- 1The Vaccine and Immunotherapy Center, Wistar Institute, Philadelphia, PA, United States
- 2Department of Pharmacology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
DNA vaccines are considered as a third-generation vaccination approach in which antigenic materials are encoded as DNA plasmids for direct in vivo production to elicit adaptive immunity. As compared to other platforms, DNA vaccination is considered to have a strong safety profile, as DNA plasmids neither replicate nor elicit vector-directed immune responses in hosts. While earlier work found the immune responses induced by DNA vaccines to be sub-optimal in larger mammals and humans, recent developments in key synthetic DNA and electroporation delivery technologies have now allowed DNA vaccines to elicit significantly more potent and consistent responses in several clinical studies. This paper will review findings from the recent clinical and preclinical studies on DNA vaccines targeting emerging infectious diseases (EID) including COVID-19 caused by the SARS-CoV-2 virus, and the technological advancements pivotal to the improved responses—including the use of the advanced delivery technology, DNA-encoded cytokine/mucosal adjuvants, and innovative concepts in immunogen design. With continuous advancement over the past three decades, the DNA approach is now poised to develop vaccines against COVID-19, as well as other EIDs.
Introduction
Vaccination is an extremely important approach that has impacted global health for the past centuries (1). DNA vaccines are considered as a third-generation vaccine approach that was first brought to the attention of scientific community in the early 1990s (2). As compared to vaccination approaches (recombinant proteins and viral vector), DNA plasmids encoding antigen transgenes can be rapidly and cost-efficiently manufactured (3, 4). Simple DNA plasmids do not provoke antigen specific immunity against the DNA backbone, enabling vaccine boosting in the same individuals with the same plasmid vector, and focusing the host immunity on the transgene (5). Synthetic DNA is highly stable, thereby obviating the need for cold chain transport or storage and facilitating global deployment of the vaccines during outbreaks (3, 6).
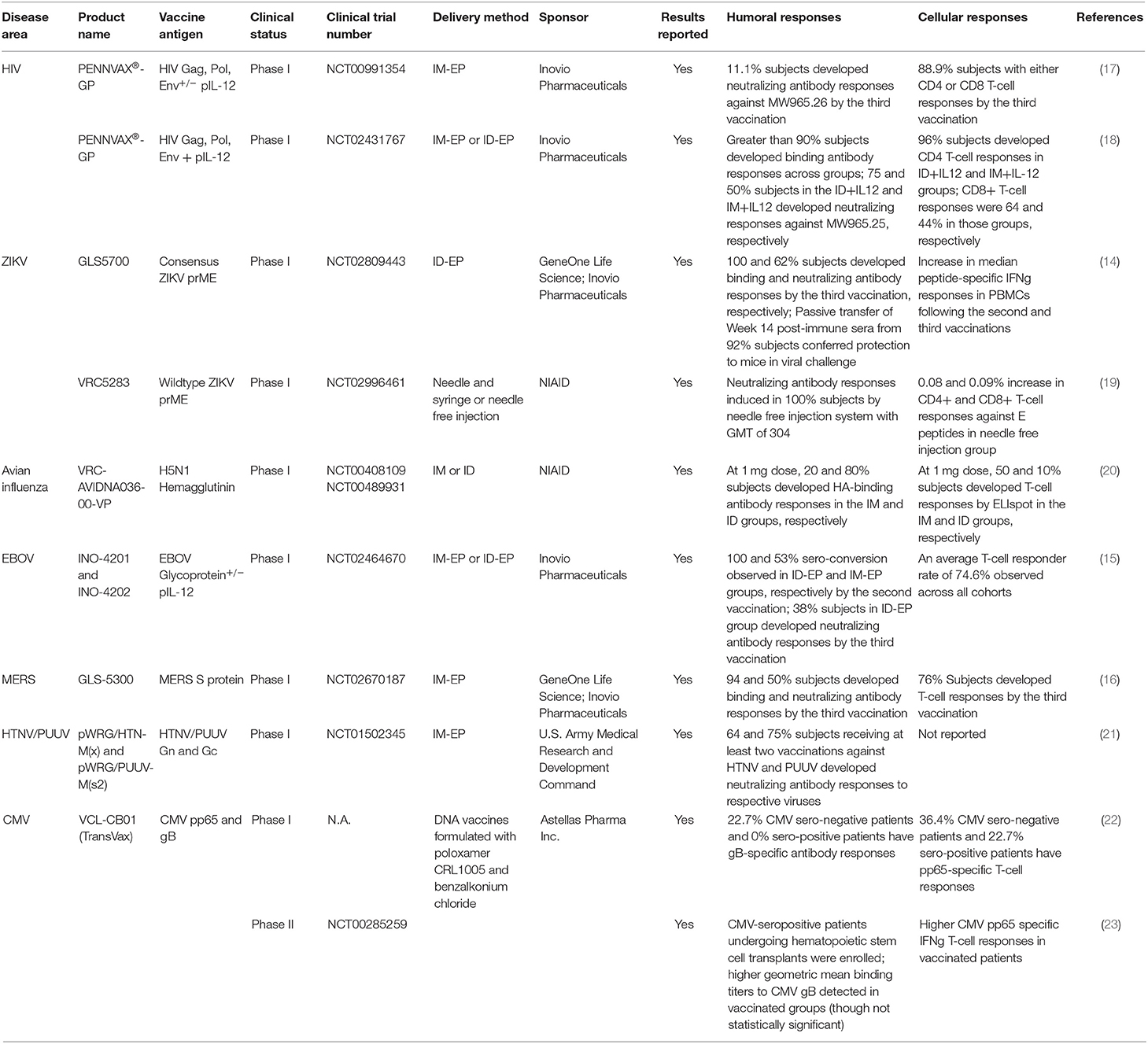
First-generation DNA vaccines had limited immunogenicity in larger mammals (NHPs) and in humans, impeding early enthusiasm for this approach (7, 8). Since these early studies, several strategies have been attempted to improve the overall immunogenicity of DNA vaccines. One such strategy is the co-delivery of DNA-vaccine with DNA-encoded cytokine adjuvant (such as IL-12) to enhance co-stimulation of the antigen-presenting cells (APCs) (9). Another important approach was the delivery of DNA vaccines alongside with adaptive electroporation (10, 11). Membrane electrochemical permeabilization and electric field created by applied voltages can significantly improve uptake of DNA plasmids into the transfected cells, improving transfection efficiency by up to 1,000-fold (12, 13). DNA vaccines, in conjunction with the newer adaptive electroporation technologies, have now been observed to induce more potent and consistent responses in a series of clinical studies against Zika, Ebola, MERS and HIV-1 [(14–17); Table 1].
As with other vaccine platforms, routes of immunization can significantly impact the immunogenicity and tolerability profiles of vaccines (24). Additional advances have been reported in the latest intradermal electroporation (ID-EP) DNA delivery technology (25). As compared to conventional DNA delivery by intramuscular electroporation (IM-EP), ID-EP was observed to be well-tolerated and dose-sparing, especially in the induction of humoral immune responses (15, 26). Additionally, there has also been a recent demonstration that DNA/EP can enable direct in vivo production of more complex and potent nanoparticle vaccines to elicit rapid sero-conversion and more potent immune responses in animal models (27). With 30 years of development, significant technological advancements have accrued in the field of DNA vaccinology, making it an attractive approach in our efforts to develop vaccines against COVID-19 and other EIDs at the pandemic speed.
DNA Vaccines Against EID
Between 1940 and 2004, over 300 new pathogens have been discovered, the majority of which originated from animals and were transmitted to humans by disease vectors (insects, birds and rodents) (28). Several of these, including Zika and SARS, caused regional epidemics or global pandemics, highlighting how the spread of EIDs can be compounded by global travel (29). Nucleic acid-based vaccinations (DNA and mRNA vaccines) represent an elegant approach for rapid development of vaccines against EID (30, 31). Unlike other vaccine platforms (such as protein or viral-vectored vaccines), nucleic acid-based vaccines do not require prior knowledge on production and purification of the vaccine antigens (3). Production of DNA plasmids, in particular, is relatively cost effective and straightforward, and does not require specialized pipelines (32). In theory, a vaccine can be rapidly designed so long as the protein sequences of the vaccine targets are known. Additionally, owning to the relative lack of plasmid size restriction, several vaccine antigens might be simultaneously encoded in a single DNA plasmid for explorative studies, even if the disease target is not clearly identified (33). DNA vaccines, being capable of inducing both antibody and CD8+ T-cell responses (4), may represent an attractive strategy to prevent disease transmission or improve patient clinical outcomes depending on the goals (34–36). This section will briefly review the clinical data for DNA vaccines against Ebola virus (EBOV), Zika virus (ZIKV), and Venezuelan equine encephalitis virus (VEEV), and also preclinical data for a DNA vaccine against Lassa Virus (LASV).
EBOV is the causative agent for Ebola Virus Disease (EVD), in which subjects first develop mild symptoms including fever, dysphagia, myalgia, nausea, and emesis, followed by more severe symptoms including bleeding from orifices and fulminant hepatic and renal failure, with a median mortality rate of around 50% (37). Several vaccine candidates have been developed to target EVD, including the adenovirus- and protein-based vaccine candidates, as well as FDA-approved ERVEBO-vaccine from Merck & Co, which consists of Vesicular Stomatitis Virus (VSV) live-attenuated vector modified to express EBOV glycoproteins (GP) (38). However, adverse effects such as arthralgia, lymphopenia, neutropenia related to the vector backbones have been reported in some vaccinees in early trials (39). In the DNA space, Patel et al. reported that a DNA-encoded synthetic consensus (SynCon) vaccine against EBOV GP could confer rapid protection following a single vaccination in mice from a heterologous mouse-adapted challenge strain (40). In NHPs, a two-dose dose-sparing ID SynCon DNA vaccine regimen conferred 100% protection from challenge, inducing durable responses in the animals a year after the final vaccination (40). In a first-in-human (FIH) DNA vaccine study, the aforementioned SynCon EBOLA GP vaccine (INO-4201) as well as another DNA vaccine encoding EBOV-GP from a 2014 outbreak Zaire Makona strain (INO-4202) were given individually, or in combination along with a DNA-encoded human IL-12 adjuvant in a three-dose regimen delivered by IM- or ID-EP (15). Robust antibody responses were induced in every arm. In particular, the ID-EP delivery of INO-4201 induced extremely rapid sero-conversion, with 100% sero-activity observed in all participants by the second vaccination. T-cell responses were observed in 70% of participants overall. Cytokine responses were detected in peripheral blood CD8+ and CD4+ T-cells, especially in subjects receiving ID DNA vaccination. Durable responses were observed in most participants, with a Geometric Mean Titer (GMT) of 42 in the ID INO-4201 group 48 weeks after the final vaccination (15).
ZIKV is a mosquito-borne illness for which patients can present with fever, malaise, rash and conjunctivitis. Additionally, ZIKV infection in pregnant women can cause significant birth defects including microcephaly and in men can cause testicular atrophy (41, 42). In preclinical studies, DNA vaccination with ZIKV pre-membrane + envelope proteins (prME) induced robust humoral and cellular responses in both mice and non-human primates (NHPs) (43, 44). In a study where mice were vaccinated intramuscularly with a ZIKV prME DNA vaccine without EP, both antibody and T-cell responses were induced, though the magnitudes were lower than those induced by a viral vectored ChAdOX1 vaccine (45). IM-EP-mediated vaccination of a different ZIKV DNA vaccine (GLS-5700) in IFNAR-/- mice decreased brain viral load and protected them from weight loss following lethal ZIKV challenges. Passive transfer of sera from GLS-5700 vaccinated NHPs similarly protected the IFNAR-/- mice in challenges (44). In a separate NHP study, two-dose intramuscular DNA vaccination of wildtype ZIKV prME (VRC5283) induced potent binding and neutralizing antibodies at both 1 and 4 mg doses and conferred complete protection to macaques from subsequent ZIKV challenges (43). Promising results have also been observed in several clinical ZIKV DNA vaccine studies. Tebas et al. reported that the three-dose ID DNA vaccination of GLS-5700 was safe and effectively elicited binding antibody responses in 100% of participants and 63% neutralizing antibody responses by the third vaccination (14). Passive transfer of post-immune sera from the participants to IFNAR-/- mice effectively protected them from lethal ZIKV challenges. T-cell responses were also induced in the subjects, particularly in subjects receiving the higher dose (2.0 mg), with a median IFNγ ELIspot counts of 58 per million PBMCs at 2 weeks post the second vaccination (as compared to 0 spots per million PBMCs observed at baseline) (14). In a separate Phase I DNA vaccine trial, participants received a three-dose DNA vaccine regimen at 4 mg dose against wildtype ZIKV prME (VRC5283) via a needle-free injection system. Sero-conversion was observed in 100% participants by the third vaccination with a neutralization GMT of 304. Relative to baseline, there was an increase in cytokine responses by 0.08 and 0.09% in peripheral blood CD4+ and CD8+ T cell, respectively (19).
VEEV is a mosquito-borne alphavirus that can cause febrile illness and progressive encephalitis in both equines and humans. Currently, there is no FDA-approved vaccine or immunotherapy against VEEV (46). Hannaman et al. reported a Phase I DNA vaccine encoding the VEEV E3-E2-6K-E1 genes (47). Subjects received a three-dose DNA vaccine regimen delivered by either IM- or ID-EP with the Ichor Delivery System. The vaccine was safe and well-tolerated. Neutralizing antibody responses were observed in all volunteers receiving the IM DNA vaccine, and 87.5 and 62.5% subjects receiving the high dose or low dose ID DNA vaccine respectively, even though the dosage used in the ID vaccinations were lower than those in the IM vaccinations (DNA doses of 0.08 or 0.3 mg for ID vs. 0.5 or 2.0 mg for IM). In this small study, robust and durable neutralizing antibody responses were observed in the high dose IM group a year after the initial vaccination (47).
There are additional preclinical studies describing the design and evaluation of DNA vaccines against other EIDs. Lassa fever, caused by LASV, is endemic in West Africa and patients can present with hemorrhages from orifices, respiratory distress and shock, and is associated with a significant mortality rate of 80% (48). Jiang et al. reported that DNA vaccines against LASV glycoprotein precursor gene, generated robust humoral and cellular immunity in both guinea pigs and NHPs and completely protected them from viremia, clinical disease, and death following lethal LASV challenges (26).
DNA Vaccines Against SARS-CoV and MERS-CoV
Coronaviruses are a group of enveloped positive-sense RNA viruses that can cause mild to severe respiratory infections in mammals and birds (49). While they are used to be associated with milder infections such as the common cold, three variants- Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and SARS-CoV-2- are associated significant morbidity and mortality in the infected individuals, causing major regional epidemics or global pandemics in the twenty-first century (50). While several vaccine candidates appear promising in preclinical animal studies and are currently being evaluated in the early phase clinical trials, there is to date no approved vaccine against these coronaviruses, highlighting the urgent need for rapid and successful development of effective vaccines to mitigate the global outbreaks (51).
SARS-CoV is the virus responsible for the SARS outbreak which originated in Guangdong, China, and subsequently spread globally to affect countries in Southeast Asia, North and South America, and Europe, with a cumulative case counts of at least 8,000 and a global death toll of at least 774 (52, 53). The virus was transmitted zoonotically from a civet cat to a human (54). Following the outbreak in 2002-2003, a preclinical SARS-CoV DNA vaccine was reported in 2004. DNA vaccine against SARS-CoV S protein administered intramuscularly by needle and syringe induced both neutralizing antibody and also T-cell responses in mice (34). Vaccinated mice had six orders of magnitudes lower lung viral load following intranasal challenge. T-cell depletion and serum passive transfer studies demonstrated that protection against SARS-CoV in vaccinated mice depended only on serum antibodies but not on T cells (34). This vaccine candidate was evaluated in a Phase I trial between 2004 and 2005 (55). Subjects received a three-dose IM DNA vaccine regimen at the 4 mg dose. Binding and neutralizing antibody responses were detected in 80 and 50% of vaccinated individuals, respectively. CD4+ T-cell responses were detected in all vaccinees, whereas CD8+ T-cell responses were detected in 20% of subjects. This study showed 30% vaccinees remained sero-positive 24 weeks following the final vaccination.
MERS-CoV was responsible for the local outbreak of MERS in the Arabian Peninsula in 2012, and the local epidemic in Seoul, South Korea, in 2015 (56). MERS-CoV is considered as a zoonotic virus with a mortality rate of 35%, transmitted originally from an infected camel to a human (57). Muthumani et al. described the development of DNA vaccine encoding MERS spike protein, which induced potent humoral and cellular responses in mice, camels and NHPs. NHPs receiving the IM DNA vaccines were significantly protected in the subsequent MERS challenge, having lower viral load, fewer clinical symptoms and lung pathology as compared to the control macaques. Importantly upon challenge, histological examinations did not reveal any signs of disease enhancement in macaques receiving either high or low dose DNA vaccines (58). This DNA vaccine (GLS-5300) was subsequently evaluated in a Phase I dose-escalation study. Subjects received three IM vaccinations at the low (0.67 mg), medium (2.0 mg), and high (6.0 mg) doses (16). The vaccines were observed to be safe and well-tolerated, and induced sero-conversion in 94%, neutralizing antibody responses in 50%, and T-cell responses in 50% individuals by the third vaccination. Importantly, 48 weeks post the final vaccination, humoral, and cellular responses were still observed in 77 and 64% of subjects, respectively (16).
Developing DNA Vaccines Against SARS-CoV-2
Overview of SARS-CoV-2
SARS-CoV-2 is the virus responsible for causing COVID-19, which swept across the globe to affect over 200 countries between 2019 and 2020, and is associated with more than 13 million cases globally and a death toll over 550,000 as of July 13th, 2020 (59, 60). Similar to SARS-CoV, SARS-CoV-2 gains entry into the target cells through viral S protein and host ACE-2 interaction. Priming of the viral S protein through proteolytic cleavage with host serine protease TMPRSS2 is also known to be important for viral entry (61, 62). ACE-2 is known to be abundantly expressed in many tissues, including small intestines, kidneys, and cardiac muscles (63). Clinically, COVID-19 patients can present with cardiac failure, hepatic and renal failure, as well as acute respiratory distress syndrome, and have an estimated disease mortality rate of approximately 1.3% (64). Other than significant morbidity and mortality associated with the global pandemic, efforts to mitigate the virus, including social distancing and lockdown, have significantly disturbed the global economic activity (65). There is an urgent need to develop effective and sustainable approaches to contain the global spread of the virus.
Vaccine Strategies Against SARS-CoV-2
Development of effective vaccines against SARS-CoV-2 is considered to be one of the most important mitigation measures (51). In the NHP model, prior infection has been observed to protect animals from subsequent SARS-CoV-2 viral exposure (66, 67). Several vaccine strategies against SARS-CoV-2, including RNA vaccines (68–71), DNA vaccines (72, 73), inactivated virus vaccines (74), viral vectored vaccines (75), and recombinant protein vaccines (76), are concurrently being explored and are at different stages of clinical development (77). Most vaccine candidates target the SARS-CoV-2 spike (S) protein (78). The mRNA vaccine candidate mRNA-1273, in particular, has demonstrated protective efficacy in mice challenged with mouse-adapted SARS-CoV-2 virus (68) and has also been shown to induce consistent binding and neutralizing antibody responses in vaccinated individuals (70); whereas the inactivated vaccine PiCoVacc and viral-vectored vaccine ChAdOx1 nCoV-19 demonstrated complete and partial protection in rhesus macaques challenged with SARS-CoV-2 (74, 76). Importantly, while antibody-dependent enhancement (ADE), a process in which non-neutralizing antibodies induced in a host from vaccination or prior infection subsequently exacerbate viral infection in the host from a second exposure, had been observed in macaques vaccinated with a MVA-vectored SARS-CoV vaccine and challenged with SARS-CoV (79), it had not been observed in PiCoVacc or ChAdOx1 nCoV-19 vaccinated macaques that were subsequently challenged with SARS-CoV-2 (74, 76). At present, there are no known clinical findings that differentiate severe viral infections from immune-enhanced diseases (80, 81).
In terms of immune correlates of protection, neutralizing antibodies (NAbs) are considered important in preventing transmission of SARS-CoV-2. For example, passive transfer of a human SARS-CoV-2 neutralizing antibody from a convalescent patient into Syrian hamsters was observed to confer protection from SARS-CoV-2 exposure in a dose-dependent manner (82). Increasingly, however, there is an appreciation of the importance of cellular immunity in the resolution of SARS-CoV-2 infection. Two patients with agammaglobulinemia, for example, successfully recovered from COVID-19 (83). Additionally, other serological surveys of convalescent COVID-19 patients demonstrate that there is a surprisingly large proportion of recovered patients without robust neutralizing antibody titers during the early convalescent phase (84). Additionally, unexpectedly, higher titers of neutralizing antibodies were observed in patients with more severe disease (85). In critically ill patients, it was observed that CD4+ T-cell responses were relatively impaired, while IgG antibody responses were surprisingly robust (86). These studies highlight the importance of developing vaccine approaches that can induce both humoral and cellular immunity, and the need for continuous ongoing efforts to monitor the vaccine safety profiles during the current pandemic.
DNA Vaccines Against SARS-CoV-2 Currently Under Development
Recently, Smith et al. reported rapid development of a DNA vaccine candidate (INO-4800) against the SARS-CoV-2 S protein. The design of this vaccine candidate leveraged previous understanding of SARS-CoV and MERS-CoV S protein folding. INO-4800 induced robust binding and neutralizing antibody responses as well as antigen-specific T-cell responses in both mice and guinea pigs (72). The vaccine candidate also induced strong binding and neutralizing antibody responses as well as T-cell responses in an NHP study at both 1 and 2 mg doses (87). When the macaques were challenged 3 months post-vaccination, they developed significantly lower lung viral load and faster viral clearance in the nose as compared to control macaques. Additionally, vaccinated macaques were observed to have fast recall responses, in which binding and neutralizing antibody titers rise rapidly 7 days post-viral inoculation, as compared to control macaques (87). In a parallel study, Yu et al. demonstrated in an NHP challenge model that two intramuscular DNA vaccinations at 5 mg dose of variants of SARS-CoV-2 S protein induced binding and neutralizing antibody responses, CD4+ and CD8+ T-cell responses, and decreased viral shedding when macaques were challenged (73). Vaccine induced neutralizing antibodies at the time of challenge was observed to be strongly correlated with challenge protection. Of note, INO-4800 is currently being evaluated in a Phase I clinical study (NCT04336410) in which subjects received two EP-mediated ID DNA vaccinations at the low (1.0 mg) or regular (2.0 mg) doses in the US. Concurrently, the vaccine candidate will be evaluated in a Phase I/IIa study in the Republic of Korea (NCT04447781). Safety and immunogenicity data from this trial will provide valuable insights to understand and evaluate the DNA vaccine approach against SARS-CoV-2 during the current pandemic.
Latest Advancement in the DNA Vaccine Technology
The observation that DNA vaccines induce more potent and consistent responses in clinical trials can be attributed to the recent advances in synthetic DNA and electroporation technology. In fact, enhanced DNA/EP parameters have also allowed biologics to be delivered systematically to achieve potent and durable in vivo expression in animals (88–91). An DNA-encoded Monoclonal Antibody (DMAb) against Zika virus, in particular, is currently under clinical evaluation in a Phase I study (NCT03831503). The next section will highlight some additional recent developments in DNA vaccine technology.
DNA-Encoded Cytokine Adjuvants
Several studies have reported that incorporating DNA-encoded cytokines with DNA vaccines could adjuvant vaccine induced responses (6). Co-delivery of IL-2, for example, was observed to improve the immunogenicity of DNA vaccines against SARS-CoV S and N proteins, influenza H1N1 hemagglutinin and neuraminidase, and HIV gp120 and Nef (92–94). Co-delivery of DNA vaccine against liver-stage malaria antigens with IL-33 was found to improve liver-localized CD8+ T-cell responses and confer improved protection to mice from Plasmodium challenge (95). Co-administration of IL-36γ, on the other hand, was found to improve immune responses induced by DNA-encoded Zika, HIV and influenza vaccines, and reduced dose requirement of DNA vaccines in mice to protect against ZIKV challenge (96). Co-delivery of DNA vaccines with plasmid-encoded IL-12 (pIL-12) in particular has drawn significant interests recently. The importance of IL-12 in inducing Th1-biased immunity and augmenting CD8+ cytotoxic T-cell activity is well-established (97, 98). In preclinical studies, plasmid-encoded IL-12 was found to increase IFNγ+ T-cell responses induced by DNA vaccines against HIV, HCV, HSV-2, and Toxoplasma gondii (98–101). In a Phase I study (HVTN098), coformulation of PENNVAX®-GP with pIL-12 significantly increased the proportion CD4+ T-cell responders from 56 to 96% (18).
Targeted DNA Vaccine Delivery
Intracellular barriers can negatively impact expression and presentation of a DNA-encoded antigen (102). Approaches to enhance targeting of DNA-encoded transgene to the desired cellular compartments have been quite successfully explored. Given the observation that secreted antigens elicit enhanced antigen-specific antibody responses than cytosolic antigens in some studies (102), leader sequences have been designed and used to facilitate secretion of DNA-encoded antigens. The incorporation of a tissue plasminogen activator (TPA) leader sequence, for example, has been shown to enhance antibody responses, cellular responses, and protection against mycobacterial antigens (103), and also increase antibody responses to a major birch pollen antigen (104). The use of human IgE leader sequence has similarly been demonstrated to improve trafficking of a DNA-encoded transgene to the secretory network (88). In the scenarios where the induction of CD8+ T-cell responses are preferred, DNA vaccines are designed to encode antigens fused to ubiquitin (102). Ubiquitin fusion facilitates proteasomal turnover of the tagged antigens into epitope peptides and enhances targeting of the antigens to the MHC Class I pathway. This strategy has been explored for several disease targets, including LCMV, influenza and melanoma (105–107). All studies consistently demonstrated that ubiquitin-conjugation enhanced CTL responses. Interestingly, antibody responses were observed to simultaneously decrease (102, 106). In other scenarios where the induction of CD4+ T-cell responses and humoral responses are desired, DNA-encoded antigens are designed to incorporate a lysosomal targeting moiety, given the abundance of MHC Class II complexes in the cellular endosomal/lysosomal compartment (108). Linkage of antigen to Lysosomal Associated Membrane Protein type 1 (LAMP-1), for example, has been found to increase the DNA-vaccine induced neutralizing antibody responses against Dengue virus (109) and the binding antibody responses against West Nile virus (110). It can be envisioned that the immune responses induced by a SARS-CoV-2 DNA vaccine may be further tailored with the aforementioned approaches.
Intradermal vs. Intramuscular DNA Vaccination
In terms of DNA vaccine delivery, the ID-EP technology has recently drawn significant interests. This approach harnessed the biology of antigen presenting cells, enriched in human skin tissues, for the induction of immune responses (111). Delivery of DNA vaccines by ID-EP was observed to result in direct transfection of dermal dendritic cells (DCs), which subsequently migrated to the draining lymph nodes (112). In rats, single delivery of low-dose DNA vaccine against RSV F protein by ID-EP conferred complete protection from RSV/A challenge (113). In comparison, intramuscular DNA vaccination can result in efficient transfection of myocytes, which may in turn secrete soluble antigen through the lymphatic drainage systems (6). Additionally, especially in the context of electroporation, DNA vaccination can result in formation of apoptotic muscles cells harboring plasmid-encoded antigens, which can then be taken up by muscle-infiltrating APCs for cross presentation (6). ID DNA vaccines were found to be more well-tolerated than IM DNA vaccines in humans. In terms of induction of humoral responses, some preliminary studies suggest that ID vaccination enhanced humoral responses and was dose sparing, with or without EP (20, 47, 114). In terms of cellular responses, IM DNA vaccination was observed to induce stronger T-cell responses than ID DNA vaccination in some studies (20), and similar responses in other studies (18). In the Phase I EBOV DNA vaccine study, ID-EP vaccination of INO-4201 induced 100% sero-conversion post-dose two, whereas 53% sero-conversion was induced by the IM delivery of INO-4201. Upon the completion of the 3-dose regimen, overall T-cell responses were induced in 53.3% subjects receiving IM INO-4201 and 73.3% subjects receiving ID INO-4201 (15). In the Phase I HIV DNA vaccine study (HVTN098), ID delivery of PENNVAX®-GP at a lower dose of 1.6 mg elicited higher magnitude of gp140-specific binding antibody responses in subjects than IM delivery of the vaccine at 8.0 mg dose. In terms of cellular responses, 96% CD4+ T-cell response rates were observed in both ID+IL12 and IM+IL12 groups, whereas 64 and 44% CD8+ T-cell response rates were observed in the ID+IL12 and IM+IL12 groups, respectively (18).
Intranasal DNA Vaccination and Other Strategies to Enhance Mucosal Immunity
Transmission of several viral pathogens, such as HIV-1, influenza and SARS-CoV-2, can occur through mucosal sites (115–117). Strategies to enhance mucosal immunity for DNA vaccines, therefore, can be important. Secretory IgAs play a special role in host mucosal defense. As compared to IgG, IgA is typically 30–100 times more concentrated at the mucosal site, due to its ability to resist protease degradation (118). Intranasal DNA vaccination is one such strategy to promote trafficking of antigens to mucosal associated lymphoid tissue to prime mucosal immunity. In one study, it was observed that an intranasal (IN) H5N1 HA DNA vaccine co-administered with PEI induced comparable serum HAI titers as compared to an ID H5N1 DNA vaccine, but the IN vaccination elicited significantly higher HAI titers in Bronchoalveolar lavage (BAL) fluid as well as higher serum and BAL IgA levels (119). In a separate study, Kumar et al. reported the use of chitosan nanoparticles to intranasally deliver DNA vaccine against acute respiratory syncytial virus (RSV) infection. High levels of serum IgG and mucosal IgA antibodies were induced following mucosal vaccination. Strong CD8+ T-cell responses were also induced systemically and in the lung following viral challenge (120). While promising, this approach may be associated with certain pitfalls. Certain agents used to facilitate mucosal DNA delivery, such as PEI, are non-biodegradable and can be associated with significant toxicity (121). Additionally, there may be concerns and required additional safety studies to investigate the effects of targeting the nasal mucosa, considering the olfactory epithelium is the only part of the central nervous system (CNS) exposed to the external environment. Components of intranasal vaccines may therefore gain easy access to the CNS, bypassing blood brain barrier (121). In light of these challenges, alternative approaches can be considered to increase mucosal responses for DNA vaccines, such as through the use of mucosal adjuvants. For example, in the HIV and influenza model, combined delivery of DNA vaccines with plasmids encoding a mucosal chemokine, CCL25, was found to increase antigen-specific responses in the lung and mesenteric lymph nodes, and also increase antigen-specific CD4+, CD8+ T-cell responses as well as IgA responses at the mucosal sites (122). Additionally, in the NHP model, co-administration of an SIV DNA vaccine with plasmid-encoded CCR10L increased serum and vaginal IgA levels and conferred improved protection to macaques from SIVsmE660 challenge (123). Further exploration of approaches that may adjuvant mucosal responses of a SARS-CoV-2 DNA vaccine is likely of relevance.
DNA Launched Nano-Vaccines
While genetic adjuvants have been demonstrated to enhance immunogenicity of DNA vaccines in several cases, their use is highly contingent upon correct dose titration for adjuvants vs. vaccines in both animals and humans (124). Additionally, each plasmid-encoded adjuvant will likely need to undergo additional preclinical toxicology evaluation (125). The use of DNA-launched nano-vaccine technology to enhance DNA vaccine responses is potentially a viable and convenient alternative to these challenges. As compared to monomeric antigens, nanoparticle vaccines have been demonstrated to induce significantly stronger humoral responses to various targets including HIV, influenza, RSV, and malaria (126–130). However, assembly of these nano-vaccines is technologically cumbersome and costly with the conventional techniques (131). Xu et al. recently demonstrated that DNA/EP can enable direct de novo assembly of nanoparticle vaccines in the hosts to bypass such complex production processes (27, 132). As compared to DNA-encoded monomeric vaccines, DNA-launched nano-vaccines induced more rapid sero-conversion, higher binding, and neutralizing antibody titers, stronger CD8+ T-cell responses and conferred improved protection in a mouse influenza challenge model in a dose-sparing fashion and additional study is warranted (27, 133).
Concluding Remarks
This paper reviewed some major advancements in the DNA vaccine field, which support the findings that the newer DNA vaccines are now inducing more potent, consistent, and durable immune responses in several recent clinical studies. Advances in synthetic DNA and EP technology were harnessed for rapid design and evaluation of a novel DNA vaccine against SARS-CoV-2. Within 10 weeks from publication of the viral sequences, the vaccine is now being evaluated in a Phase I clinical study. The Phase I study was expanded to included older participants (134). It remains to be explored how the immune outcomes induced in the older participants compared to younger participants, as it has previously been observed that less potent humoral responses were observed in older volunteers by a mRNA vaccine candidate, and for protein based approaches higher antigen doses can improve immune responses in this population (135).
Another foreseeable challenge is the durability of vaccine responses. Immune responses against SARS-CoV-2 in natural infection were reported to taper relatively quickly. In one study, 40% of asymptomatic patients become seronegative for N protein and an S2 peptide during early convalescence (136). In a separate study, antibody titers in exposed healthcare workers were observed to quickly decline over two-month period (81). For DNA vaccines, some early clinical studies found that the vaccine responses were more limited in durability. For a SARS DNA vaccine candidate, seropositivity rate declined from 80% at the peak to 30% 24 weeks following the final vaccination (34). In some more recent studies, durability was found to be improved. For a MERS DNA vaccine candidate, seropositivity rate declined from 94% at the peak to 77% 48 weeks post the final vaccination, while the cellular responses increased from 50% during the final vaccination to 64% 48 weeks post the final vaccination (16). It will therefore be pertinent to evaluate the durability of vaccine responses of INO-4800 and other SARS-CoV-2 vaccine candidates in comparison with the kinetics of immune responses induced following a natural SARS-CoV-2 infection. Furthermore, milligram levels of DNA are currently required in DNA vaccine regimen. While DNA can be quickly and inexpensively manufactured, studies to further reduce the dose are of continued importance.
Finally, another important consideration for DNA vaccination is the heterologous DNA prime protein/viral vector boost strategy, which has been extensively explored for many disease targets, including HIV (137, 138), influenza (139, 140), malaria (141), and tuberculosis (142), and has been demonstrated to improve vaccine immunogenicity and protection in many cases (143). The utility of the DNA platform to boost many other platforms in a highly tolerable approach could be advantageous for expanding immunity and memory responses from other vaccine platforms.
In summary, owning to the strong clinical safety profile, low costs of production and transportation, and the unique ability to induce both humoral and cellular responses, newer DNA vaccine will be an extremely important tool as part of the current COVID-19 pandemic and will possibly help address other EIDs. Continuous development in this technology, especially in such areas as DNA-encoded cytokine adjuvants, DNA-launched nano-vaccines, advanced plasmid delivery technologies, and innovative prime-boost strategies will have significant impact on continually advancing this platform to impact human and animal health.
Author Contributions
ZX and DW conceptualized the paper. ZX, AP, NT, XZ, KM, DK, and DW wrote the paper. All authors contributed to the article and approved the submitted version.
Funding
This research is supported by NIH IPCAVD Grant U19 Al109646-04, NIH/NIAID Collaborative Influenza Vaccine Innovation Centers (CIVIC) contract 75N93019C00051, Inovio Pharmaceuticals Virus Grant 5181101374 and Wistar Coronavirus Discovery Fund awarded to DW, W. W. Smith Charitable Trust 68112-01-383 awarded to DK, and by Wistar Monica H.M. Shander Memorial Fellowship awarded to ZX.
Conflict of Interest
ZX, DW, and DK have a pending patent US.62784318. KM receives grants and consulting fees from Inovio related to DNA vaccine development. DW has received grant funding, participates in industry collaborations, has received speaking honoraria, and has received fees for consulting, including serving on scientific review committees and board series. Remuneration received by DW includes direct payments, stock or stock options, and in the interest of disclosure he notes potential conflicts associated with his work with Inovio and possible others.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Gessner BD, Kaslow D, Louis J, Neuzil K, O'Brien KL, Picot V, et al. Estimating the full public health value of vaccination. Vaccine. (2017) 35:6255–63. doi: 10.1016/j.vaccine.2017.09.048
2. Kutzler MA, and Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. (2008) 9:776–88. doi: 10.1038/nrg2432
3. Hobernik D, and Bros M. DNA vaccines-how far from clinical use? Int J Mol Sci. (2018) 19(11). doi: 10.3390/ijms19113605
4. Gary EN, and Weiner DB. DNA vaccines: prime time is now. Curr Opin Immunol. (2020) 65:21–7. doi: 10.1016/j.coi.2020.01.006
5. Villarreal DO, Talbott KT, Choo DK, Shedlock DJ, and Weiner DB. Synthetic DNA vaccine strategies against persistent viral infections. Expert Rev Vaccines. (2013) 12:537–54. doi: 10.1586/erv.13.33
6. Saade F, and Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. (2012) 11:189–209. doi: 10.1586/erv.11.188
7. Suschak JJ, Williams JA, and Schmaljohn CS. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccin Immunother. (2017) 13:2837–48. doi: 10.1080/21645515.2017.1330236
8. Redding L, and Weiner DB. DNA vaccines in veterinary use. Expert Rev Vaccines. (2009) 8:1251–76. doi: 10.1586/erv.09.77
9. Kalams SA, Parker S, Jin X, Elizaga M, Metch B, Wang M, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PLoS ONE. (2012) 7:e29231. doi: 10.1371/journal.pone.0029231
10. Mann JF, McKay PF, Fiserova A, Klein K, Cope A, Rogers P, et al. Enhanced immunogenicity of an HIV-1 DNA vaccine delivered with electroporation via combined intramuscular and intradermal routes. J Virol. (2014) 88:6959–69. doi: 10.1128/JVI.00183-14
11. Todorova B, Adam L, Culina S, Boisgard R, Martinon F, Cosma A, et al. Electroporation as a vaccine delivery system and a natural adjuvant to intradermal administration of plasmid DNA in macaques. Sci Rep. (2017) 7:4122. doi: 10.1038/s41598-017-04547-2
12. Donate A, Coppola D, Cruz Y, and Heller R. Evaluation of a novel non-penetrating electrode for use in DNA vaccination. PLoS ONE. (2011) 6:e19181. doi: 10.1371/journal.pone.0019181
13. Roos AK, Moreno S, Leder C, Pavlenko M, King A, and Pisa P. Enhancement of cellular immune response to a prostate cancer DNA vaccine by intradermal electroporation. Mol Ther. (2006) 13:320–7. doi: 10.1016/j.ymthe.2005.08.005
14. ITebas P, Roberts CC, Muthumani K, Reuschel EL, Kudchodkar SB, Zaidi FI, et al. Safety and immunogenicity of an anti-zika virus DNA vaccine - preliminary report. N Engl J Med. (2017). doi: 10.1056/NEJMoa1708120
15. Tebas P, Kraynyak KA, Patel A, Maslow JN, Morrow MP, Sylvester AJ, et al. Intradermal SynCon(R) Ebola GP DNA vaccine is temperature stable and safely demonstrates cellular and humoral immunogenicity advantages in healthy volunteers. J Infect Dis. (2019) 220:400–10. doi: 10.1093/infdis/jiz132
16. Modjarrad K, Roberts CC, Mills KT, Castellano AR, Paolino K, Muthumani K, et al. Safety and immunogenicity of an anti-Middle east respiratory syndrome coronavirus DNA vaccine: a phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. (2019) 19:1013–22. doi: 10.1016/S1473-3099(19)30266-X
17. Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis. (2013) 208:818–29. doi: 10.1093/infdis/jit236
18. DeRosa S, Edupuganti S, Huang Y, Han X, Elizaga M, Swann E, et al. Robust antibody and cellular responses induced by DNA-only vaccination for HIV. JCI Insight. (2020) 5:e137079. doi: 10.1172/jci.insight.137079
19. Gaudinski MR, Houser KV, Morabito KM, Hu Z, Yamshchikov G, Rothwell RS, et al. Safety, tolerability, and immunogenicity of two zika virus DNA vaccine candidates in healthy adults: randomised, open-label, phase 1 clinical trials. Lancet. (2018) 391:552–62.
20. Ledgerwood JE, Hu Z, Gordon IJ, Yamshchikov G, Enama ME, Plummer S, et al. Influenza virus h5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans. Clin Vaccine Immunol. (2012) 19:1792–7. doi: 10.1128/CVI.05663-11
21. Hooper JW, Moon JE, Paolino KM, Newcomer R, McLain DE, Josleyn M, et al. A phase 1 clinical trial of hantaan virus and puumala virus M-segment DNA vaccines for haemorrhagic fever with renal syndrome delivered by intramuscular electroporation. Clin Microbiol Infect. (2014) 20(Suppl. 5):110–7. doi: 10.1111/1469-0691.12553
22. Wloch MK, Smith LR, Boutsaboualoy S, Reyes L, Han C, Kehler J, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. (2008) 197:1634–42. doi: 10.1086/588385
23. Kharfan-Dabaja MA, Boeckh M, Wilck MB, Langston AA, Chu AH, Wloch MK, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. (2012) 12:290–9. doi: 10.1016/S1473-3099(11)70344-9
24. Marra F, Young F, Richardson K, and Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses. (2013) 7:584–603. doi: 10.1111/irv.12000
25. Smith TR, Schultheis K, Kiosses WB, Amante DH, Mendoza JM, Stone JC, et al. DNA vaccination strategy targets epidermal dendritic cells, initiating their migration and induction of a host immune response. Mol Ther Methods Clin Dev. (2014) 1:14054. doi: 10.1038/mtm.2014.54
26. Jiang J, Banglore P, Cashman KA, Schmaljohn CS, Schultheis K, Pugh H, et al. Immunogenicity of a protective intradermal DNA vaccine against lassa virus in cynomolgus macaques. Hum Vaccin Immunother. (2019) 15:2066–74. doi: 10.1080/21645515.2019.1616499
27. Xu Z, Wise MC, Chokkalingam N, Walker S, Tello-Ruiz E, Elliott STC, et al. In vivo assembly of nanoparticles achieved through synergy of structure-based protein engineering and synthetic dna generates enhanced adaptive immunity. Adv Sci. (2020) 7:1902802. doi: 10.1002/advs.201902802
28. Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. (2008) 451:990–3. doi: 10.1038/nature06536
29. Petersen E, Petrosillo N, Koopmans M, and Panel EEITFE. Emerging infections-an increasingly important topic: review by the emerging infections task force. Clin Microbiol Infect. (2018) 24:369–75. doi: 10.1016/j.cmi.2017.10.035
30. Rauch S, Jasny E, Schmidt KE, and Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. (2018) 9:1963. doi: 10.3389/fimmu.2018.01963
31. Zhang C, Maruggi G, Shan H, and Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol. (2019) 10:594. doi: 10.3389/fimmu.2019.00594
32. Schmeer M, and Schleef M. Pharmaceutical grade large-scale plasmid DNA manufacturing process. Methods Mol Biol. (2014) 1143:219–40. doi: 10.1007/978-1-4939-0410-5_14
33. Fink TL, Klepcyk PJ, Oette SM, Gedeon CR, Hyatt SL, Kowalczyk TH, et al. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. (2006) 13:1048–51. doi: 10.1038/sj.gt.3302761
34. Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. (2004) 428:561–4. doi: 10.1038/nature02463
35. Abbink P, Larocca RA, Visitsunthorn K, Boyd M, De La Barrera RA, Gromowski GD, et al. Durability and correlates of vaccine protection against zika virus in rhesus monkeys. Sci Transl Med. (2017) 9:aao4163. doi: 10.1126/scitranslmed.aao4163
36. Zhao J, Alshukairi AN, Baharoon SA, Ahmed WA, Bokhari AA, Nehdi AM, et al. Recovery from the middle east respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. (2017) 2:aan5393. doi: 10.1126/sciimmunol.aan5393
37. MacNeil A, Farnon EC, Wamala J, Okware S, Cannon DL, Reed Z, et al. Proportion of deaths and clinical features in bundibugyo ebola virus infection, Uganda. Emerg Infect Dis. (2010) 16:1969–72. doi: 10.3201/eid1612.100627
38. Ollmann Saphire E. A vaccine against ebola virus. Cell. (2020) 181:6. doi: 10.1016/j.cell.2020.03.011
39. Sridhar S. Clinical development of Ebola vaccines. Ther Adv Vaccines. (2015) 3:125–38. doi: 10.1177/2051013615611017
40. Patel A, Reuschel EL, Kraynyak KA, Racine T, Park DH, Scott VL, et al. Protective efficacy and long-term immunogenicity in cynomolgus macaques by ebola virus glycoprotein synthetic DNA vaccines. J Infect Dis. (2019) 219:544–55. doi: 10.1093/infdis/jiy537
41. Song BH, Yun SI, Woolley M, and Lee YM. Zika virus: history, epidemiology, transmission, and clinical presentation. J Neuroimmunol. (2017) 308:50–64. doi: 10.1016/j.jneuroim.2017.03.001
42. Uraki R, Hwang J, Jurado KA, Householder S, Yockey LJ, Hastings AK, et al. Zika virus causes testicular atrophy. Sci Adv. (2017) 3:e1602899. doi: 10.1126/sciadv.1602899
43. Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, et al. Rapid development of a DNA vaccine for zika virus. Science. (2016) 354:237–40. doi: 10.1126/science.aai9137
44. Muthumani K, Griffin BD, Agarwal S, Kudchodkar SB, Reuschel EL, Choi H, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. NPJ Vaccines. (2016) 1:16021. doi: 10.1038/npjvaccines.2016.21
45. Lopez-Camacho C, Abbink P, Larocca RA, Dejnirattisai W, Boyd M, Badamchi-Zadeh A, et al. Rational Zika vaccine design via the modulation of antigen membrane anchors in chimpanzee adenoviral vectors. Nat Commun. (2018) 9:2441. doi: 10.1038/s41467-018-04859-5
46. Aguilar PV, Estrada-Franco JG, Navarro-Lopez R, Ferro C, Haddow AD, and Weaver SC. Endemic Venezuelan equine encephalitis in the Americas: hidden under the dengue umbrella. Future Virol. (2011) 6:721–40. doi: 10.2217/fvl.11.50
47. Hannaman D, Dupuy LC, Ellefsen B, and Schmaljohn CS. A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine. (2016) 34:3607–12. doi: 10.1016/j.vaccine.2016.04.077
48. Richmond JK, and Baglole DJ. Lassa fever: epidemiology, clinical features, and social consequences. BMJ. (2003) 327:1271–5. doi: 10.1136/bmj.327.7426.1271
49. Fehr AR, and Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. (2015) 1282:1–23. doi: 10.1007/978-1-4939-2438-7_1
50. Yang Y, Peng F, Wang R, Guan K, Jiang T, Xu G, et al. The deadly coronaviruses: the 2003. SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. (2020) 109:102434. doi: 10.1016/j.jaut.2020.102434
51. Amanat F, and Krammer F. SARS-CoV-2 vaccines: status report. Immunity. (2020) 52:583–9. doi: 10.1016/j.immuni.2020.03.007
52. Xu RH, He JF, Evans MR, Peng GW, Field HE, Yu DW, et al. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. (2004) 10:1030–7. doi: 10.3201/eid1006.030852
53. Wilder-Smith A, Chiew CJ, and Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. (2020) 20:e102–e7. doi: 10.1016/S1473-3099(20)30129-8
54. Wang LF, and Eaton BT. Bats, civets and the emergence of SARS. Curr Top Microbiol Immunol. (2007) 315:325–44. doi: 10.1007/978-3-540-70962-6_13
55. Martin JE, Louder MK, Holman LA, Gordon IJ, Enama ME, Larkin BD, et al. A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial. Vaccine. (2008) 26:6338–43. doi: 10.1016/j.vaccine.2008.09.026
56. Hajjar SA, Memish ZA, and McIntosh K. Middle east respiratory syndrome coronavirus (MERS-CoV): a perpetual challenge. Ann Saudi Med. (2013) 33:427–36. doi: 10.5144/0256-4947.2013.427
57. Li F, and Du L. MERS coronavirus: an emerging zoonotic virus. Viruses. (2019) 11:663. doi: 10.3390/v11070663
58. Muthumani K, Falzarano D, Reuschel EL, Tingey C, Flingai S, Villarreal DO, et al. A synthetic consensus anti-spike protein DNA vaccine induces protective immunity against Middle East respiratory syndrome coronavirus in nonhuman primates. Sci Transl Med. (2015) 7:301ra132. doi: 10.1126/scitranslmed.aac7462
59. Lai CC, Shih TP, Ko WC, Tang HJ, and Hsueh PR. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. (2020) 55:105924. doi: 10.1016/j.ijantimicag.2020.105924
60. Khafaie MA, and Rahim F. Cross-country comparison of case fatality rates of COVID-19/SARS-COV-2. Osong Public Health Res Perspect. (2020) 11:74–80. doi: 10.24171/j.phrp.2020.11.2.03
61. Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80 e8. doi: 10.1016/j.cell.2020.02.052
62. Dong Y, Dai T, Liu J, Zhang L, and Zhou F. Coronavirus in continuous flux: from SARS-CoV to SARS-CoV-2. Adv Sci. (2020). doi: 10.1002/advs.202001474. [Epub ahead of print].
63. Li MY, Li L, Zhang Y, and Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. (2020) 9:45. doi: 10.1186/s40249-020-00662-x
64. Basu A. Estimating the infection fatality rate among symptomatic COVID-19 cases in the United States. Health Aff. (2020) 39:1229–36. doi: 10.1377/hlthaff.2020.00455
65. Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, et al. The socio-economic implications of the coronavirus and COVID-19 pandemic: a review. Int J Surg. (2020) doi: 10.1016/j.ijsu.2020.04.018
66. Chandrashekar A, Liu J, Martinot AJ, McMahan K, Mercado NB, Peter L, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. (2020) 369:812-817. doi: 10.1126/science.abc477
67. Deng W, Bao L, Liu J, Xiao C, Liu J, Xue J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. (2020) 2020:eabc5343. doi: 10.1126/science.abc5343
68. Corbett KS, Edwards D, Leist SR, Abiona OM, Boyoglu-Barnum S, Gillespie RA, et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.06.11.145920
69. McKay PF, Hu K, Blakney AK, Samnuan K, Brown JC, Penn R, et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. (2020). 11:3523. doi: 10.1038/s41467-020-17409-9
70. Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. An mRNA Vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. (2020). doi: 10.1056/NEJMoa2022483. [Epub ahead of print].
71. Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart SP, et al. Phase 1/2 study to describe the safety and immunogenicity of a COVID-19 RNA vaccine candidate (BNT162b1) in adults 18 to 55 Years of age: interim report. medRxiv. (2020) 6:20142570. doi: 10.1101/2020.06.30.20142570
72. Smith TRF, Patel A, Ramos S, Elwood D, Zhu X, Yan J, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. (2020). 11:2601. doi: 10.1038/s41467-020-16505-0
73. Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, Mahrokhian SH, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. (2020) 369:806–11. doi: 10.1126/science.abc6284
74. Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Rapid development of an inactivated vaccine candidate for SARS-CoV-2. Science. (2020) 369:77–81. doi: 10.1126/science.abc1932
75. Zhu F-C, Li Y-H, Guan X-H, Hou L-H, Wang W-J, Li J-X, et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in-human trial. Lancet. (2020) 395:1845–1854. doi: 10.1016/S0140-6736(20)31208-3
76. van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. (2020) 5:093195. doi: 10.1101/2020.05.13.093195
77. Callaway E. The race for coronavirus vaccines: a graphical guide. Nature. (2020) 580:576–7. doi: 10.1038/d41586-020-01221-y
78. Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. (2020) 581:221–4. doi: 10.1038/s41586-020-2179-y
79. Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. (2019) 4:123158. doi: 10.1172/jci.insight.123158
80. Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, et al. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature. (2020) 584:353–63. doi: 10.1038/s41586-020-2538-8
81. Stephens DS, and Mcelrath MJ. COVID-19 and the path to immunity. JAMA. (2020) 324:1279–81. doi: 10.1001/jama.2020.16656
82. Rogers TF, Zhao F, Huang D, Beutler N, Burns A, He WT, et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. (2020) 369:956–63. doi: 10.1126/science.abc7520
83. Soresina A, Moratto D, Chiarini M, Paolillo C, Baresi G, Foca E, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. (2020) 31:565–9. doi: 10.1111/pai.13263
84. Robbiani DF, Gaebler C, Muecksch F, Lorenzi JCC, Wang Z, Cho A, et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. (2020) 584:437–42. doi: 10.1038/s41586-020-2456-9
85. Chen X, Pan Z, Yue S, Yu F, Zhang J, Yang Y, et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther. (2020) 5:180. doi: 10.1038/s41392-020-00301-9
86. Oja AE, Saris A, Ghandour CA, Kragten NAM, Hogema BM, Nossent EJ, et al. Divergent SARS-CoV-2-specific T and B cell responses in severe but not mild COVID-19. bioRxiv. (2020) 6:159202. doi: 10.1101/2020.06.18.159202
87. Patel A, Walters J, Reuschel EL, Schultheis K, Parzych E, Gary EN, et al. Intradermal-delivered DNA vaccine provides anamnestic protection in a rhesus macaque SARS-CoV-2 challenge model. bioRxiv. (2020) 07:225649. doi: 10.1101/2020.07.28.225649
88. Xu Z, Wise MC, Choi H, Perales-Puchalt A, Patel A, Tello-Ruiz E, et al. Synthetic DNA delivery by electroporation promotes robust in vivo sulfation of broadly neutralizing anti-HIV immunoadhesin eCD4-Ig. EBioMedicine. (2018) 35:97–105. doi: 10.1016/j.ebiom.2018.08.027
89. Esquivel RN, Patel A, Kudchodkar SB, Park DH, Stettler K, Beltramello M, et al. In vivo delivery of a DNA-encoded monoclonal antibody protects non-human primates against zika virus. Mol Ther. (2019) 27:974–85. doi: 10.1016/j.ymthe.2019.03.005
90. Wise MC, Xu Z, Tello-Ruiz E, Beck C, Trautz A, Patel A, et al. In vivo delivery of synthetic DNA-encoded antibodies induces broad HIV-1-neutralizing activity. J Clin Invest. (2020) 130:827–37. doi: 10.1172/JCI132779
91. Khoshnejad M, Patel A, Wojtak K, Kudchodkar SB, Humeau L, Lyssenko NN, et al. Development of novel DNA-encoded PCSK9 monoclonal antibodies as lipid-lowering therapeutics. Mol Ther. (2019) 27:188–99. doi: 10.1016/j.ymthe.2018.10.016
92. Aggarwal P, Kumar S, Vajpayee M, and Seth P. Adjuvant action of murine IL-2/Ig plasmid after intramuscular immunization with Indian HIV-1 subtype C recombinant env.gp 120 construct. Viral Immunol. (2005) 18:649–56. doi: 10.1089/vim.2005.18.649
93. Hu H, Lu X, Tao L, Bai B, Zhang Z, Chen Y, et al. Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice. Clin Vaccine Immunol. (2007) 14:894–901. doi: 10.1128/CVI.00019-07
94. Henke A, Rohland N, Zell R, and Wutzler P. Co-expression of interleukin-2 by a bicistronic plasmid increases the efficacy of DNA immunization to prevent influenza virus infections. Intervirology. (2006) 49:249–52. doi: 10.1159/000092487
95. Reeder SM, Reuschel EL, Bah MA, Yun K, Tursi NJ, Kim KY, et al. Synthetic DNA vaccines adjuvanted with pil-33 drive liver-localized t cells and provide protection from plasmodium challenge in a mouse model. Vaccines. (2020) 8:21. doi: 10.3390/vaccines8010021
96. Louis L, Wise MC, Choi H, Villarreal DO, Muthumani K, and Weiner DB. Designed DNA-encoded il-36 gamma acts as a potent molecular adjuvant enhancing zika synthetic dna vaccine-induced immunity and protection in a lethal challenge model. Vaccines. (2019) 7:42. doi: 10.3390/vaccines7020042
97. Sin JI, Kim JJ, Arnold RL, Shroff KE, McCallus D, Pachuk C, et al. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol. (1999) 162:2912–21.
98. Jalah R, Patel V, Kulkarni V, Rosati M, Alicea C, Ganneru B, et al. IL-12 DNA as molecular vaccine adjuvant increases the cytotoxic T cell responses and breadth of humoral immune responses in SIV DNA vaccinated macaques. Hum Vaccin Immunother. (2012) 8:1620–9. doi: 10.4161/hv.21407
99. Naderi M, Saeedi A, Moradi A, Kleshadi M, Zolfaghari MR, Gorji A, et al. Interleukin-12 as a genetic adjuvant enhances hepatitis C virus NS3 DNA vaccine immunogenicity. Virol Sin. (2013) 28:167–73. doi: 10.1007/s12250-013-3291-z
100. Bagley KC, Schwartz JA, Andersen H, Eldridge JH, Xu R, Ota-Setlik A, et al. An interleukin 12 adjuvanted herpes simplex virus 2 dna vaccine is more protective than a glycoprotein d subunit vaccine in a high-dose murine challenge model. Viral Immunol. (2017) 30:178–95. doi: 10.1089/vim.2016.0136
101. Ghaffarifar F, Jafarimodrek M, Vazini H, Sharifi Z, Dalimi A, and Dayer MS. Assessment of DNA vaccine encoding Toxoplasma gondii microneme complete gene and IL-12 as adjuvant in BALB/c mice. Iran J Basic Med Sci. (2019) 22:901–7. doi: 10.22038/ijbms.2019.34872.8276
102. Wang G, Pan L, and Zhang Y. Approaches to improved targeting of DNA vaccines. Hum Vaccin. (2011) 7:1271–81. doi: 10.4161/hv.7.12.17983
103. Delogu G, Li A, Repique C, Collins F, and Morris SL. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect Immun. (2002) 70:292–302. doi: 10.1128/IAI.70.1.292-302.2002
104. Weinberger EE, Isakovic A, Scheiblhofer S, Ramsauer C, Reiter K, Hauser-Kronberger C, et al. The influence of antigen targeting to sub-cellular compartments on the anti-allergic potential of a DNA vaccine. Vaccine. (2013) 31:6113–21. doi: 10.1016/j.vaccine.2013.08.005
105. Rodriguez F, An LL, Harkins S, Zhang J, Yokoyama M, Widera G, et al. DNA immunization with minigenes: low frequency of memory cytotoxic T lymphocytes and inefficient antiviral protection are rectified by ubiquitination. J Virol. (1998) 72:5174–81. doi: 10.1128/JVI.72.6.5174-5181.1998
106. Fu TM, Guan L, Friedman A, Ulmer JB, Liu MA, and Donnelly JJ. Induction of MHC class I-restricted CTL response by DNA immunization with ubiquitin-influenza virus nucleoprotein fusion antigens. Vaccine. (1998) 16:1711–7. doi: 10.1016/S0264-410X(98)00134-0
107. Zhang M, Obata C, Hisaeda H, Ishii K, Murata S, Chiba T, et al. A novel DNA vaccine based on ubiquitin-proteasome pathway targeting 'self'-antigens expressed in melanoma/melanocyte. Gene Ther. (2005) 12:1049–57. doi: 10.1038/sj.gt.3302490
109. Lu Y, Raviprakash K, Leao IC, Chikhlikar PR, Ewing D, Anwar A, et al. Dengue 2 PreM-E/LAMP chimera targeted to the MHC class II compartment elicits long-lasting neutralizing antibodies. Vaccine. (2003) 21:2178–89. doi: 10.1016/S0264-410X(03)00009-4
110. Anwar A, Chandrasekaran A, Ng ML, Marques E, and August JT. West Nile premembrane-envelope genetic vaccine encoded as a chimera containing the transmembrane and cytoplasmic domains of a lysosome-associated membrane protein: increased cellular concentration of the transgene product, targeting to the MHC II compartment, and enhanced neutralizing antibody response. Virology. (2005) 332:66–77. doi: 10.1016/j.virol.2004.11.022
111. Kashem SW, Haniffa M, and Kaplan DH. Antigen-presenting cells in the skin. Annu Rev Immunol. (2017) 35:469–99. doi: 10.1146/annurev-immunol-051116-052215
112. Amante DH, Smith TR, Mendoza JM, Schultheis K, McCoy JR, Khan AS, et al. Skin transfection patterns and expression kinetics of electroporation-enhanced plasmid delivery using the cellectra-3p, a portable next-generation dermal electroporation device. Hum Gene Ther Methods. (2015) 26:134–46. doi: 10.1089/hgtb.2015.020
113. Smith TRF, Schultheis K, Morrow MP, Kraynyak KA, McCoy JR, Yim KC, et al. Development of an intradermal DNA vaccine delivery strategy to achieve single-dose immunity against respiratory syncytial virus. Vaccine. (2017) 35:2840–7. doi: 10.1016/j.vaccine.2017.04.008
114. Haidari G, Cope A, Miller A, Venables S, Yan C, Ridgers H, et al. Combined skin and muscle vaccination differentially impact the quality of effector T cell functions: the CUTHIVAC-001 randomized trial. Sci Rep. (2017) 7:13011. doi: 10.1038/s41598-017-13331-1
115. Yu M, and Vajdy M. Mucosal HIV transmission and vaccination strategies through oral compared with vaginal and rectal routes. Expert Opin Biol Ther. (2010) 10:1181–95. doi: 10.1517/14712598.2010.496776
116. McMahon M, Kirkpatrick E, Stadlbauer D, Strohmeier S, Bouvier NM, and Krammer F. Mucosal immunity against neuraminidase prevents influenza b virus transmission in guinea pigs. mBio. (2019) 10:19. doi: 10.1128/mBio.00560-19
117. Sun CB, Wang YY, Liu GH, and Liu Z. Role of the eye in transmitting human coronavirus: what we know and what we do not know. Front Public Health. (2020) 8:155. doi: 10.3389/fpubh.2020.00155
118. Neutra MR, and Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. (2006) 6:148–58. doi: 10.1038/nri1777
119. Torrieri-Dramard L, Lambrecht B, Ferreira HL, Van den Berg T, Klatzmann D, and Bellier B. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol Ther. (2011) 19:602–11. doi: 10.1038/mt.2010.222
120. Kumar M, Behera AK, Lockey RF, Zhang J, Bhullar G, De La Cruz CP, et al. Intranasal gene transfer by chitosan-DNA nanospheres protects BALB/c mice against acute respiratory syncytial virus infection. Hum Gene Ther. (2002) 13:1415–25. doi: 10.1089/10430340260185058
121. Xu Y, Yuen PW, and Lam JK. Intranasal DNA vaccine for protection against respiratory infectious diseases: the delivery perspectives. Pharmaceutics. (2014) 6:378–415. doi: 10.3390/pharmaceutics6030378
122. Kathuria N, Kraynyak KA, Carnathan D, Betts M, Weiner DB, and Kutzler MA. Generation of antigen-specific immunity following systemic immunization with DNA vaccine encoding CCL25 chemokine immunoadjuvant. Hum Vaccin Immunother. (2012) 8:1607–19. doi: 10.4161/hv.22574
123. Kutzler MA, Wise MC, Hutnick NA, Moldoveanu Z, Hunter M, Reuter M, et al. Chemokine-adjuvanted electroporated DNA vaccine induces substantial protection from simian immunodeficiency virus vaginal challenge. Mucosal Immunol. (2016) 9:13–23. doi: 10.1038/mi.2015.31
124. Rose AH, Hoffmann FW, Hara JH, Urschitz J, Moisyadi S, Hoffmann PR, et al. Adjuvants may reduce in vivo transfection levels for DNA vaccination in mice leading to reduced antigen-specific CD8+ T cell responses. Hum Vaccin Immunother. (2015) 11:2305–11. doi: 10.1080/21645515.2015.1047567
125. Petrovsky N. Comparative safety of vaccine adjuvants: a summary of current evidence and future needs. Drug Saf. (2015) 38:1059–74. doi: 10.1007/s40264-015-0350-4
126. Xu Z, and Kulp DW. Protein engineering and particulate display of B-cell epitopes to facilitate development of novel vaccines. Curr Opin Immunol. (2019) 59:49–56. doi: 10.1016/j.coi.2019.03.003
127. Tokatlian T, Read BJ, Jones CA, Kulp DW, Menis S, Chang JYH, et al. Innate immune recognition of glycans targets HIV nanoparticle immunogens to germinal centers. Science. (2019) 363:649–54. doi: 10.1126/science.aat9120
128. Kanekiyo M, Wei CJ, Yassine HM, McTamney PM, Boyington JC, Whittle JR, et al. Self-assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature. (2013) 499:102–6. doi: 10.1038/nature12202
129. Marcandalli J, Fiala B, Ols S, Perotti M, de van der Schueren W, Snijder J, et al. Induction of potent neutralizing antibody responses by a designed protein nanoparticle vaccine for respiratory syncytial virus. Cell. (2019) 176:1420–31.e17. doi: 10.1016/j.cell.2019.01.046
130. Wilson KL, Pouniotis D, Hanley J, Xiang SD, Ma C, Coppel RL, et al. A synthetic nanoparticle based vaccine approach targeting msp4/5 is immunogenic and induces moderate protection against murine blood-stage malaria. Front Immunol. (2019) 10:331. doi: 10.3389/fimmu.2019.00331
131. Lopez-Vidal J, Gomez-Sebastian S, Barcena J, Nunez Mdel C, Martinez-Alonso D, Dudognon B, et al. Improved production efficiency of virus-like particles by the baculovirus expression vector system. PLoS ONE. (2015) 10:e0140039. doi: 10.1371/journal.pone.0140039
132. Xu Z, Chokkalingam N, Tello-Ruiz E, Walker S, Kulp DW, and Weiner DB. Incorporation of a novel cd4+ helper epitope identified from aquifex aeolicus enhances humoral responses induced by dna and protein vaccinations. iScience. (2020) 2020:101399. doi: 10.1016/j.isci.2020.101399
133. Xu Z, Chokkalingam N, Tello-Ruiz E, Wise MC, Bah MA, Walker S, et al. A DNA-launched nanoparticle vaccine elicits CD8+ T-cell immunity to promote in vivo tumor control. Cancer Immunol Res. (2020). doi: 10.1158/2326-6066.CIR-20-0061. [Epub ahead of print].
134. Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. (2020) 8:e1003–17. doi: 10.1016/S2214-109X(20)30264-3
135. Walsh EE, Frenck R, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. RNA-Based COVID-19 vaccine BNT162b2 selected for a pivotal efficacy study. medRxiv. (2020) 08:20176651. doi: 10.1101/2020.08.17.20176651
136. Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. (2020) 26:1200–4. doi: 10.1038/s41591-020-0965-6
137. Rouphael NG, Morgan C, Li SS, Jensen R, Sanchez B, Karuna S, et al. DNA priming and gp120 boosting induces HIV-specific antibodies in a randomized clinical trial. J Clin Invest. (2019) 129:4769–85. doi: 10.1172/JCI128699
138. Joseph S, Quinn K, Greenwood A, Cope AV, McKay PF, Hayes PJ, et al. A comparative phase i study of combination, homologous subtype-C DNA, MVA, and Env gp140 protein/adjuvant hiv vaccines in two immunization regimes. Front Immunol. (2017) 8:149. doi: 10.3389/fimmu.2017.00149
139. Le Gall-Recule G, Cherbonnel M, Pelotte N, Blanchard P, Morin Y, and Jestin V. Importance of a prime-boost DNA/protein vaccination to protect chickens against low-pathogenic H7 avian influenza infection. Avian Dis. (2007) 51:490–4. doi: 10.1637/7592-040206R.1
140. Wang S, Parker C, Taaffe J, Solorzano A, Garcia-Sastre A, and Lu S. Heterologous HA DNA vaccine prime–inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine. (2008) 26:3626–33. doi: 10.1016/j.vaccine.2008.04.073
141. Dunachie SJ, Walther M, Epstein JE, Keating S, Berthoud T, Andrews L, et al. A DNA prime-modified vaccinia virus ankara boost vaccine encoding thrombospondin-related adhesion protein but not circumsporozoite protein partially protects healthy malaria-naive adults against plasmodium falciparum sporozoite challenge. Infect Immun. (2006) 74:5933–42. doi: 10.1128/IAI.00590-06
142. Magalhaes I, Sizemore DR, Ahmed RK, Mueller S, Wehlin L, Scanga C, et al. rBCG induces strong antigen-specific T cell responses in rhesus macaques in a prime-boost setting with an adenovirus 35 tuberculosis vaccine vector. PLoS ONE. (2008) 3:e3790. doi: 10.1371/journal.pone.0003790
Keywords: DNA vaccines, intradermal electroporation, coronaviruses, COVID-19, SARS-CoV-2, emerging infectious diseases (EIDs), DNA-launched nanoparticle vaccines, intranasal vaccines
Citation: Xu Z, Patel A, Tursi NJ, Zhu X, Muthumani K, Kulp DW and Weiner DB (2020) Harnessing Recent Advances in Synthetic DNA and Electroporation Technologies for Rapid Vaccine Development Against COVID-19 and Other Emerging Infectious Diseases. Front. Med. Technol. 2:571030. doi: 10.3389/fmedt.2020.571030
Received: 09 June 2020; Accepted: 08 September 2020;
Published: 21 October 2020.
Edited by:
Ada Maria De Barcelos Alves, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Roberto Lins, Aggeu Magalhães Institute (IAM), BrazilAnnie Elong Ngono, La Jolla Institute for Immunology (LJI), United States
Copyright © 2020 Xu, Patel, Tursi, Zhu, Muthumani, Kulp and Weiner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David B. Weiner, ZHdlaW5lckB3aXN0YXIub3Jn
 Ziyang Xu
Ziyang Xu Ami Patel1
Ami Patel1 Nicholas J. Tursi
Nicholas J. Tursi Kar Muthumani
Kar Muthumani David B. Weiner
David B. Weiner