
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. Technol., 28 November 2022
Sec. Diagnostic and Therapeutic Devices
Volume 4 - 2022 | https://doi.org/10.3389/fmedt.2022.1038087
This article is part of the Research TopicMedical Technologies in Diagnostic & TherapeuticsView all 8 articles
 Carlo Calabrese1,2
Carlo Calabrese1,2 Dania Gelli1,2
Dania Gelli1,2 Fernando Rizzello1,2
Fernando Rizzello1,2 Paolo Gionchetti1,2
Paolo Gionchetti1,2 Rafael Torrejon Torres3
Rafael Torrejon Torres3 Rhodri Saunders3*
Rhodri Saunders3* Jason Davis3
Jason Davis3
Background: Crohn's disease (CD) is a potentially debilitating condition that burdens Italian healthcare substantially. The symptomatic management relies on prompt therapy adjustment to reduce flares and follow-up diagnostic inputs to maximise remission. Capsule endoscopy (CE) has introduced advantages in CD diagnostics, allowing the direct inspection of the entire gastrointestinal mucosa. The diagnostic procedure is comparable in effort to standard ileocolonoscopy (IC) but requires no anaesthesia. Whether CE follow-up improves clinical outcomes remains to be defined.
Objectives: To provide a preliminary evaluation of CE in terms of clinical outcomes with respect to the standard of care ileocolonoscopy/MRE in Italy.
Methods: This retrospective analysis utilises anonymised, monocentric data from the S. Orsola-Malpighi Hospital IBD database in Bologna, Italy, collected between 1999 and 2019. Out of 421 adult patient records, 100 were included in the analysis (50 per arm, matched per demographic and clinical characteristics). The CE represented the intervention arm, whereas ileocolonoscopy/magnetic resonance enterography was the standard of care. The use of biologics, symptomatology course, and surgery were the outcomes.
Results: The two techniques performed similarly overall. In general, no significant difference emerged in the use of biologics. The use of biologics appears reduced in the CE group, only in L4 patients after the first follow-up year. Similarly, surgery was seemingly less frequent among L4 patients in the CE group. No difference was found between groups in flare occurrence and duration. CE patients might have experienced longer and earlier first remissions, but no long-term difference persisted.
Conclusions: The CE group showed an apparent reduction in biologics and surgery, limiting to L4 diagnoses. More extensive, prospective, multicentre, randomised studies must corroborate these preliminary findings.
Crohn's disease (CD) is a potentially debilitating, idiopathic chronic inflammatory disorder of the gastrointestinal tract (GI) (1) and a considerable burden on the Italian healthcare system. In 2009, the prevalence was estimated to be 81 (female) and 91 (male) cases per 100,000 inhabitants, with an incidence of 6.5 and 7.4, respectively (2). More recent estimates increased these figures to 9.4 cases per 100,000 inhabitants a year (3). In terms of healthcare resources, ∼17,000 CD admissions were registered in Italy in 2005, 2008, and 2011 (4). The annual cost of disease has been appraised at €15,000 per patient, with direct costs comprising treatments and hospitalisations accounting for 76% of the total (5, 6).
From the patients' perspective, CD has a detrimental impact on quality of life, scoring between 0.52 and 0.76 on the EuroQol 5D scale for severe and milder forms, respectively (7). Although any section of the GI can be affected, the main foci are localised at the colon and small bowel. The aetiology remains unclear, and therapy has historically been symptomatic (8, 9). Long-term consequences caused by uncontrolled inflammation include fibrotic strictures, enteric fistulae, and intestinal neoplasia (10). Early diagnosis, treatment of inflammation, iterative disease assessment, and therapy adjustment effectively contribute to achieving clinical and endoscopic remission (10–13). These measures are also associated with reduced complications, shorter hospitalisations, and diminished resort to steroids, biologics, and surgical procedures (12, 14–16).
The diagnosis and continued surveillance of CD have relied on markers such as C-reactive protein (CRP) and faecal calprotectin (fCal), instrumental assessment by cross-sectional imaging, and direct visual mucosal inspection by ileocolonoscopy (IC), depending on the disease location and comorbidities. However, molecular markers and cross-sectional imaging have limited efficacy: The global magnetic resonance index of activity (MaRIA) and fCal values correlate moderately with endoscopic activity and do not correlate with each other (17). One in three CD patients does not exhibit high CRP levels during full-blown disease (18, 19), and ileal CD is not correlated with calprotectin levels (20, 21). Currently, IC remains the gold standard for mucosal assessment. However, the procedure is invasive, involves sedation and allows access to only a modest segment of the terminal ileum, while most of the small bowel is not accessible. Imaging the upper sections of the ileum is viable only with complementary ultrasonography, magnetic resonance enterography (MRE), and computed tomography (22).
Direct imaging of the small bowel became possible with the introduction of capsule endoscopy (CE), currently included in the ECCO Guidelines recommendations (9, 13, 23, 24). CE permits the direct imaging of the entire GI mucosa in a single procedure and was found to be better accepted by patients than IC (25–27). A multicentre study determined that the sensitivity of CE is equivalent to IC and/or MRE in each segment of the lower GI (28). Regarding specificity, CE outperforms MRE in the small bowel and is comparable to IC in the terminal ileum and colon (28–31). CE was previously shown also to yield comparable diagnostics to other non-invasive techniques (32–35). This is attributed to CE's greater sensitivity to lesions or defects in the bowel mucosa below the detection threshold of alternative imaging techniques and its significantly deeper coverage of the small bowel length than IC alone (32).
The management of CD articulates through early symptomatic treatment with corticosteroids to achieve rapid remission and long-term maintenance therapy to prevent flares and reduce the risk of complications (10, 36, 37). The maintenance treatment comprises immunomodulators and biologics for preventing flares and complications (10, 38). Despite these notable pharmacological advances, surgery remains necessary to treat approximately two-thirds of CD cases (39, 40).
However, the practical clinical implications of CE diagnostics and follow-up in guiding the course of treatment and remission have yet to be comprehensively characterised, i.e., whether CE-based diagnostics and follow-up have any real, practical benefit compared to IC/MRE in CD management. From this perspective, this study aims to provide a preliminary, retrospective, and monocentric gauge of the impact of CE on clinical outcomes in Italy.
Retrospective, single-centre data were extracted from the IBD registry of the Azienda Ospedaliero-Universitaria Policlinico S. Orsola-Malpighi Hospital, Bologna, Italy. Data included limited patients' demographics (age, sex, disease parameters, and diagnostic method) in 6-month intervals over a five-year follow-up. An IC/MRE diagnosis qualified as the standard of care (SOC), whereas CE (Medtronic PillCam™ SB2/PillCam2) was the intervention group. Diagnoses in both arms were grouped per location according to the Vienna classification (41). No patients in the SOC group underwent CE, neither at first diagnosis nor during follow-up. Endoscopies of the upper GI were not performed in L4 diagnoses in the SOC group. All SOC patients received MRE at least once a year.
Data were pseudonymised by the data controllers (CC and DG) before transmission to the data processor (Coreva Scientific) and handled according to the General Data Protection Regulation (GDPR). The Sant'Orsola-Malpighi Hospital Ethics Committee reviewed and approved the study (approbation number 173/2017/O/OssN).
The analysis included CD patients aged 18 or older with at least five years of available follow-up data from entry in the database. Patients who entered the database before 1999, missing diagnosed CD location or initial CD activity index (CDAI), were excluded. Patients with a history or symptoms of suspected strictures were ineligible for inclusion in the CE group and assigned to SOC.
The outcomes investigated were the incidence of surgery, evolution of symptomatology in terms of CDAI score, and biologics therapy (prescription, initiation, and duration). Symptomatology was classified as none for CDAI < 150, mild for 150 ≤ CDAI < 220, moderate for 220 ≤ CDAI < 450 and severe for CDAI ≥ 450. Remission was defined as two consecutive asymptomatic intervals (one year, CDAI < 150). A flare was defined as the recurrence of moderate or severe symptoms (CDAI ≥ 220) following at least an asymptomatic (CDAI < 150) or mildly symptomatic year (150 ≤ CDAI < 220). Gaps in the CDAI data were excluded as missing data. Prescription data did not include the specific medication consistently and, therefore, did thus not allow stratifying per type of biologics.
The preparation for CE included a clear liquid diet for 24 h plus 12 h fasting and receiving 2 L of polyethylene glycol (PEG) solution 2–8 h before capsule ingestion (PillCam SB2/PillCam2). An additional fluid bolus was given after 2 h from capsule ingestion to facilitate transit through the small bowel (SB). All images were reviewed using the RAPID 8 software (Given Imaging, Yokneam, Israel). SB was divided into three main segments: proximal, medial, and distal. Mucosal inflammation was quantified using the Lewis Score (LS) (42, 43). Using the software application to calculate the LS, the SB was automatically divided into equal thirds (tertiles). SB lesions were considered proximal if located in the upper two-thirds of the SB (first two tertiles of the CE) and had an LS ≥ 135. SB inflammatory activity was classified as mild (135 ≤ LS < 790) or moderate-to-severe (LS ≥ 790). Gastric transit time and SB transit time were collected from the CE studies. In the event of an incomplete study, SB transit time was calculated as 480 min minus gastric transit time. A CE study was defined as complete if the capsule reached the cecum. Cleanliness in SB scored from 1 to 3 (1, free of stool and debris; 2, some stool and debris; 3, full of stool and debris). A board-certified gastroenterologist (CC) read the capsule videos.
Examinations were performed with a single oral contrast preparation consisting of 1500 ml PEG solution ingested gradually for 45 min. MRE was carried out with an Intera 1.5 T MR system with a 5-element Synbody coil (Philips Medical Systems, Eindhoven, The Netherlands). Patients were examined supine. The protocol contained the sequences Cor T2 (B-FFE; TR/TE, 4.1/2.0 milliseconds; flip angle, 60°; slice thickness, 5 mm; 224 matrix; FOV 400), and axial T1W (TR/TE, 7/3.4; flip angle, 15°; slice thickness, 4 mm; 208 matrix; FOV 375), with discontinuous breath-hold before and after contrast. Gadodiamid 0.1 mmol/kg (GE Healthcare, Medical Diagnostics, Oslo, Norway) was given intravenously, and hyoscinbutyl-bromide 20 mg (Buscopan; Boehringer Ingelheim, Basel, Switzerland) was administered to reduce peristalsis during the procedure. CTE was performed with a 64-slice CT system (Somatom Sensation; Siemens, Erlangen, Germany) using: CARE-Dose: on, 120 kV, up to 150 mA; rotation time, 0.5 s; pitch, 1.5; collimation, 0.6 mm; increment 2. Contrast-enhanced CT scanning in the portal phase was performed after intravenous 100 ml iomeprol (Iomeron; Bracco, Milan, Italy) 300 mg/ml using an automatic injector OptiVantage DH (Mallinckrodt, Cincinnati, OH) at an injection rate of 4 ml/s. Patients with a body weight greater than 80 kg received 150 ml iomeprol at the same injection rate. All images were evaluated by using an Impax PACS workstation (Agfa, Mortsel, Belgium) with two Coronis monitors (1,600 × 200 pixels) (Megapixels Diagnostic Display System; Barco, Kortrijk, Belgium). CST readers identified active CD by segmental mural hyperenhancement, mural stratification, increased density in perienteric fat, sinus tract, or fistula. Fibrofatty proliferation and luminal narrowing without hyperenhancement were considered to represent inactive CD. Small bowel distension was rated on a two-point scale: sufficient (≥50%, score 1) or poor (<50%, score 0) for each examined segment. The image quality was rated as good (diagnostic images without artefacts, score 3), sufficient (diagnostic images with artefacts, score 2), or poor (non-diagnostic images, score 1). A bowel wall of 4 mm or more measured perpendicular to the bowel surface was considered thickened. Small bowel stenosis was defined as a change in bowel calibre with dilatation of the proximal segment above 2.5 cm and/or a collapse of the distal segment. MRE was interpreted at the time of examination by two experienced gastrointestinal radiologists. All SOC patients received MRE at least once a year.
Data were processed using R v4.0.2 without a priori power calculation. Medians and interquartile ranges are provided for demographic data and outcomes, where applicable. Inference testing was performed using non-parametric tests (Fisher's exact test, Wilcox's test). A group matching algorithm was run using the R “MatchIt” package v3.0.2. Matching was performed over sex, age, disease duration, disease location, initial CDAI score, surgery history, and gastrointestinal bleeding.
After applying exclusion criteria to 421 patient records, 325 were included in the study: 270 were diagnosed by IC/MRE (SOC group) and 55 by CE (intervention, CE group). After matching the groups, fifty patients per arm were ultimately included in the analysis. There was no statistically significant distinction between the groups' demographics after matching (Table 1). Subsequent analyses, stratified per CD location, excluded the L2 group due to low numbers.
The use of biologics in the two groups is summarised in Figure 1. Overall, the SOC group appeared to require more extensive use of biologics (71 patient years) than the CE group (53.5 patient years). However, this could be justified by the longer average disease duration in the SOC group (5.5 vs. 4.0 years); adjusting for disease duration rendered the difference marginal (CE, 73.5 patient years). Similar results emerged stratifying by the site of diagnosis. The two groups were comparable also in terms of initiation of biologics and time course throughout the follow-up, except for L4 diagnoses (22.5 patient years SOC vs. 14.0 patient years CE—Figure 1). Although L4 patients started therapy at the same time in both arms, the CE group seemed to make a reduced use of biologics after the first follow-up year (89.5% in SOC, 56.8% in CE at 5-year follow up).
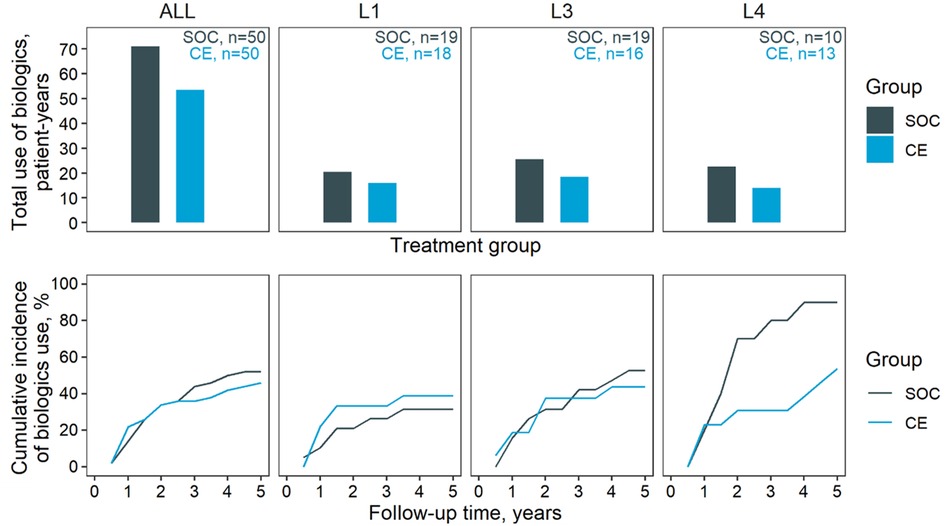
Figure 1. Biologics use. Biologics outcomes for the two management groups. Top, cumulative use of biologics through the five-year follow-up; bottom, cumulative incidence of biologics prescription. Results are stratified horizontally by diagnosed location: ALL, all diagnosed sites; L1, L3, and L4, disease location according to the Vienna classification. Note that L2 patients were excluded due to the low count.
The reliance on surgery in the SOC and CE groups is summarised in Figure 2. Except for L1 diagnoses, the CE group apparently underwent less frequent surgery than SOC (67.3% in SOC, 53.7% in CE at five years). This effect was most distinct in L4 patients (88.1% in SOC, 53.4% in CE at year five). The course of symptoms (expressed as CDAI) between arms was compared depending on symptoms at baseline: Asymptomatic or mildly symptomatic patients at baseline were compared in terms of occurrence and duration of flares (Figure 3), whereas moderate-to-severe patients were compared regarding time in remission. This subdivision of the groups further thinned the numbers, making the comparison by diagnostic site impracticable. The total time in flare was comparable between the SOC and CE groups (34 patient years SOC vs. 33 patient years CE), as was the progression of flare incidence. Towards the follow-up conclusion, there was only a modest difference in the proportion of patients who had ever experienced a flare after a period of moderate or no symptoms (55.6% in SOC, 48.1% in CE). Patients in the CE group experienced a more prolonged cumulative remission than the SOC group (34.5 vs. 28.0 patient years). After the first year, CE patients achieved their first remission sooner. However, an equivalent fraction of patients had achieved at least one remission in both groups by the end of follow-up (68.4% CE, 65.2% SOC).
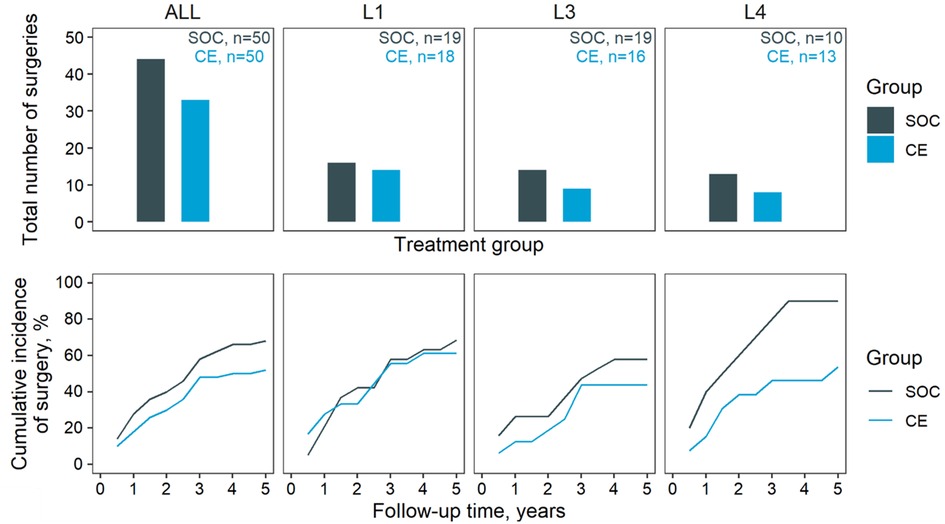
Figure 2. Surgery. Occurrence of surgery in the two groups. Top, cumulative number of surgeries through the five-year follow-up; bottom, cumulative incidence of surgery. Results are stratified horizontally by diagnosed location: ALL, all diagnosed sites; L1, L3, and L4 disease location according to the Vienna classification. Note that L2 patients were excluded due to the low count.
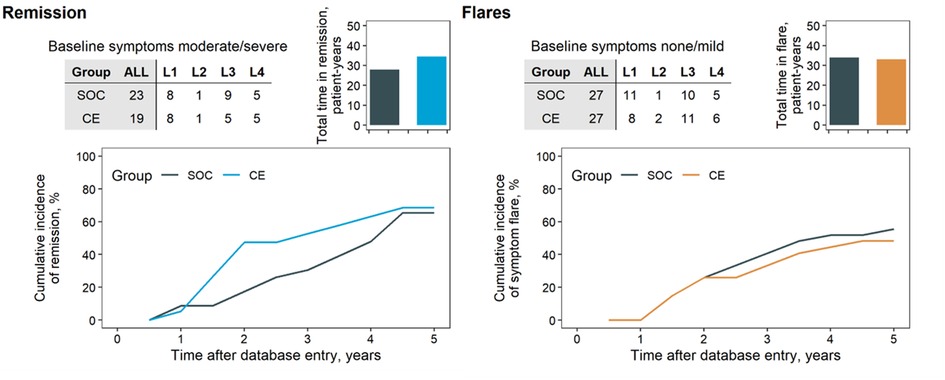
Figure 3. Cumulative incidence of remission and flares. Patients’ remission (top) and flare (bottom) analyses. Patients were divided by CDAI at baseline in moderate/severe symptoms at baseline for analysis of remission outcomes (top) and those with no or mild symptoms for analysis of flares (bottom). Analyses were not stratified per disease location because of the limited number of patients (tables, inset). Shaded columns indicate the total in each group in the analysis—four patients from the CE group due to missing CDAI score. Remission was defined as patients’ experiencing at least two consecutive intervals of no symptoms (CDAI score < 150). Flare was defined as the transition from a period of at least two consecutive intervals of no or mild symptoms (CDAI < 220) to at least one interval of moderate (CDAI score ≥ 220) or severe (CDAI score ≥ 450).
Capsule endoscopy has entered the field of IBD diagnoses and offers a competitive alternative to traditional IC/MRE imaging. Beyond indicators of increased sensitivity, technical performance, and improved patient acceptance, all of which are not to be overlooked, we questioned whether CE diagnostics alone brings tangible benefits to clinical outcomes. A substantial equivalence emerged between the two diagnostic approaches in the continued surveillance of CD patients. In general, neither the treatment choice nor the prognosis appeared to depend on the diagnostic approach. Nonetheless, despite the restrictions imposed by the unicentric, retrospective nature of the study and the small population size, some tendencies worthy of future investigations emerged. A preliminary, qualitative consideration concerns the apparent dichotomy in the course of biologics use among L4 patients. Although biologics were initiated at approximately the same time in the two arms, the cumulative incidence of biologics progressed slower among L4 patients in the CE group from the first year of follow-up. A similar reduction in the overall incidence of surgery is observed among L4 patients in the CE group throughout follow-up. A hypothesis to explain the decreased use of biologics and surgery in the CE group, notably among L4 patients, could be a superior clinical efficacy of CE in continued vigilance, i.e., CE might allow for a prompter and more flexible therapy remodulation (28), in particular in the medium-to-long term management of upper GI lesions. However, the data at hand and its limitations in statistical significance do not allow for numerically elaborating nor corroborating this observation's determinants nor discussing the role of cofounders, such as a history of flare-ups or smoking. The absence of upper GI endoscopies, as recommended by ECCO-ESGAR guidelines in 2018 for patients with CD with upper GI symptoms (44), may constitute an additional limitation of this study.
Any definitive conclusions necessitate a larger sample. Nonetheless, this hypothesis is in line with our recent experience in the proactive use of CE in CD patient management, including active CD (31) and evidence of proximal bowel CD as a poor prognostic factor for therapeutic escalation (45, 46). Ultimately, this analysis does not consider aspects for a comprehensive evaluation of the two techniques from the healthcare standpoint. Policymaking is heavily reliant on patient acceptance, procedural simplification and, not least, monetary considerations. While the literature supports the general findings on the clinical benefits of CE in the local context (28, 46, 47), the monetary aspects have yet to be quantified within the standards of the Italian healthcare system. In conclusion, we interpret these data qualitatively, wary of the existing constraints, and urge additional multicentre studies in Italy.
In this retrospective, matched cohort analysis, we observed a reduced use of biologics and surgery in patients with L4 disease in the CE group. In addition, the CE group also showed greater incidence and duration of remission independent of disease location for patients symptomatic at baseline. More extensive, prospective, multicentre, randomised studies are required to corroborate these preliminary findings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Sant'Orsola-Malpighi Hospital Ethics Committee approbation number 173/2017/O/OssN. The patients/participants provided their written informed consent to participate in this study.
CC, DG, JD, and RS designed research and wrote the paper; CC, JD, RS, and DG performed research; JD, RS, and RTT analysed data; CC and DG patient data extraction; CC, DG, PG, and FR patient management and database entry; PG and FR, supervision of the paper. All authors contributed to the article and approved the submitted version.
CC, DG, PG, and FR report no conflicts of interest. JD was employed at Coreva Scientific at the time the analysis was conducted. RTT is an employee, and RS is the owner of Coreva Scientific GmbH & Co KG, which has previously received consultancy fees from the manufacturer of a CE product.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's Disease. Dis Mon. (2018) 64:20–57. doi: 10.1016/j.disamonth.2017.07.001
2. Di Domenicantonio R, Cappai G, Arcà M, Agabiti N, Kohn A, Vernia P, et al. Occurrence of inflammatory bowel disease in central Italy: a study based on health information systems. Dig Liver Dis. (2014) 46:777–82. doi: 10.1016/j.dld.2014.04.014
3. Crocetti E, Bergamaschi W, Russo AG. Population-based incidence and prevalence of inflammatory bowel diseases in Milan (Northern Italy), and estimates for Italy. Eur J Gastroenterol Hepatol. (2021) 33:e383–9. doi: 10.1097/MEG.0000000000002107
4. Meregaglia M, Banks H, Fattore G. Hospital burden and gastrointestinal surgery in inflammatory bowel disease patients in Italy: a retrospective observational study. J Crohns Colitis. (2015) 9:853–62. doi: 10.1093/ecco-jcc/jjv104
5. Benedini V, Caporaso N, Corazza GR, Rossi Z, Fornaciari G, Cottone M, et al. Burden of Crohn's Disease: economics and quality of life aspects in Italy. Clinicoecon Outcomes Res. (2012) 4:209–18. doi: 10.2147/CEOR.S31114
6. Capri S, Russo A. I costi dei pazienti con patologie infiammatorie dell’intestino (Malattia di Crohn e colite ulcerosa). Studio real world in Italia. Global & Regional Health Technology Assessment. (2018) 2018:228424031879328. doi: 10.1177/2284240318793281
7. Mozzi A, Meregaglia M, Lazzaro C, Tornatore V, Belfiglio M, Fattore G. A comparison of EuroQol 5-dimension health-related utilities using Italian, UK, and US preference weights in a patient sample. Clinicoecon Outcomes Res. (2016) 8:267–74. doi: 10.2147/CEOR.S98226
8. Iacucci M, Ghosh S. Looking beyond symptom relief: evolution of mucosal healing in inflammatory bowel disease. Therap Adv Gastroenterol. (2011) 4:129–43. doi: 10.1177/1756283X11398930
9. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO Guidelines on therapeutics in Crohn's Disease: medical treatment. J Crohns Colitis. (2020) 14:4–22. doi: 10.1093/ecco-jcc/jjz180
10. Cushing K, Higgins PD. Management of crohn disease: a review. JAMA. (2021) 325:69–80. doi: 10.1001/jama.2020.18936
11. Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical guideline: management of Crohn's Disease in adults. Am J Gastroenterol. (2018) 113:481–517. doi: 10.1038/ajg.2018.27
12. Watanabe K. Clinical management for small bowel of Crohn's Disease in the treat-to-target era: now is the time to optimize treatment based on the dominant lesion. Intest Res. (2020) 18:347–54. doi: 10.5217/ir.2020.00032
13. Hart AL, Lomer M, Verjee A, Kemp K, Faiz O, Daly A, et al. What are the top 10 research questions in the treatment of inflammatory bowel disease? A priority setting partnership with the james lind alliance. J Crohns Colitis. (2017) 11:204–11. doi: 10.1093/ecco-jcc/jjw144
14. Colombel J-F, Panaccione R, Bossuyt P, Lukas M, Baert F, Vaňásek T, et al. Effect of tight control management on Crohn's Disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. (2017) 390:2779–89. doi: 10.1016/S0140-6736(17)32641-7
15. Khanna R, Bressler B, Levesque BG, Zou G, Stitt LW, Greenberg GR, et al. Early combined immunosuppression for the management of Crohn's Disease (REACT): a cluster randomised controlled trial. Lancet. (2015) 386:1825–34. doi: 10.1016/S0140-6736(15)00068-9
16. Colombel J-F, D'Haens G, Lee W-J, Petersson J, Panaccione R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. (2020) 14:254–66. doi: 10.1093/ecco-jcc/jjz131
17. Ye L, Cheng W, Chen B-Q, Lan X, Wang S-D, Wu X-C, et al. Levels of faecal calprotectin and magnetic resonance enterocolonography correlate with severity of small bowel Crohn's Disease: a retrospective cohort study. Sci Rep. (2017) 7:1970. doi: 10.1038/s41598-017-02111-6
18. Kopylov U, Rosenfeld G, Bressler B, Seidman E. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis. (2014) 20:742–56. doi: 10.1097/01.MIB.0000442681.85545.31
19. Burri E, Beglinger C, Lehmann FS. Monitoring of therapy for inflammatory bowel disease. Digestion. (2012) 86(Suppl 1):1–5. doi: 10.1159/000341953
20. Laharie D, Mesli S, El Hajbi F, Chabrun E, Chanteloup E, Capdepont M, et al. Prediction of Crohn's Disease relapse with faecal calprotectin in infliximab responders: a prospective study. Aliment Pharmacol Ther. (2011) 34:462–9. doi: 10.1111/j.1365-2036.2011.04743.x
21. Koulaouzidis A, Douglas S, Rogers MA, Arnott ID, Plevris JN. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. (2011) 46:561–6. doi: 10.3109/00365521.2011.551835
22. Fidder HH LA. Bowel imaging in IBD patients: review of the literature and current recommendations. J Gastrointest Dig Syst. (2014) 04:1–6. doi: 10.4172/2161-069X.1000189
23. Rondonotti E, Spada C, Adler S, May A, Despott EJ, Koulaouzidis A, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: european society of gastrointestinal endoscopy (ESGE) technical review. Endoscopy. (2018) 50:423–46. doi: 10.1055/a-0576-0566
24. Cortegoso Valdivia P, Skonieczna-Żydecka K, Elosua A, Sciberras M, Piccirelli S, Rullan M, et al. Indications, detection, completion and retention rates of capsule endoscopy in two decades of use: a systematic review and meta-analysis. Diagnostics (Basel). (2022) 12:1005–17. doi: 10.3390/diagnostics12051105
25. Buisson A, Gonzalez F, Poullenot F, Nancey S, Sollellis E, Fumery M, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. (2017) 23:1425–33. doi: 10.1097/MIB.0000000000001140
26. Spiceland CM, Lodhia N. Endoscopy in inflammatory bowel disease: role in diagnosis, management, and treatment. World J Gastroenterol. (2018) 24:4014–20. doi: 10.3748/wjg.v24.i35.4014
27. Leighton JA, Helper DJ, Gralnek IM, Dotan I, Fernandez-Urien I, Lahat A, et al. Comparing diagnostic yield of a novel pan-enteric video capsule endoscope with ileocolonoscopy in patients with active Crohn's Disease: a feasibility study. Gastrointest Endosc. (2017) 85:196–205.e1. doi: 10.1016/j.gie.2016.09.009
28. Bruining DH, Oliva S, Fleisher MR, Fischer M, Fletcher JG. Panenteric capsule endoscopy versus ileocolonoscopy plus magnetic resonance enterography in Crohn's Disease: a multicentre, prospective study. BMJ Open Gastroenterol. (2020) 7. doi: 10.1136/bmjgast-2019-000365
29. González-Suárez B, Rodriguez S, Ricart E, Ordás I, Rimola J, Díaz-González Á, et al. Comparison of capsule endoscopy and magnetic resonance enterography for the assessment of small bowel lesions in Crohn's Disease. Inflamm Bowel Dis. (2018) 24:775–80. doi: 10.1093/ibd/izx107
30. Leighton JA, Gralnek IM, Cohen SA, Toth E, Cave DR, Wolf DC, et al. Capsule endoscopy is superior to small-bowel follow-through and equivalent to ileocolonoscopy in suspected Crohn's Disease. Clin Gastroenterol Hepatol. (2014) 12:609–15. doi: 10.1016/j.cgh.2013.09.028
31. Calabrese C, Diegoli M, Dussias N, Salice M, Rizzello F, Cappelli A, et al. Performance of capsule endoscopy and cross-sectional techniques in detecting small bowel lesions in patients with Crohn's Disease. Crohn's Colitis 360. (2020) 2:otaa046. doi: 10.1093/crocol/otaa046
32. Lahat A, Veisman I. Capsule endoscopy in Crohn's Disease-From a relative contraindication to habitual monitoring tool. Diagnostics (Basel). (2021) 11:1737–49. doi: 10.3390/diagnostics11101737
33. Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn's Disease: a meta-analysis. Am J Gastroenterol. (2010) 105:1240–8; quiz 1249. doi: 10.1038/ajg.2009.713
34. Choi M, Lim S, Choi M-G, Shim K-N, Lee SH. Effectiveness of capsule endoscopy compared with other diagnostic modalities in patients with small bowel Crohn's Disease: a meta-analysis. Gut Liver. (2017) 11:62–72. doi: 10.5009/gnl16015
35. Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn's Disease. Off J Am College Gastroenterol. (2006) 101:954–64. doi: 10.1111/j.1572-0241.2006.00506.x
36. Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn's Disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis. (2014) 8:1610–5. doi: 10.1016/j.crohns.2014.02.018
37. Dorrington AM, Selinger CP, Parkes GC, Smith M, Pollok RC, Raine T. The historical role and contemporary use of corticosteroids in inflammatory bowel disease. J Crohns Colitis. (2020) 14:1316–29. doi: 10.1093/ecco-jcc/jjaa053
38. Samaan M, Campbell S, Cunningham G, Tamilarasan AG, Irving PM, McCartney S. Biologic therapies for Crohn's Disease: optimising the old and maximising the new. F1000Res. (2019) 8. doi: 10.12688/f1000research.18902.1
39. Kavalukas SL, Scheurlen KM, Galandiuk S. State-of-the-art surgery for Crohn's Disease: part I-small intestine/ileal disease. Langenbeck's Arch Surg. (2022) 407:885–95. doi: 10.1007/s00423-021-02324-4
40. Macleod A, Kavalukas SL, Scheurlen KM, Galandiuk S. State-of-the-art surgery for Crohn's Disease: part II-colonic Crohn's Disease and associated neoplasms. Langenbeck's Arch Surg. (2022) 407:2595–605. doi: 10.1007/s00423-022-02572-y
41. Gasche C, Scholmerich J, Brynskov J, D'Haens G, Hanauer SB, Irvine EJ, et al. A simple classification of Crohn's Disease: report of the working party for the world congresses of gastroenterology, Vienna 1998. Inflamm Bowel Dis. (2000) 6:8–15. doi: 10.1097/00054725-200002000-00002
42. Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. (2008) 27:146–54. doi: 10.1111/j.1365-2036.2007.03556.x
43. Rosa B, Moreira MJ, Rebelo A, Cotter J. Lewis score: a useful clinical tool for patients with suspected Crohn's Disease submitted to capsule endoscopy. J Crohns Colitis. (2012) 6:692–7. doi: 10.1016/j.crohns.2011.12.002
44. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO-ESGAR Guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. (2019) 13:144–64. doi: 10.1093/ecco-jcc/jjy113
45. Lazarev M, Huang C, Bitton A, Cho JH, Duerr RH, McGovern DP, et al. Relationship between proximal Crohn's Disease location and disease behavior and surgery: a cross-sectional study of the IBD genetics consortium. Am J Gastroenterol. (2013) 108:106–12. doi: 10.1038/ajg.2012.389
46. Tai FW, Ellul P, Elosua A, Fernandez-Urien I, Tontini GE, Elli L, et al. Panenteric capsule endoscopy identifies proximal small bowel disease guiding upstaging and treatment intensification in Crohn's Disease: a European multicentre observational cohort study. United European Gastroenterol J. (2021) 9:248–55. doi: 10.1177/2050640620948664
Keywords: crohn's disease, capsule endoscopy, biological treatment, symptomatology, outcome, real-world data, retrospective analysis
Citation: Calabrese C, Gelli D, Rizzello F, Gionchetti P, Torrejon Torres R, Saunders R and Davis J (2022) Capsule endoscopy in Crohn's disease surveillance: A monocentric, retrospective analysis in Italy. Front. Med. Technol. 4:1038087. doi: 10.3389/fmedt.2022.1038087
Received: 6 September 2022; Accepted: 11 November 2022;
Published: 28 November 2022.
Edited by:
Rubina Shaikh, TU Dublin, IrelandReviewed by:
Amir Ahari, University of California, Los Angeles, United States© 2022 Calabrese, Gelli, Rizzello, Gionchetti, Torrejon Torres, Saunders and Davis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rhodri Saunders cnNAY29yZXZhLXNjaWVudGlmaWMuY29t
Specialty Section: This article was submitted to Diagnostic and Therapeutic Devices, a section of the journal Frontiers in Medical Technology
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.