural and cellular processing mechanisms and, under pathological conditions, would be unbalanced (11). According to Rinaldi et al. (2011), the REAC technology, especially its neuromodulation protocols, allows the regulation and modulation of endogenous bioelectric fields and, in this way, promotes better functioning of neuronal circuits (12–14). In addition, a balanced electric field is fundamental for cellular metabolism since cell activity is directly linked to electricity in the intra and extracellular environments (5).
The REAC technology works through the interaction of the radioelectric field produced by the REAC device with the endogenous bioelectric fields produced by the cells. After establishing this interaction, a series of cellular ionic fluxes are generated, triggering transcriptional and signaling events that drive reprogramming and differentiation decisions of cells affected by this interaction (11, 15, 16).
Scientific research published to date has shown that REAC neuromodulation technology has positive effects on the treatment of central neurological disorders such as depressive syndromes (17, 18), anxiety (17–23), stress (2, 18, 19, 21–28), Parkinson's (9, 10) and Alzheimer's diseases (3, 29–32).
In addition, the effects of REAC protocols seem to be beneficial for slowing down the processes of neurodegenerative diseases (32) and the aging process (6, 33–36), which inextricably cause changes in motor control mechanisms.
Although some studies (3, 29, 31, 37–39) have investigated the impact of REAC protocols on motor control, the topic needs to be explored. It is known that neurological conditions that impair motor control possibly modify the electric field produced by neuronal circuits, altering the communication and integration of information (40). Motor control depends on the integration of somatosensory information in the supraspinal centers, and the REAC protocols might contribute to this process through the functional optimization of neuronal circuits (14, 38). Given this scenario, this study aims to review the literature that explored the effects of REAC protocols on motor control and to identify which mechanisms would be involved.
Materials and methods
The present study is an integrative review (41). We have searched for scientific articles published in PubMed/MEDLINE, Bireme/Brisa, SCIELO, Capes Periodicals, Clinical Trials, Scopus, and IEEE databases without time restriction. The keywords used for the search were “radio electric asymmetric conveyer” and “REAC” without linguistic restrictions.
To be included in this review, the studies should have applied REAC protocols and observed outcomes related to motor control. Studies that did not provide information on the REAC protocol or describe how it was applied would be excluded.
The screening process began with the title analysis to verify the use of REAC protocols. After the initial screening, the selected studies were retained for analytical reading of the abstract and verification of eligibility criteria. Those potentially eligible were analyzed in full detail, and those that did not fit the eligibility criteria were excluded.
The information extracted from the included studies were: title, author, year, country of origin, and type of study. Descriptive data from clinical studies were: sample (number, age, and gender), assessed motor task, outcomes, and assessment methods. In experimental studies, the animal model, the model's characteristics, the motor task, and the outcome evaluation methods were considered. Additionally, information about REAC protocols was extracted from both clinical and experimental studies.
Two reviewers carried out the entire methodological process of this review and the inconsistencies would be resolved by a third reviewer.
Results
Through the search engine, 283 studies were found, of which 234 were excluded by duplication and title because they did not use the REAC technology. Of the remaining 49 studies, 39 were excluded because they did not present motor control as a primary or secondary outcome. As a result, ten studies were reviewed. The process of identification, screening, and inclusion of studies is summarized in Figure 1.

Figure 1. Flowchart of the identification, screening, analysis and inclusion of studies in the review process.
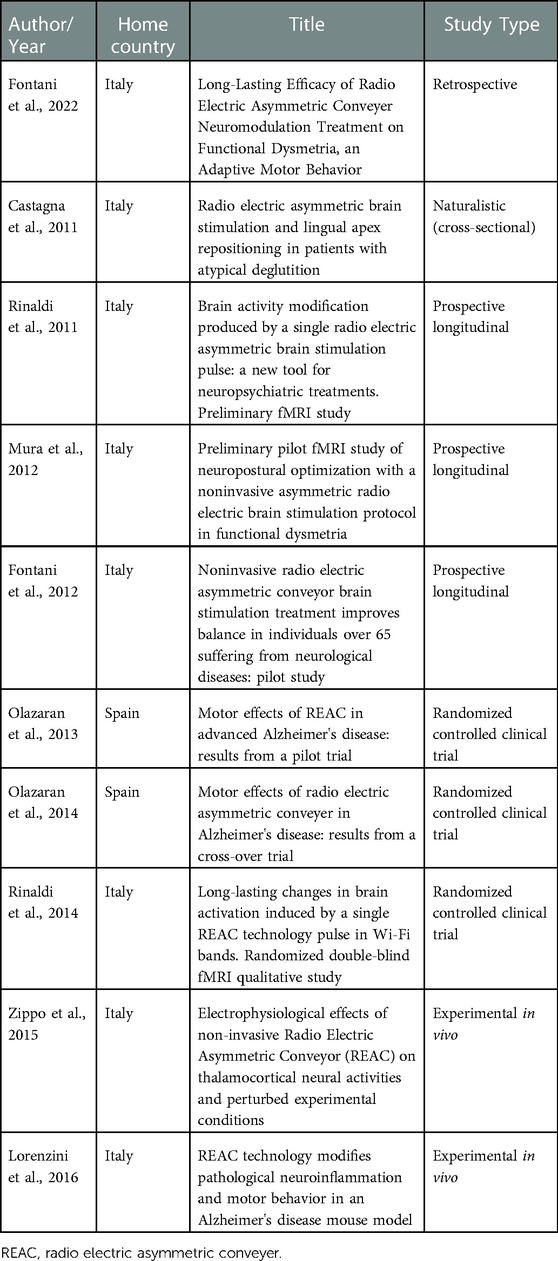
Out of the ten studies included in this review, eight were clinical studies and two were experimental, with animal models. Three of the clinical studies had a prospective longitudinal design, three were randomized controlled trials and one was retrospective.
The reviewed articles were published in the last 10 years, most of them between 2011 and 2015. Most of the studies were developed in Italy, specifically at the Instituto Rinaldi Fontani located in the city of Florence. Two studies were carried out in Spain at the Alzheimer's Center of the Reiná Sofía Foundation.
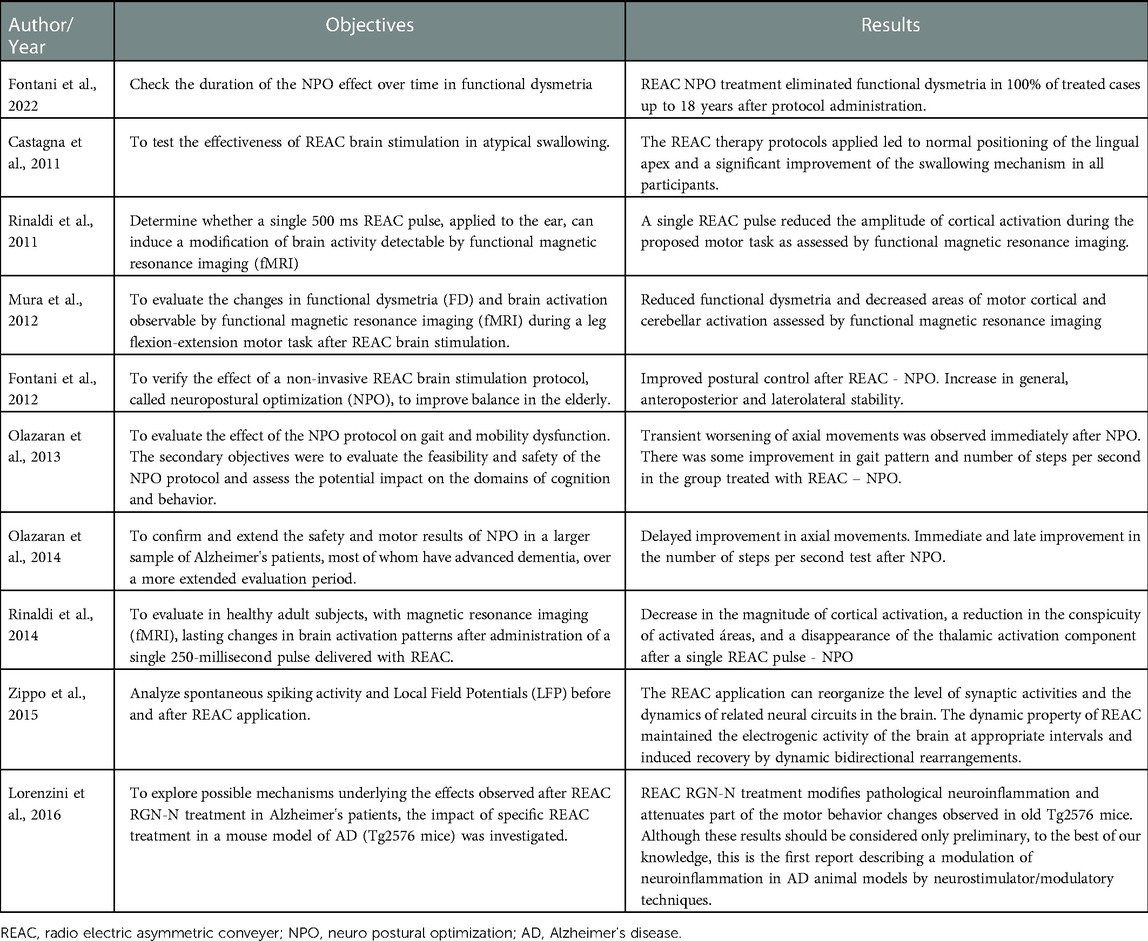
The characteristics of the studies regarding the type and country are given in Table 1, while their objectives and main results are in Table 2.
The clinical studies evaluated 473 patients. The age of the research subjects was quite heterogeneous among the studies and the averages ranged from 9 to 86 years. There was also great variability in terms of clinical, functional, cognitive, and psychological characteristics. Altogether, the studies evaluated 53 healthy subjects, 91 with Alzheimer's disease, 128 patients with atypical swallowing, 12 subjects with neurological disorders (three with Parkinson's disease, three with chronic cerebral vasculopathy, four with post-stroke spastic syndrome, one with multi-infarct encephalopathy and one with ataxia caused by pontine glioma) and 189 without specification of the disease.
Details of the clinical studies with humans are presented in Table 3 and those of the experimental studies, in Table 4.
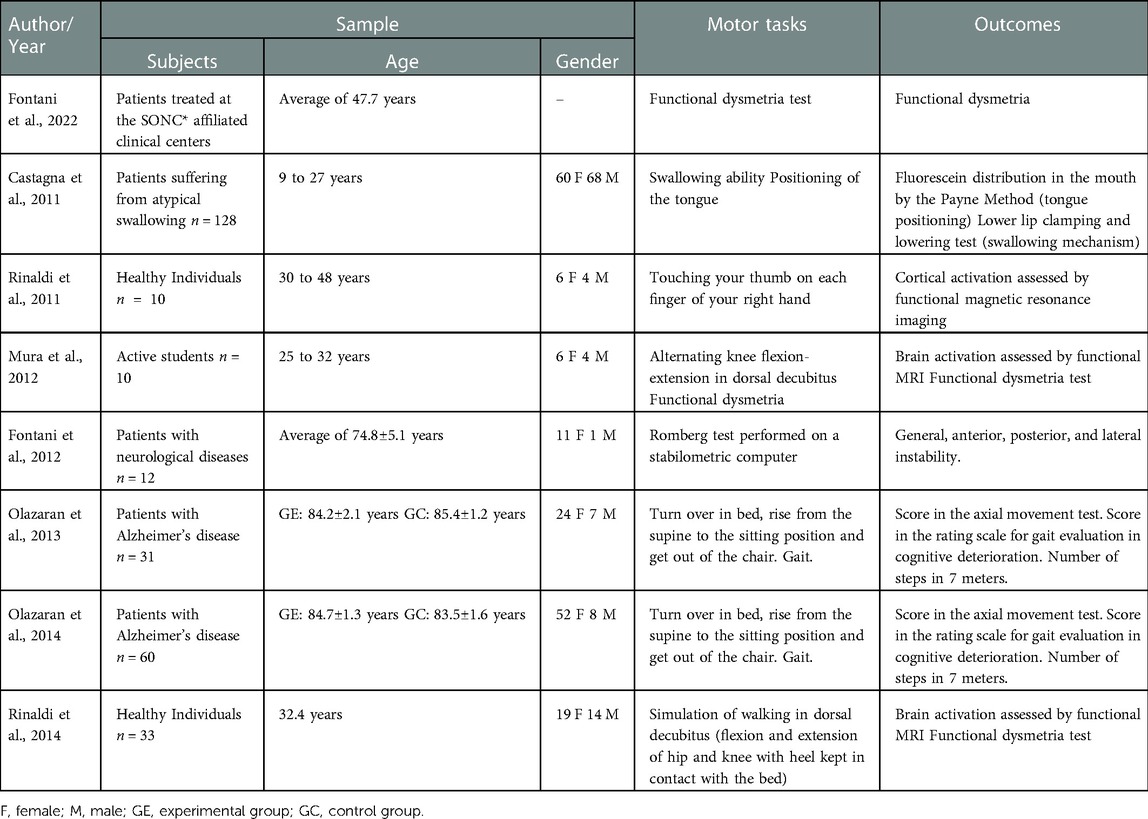
Table 3. Characterization of the included clinical studies regarding the sample, motor tasks, and outcomes.
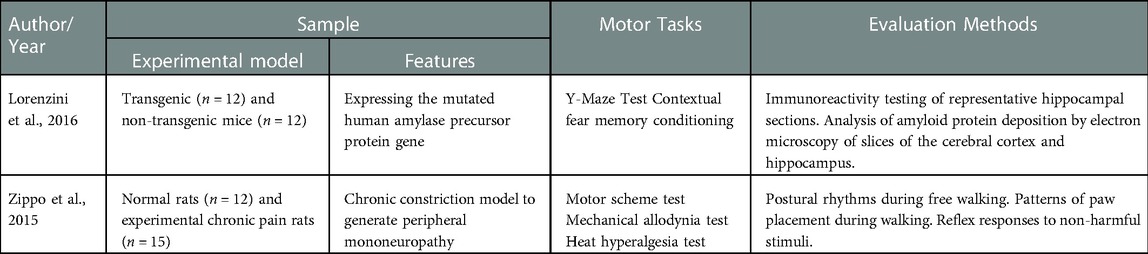
Table 4. Characterization of the experimental studies included in relation to the sample, motor tasks and evaluation methods.
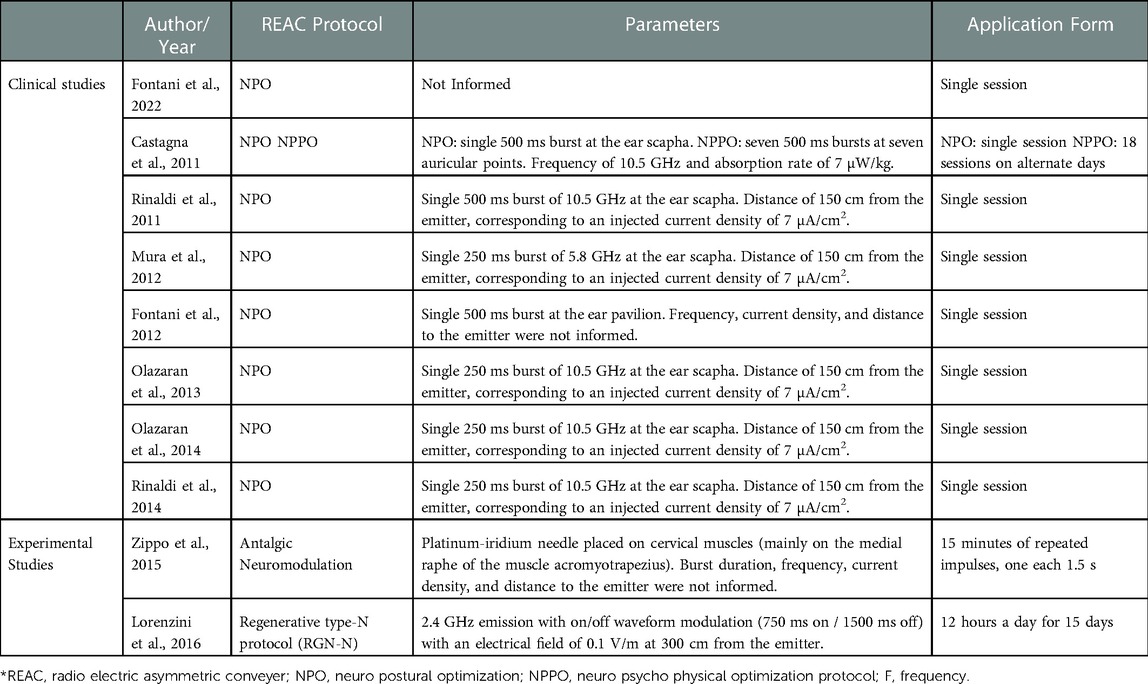
Regarding the REAC protocols, all the clinical studies used the neuro postural optimization (NPO) protocol. Castagna et al. used the neuro psycho physical optimization (NPPO) protocol, in addition to NPO (42). The experimental studies by Zippo et al., 2015 and Lorenzini et al., 2016 used the antalgic neuromodulation and neuroregeneration protocols respectively (43) and (29). The protocols and parameters used in the reviewed studies are shown in Table 5.
According to Rinaldi et al. the NPO protocol consists of a single radio electric burst of 500 ms at 10.5 GHz with REAC CRM devices, and of 250 ms at 5.8 GHz with REAC BENE devices (32). The radio electric burst is dispersed in the environment, while the result of its interaction with the endogenous bioelectrical activity of the subject being treated is conveyed to the area to be treated via the specific probe called asymmetric conveyer probe (ACP). In the REAC NPO treatment a specific ACP is positioned on a precise area of the auricular scapha. In the NPO protocol the specific absorption rate of 7 µW/kg for a current density of 7 µA/cm2 and a distance of 150 cm from the emitter. With these parameters, a radio electric field of approximately 20 µW/m2 is generated (32). The NPO protocol is normally used at the beginning of treatments with REAC in association with other protocols. According to Fontani et al., NPO improves postural attitude (38), reduces pain symptoms, and optimizes motor strategies in healthy individuals (14, 26) and also in those with motor impairment (44). The NPO thanks to the long duration of its effect (14), controllable through the stable disappearance of the functional dysmetria does not require further administrations (38).
The neuro psycho physical optimization protocol (NPPO), according to Rinaldi et al., (27), consists of seven radiofrequency pulses of 500 ms conveyed by a specific ACP at precise points of the auricle (30, 45). According to Rinaldi et al., the NPPO protocol is performed in cycles of 18 therapy sessions administered on alternate days (32). Usually, each session lasts around 5 s.
The NPPO treatment aims to reorganize the overall dysfunctional adaptive changes, also on an epigenetic basis, which are stratified during life. Furthermore, NPPO treatment is intended to prevent them. To try to achieve and maintain this goal it is necessary to repeat the treatment cycles as environmental interaction and allostatic pressure are unavoidable. The generally recommended interval between cycles of the NPPO protocol is approximately six months.
The study by Zippo et al., 2015, used the antalgic neuromodulation protocol (ANM) in animal models, with pulses repeated every 1.5 s (43). The aim of the ANM is to alleviate pain by determining and sustaining the progressive process of optimizing epigenetic adaptive responses at the level of pain circuits. Clinically, it can be applied to the specific site of pain, in the cervico-brachial region and in the cervico-dorsal-lumbar region. It is usually applied in cycles, of 18 sessions and the recommendation is to have one to three cycles/year in relation to the state of suffering and the determining cause of the pain. The tissue regeneration optimization protocol (TO-RGN) was used in the study of Lorenzini et al. and consists of a radiofrequency emission of 2.4 GHz with a very low intensity at a distance 300 cm from the emitter (29). The radio electric burst modulation is done through a waveform on/off system, where there will be 750 ms on and 1,500 ms off (25).
Discussion
The purpose of this integrative review was to seek evidence to explain the mechanisms of action of REAC technology to modulate neurophysiological aspects involved in motor control. In addition, it was also aimed to identify in the studies the protocols, parameters, and forms of application of the REAC used to act in the mechanisms of motor control. For this, we sought clinical and experimental research in the databases that used REAC and investigated motor outcomes. As a result, it was possible to select 10 studies, 8 clinical and 2 experimental.
The role of endogenous bioelectricity in neuronal physiology
According to Frohlich (46) synchronized neuronal activity in the cerebral cortex generates weak electrical fields that are routinely measured in humans and animal models by electroencephalography and local field potential recordings (46). This neuronal synchronization was analyzed in the study by Zippo et al., 2015, which used local field potential records to determine the coherence of neuronal activity before and after the use of REAC. The authors verified that after REAC treatments there seems to be a reduction in local field potentials which can be interpreted as a temporal redistribution of synaptic signals related to the peaks of neuronal firing rate observed in the studied animals (43).
Until recently, these endogenous electric fields were considered an epiphenomenon of brain activity, but it is currently understood that this energy is able to change protein metabolism, the process of cell differentiation, electrical and chemical synaptic signaling, the process of inflammation, tissue repair and pain perception (47). Some studies have shown that possibly endogenous electric fields may play an active role in neuronal circuits since neuron signaling depends on the generation and transmission of transient electrical impulses that represent the fundamental unit of information in the brain (48, 49).
So, is the presence of endogenous electric fields just an epiphenomenon? Or can it be considered a necessary event for the amplification of neuronal communication?.
According to Fries et al. (50), the neural information processing model is based on the notion that changes in the electrical potential inside the neurons in relation to the constant electrical potential outside the neuron determine the membrane voltage and, therefore, the functional state of individual neurons. Consequently, this compartmentalization of electric fields has been postulated as a form of cellular communication and transmission of neuronal signals (51).
Therefore, endogenous bioelectric fields seem to be fundamental to aiding synchronization in neuron communication, especially when it comes to motor control. Motor control, therefore, depends on the coordinated and stable cortical activity that is partly due to the electric field generated by the simultaneous activity of the cells that make up the brain tissue, both neurons and cells of the glial system (12).
Therefore, the normality of the bioelectric field in the central nervous system depends on the morphological and functional integrity of the cells and, thus, neurological and psychiatric diseases are related to changes in bioelectricity, which contribute to stereotyped responses of brain functions (46, 51, 52). This leads to clinical, psychological, and functional manifestations that characterize a given disease (17, 18, 20, 28, 37).
The effect of REAC technology on endogenous bioelectricity
Three studies included in this review (12–14) showed that the NPO protocol can modify brain activation in healthy individuals. Mura et al. (2012) showed that REAC could reduce activation in cortical motor areas using magnetic resonance imaging (fMRI) during the sit and stand task. Furthermore, the study's results showed the disappearance of right thalamic activation and a decrease in the activation of the cerebellar vermis and the pontine and midbrain regions (13).
Thus, reduced brain activation in the motor cortex detected by fMRI may be related to a more efficient motor strategy with fewer brain areas being activated, supposedly only those which are strictly necessary. In fMRI, brain (cortical) activation during motor activity is represented by increased or decreased BOLD signals (53). This signal appears in the resonance image due to the magnetic properties of hemoglobin that determine distortions in the magnetic field, especially when the hemoglobin is deoxygenated (54).
Therefore, reductions in the BOLD signal can represent a decrease in brain activity determined by the reduction of local blood flow or an increase in the neuronal metabolic rate that determines the presence of deoxygenated hemoglobin (53, 55). Whatever the reason for the reduction of the BOLD signal, the findings suggest that the REAC technology seems to induce activation of regions that are strictly necessary for the accomplishment of the motor task, excluding or inhibiting secondary neuronal circuits that would not be essential for the motor execution (12–14).
It is important to highlight that reducing brain activation means, in this case, a “functional optimization” of the neuronal circuits as, to perform the same task, the subject uses fewer neural resources, increasing the system's efficiency.
One of the studies included in this review (43) sought to analyze the firing rate and Local Field Potentials (PFL) before and after the application of the REAC protocol in normal mice and those with chronic neuropathy. According to the authors, the results obtained were interpreted as a “functional optimization” of the brain structures that govern the coordination of motor control and balance (43). This is because it was possible to observe that REAC reduced the firing rate of the thalamic and cortical regions both in spontaneous activities and in the tactile evoked stimulation of the animals. It was also observed that the firing rate of the thalamic and cortical circuits was significantly reduced in the animals with chronic pain (43).
According to Berge, the neuronal processing of pain hinders the segregation and integration of information in the thalamic cortical circuits due to the sensory overload that determines changes in the electrical fields around the neurons responsible for this processing (56). Pain processing, therefore, determines increases in the firing rates of higher centers and changes in the synchronization of local field potentials. In the study by Zippo et al., present in this review, it was possible to verify that REAC was able to increase the synchronization of potentials of local fields, possibly indicating that the technology can rebalance the endogenous bioelectric fields (43). The segregation and integration of information are related to neuronal communication, and REAC seems to increase the efficiency of this system, especially in animals with chronic pain (57).
The studies of Rinaldi et al. (2011) and (2014) found similar results in brain activation, especially the disappearance of the activation signal of the thalamic component after the NPO protocol of REAC (12, 14). It is known that the thalamus is considered a fundamental processing center of the central nervous system, responsible for the integration and reorganization of stimuli from the periphery, brainstem, and higher centers to filter information and modulate signals for a motor or autonomic response to be reproduced more efficiently (58). The reduction in thalamic activity possibly indicates an adjustment of the somatosensory processing involved in the planning and executing a motor task. It is as if the thalamus better integrates sensory information and filters stimuli from the periphery or brainstem better before sending the information to the motor cortex (59).
The reorganization of endogenous electric fields seems to be responsible for these effects on the nervous system, as it is known that these fields are fundamental for synaptic communication, especially when it comes to motor control (46). Lorenzini et al. demonstrated that REAC treatment improved locomotion in aged mice carrying the human amyloid precursor protein mutation (model for Alzheimer's disease) (29). In Alzheimer's disease, the deposition of amyloid protein in the hippocampus, thalamus, and motor cortex hinders the communication of neurons responsible for integrating decisive information for the processing of locomotion. It probably alters the local electric field (60). According to Lorenzini et al., REAC treatment increased glial fibrillary acidic protein, a molecule part of the cytoskeleton of several cells, including astrocytes. In this study, REAC favored the increase in the proliferation of astrocytes around the amyloid plaques determined by the higher expression of the glial fibrillary acidic protein. In vitro and in vivo studies (49) indicate that astrocytes may play a role in removing debris from injury and amyloid plaque deposits (61).
Thus, it can be assumed that the application of REAC technology protocols can optimize neuronal communication by favoring the containment or removal of amyloid plaques. Thus, the establishment of a balanced electric field possibly influences the activity of neurons inducing the activation of cells that would be responsible for transmitting information (12, 13). This mechanism may explain the results of the study by Olazarán et al., 2013 and 2014, which showed that REAC delayed axial movements in patients with Alzheimer's disease. In addition, in this study, there was an immediate and delayed improvement in the steps per second and gait test after using the NPO-REAC (3, 31).
These explanations may be related, for example, to the results of one of the studies included in this review: Castagna et al. aimed to test the effectiveness of REAC brain stimulation in atypical swallowing (42). Atypical swallowing is mainly characterized by the malfunction of the tongue and other muscles during the swallowing reflex, often caused by neurological or structural dysfunctions of the stomatognathic system (62). The motor response of the masticatory muscles in this situation consists of the interposition of the tongue between the teeth or lips since the possible hypotonia of the masticatory muscles makes the mouth open at the time of the oral phase of swallowing (63).
According to Hebling et al., stress and anxiety seem to be related to swallowing abnormalities, especially in the oropharyngeal phase of the process. The oropharyngeal phase consists of the sequence of events that begins with the voluntary contraction of the muscles of mastication and gradually passes into involuntary control. This process is mediated by mechanisms that involve integrating information from the swallowing center in the brainstem with the motor cortex through the basal ganglia (64). Therefore, changes in endogenous electrical fields determined by anxiety disorder (18), for example, can influence the synchronization of information necessary for normal swallowing control. Barcessat et al. demonstrated that REAC technology appears to be effective in reducing anxiety and stress. Thus, if we consider anxiety and stress as factors involved in atypical swallowing, we can assume that the bioelectrical modulation provided by REAC may favor the correction of the swallowing mechanism in patients with atypical swallowing (5).
According to the developers of the REAC treatment, Rinaldi and Fontani, environmental stress is responsible for causing morpho-functional changes in the central nervous system capable of inducing the development of functional dysmetria (38). This motor behavior is defined as a deviation from perfect bilateral symmetry. It can be considered a manifestation of the deterioration of the allostatic mechanisms of development influenced by genetic alterations and environmental stress factors (65). The study by Fontani et al., identified that all patients who received REAC NPO treatment showed the disappearance of functional dysmetria up to 18 years after administration of the protocol (37, 38). This phenomenon can be explained by the functional optimization of neuronal communication, where an increase in the efficiency of the activation pattern substantiates the participation of neural circuits strictly necessary to perform the motor task (12–14). In this context, functional dysmetria has been an evaluation parameter inherent to the treatment process with REAC since the evidence of its disappearance possibly indicates the effectiveness of the treatment.
Conclusion
Clinical studies that evaluated the effect of REAC on motor tasks showed that the technology is able to improve motor response through functional optimization of neural circuits. The experimental studies included in this review indicated that REAC technology influences glial cell activity and improves neuronal synchronization by reducing local field strengths. These findings may be a promising basis for elucidating the mechanisms involved in the modulation of technology-induced neuronal bioelectrical activity.
Author contributions
VGM: conception of the research, methodology, analysis and interpretation, data curation and writing. ABSB: Formal analysis, analysis and interpretation. EFM: project administration, analysis and interpretation, supervision, funding acquisition, writing, and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
We thank the SONC Scientific Society for supporting the costs of the open access fees. VGM and ABSB are thankful to the Brazilian National Council of Scientific and Technological Development (CNPq) for their fellowships (MAI/DAI CP 12/2020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elio C, Fontani V, Rinaldi S, Gasbarro V. Reac-induced endogenous bioelectric currents in the treatment of venous ulcers: a three-arm randomized controlled prospective study. Acta Dermatovenerol Alp Pannonica Adriat. (2020) 29(3):109–13. doi: 10.15570/actaapa.2020.24
2. Fontani V, Aravagli L, Margotti ML, Castagna A, Mannu P, Rinaldi S. Neuropsychophysical optimization by reac technology in the treatment of: sense of stress and confusion. Psychometric evaluation in a randomized, single blind, sham-controlled naturalistic study. Patient Prefer Adherence. (2012) 6:195–9. doi: 10.2147/PPA.S29734
3. Olazaran J, Gonzalez B, Osa-Ruiz E, Felipe-Ruiz S, Boyano I, Fontani V, et al. Motor effects of radio electric asymmetric conveyer in Alzheimer’s disease: results from a cross-over trial. J Alzheimers Dis. (2014) 42(1):325–32. doi: 10.3233/JAD-140417
4. Pellegata G, Caracci S, Medaglini S. Radio electric asymmetric conveyer neurobiological treatments in non-specific neck pain: a retrospective study. J Pain Res. (2020) 13:2451–9. doi: 10.2147/JPR.S271537
5. Barcessat ARP, Bittencourt MN, Pereira JAC, Castagna A, Fontani V, Rinaldi S. Reac neurobiological treatments in acute post-traumatic knee medial collateral ligament lesion. Heliyon. (2020) 6(7):e04539. doi: 10.1016/j.heliyon.2020.e04539
6. Maioli M, Rinaldi S, Pigliaru G, Santaniello S, Basoli V, Castagna A, et al. Reac technology and hyaluron synthase 2, an interesting network to slow down stem cell senescence. Sci Rep. (2016) 6:28682. doi: 10.1038/srep28682
7. Basoli V, Santaniello S, Rinaldi S, Fontani V, Pigliaru G, Wieser M, et al. Physical stimulation by reac and Bmp4/Wnt-1 inhibitor synergistically enhance cardiogenic commitment in Ipscs. PLoS One. (2019) 14(1):e0211188. doi: 10.1371/journal.pone.0211188
8. Collodel G, Fioravanti A, Pascarelli NA, Lamboglia A, Fontani V, Maioli M, et al. Effects of regenerative radioelectric asymmetric conveyer treatment on human normal and osteoarthritic chondrocytes exposed to Il-1beta. A biochemical and morphological study. Clin Interv Aging. (2013) 8:309–16. doi: 10.2147/CIA.S42229
9. Maioli M, Rinaldi S, Migheli R, Pigliaru G, Rocchitta G, Santaniello S, et al. Neurological morphofunctional differentiation induced by reac technology in Pc12. A neuro protective model for Parkinson’s disease. Sci Rep. (2015) 5:10439. doi: 10.1038/srep10439
10. Panaro MA, Aloisi A, Nicolardi G, Lofrumento DD, De Nuccio F, La Pesa V, et al. Radio electric asymmetric conveyer technology modulates neuroinflammation in a mouse model of neurodegeneration. Neurosci Bull. (2018) 34(2):270–82. doi: 10.1007/s12264-017-0188-0
11. Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, et al. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regeneration. Cell Transplant. (2012) 21(6):1225–33. doi: 10.3727/096368911X600966
12. Rinaldi S, Fontani V, Castagna A. Brain activity modification produced by a single radioelectric asymmetric brain stimulation pulse: a new tool for neuropsychiatric treatments. Preliminary fmri study. Neuropsychiatr Dis Treat. (2011) 7:649–54. doi: 10.2147/NDT.S26123
13. Mura M, Castagna A, Fontani V, Rinaldi S. Preliminary pilot fmri study of neuropostural optimization with a noninvasive asymmetric radioelectric brain stimulation protocol in functional dysmetria. Neuropsychiatr Dis Treat. (2012) 8:149–54. doi: 10.2147/NDT.S29971
14. Rinaldi S, Mura M, Castagna A, Fontani V. Long-lasting changes in brain activation induced by a single reac technology pulse in Wi-Fi bands. Randomized double-blind fmri qualitative study. Sci Rep. (2014) 4:5668. doi: 10.1038/srep05668
15. Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, et al. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: a novel approach to multipotency. Cell Transplant. (2014) 23(12):1489–500. doi: 10.3727/096368913X672037
16. Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, et al. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant. (2013) 22(7):1227–35. doi: 10.3727/096368912X657297
17. Olivieri EB, Vecchiato C, Ignaccolo N, Mannu P, Castagna A, Aravagli L, et al. Radioelectric brain stimulation in the treatment of generalized anxiety disorder with comorbid major depression in a psychiatric hospital: a pilot study. Neuropsychiatr Dis Treat. (2011) 7:449–55. doi: 10.2147/NDT.S23420
18. Rinaldi A, Rinaldi C, Coelho Pereira JA, Lotti Margotti M, Bittencourt MN, Barcessat ARP, et al. Radio electric asymmetric conveyer neuromodulation in depression, anxiety, and stress. Neuropsychiatr Dis Treat. (2019) 15:469–80. doi: 10.2147/NDT.S195466
19. Rinaldi S, Fontani V, Aravagli L, Margotti ML. Psychological and symptomatic stress-related disorders with radio-electric treatment: psychometric evaluation. Stress Health. (2010) 26(5):350–8. doi: 10.1002/smi.1298
20. Fontani V, Mannu P, Castagna A, Rinaldi S. Social anxiety disorder: radio electric asymmetric conveyor brain stimulation versus sertraline. Patient Prefer Adherence. (2011) 5:581–6. doi: 10.2147/PPA.S27409
21. Pinheiro Barcessat AR, Nolli Bittencourt M, Duarte Ferreira L, de Souza Neri E, Coelho Pereira JA, Bechelli F, et al. Reac cervicobrachial neuromodulation treatment of depression, anxiety, and stress during the COVID-19 pandemic. Psychol Res Behav Manag. (2020) 13(13):929–37. doi: 10.2147/PRBM.S275730
22. Pinheiro Barcessat AR, Nolli Bittencourt M, Goes Goncalves R, Goncalves de Oliveira Cruz AV, Coelho Pereira JA, Bechelli FA, et al. Reac neuromodulation treatments in depression, anxiety and stress. A comparative retrospective study. Psychol Res Behav Manag. (2020) 13(13):1247–56. doi: 10.2147/PRBM.S287143
23. Rinaldi S, Fontani V, Moretti E, Rosettani B, Aravagli L, Sarago G, et al. A new approach on stress-related depression and anxiety: neuro-psycho-physical-optimization with radio electric asymmetric-conveyer. Indian J Med Res. (2010) 132:189–94.20716819
24. Collodel G, Moretti E, Fontani V, Rinaldi S, Aravagli L, Sarago G, et al. Effect of emotional stress on sperm quality. Indian J Med Res. (2008) 128(3):254–61.19052335
25. Fontani V, Rinaldi S, Aravagli L, Mannu P, Castagna A, Margotti ML. Noninvasive radioelectric asymmetric brain stimulation in the treatment of stress-related pain and physical problems: psychometric evaluation in a randomized, single-blind placebo-controlled, naturalistic study. Int J Gen Med. (2011) 4:681–6. doi: 10.2147/IJGM.S24628
26. Goncalves de Oliveira Cruz AV, Goes Goncalves R, Nunes L, Douglas Quaresma de Oliveira J, Lima Monteiro ES, Soares Eneias I, et al. Neuro postural optimization neuromodulation treatment of radio electric asymmetric conveyer technology on stress and quality of life in institutionalized children in a capital city of the Brazilian Amazon. Cureus. (2022) 14(7):e26550. doi: 10.7759/cureus.26550
27. Rinaldi S, Fontani V, Aravagli L, Mannu P. Psychometric evaluation of a radio electric auricular treatment for stress related disorders: a double-blinded, placebo-controlled controlled pilot study. Health Qual Life Outcomes. (2010) 8:31. doi: 10.1186/1477-7525-8-31
28. Rinaldi S, Fontani V, Aravagli L, Mannu P, Castagna A, Margotti ML, et al. Stress-related psycho-physiological disorders: randomized single blind placebo controlled naturalistic study of psychometric evaluation using a radio electric asymmetric treatment. Health Qual Life Outcomes. (2011) 9:54. doi: 10.1186/1477-7525-9-54
29. Lorenzini L, Giuliani A, Sivilia S, Baldassarro VA, Fernandez M, Lotti Margotti M, et al. Reac technology modifies pathological neuroinflammation and motor behaviour in an Alzheimer’s disease mouse model. Sci Rep. (2016) 6(1):35719. doi: 10.1038/srep35719
30. Mannu P, Rinaldi S, Fontani V, Castagna A. Radio electric asymmetric brain stimulation in the treatment of behavioral and psychiatric symptoms in Alzheimer disease. Clin Interv Aging. (2011) 6:207–11. doi: 10.2147/CIA.S23394
31. Olazaran J, Gonzalez B, Lopez-Alvarez J, Castagna A, Osa-Ruiz E, Herrero-Cano V, et al. Motor effects of reac in advanced Alzheimer’s disease: results from a pilot trial. J Alzheimers Dis. (2013) 36(2):297–302. doi: 10.3233/JAD-130077
32. Rinaldi S, Calza L, Giardino L, Biella GE, Zippo AG, Fontani V. Radio electric asymmetric conveyer: a novel neuromodulation technology in Alzheimer’s and other neurodegenerative diseases. Front Psychiatry. (2015) 6:22. doi: 10.3389/fpsyt.2015.00022
33. Rinaldi S, Maioli M, Santaniello S, Castagna A, Pigliaru G, Gualini S, et al. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: an in vitro beta-galactosidase study. Clin Interv Aging. (2012) 7:191–4. doi: 10.2147/CIA.S33312
34. Maioli M, Rinaldi S, Cruciani S, Necas A, Fontani V, Corda G, et al. Antisenescence effect of reac biomodulation to counteract the evolution of myelodysplastic syndrome. Physiol Res. (2022) 71(4):539–49. doi: 10.33549/physiolres.934903
35. Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, et al. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age. (2014) 36(1):9–20. doi: 10.1007/s11357-013-9537-8
36. Rinaldi S, Maioli M, Pigliaru G, Castagna A, Santaniello S, Basoli V, et al. Stem cell senescence. Effects of reac technology on telomerase-independent and telomerase-dependent pathways. Sci Rep. (2014) 4:6373. doi: 10.1038/srep06373
37. Fontani V, Rinaldi A, Castagna A, Rinaldi S. Calcific tendinitis of the shoulder: a neuro-psychomotor behavioral diagnostic and therapeutic approach with radioelectric asymmetric conveyer neurobiological stimulation treatments. Cureus. (2022) 14(7):e26770. doi: 10.7759/cureus.26770
38. Fontani V, Rinaldi A, Rinaldi C, Araldi L, Azzara A, Carta AM, et al. Long-lasting efficacy of radio electric asymmetric conveyer neuromodulation treatment on functional dysmetria, an adaptive motor behavior. Cureus. (2022) 14(6):e25768. doi: 10.7759/cureus.25768
39. Rinaldi S, Rinaldi C, Fontani V. Regenerative radio electric asymmetric conveyer treatment in generalized cerebral and cerebellar atrophy to improve motor control: a case report. Cureus. (2022) 14(8):e28245. doi: 10.7759/cureus.28245
40. Cano-de-la-Cuerda R, Molero-Sanchez A, Carratala-Tejada M, Alguacil-Diego IM, Molina-Rueda F, Miangolarra-Page JC, et al. Theories and control models and motor learning: clinical applications in neuro-rehabilitation. Neurologia. (2015) 30(1):32–41. doi: 10.1016/j.nrl.2011.12.010
41. Souza MT, Silva MD, Carvalho R. Integrative review: what is it? How to do it? Einstein (Sao Paulo). (2010) 8(1):102–6. doi: 10.1590/S1679-45082010RW1134
42. Castagna A, Rinaldi S, Fontani V, Mannu P. Radioelectric asymmetric brain stimulation and lingual apex repositioning in patients with atypical deglutition. J Multidiscip Healthc. (2011) 4:209–13. doi: 10.2147/JMDH.S22830
43. Zippo AG, Rinaldi S, Pellegata G, Caramenti GC, Valente M, Fontani V, et al. Electrophysiological effects of non-invasive radio electric asymmetric conveyor (reac) on thalamocortical neural activities and perturbed experimental conditions. Sci Rep. (2015) 5:18200. doi: 10.1038/srep18200
44. Fontani V, Rinaldi S, Castagna A, Margotti ML. Noninvasive radioelectric asymmetric conveyor brain stimulation treatment improves balance in individuals over 65 suffering from neurological diseases: pilot study. Ther Clin Risk Manag. (2012) 8:73–8. doi: 10.2147/TCRM.S28812
45. Mannu P, Rinaldi S, Fontani V, Castagna A. Long-term treatment of bipolar disorder with a radioelectric asymmetric conveyor. Neuropsychiatr Dis Treat. (2011) 7:373–9. doi: 10.2147/NDT.S22007
46. Frohlich F. Endogenous and exogenous electric fields as modifiers of brain activity: rational design of noninvasive brain stimulation with transcranial alternating current stimulation. Dialogues Clin Neurosci. (2014) 16(1):93–102. doi: 10.31887/DCNS.2014.16.1/ffroehlich
47. Baer ML, Henderson SC, Colello RJ. Elucidating the role of injury-induced electric fields (Efs) in regulating the astrocytic response to injury in the mammalian central nervous system. PLoS One. (2015) 10(11):e0142740. doi: 10.1371/journal.pone.0142740
48. Suzuki T, Sakata H, Kato C, Connor JA, Morita M. Astrocyte activation and wound healing in intact-skull mouse after focal brain injury. Eur J Neurosci. (2012) 36(12):3653–64. doi: 10.1111/j.1460-9568.2012.08280.x
49. Cao L, Wei D, Reid B, Zhao S, Pu J, Pan T, et al. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep. (2013) 14(2):184–90. doi: 10.1038/embor.2012.215
50. Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. (2005) 9(10):474–80. doi: 10.1016/j.tics.2005.08.011
51. Floyd CL, Lyeth BG. Astroglia: important mediators of traumatic brain injury. Prog Brain Res. (2007) 161:61–79. doi: 10.1016/S0079-6123(06)61005-4
52. Baer ML, Colello RJ. Endogenous bioelectric fields: a putative regulator of wound repair and regeneration in the central nervous system. Neural Regen Res. (2016) 11(6):861–4. doi: 10.4103/1673-5374.184446
53. Viswanathan A, Freeman RD. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat Neurosci. (2007) 10(10):1308–12. doi: 10.1038/nn1977
54. Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. (1990) 87(24):9868–72. doi: 10.1073/pnas.87.24.9868
55. Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. (1998) 40(3):383–96. doi: 10.1002/mrm.1910400308
56. Berge OG. Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol. (2011) 164(4):1195–206. doi: 10.1111/j.1476-5381.2011.01300.x
57. Zippo A, Storchi R, Valente M, Caramenti GC, Biella G. Neural substrates of chronic pain in the thalamocortical circuit. Nat Prec. (2011). doi: 10.1038/npre.2011.6548.1
58. Kao TC, Sadabadi MS, Hennequin G. Optimal anticipatory control as a theory of motor preparation: a thalamo-cortical circuit model. Neuron. (2021) 109(9):1567–81 e12. doi: 10.1016/j.neuron.2021.03.009
59. Hou JM, Yan RB, Xiang ZM, Zhang H, Liu J, Wu YT, et al. Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience. (2014) 266:208–15. doi: 10.1016/j.neuroscience.2014.02.013
60. Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. (2015) 14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5
61. Maragakis NJ, Rothstein JD. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. (2006) 2(12):679–89. doi: 10.1038/ncpneuro0355
62. Gisfrede TK, Kimura JS, Reyes A, Bassi J, Drugowick R, Matos R, et al. Deleterious oral habits and its consequences in pediatric dentistry. Revista Brasileira de Odontolologia. (2016) 73(2):144–9. doi: 10.18363/rbo.v73n2.p.144
63. Akin E, Sayin MO, Karacay S, Bulakbasi N. Real-time balanced turbo field echo cine-magnetic resonance imaging evaluation of tongue movements during deglutition in subjects with anterior open bite. Am J Orthod Dentofacial Orthop. (2006) 129(1):24–8. doi: 10.1016/j.ajodo.2005.10.002
64. Hebling SR, Cortellazzi KL, Tagliaferro EP, Hebling E, Ambrosano GM, Meneghim Mde C, et al. Relationship between malocclusion and behavioral, demographic and socioeconomic variables: a cross-sectional study of 5-year-olds. J Clin Pediatr Dent. (2008) 33(1):75–9. doi: 10.17796/jcpd.33.1.3457qg88w37h2405
Keywords: rehabilitation, motor disorders, radio electric fields, electrophysiology, neurophysiology, endogenous bioelectric fields
Citation: Machado VG, Brun ABS and Manffra EF (2023) Effects of the radio electric asymmetric conveyer (REAC) on motor disorders: An integrative review. Front. Med. Technol. 5:1122245. doi: 10.3389/fmedt.2023.1122245
Received: 12 December 2022; Accepted: 25 January 2023;
Published: 27 February 2023.
Edited by:
Laura Calza, University of Bologna, ItalyReviewed by:
Alessandro Castagna Rinaldi Fontani Institute, ItalySara Cruciani, University of Sassari, Italy
© 2023 Machado, Brun and Manffra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisangela Ferretti Manffra ZWxpc2FuZ2VsYS5tYW5mZnJhQHB1Y3ByLmJy ZWxpc2FuZ2VsYWZlcnJldHRpQGdtYWlsLmNvbQ==
Specialty Section: This article was submitted to Regenerative Technologies, a section of the journal Frontiers in Medical Technology
 Vinícius Gomes Machado
Vinícius Gomes Machado Ana Beatriz Sorgenfrei Brun2
Ana Beatriz Sorgenfrei Brun2 Elisangela Ferretti Manffra
Elisangela Ferretti Manffra