- 1Pulmonary Department, General Clinic Euromedica, Thessaloniki, Greece
- 23rd Surgery Department, AHEPA University Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 3Sana Clinic Group Franken, Department of Cardiology/Pulmonology/Intensive Care/Nephrology, “Hof” Clinics, University of Erlangen, Hof, Germany
- 4Oncology Department, University General Hospital of Larissa, Larissa, Greece
- 5Oncology Department, General Hospital of Volos, Volos, Greece
- 6Radiology Department, Democritus University of Thrace, Alexandroupolis, Greece
- 7Pulmonary Department, `G. Papanikolaoù` General Hospital, Aristotle University of Thessaloniki, Thessaloniki, Greece
- 8Oncology Department, General Hospital of Rhodes, Rhodes, Greece
- 9Vascular Surgery Department, General Clinic Euromedica, Thessaloniki, Greece
Background: Single pulmonary nodules are a common issue in everyday clinical practice. Currently, there are navigation systems with radial-endobronchial ultrasound and electromagnetic navigation for obtaining biopsies. Moreover, rapid on-site evaluation can be used for a quick assessment. These small lesions, even when they do not have any clinically significant information with positron emission tomography, are important to investigate.
Case description: Radiofrequency and microwave ablation have been evaluated as local treatment techniques. These techniques can be used as therapy for a patient population that cannot be operated on. Currently, one verified operating system is used for endoscopic radiofrequency ablation through the working channel of a bronchoscope.
Conclusion: In our case, a new system was used to perform radiofrequency ablation with long-term follow-up.
Introduction
Single pulmonary nodules are a clinical issue that needs special attention. A nodule is defined as a lesion up to 3 cm. Currently, we can use imaging techniques with computed tomography (CT) and positron emission tomography (PET-CT). If a nodule is ≤1 cm, then positron emission tomography will not provide us with useful clinical information unless the uptake of SUV is ≥3. In the case of these very small lesions, we can order a follow-up every 2–3 months, and if they are increased in size, we can proceed to biopsy or surgery. In the case of nodules ≥1.1 cm, positron emission tomography can provide us with useful clinical information based on the SUV uptake; however, again such a lesion might be a cancerous lesion with a low metabolic rate, and we might have a false-negative result (1, 2). However, all these guidelines have not been updated according to the new endoscopic techniques that have been developed in recent years. Nowadays, radial-endobronchial ultrasound (EBUS) and electromagnetic navigation can be used to perform a biopsy in pulmonary nodules with a high rate of efficiency (3–6). Moreover, over the past decade, rapid on-site evaluation (ROSE) has been used to identify malignant lesions (7). In the case of a single nodule without any other cancer metastatic site, surgery or ablation is performed. Currently, ablation is performed under computed tomography guidance with radiofrequency, microwave, and thermosphere probes. In our case, a Covidien radiofrequency system was used.
Case presentation
A 65-year-old woman, nonsmoker, was diagnosed in our outpatient cabinet with a single pulmonary nodule in the left lower lobe. Positron emission tomography was performed, and a significant SUV uptake of 8.9 was observed in the nodule (Figure 1). The patient had a persistent cough for 3 weeks, she did not report fever, and her laboratory findings were within normal range. It was decided to have a radial-endobronchial ultrasound with C-ARM for diagnosis with ROSE and convex-endobronchial ultrasound with rapid on-site evaluation for staging (Figure 2). Indeed, the ROSE technique evaluated non-small-cell lung cancer (NSCLC) for the single pulmonary nodule, and lymph node staging by ROSE revealed no metastasis in the lymph nodes or other organs. Local radiofrequency ablation with an endoscopic radiofrequency probe was proposed.
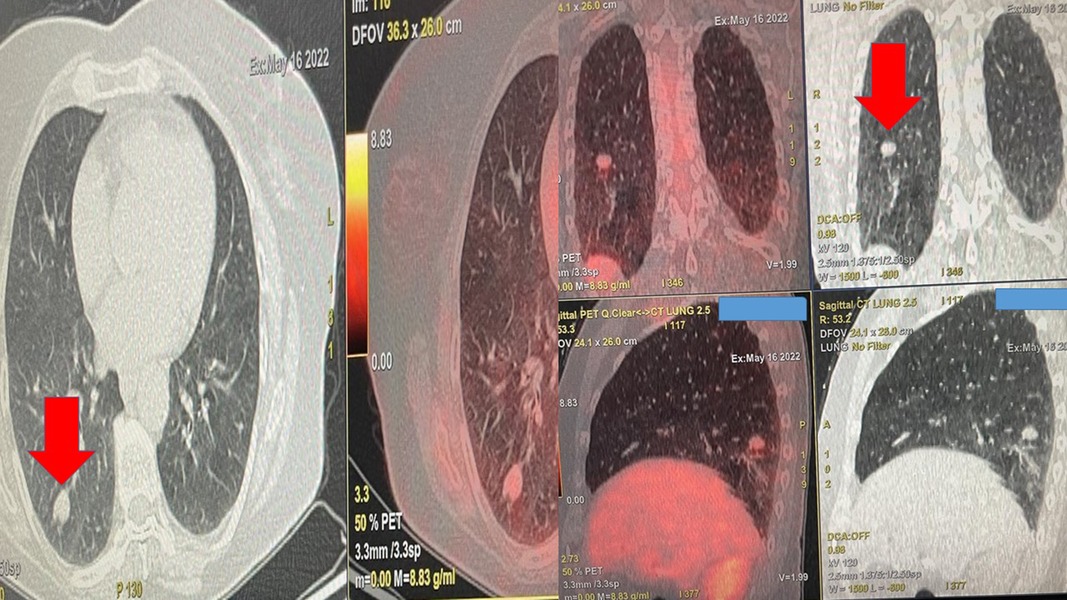
Figure 1. Positron emission tomography findings upon diagnosis. The red arrow indicates the single nodule.
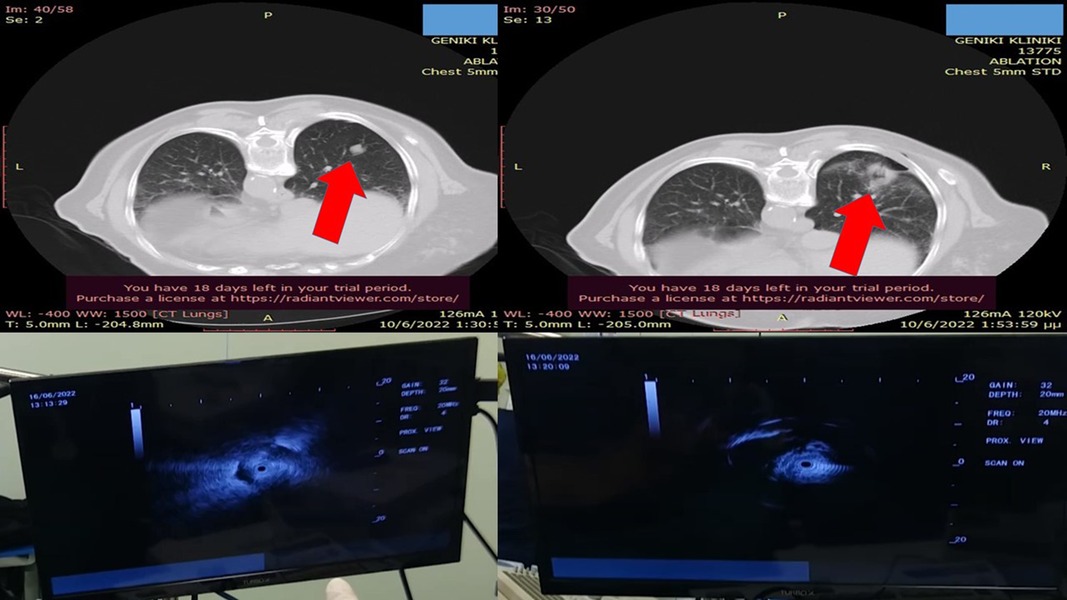
Figure 2. Left upper-row red arrow indicates the lesion before ablation, the left lower row demonstrates the lesion sign with radial-EBUS, the right upper-row red arrow indicates the area after two sessions of thermal effect with radiofrequency ablation, and the right lower row demonstrates the lesion sign after the ablation. EBUS, endobronchial ultrasound.
Treatment procedure
The patient agreed to the minimally invasive procedure of endoscopic radiofrequency ablation. We used two sessions of 20 s each with 40 W (Figure 3). The patient was intubated with a 7.5-mm tracheal tube with a high-volume, low-pressure cuff and was under jet ventilation. The consistency of the nodule was evaluated before and after the procedure with radial-endobronchial ultrasound, and computed tomography verified the results. The patient had a spirometry and heart examination before the procedure to be prepared in the case of lobectomy. We had, at our disposal, balloon blockers and hemostatic dry powder in the case of hemorrhage. After 1 year of follow-up, the patient is disease-free. We performed computed tomography scanning with i.v. contrast infusion after 40 days for disease evaluation and PET-CT after 3 months of the procedure according to guidelines.
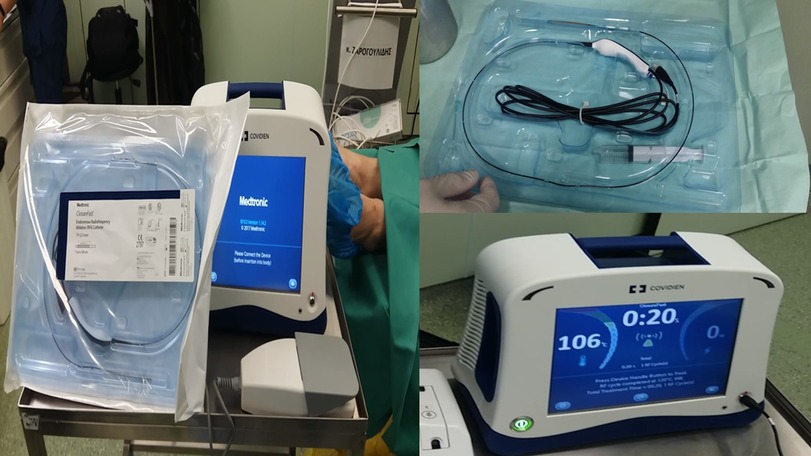
Figure 3. Covidien endovascular radiofrequency catheter. On the left, one can see both the generator and the ablation probe. On the upper right is the probe in magnification, and on the lower right is the Covidien generator.
Discussion
To perform a biopsy on a single pulmonary nodule, we use computed tomography-guided needles, usually 18G, or radial-endobronchial ultrasound through bronchoscopes. In the second method, we can use biopsy forceps for histology samples, needles for a fine needle biopsy, usually 22G, or brushes for cytology samples (3, 4). In the case of cytology material, rapid on-site evaluation can be performed, and a positive result is enough for diagnosis (8). Electromagnetic navigation is another technique that can be used, and currently, we have two platforms, the Covidien endovascular radiofrequency catheter, formerly Medtronic, and the ARCHIMEDES system from Bronchus (9, 10). The ARCHIMEDES system has the advantage of additionally providing information regarding the surrounding vessels next to the lesion. Monarch is another platform regarded as a robotic-assisted method that we use under the guidance of computed tomography (11). In the case of a single nodule positive for NSCLC without distant metastasis, we can perform surgery or ablation. Regarding surgery, we have two techniques, video-assisted thoracic surgery (VATS) and robotic-assisted thoracic surgery (RATS) (12, 13). However, ablation is another solution for these patients or those who cannot undergo surgery due to comorbidities. Until recently, we performed ablation under computed tomography with radiofrequency or microwave needles. Both probes are equally efficient; however, the ablation time is less with microwave needles. Each probe has its advantages and disadvantages, which will not be discussed here. The main adverse effects are pneumothorax, hemothorax, or bleeding (14). Currently, we have evaluated one system for endoscopic radiofrequency ablation (15). There are technical differences between the percutaneous ablation systems and the endobronchial ultrasound; however, the result is the same. Moreover, the same system that produces steam for emphysema treatment has been used as a thermal endoscopic ablation system in Australia (16). For the first time, a Covidien endovascular radiofrequency system was used with more watts and double time based on the previous knowledge from the radiofrequency probes used for computed tomography ablation. The effect of ablation was evaluated with radial-EBUS. The results were encouraging based on our long-term follow-up, and technical aspects of the procedure will be improved since we have two catheters of short and long lengths, 3 and 7 mm, respectively. Finally, computed tomography scanning with i.v. contrast infusion was used to evaluate disease relapse after 30–40 days of ablation. Positron emission tomography was avoided under several weeks since there is going to be a pseudo-positive result of increased SUV due to local inflammation.
Conclusion
The most important issue is that we can evaluate the effect of radial-EBUS on site after every session of ablation and decide whether to continue or not. We did not have any adverse effects, and the procedures were terminated after 45 min. The only reason for the delay was the approach of the nodule.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This case report was approved by the ethical committee of the General Clinic Private Hospital, Thessaloniki, Greece, ID number 11/2022. Written informed consent was obtained from the participant for publication of the details of her medical case and any accompanying images. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PZ, WH-S, and VP wrote the manuscript and performed the procedure. E-IP, DM, NC, and KP wrote the manuscript and assisted in the radiofrequency ablation sequence. KT wrote the manuscript, collected data, and was involved in the conception of the procedure. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer TK declared a shared affiliation with the author VP to the handling editor at the time of review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Meng Q, Li B, Gao P, Liu W, Zhou P, Ding J, et al. Development and validation of a risk stratification model of pulmonary ground-glass nodules based on complementary lung-RADS 1.1 and deep learning scores. Front Public Health. (2022) 10:891306. doi: 10.3389/fpubh.2022.891306
2. Zhang K, Wei Z, Nie Y, Shen H, Wang X, Wang J, et al. Comprehensive analysis of clinical logistic and machine learning-based models for the evaluation of pulmonary nodules. JTO Clin Res Rep. (2022) 3(4):100299. doi: 10.1016/j.jtocrr.2022.100299
3. Zaric B, Stojsic V, Carapic V, Kovacevic T, Stojanovic G, Panjkovic M, et al. Radial endobronchial ultrasound (EBUS) guided suction catheter-biopsy in histological diagnosis of peripheral pulmonary lesions. J Cancer. (2016) 7(1):7–13. doi: 10.7150/jca.13081
4. Haidong H, Yunye N, Wei Z, Zarogoulidis P, Hohenforst-Schmidt W, Man YG, et al. Multiple guided technologies based on radial probe endobronchial ultrasound for the diagnosis of solitary peripheral pulmonary lesions: a single-center study. J Cancer. (2017) 8(17):3514–21. doi: 10.7150/jca.20035
5. Huang Z, Huang H, Ning Y, Han J, Shen Y, Shi H, et al. Radial probe endobronchial ultrasound assisted conventional transbronchial needle aspiration in the diagnosis of solitary peribronchial pulmonary lesion located in the segmental bronchi. J Cancer. (2019) 10(3):634–42. doi: 10.7150/jca.28755
6. Zarogoulidis P, Kosmidis CS, Hohenforst-Schmidt W, Matthaios D, Sapalidis K, Petridis D, et al. Radial-EBUS: CryoBiopsy versus conventional biopsy: time-sample and C-arm. Int J Environ Res Public Health. (2022) 19(6):1–11. doi: 10.3390/ijerph19063569
7. Verhoeven RLJ, Vos S, van der Heijden E. Multi-modal tissue sampling in cone beam CT guided navigation bronchoscopy: comparative accuracy of different sampling tools and rapid on-site evaluation of cytopathology. J Thorac Dis. (2021) 13(7):4396–406. doi: 10.21037/jtd-21-518
8. Yiminniyaze R, Zhang X, Zhang Y, Chen K, Li C, Zhu N, et al. Diagnostic efficiency and safety of rapid on-site evaluation combined with CT-guided transthoracic core needle biopsy in suspected lung cancer patients. Cytopathology. (2022) 33(4):439–44. doi: 10.1111/cyt.13123
9. Dunn BK, Blaj M, Stahl J, Speicher J, Anciano C, Hudson S, et al. Evaluation of electromagnetic navigational bronchoscopy using tomosynthesis-assisted visualization, intraprocedural positional correction and continuous guidance for evaluation of peripheral pulmonary nodules. J Bronchology Interv Pulmonol. (2022) 1:16–23. doi: 10.1097/LBR.0000000000000839
10. Sun J, Criner GJ, Dibardino D, Li S, Nader D, Lam B, et al. Efficacy and safety of virtual bronchoscopic navigation with fused fluoroscopy and vessel mapping for access of pulmonary lesions. Respirology. (2022) 27(5):357–65. doi: 10.1111/resp.14224
11. Lu M, Nath S, Semaan RW. A review of robotic-assisted bronchoscopy platforms in the sampling of peripheral pulmonary lesions. J Clin Med. (2021) 10(23):1–7. doi: 10.3390/jcm10235678
12. Aguinagalde B, Insausti A, Lopez I, Sanchez L, Bolufer S, Embun R, et al. VATS Lobectomy morbidity and mortality is lower in patients with the same ppoDLCO: analysis of the database of the Spanish video-assisted thoracic surgery group. Arch Bronconeumol. (2021) 57(12):750–56. doi: 10.1016/j.arbr.2021.10.005
13. Tang Q, Liu W, Jiang D, Tang J, Zhou Q, Zhang J. Perioperative and survival outcomes of robotic-assisted surgery, comparison with laparoscopy and laparotomy, for ovarian cancer: a network meta-analysis. J Oncol. (2022) 2022:2084774. doi: 10.1155/2022/2084774
14. Shah M, NeMoyer RE, Kashyap R, Lin Y, Sarmiento J, Kooby DA, et al. Surgical resection for adrenocortical carcinoma: current trends affecting survival. J Surg Oncol. (2022) 125(8):1224–30. doi: 10.1002/jso.26845
15. de Lima A, Vidal B, Kheir F, VanderLaan PA, Mallur PS, Gangadharan SP, et al. Thermoablative techniques for excessive central airway collapse: an ex vivo pilot study on sheep tracheal tissue. J Bronchology Interv Pulmonol. (2020) 27(3):195–99. doi: 10.1097/LBR.0000000000000647
Keywords: lung cancer, ablation, radial-EBUS, ROSE, navigation, FNA
Citation: Zarogoulidis P, Hohenforst-Schmidt W, Papadopoulos V, Perdikouri E, Courcoutsakis N, Porpodis K, Matthaios D and Trigonakis K (2023) Case Report: Endoscopic radiofrequency ablation with radial-EBUS and ROSE. Front. Med. Technol. 5:1022220. doi: 10.3389/fmedt.2023.1022220
Received: 18 August 2022; Accepted: 3 January 2023;
Published: 20 January 2023.
Edited by:
Sharmilee Sawant, Becton Dickinson, United StatesReviewed by:
Jianfeng Yang, Hangzhou First People's Hospital, ChinaTheodora Kerenidi, University of Thessaly, Greece
Aggeliki Rapti, Sotiria General Hospital, Greece
© 2023 Zarogoulidis, Hohenforst-Schmidt, Papadopoulos, Perdikouri, Courcoutsakis, Porpodis, Matthaios and Trigonakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul Zarogoulidis cHphcm9nQGhvdG1haWwuY29t
Specialty Section: This article was submitted to Diagnostic and Therapeutic Devices, a section of the journal Frontiers in Medical Technology
 Paul Zarogoulidis
Paul Zarogoulidis Wolfgang Hohenforst-Schmidt
Wolfgang Hohenforst-Schmidt Vasileios Papadopoulos4
Vasileios Papadopoulos4 Konstantinos Porpodis
Konstantinos Porpodis