- 1Department of Engineering, University of Cambridge, Cambridge, United Kingdom
- 2Cambridge Centre for Advanced Research and Education in Singapore (CARES), Singapore, Singapore
This paper presents a zero-dimensional Doubly Conditional Moment Closure (0D-DCMC) methodology for investigating dual-fuel combustion involving ammonia and diesel. The approach uses two mixture fractions as conditioning variables, one for each fuel, to effectively model ignition and reveal the flame structure in mixture fraction space. Initially, 0D reactor calculations are performed using Cantera, exploring the chemical mechanism, identifying the most reactive mixture fractions, and determining key species involved in the ignition process. Following that, the 0D-DCMC simulations carried out provide understanding into the effects of the scalar and cross-scalar dissipation rates on autoignition. The results show that higher scalar dissipation rates delay ignition, while a negative cross-scalar dissipation rate reduces ignition delay compared to a positive rate. The ignition is shown to occur near the most reactive mixture fraction of the most reactive fuel, at lower conditional values of the less reactive fuel’s mixture fraction. The species fronts formed are observed to follow a trajectory between the stoichiometric mixture fractions of the fuels. The results establish a robust computational framework for modeling dual-fuel combustion.
1 Introduction
Following the Paris Agreement in 2015 (UNFCCC, 2024), the International Maritime Organisation is aiming at 50% greenhouse gas emissions reduction compared to 2008 by 2050 (Joung et al., 2020). Shipping is responsible for carrying around 80% of global trade by volume (United Nations, 2018) producing however an estimated 2.2% of global emissions
However, ammonia is a difficult fuel to burn. The flame speed is low and its ignition time long (Valera-Medina et al., 2018), which necessitates special measures to consume it successfully. One such approach is dual-fuel combustion and in particular pilot ignition, where an easy-to-ignite hydrocarbon fuel is injected into the cylinder first, so that its ignition provides a distributed ignition source for the ammonia. Reciprocating engines are expected to be one the key technologies for using ammonia in shipping in the future (Curran et al., 2024). If the ammonia is already premixed with air, then an ammonia premixed flame will initiate, but if the ammonia is injected separately as in a direct injection concept, there will be a non-premixed ammonia-air system that must be ignited. This paper deals with the latter possibility and provides some fundamental understanding of the underlying chemistry and how this is affected by diffusion.
Revealing the fundamental effects of simultaneous mixing and chemical reaction is often done by resort to the mixture fraction, as in the flamelet method [for a review, see the textbooks (Poinsot and Veynante, 2005; Peters, 2001)]. In our dual-fuel case, however, we need two mixture fractions. In the current analysis, the Doubly Conditional Moment Closure method presented by Sitte and Mastorakos (Philip, 2019) is extended to a two-mixture fraction approach. There have been previous efforts in 2D flamelet calculations using the two mixture fraction approach in staged combustion (secondary oxidizer stream) (Perry et al., 2017; Yu and Watanabe, 2023) accurately capturing the flame structure of the combustion system, but there have not been explorations of flame structures in mixture fraction space for hydrocarbon-ammonia systems. Here, the general features of hydrocarbon ignition will be mimicked by decane, for which detailed chemistry is available.
The objectives of this paper are to study decane-ammonia combustion systems, and in particular decane autoignition and the subsequent ignition of the ammonia-air flame, through a Doubly-Conditional Moment Closure formulation. The structure of the flames is explored and the sensitivity to scalar dissipation quantified.
2 Numerical methods
2.1 Flamelet and CMC
In the standard flamelet approach, the functions T (
Neglecting the terms associated with gradients along the flame front (
The only term depending on spatial variables (
The flamelet equations can also be understood as a subset of the Conditional Moment Closure approach, reviewed in Bilger (1993), where the conditioning is performed based on the mixture fraction (Peters, 2001). Rather than focusing on flame surface statistics and the laminar reactive-diffusive structure tied to the flame surface as is the case in the flamelet model, CMC employs conditional moments at a particular location x and time t within the flow field, using the conditional probability density function (Peters, 2001). The flame structure is characterized by the conditional averages of the reactive scalars and the conditional mean
Through this averaging process, the instantaneous reactive scalar value can be recovered into the conditional mean and a deviation from the conditional mean as presented in Equation 5 (Bilger, 1993):
The core concept of the CMC method involves solving transport equations for the conditional averages of reactive scalars in order to determine the flame structure (Philip, 2019). Furthermore, an exact transport equation for these conditional moments can be derived. That is by adopting a set of assumptions, including high Reynolds number, the primary closure hypothesis, molecular-level Fickian mass diffusion, a Lewis number of unity, and negligible conditional density fluctuations, the equation can be simplified to the form of Equation 6 (Klimenko and Bilger, 1999):
The unconditional mean of a variable can be determined from its conditional means as follows:
2.2 DCMC equations
The DCMC equations presented in this sub-section are adapted from the work of Philip (2019). The rationale for using double conditioning is that if most fluctuations around the single-conditional mean can be linked to the second conditional variable, then the fluctuations around the double-conditional mean, determined by two mixture fractions, should be small (Philip, 2019). As singly-conditioned CMC models are limited to cases that are either premixed or predominantly non-premixed (Philip Sitte and Mastorakos, 2019) the double conditioning allows for a wider description of phenomena present in dual fuel systems. The DCMC method extends the parameter space and incorporates the cross-scalar dissipation rate, while remaining a straightforward generalization of the CMC approach. Additionally, it is expected to describe well all combustion modes relevant to dual-fuel engines (Harikrishnan et al., 2024). The doubly conditional space is as follows in Equation 8:
The conditional mass fractions are given in Equation 9 and the conditional enthalpy in Equation 10. The conditional temperature equation also exists but only one of the temperature and enthalpy need to be solved choosing the latter:
where, according to Harikrishnan et al. (2024), the following are true:
The conditional expectation of the mixture fraction SDR,
where,
where
The ratio
2.3 0D-DCMC equations
To initially explore flame structures in dual-fuel systems, as in this paper, a simplification process is followed reducing the DCMC equations to zero dimension and essentially going from three spatial and two mixture fraction dimensions to only two mixture fraction dimensions. The underlying assumptions of the 0D-CMC equations are.
Under these assumptions, the 0D-DCMC equations assume the form of Equations 17, 18.
In this paper, 0D-DCMC simulations will be performed to understand the basic structure of dual-fuel autoignition systems, as a first step in a full implementation in a turbulent reacting flow multi-dimensional simulation.
2.4 Initial conditions
In this section, the initial conditions of the solution of the equations are discussed. Two mixture fractions are used, one for each fuel,
With the presence of two fuels, it is not clear what to do in the top right corner. The points
As the chemistry is detailed and the
2.5 Numerical procedure
The 0D-DCMC equation is a parabolic PDE in two dimensions (the two mixture fractions) and time. In a full 3D simulation in the future, we would add the 3 spatial dimensions, as in Philip, (2019), but this is not done here. The mixture fraction spaces are discretised with a 71 x 71 nonuniform grid covering the whole range of 0–1. The choice of its size is dictated by the trade-off between accuracy and computational effort. The grid is denser around the stoichiometric mixture fraction of each fuel as the flame and most reactions are expected to lie there. Due to the steep gradients and the rapid construction and destruction of species around stoichiometry, a very dense grid is required around there for accuracy. As a result, using a non-uniform grid allows for significant computational improvements. The diffusion in mixture fraction space is solved first with the VODPK solver (Alan, 1983), using the non-stiff Adam’s method which solves the set of non-linear ODEs. Central-differencing schemes for non-uniform grids, which are second-order accurate, are used for the numerical discretisation of the
Chemical source terms are addressed last using the SpeedCHEM solver (Perini et al., 2012). This solver, referred to as LIBSC, utilizes a sparse Jacobian formulation and precomputed, temperature-dependent properties to greatly enhance computational speed. It also supports the use of an analytical Jacobian, which approximates the Jacobian matrix of the ODE system. For most chemical mechanisms, this approach offers significant performance enhancements. Timesteps equal or smaller than
2.6 Chemical mechanism
This section briefly discusses the selection of the chemical mechanism used for the simulations. Typically, the physical properties of diesel, such as density and evaporation rate, are modeled using decane or dodecane, while heptane is used to mimic diesel’s chemistry. Most current chemical mechanisms for ammonia/hydrocarbon fuels are derived by merging databases for each fuel and as a result in general further research is required in this area.
A recently published chemical scheme, the Aalto75 model (Tolga Kurumus et al., 2024), includes 75 species and 451 reactions and is reported to be well-validated across various experimental datasets, including ignition delay times, laminar flame speeds, and species profiles. However, it was not used in this study as it was released after the project had already begun and is left for future work. Another chemical mechanism for ammonia/n-heptane mixtures was published by (Thorsen et al., 2023), offering good predictions of ignition delay times at pressures up to 100 bar. However, this mechanism was not selected due to its complexity, as it has too many species and reactions, making it very difficult to handle computationally for the current project.
The chemical mechanism ultimately chosen for this study is the one provided by Lin Tay et al. (2017) for decane-ammonia combustion, consisting of 80 species and 374 reactions. The ignition delay times for diesel and ammonia were found to be very similar with the parent mechanisms, indicating that this mechanism is sufficiently robust for use in diesel engine simulations and should contain the key aspects of the problem.
3 Results and discussion
3.1 0D reactor evaluation
In this section, homogeneous reactor calculations with the use of Cantera are performed. The motivation is twofold: to validate that the chemical mechanism produces reasonable results for homogeneous mixtures and to locate the “most reactive” mixture fraction
The temperature of the oxidizer is left as a parameter and the fuel temperature is set constant to 300 K. The calculations are based on a series of batch reactors for the different temperatures and pressures with the mixture fraction being varied with the initial condition as above. For each temperature or pressure, the most reactive mixture fraction is the one producing the smallest ignition delay.
A typical batch reactor simulation for the ignition of homogeneous ammonia/air mixtures is presented in Figure 3 for an oxidiser temperature of 1200 K and a pressure of 2 atm and it is found that ignition takes place at approximately 1.1 ms. We notice that the rapid temperature increase is correlated with the rise of the species OH and
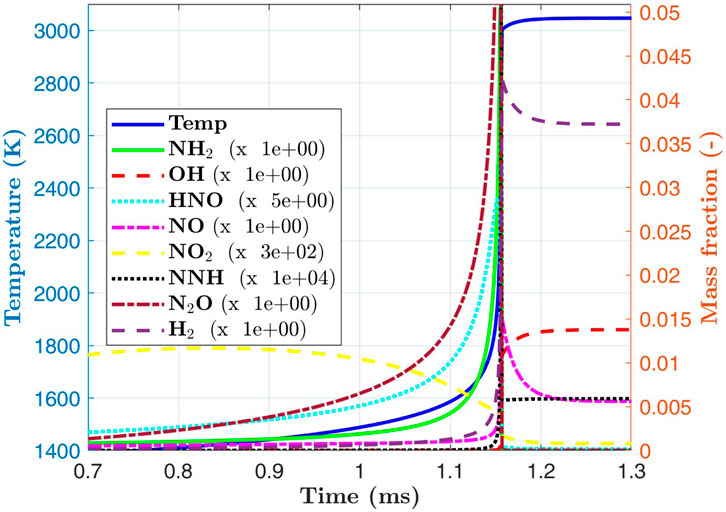
Figure 3. Temperature and various species during autoignition of ammonia/air for
For the case of ammomia/air mixing layers, i.e., where the initial condition of species and temperature are the inert mixing between pure ammonia and pure air, Figures 4, 5 show the variation of ignition delay time with mixture fraction for different pressures and temperatures. These curves serve to locate the most reactive mixture fraction which is the minimum of those U-bell shapes. For low values of mixture fraction, despite the high temperatures, the mixture is too lean while for high mixture fractions, the temperature is low and the mixture too rich, and hence the minimum autoignition time is somewhere between and this characterises the most reactive mixture fraction. The most reactive mixture fraction
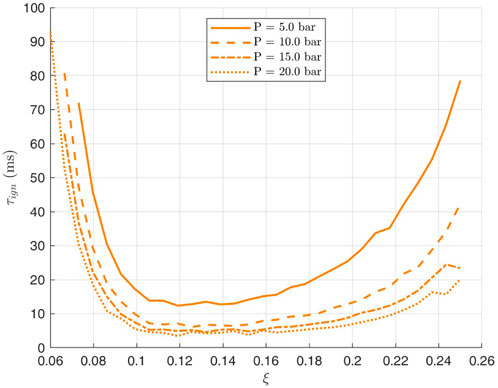
Figure 4. Variation of ammonia ignition delay with mixture fraction
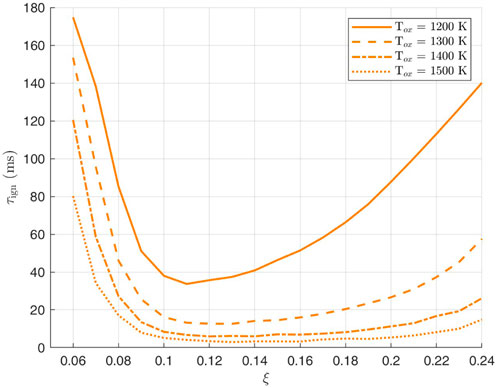
Figure 5. Variation of ammonia ignition delay with mixture fraction
The same procedure is followed for the decane-air mixture. In Figures 6, 7 ignition delay in terms of the mixture fraction
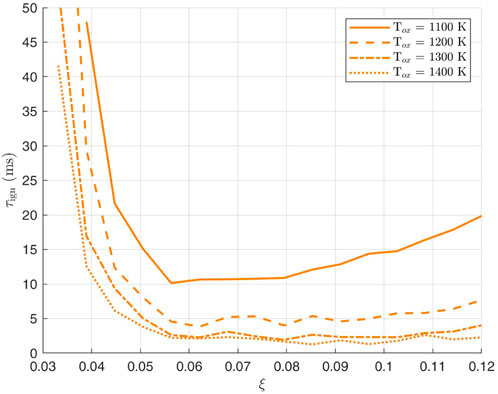
Figure 6. Variation of Decane Ignition delay with Mixture fraction
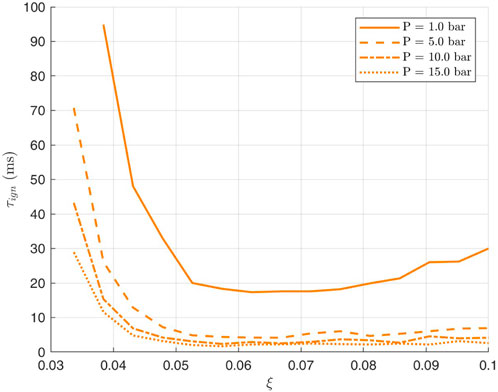
Figure 7. Variation of decane ignition delay with mixture fraction
3.2 Autoignition and evolution in 0D-DCMC
In this section, the 0D-DCMC exploration takes place to understand the effects of the scalar dissipation rates on the ignition of the decane-ammonia mixture. An overall description is given first, followed by a more detailed presentation of the structure of the autoignition spot and the evolution of the reaction fronts. The sensitivity to scalar dissipation rates is also shown.
3.2.1 General behaviour
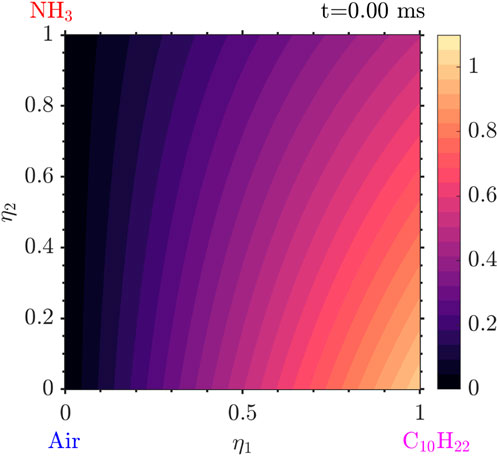
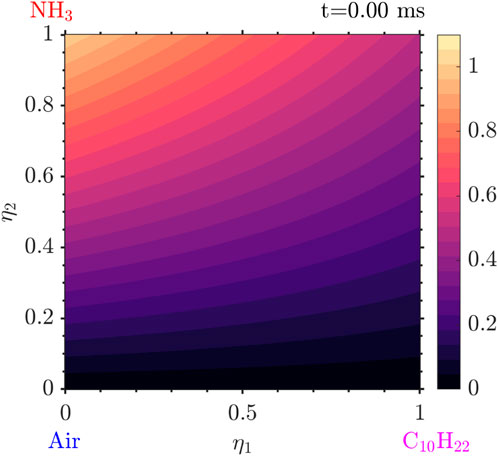
The results presented in this section are from a simulation for
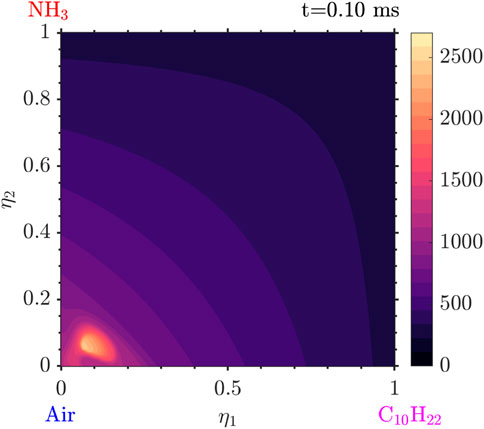
The evolution of the solution starts with the ignition of the decane and low
Following that, very quickly, the
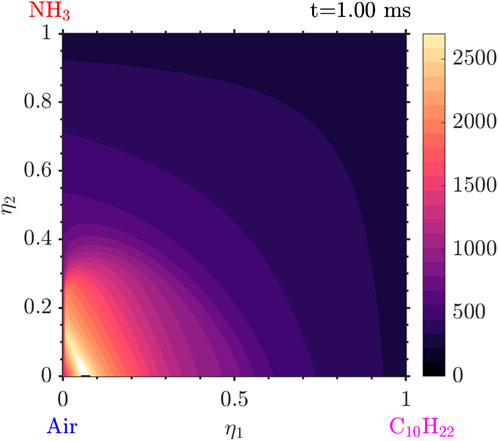
Figure 9. Temperature distribution at
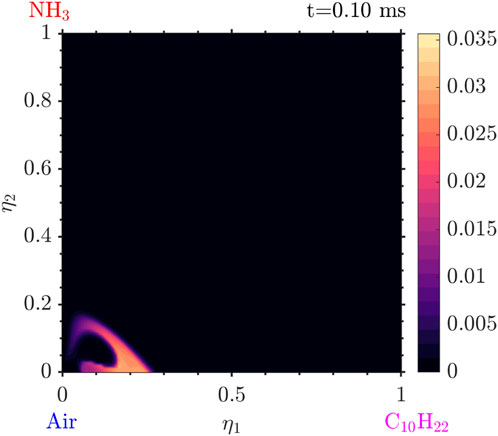
Figure 10.
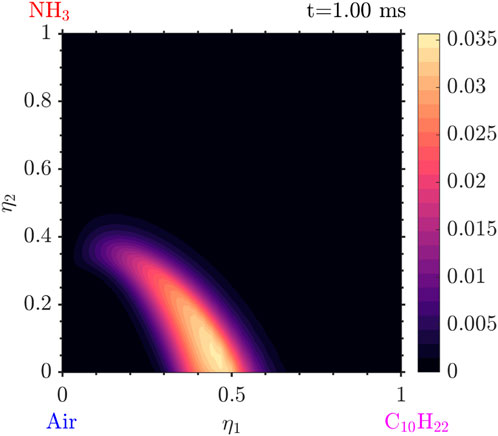
Figure 11.
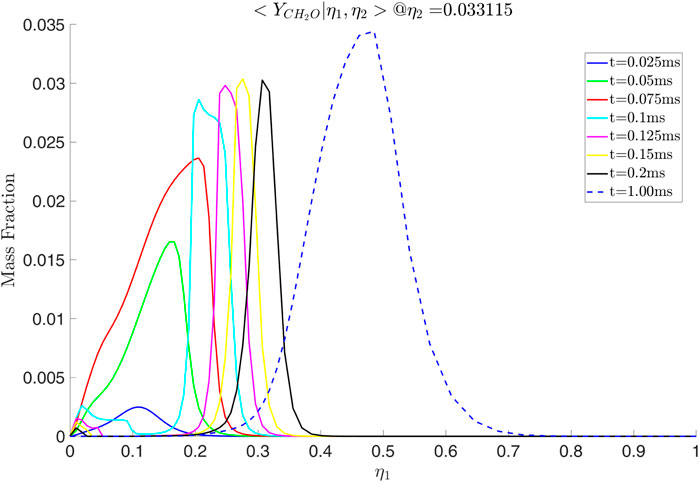
Figure 12. Conditional mass fraction of
Tracking the temperature as well as the mass fraction for the values of

Figure 13. Time evolution of the temperature and the OH mass fraction at (
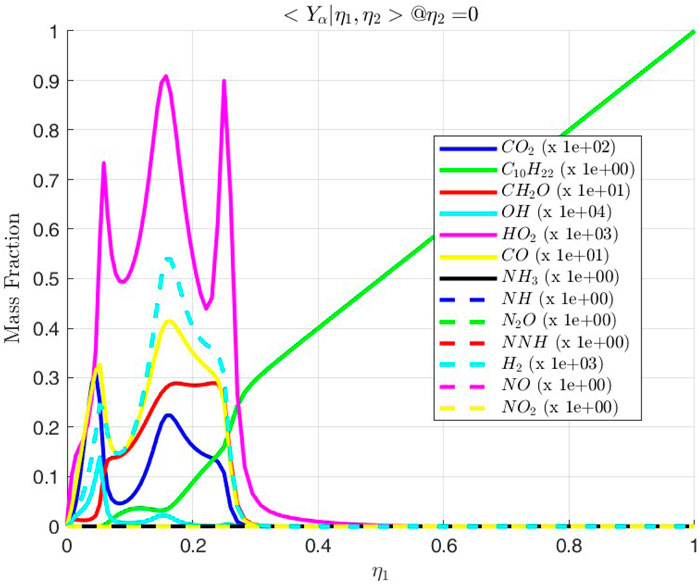
Figure 14. Conditional mass fractions of various species along the line
Various species due to the presence of ammonia are also observed. In Figure 15 it is seen that the
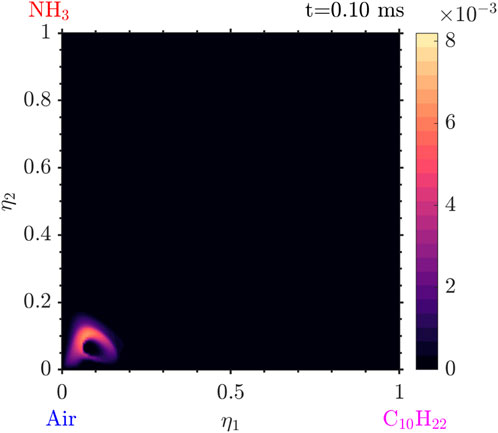
Figure 15. Mass fraction of
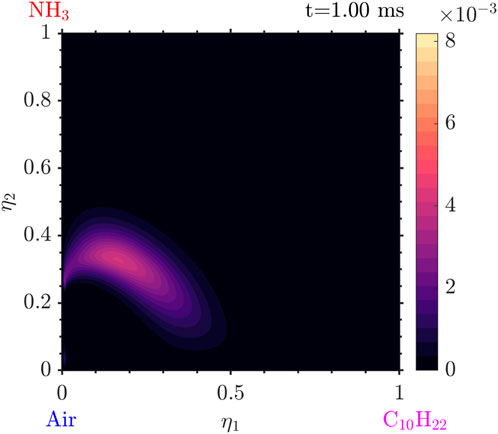
Figure 16. Mass fraction of
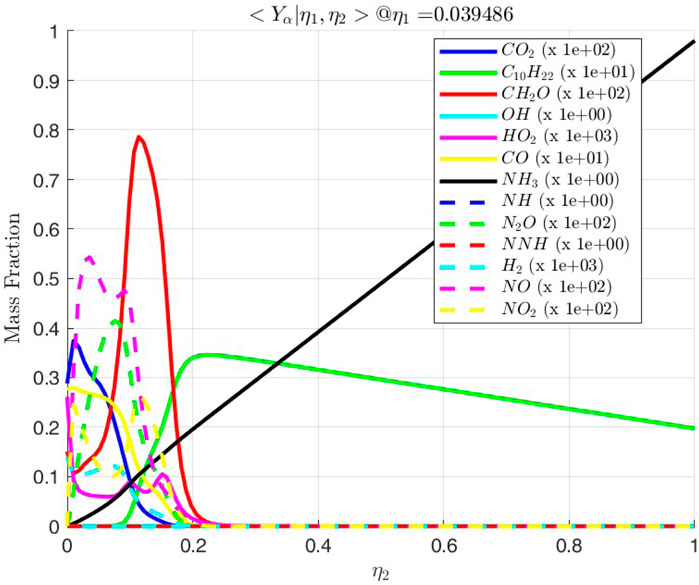
Figure 17. Conditional mass fractions as a function of
3.2.2 Intermediate phase
After autoignition, the flame grows across both mixture fractions. The diffusion flame of decane-air with no
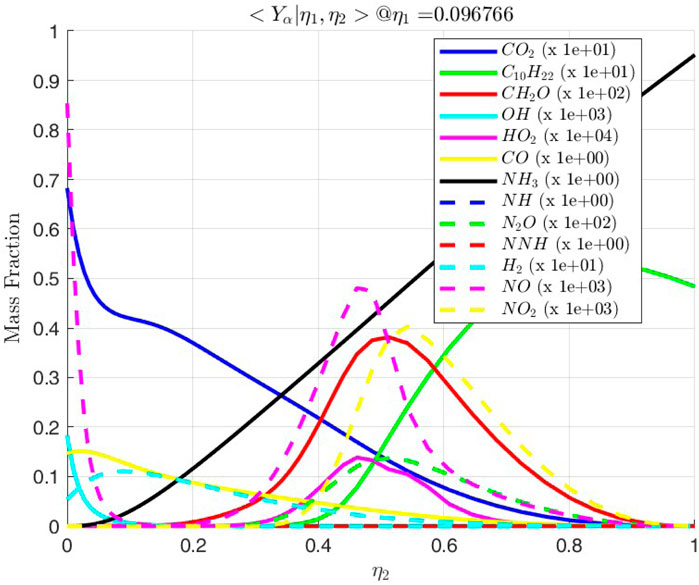
Figure 18. Conditional mass fractions vs.
3.2.3 Ignition of line
The line
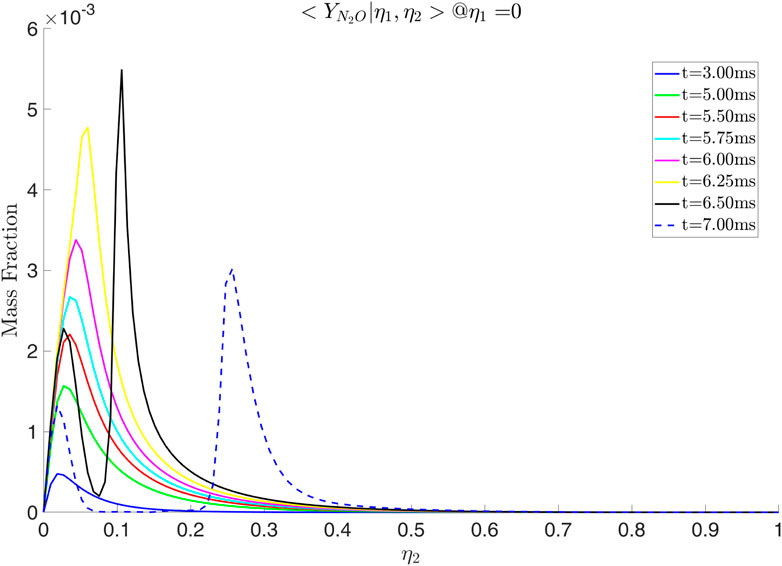
Figure 19.
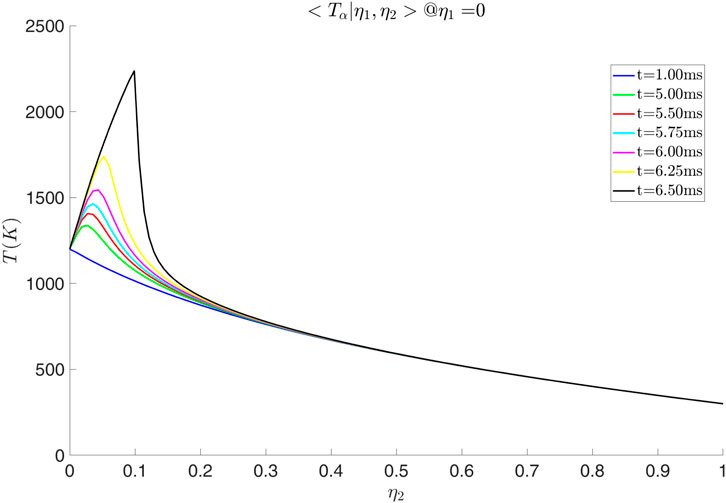
Figure 20. Temperature distribution along
3.2.4 Effects of scalar dissipation rates
Simulations with various values of scalar dissipation were performed. Examining Figures 21, 22 we observe that a positive cross-scalar dissipation rate leads to later ignition in comparison to a negative cross scalar dissipation rate. Additionally, we observe that for a higher magnitude of scalar dissipation rate the ignition is delayed in comparison to the smaller ones. Moreover, the difference in ignition time when the scalar dissipation rate is small is insignificant, as suggested by Mastorakos (2009) from analysis of autoignition in single mixture fraction. As the scalar dissipation increases, eventually autoignition does not occur, as expected (not shown here). Analyzing the evolution of the solution from Figure 23 it is evident that the diffusion of radicals is altered judging from the conditional mass fraction of
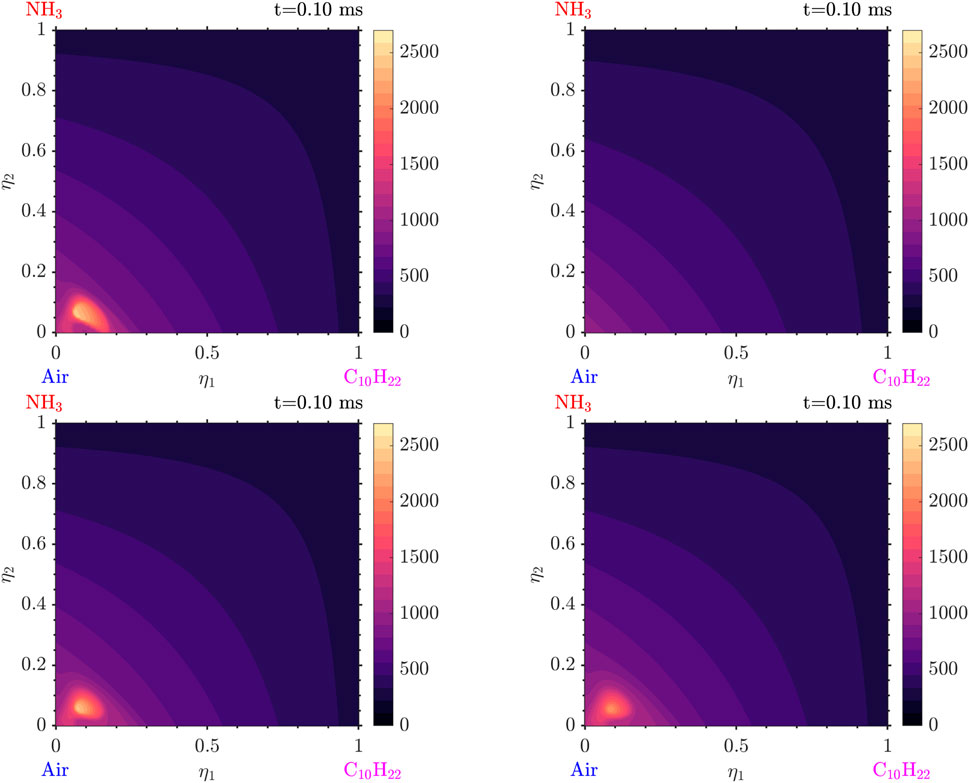
Figure 21. Distributions in
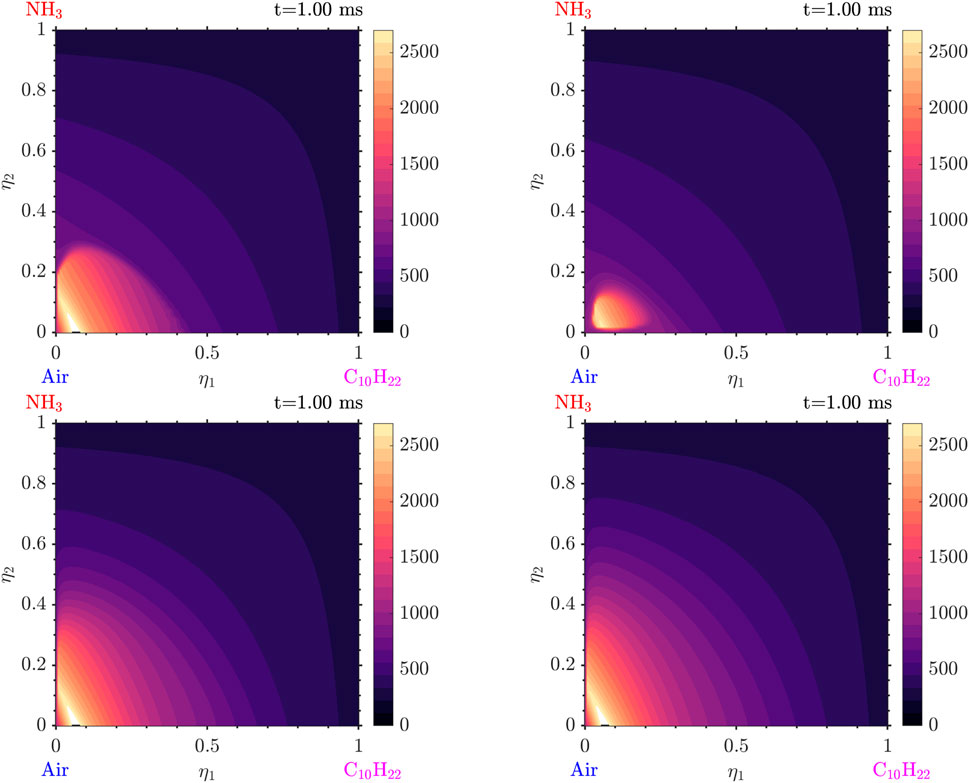
Figure 22. Distributions in
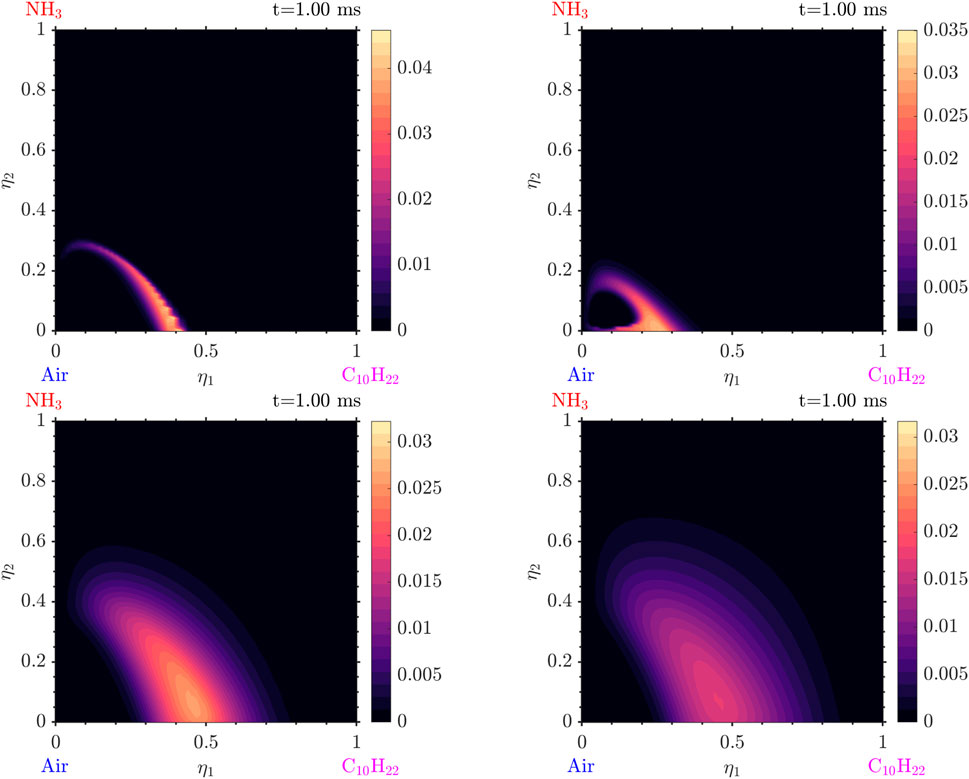
Figure 23. Distributions in
Other findings include the following: as the scalar dissipation rate increases slightly smaller temperatures and slightly reduced amounts of radicals are observed. An interesting point is that, due to the different scalar dissipation rates, each mixture ignites in a different
In general, it is observed that high values of scalar dissipation rates can slow down reactions and therefore delay autoignition in those areas. On the contrary, the low scalar dissipation rates allow for the accumulation of species with time and temperature for the reactions to occur faster and more strongly. As a result in the general case of a flow, the mixture is expected to firstly ignite in the regions with low scalar dissipation rates and less intense mixing.
It is evident that for use in a turbulent reacting flow simulation, the magnitude of the two scalar dissipation rates, but also of the cross scalar dissipation rate, can make a large difference to the autoignition time and the evolution of species, and hence pollutants, as a function of time. Further research is needed in this area.
4 Conclusion
Simulations in a two-dimensional mixture fraction space with prescribed scalar dissipation and cross scalar dissipation rates were performed to provide insights to non-premixed autoignition of a decane-ammonia-air system. Such situations may be expected in novel ammonia engines with direct injection of both fuels and the present results offer some basic understanding of the underlying canonical problems. Due to its higher reactivity, decane ignites first and then this creates reaction fronts that move across both mixture fractions to establish eventually the non-premixed flames. For low values of the scalar dissipation, its value does not affect the autoignition time significantly, however for large values autoignition is delayed. The cross-scalar dissipation (sign and magnitude) can make a large difference in the evolution of the system, as it affects the diffusion of heat and species from the autoignition spot to the remaining unburnt fluid. Positive cross scalar dissipation leads to later autoignition compared to negative values, suggesting a diffusion of radicals into the ammonia-air mixture that act as a sink, hence delaying thermal runaway.
The present results provide a first evaluation of Doubly-Conditioned Moment Closure for hydrocarbon-ammonia systems and the results suggest that care is needed with the modelling of the cross scalar dissipation rate.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AK: Data curation, Formal Analysis, Investigation, Methodology, Writing–original draft. EM: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was partly funded by the National Research Foundation (NRF), Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) programme.
Acknowledgments
We wish to thank Drs. S. Gkantonas and B. Hariskrishnan for their help with the code. This research was partly funded by the National Research Foundation (NRF), Prime Minister’s Office, Singapore, under its Campus for Research Excellence and Technological Enterprise (CREATE) programme. For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising from this submission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alan, C. H. (1983). Odepack, a systemized collection of ode solvers. Washington, Scientific computing.
Bilger, R. W. (1993). Conditional moment closure for turbulent reacting flow. Phys. Fluids A Fluid Dyn. 5 (2), 436–444. doi:10.1063/1.858867
Chakraborty, N., Mastorakos, E., and Cant, R. S. (2007). Effects of turbulence on spark ignition in inhomogeneous mixtures: a direct numerical simulation (dns) study. Combust. Sci. Technol. 179 (1-2), 293–317. doi:10.1080/00102200600809555
Chin, L. L., Foscoli, B., Mastorakos, E., and Evans, S. (2021). A comparison of alternative fuels for shipping in terms of lifecycle energy and cost. Energies 14 (24), 8502. doi:10.3390/en14248502
Curran, S., Onorati, A., Payri, R., Kumar Agarwal, A., Arcoumanis, C., Bae, C., et al. (2024). The future of ship engines: renewable fuels and enabling technologies for decarbonization. Int. J. Engine Res. 25 (1), 85–110. doi:10.1177/14680874231187954
Harikrishnan, B., Gkantonas, S., and Mastorakos, E. (2024). LES-DCMC of dual-fuel ignition problems. American Institute of Aeronautics and Astronautics.doi:10.17863/CAM.104482
International Maritime Organization (2015). Third IMO GHG study 2014. London: International Maritime Organization.
Joung, T.-H., Kang, S.-G., Lee, J.-K., and Ahn, J. (2020). The imo initial strategy for reducing greenhouse gas (ghg) emissions, and its follow-up actions towards 2050. J. Int. Marit. Saf. Environ. Aff. Shipp. 4 (1), 1–7. doi:10.1080/25725084.2019.1707938
Klimenko, A. Y., and Bilger, R. W. (1999). Conditional moment closure for turbulent combustion. Prog. Energy Combust. Sci. 25 (6), 595–687. doi:10.1016/s0360-1285(99)00006-4
Kronenburg, A. (2004). Double conditioning of reactive scalar transport equations in turbulent nonpremixed flames. Phys. Fluids 16 (7), 2640–2648. doi:10.1063/1.1758219
Lin Tay, K., Yang, W., Li, J., Zhou, D., Yu, W., Zhao, F., et al. (2017). Numerical investigation on the combustion and emissions of a kerosene-diesel fueled compression ignition engine assisted by ammonia fumigation. Appl. Energy 204, 1476–1488. doi:10.1016/j.apenergy.2017.03.100
MAN Energy Solutions (2023). Man bw two-stroke engine operating on ammonia. Available at: https://www.man-es.com/docs/default-source/marine/tools/man-b-w-two-stroke-engine-operating-on-ammonia.pdf, (Accessed August 21, 2024).
Mastorakos, E. (2009). Ignition of turbulent non-premixed flames. Prog. Energy Combust. Sci. 35 (1), 57–97. doi:10.1016/j.pecs.2008.07.002
O’Brien, E. E., and Jiang, T.-L. (1991). The conditional dissipation rate of an initially binary scalar in homogeneous turbulence. Phys. Fluids A Fluid Dyn. 3 (12), 3121–3123. doi:10.1063/1.858127
Perini, F., Galligani, E., and Reitz, R. D. (2012). An analytical jacobian approach to sparse reaction kinetics for computationally efficient combustion modeling with large reaction mechanisms. Energy and Fuels 26 (8), 4804–4822. doi:10.1021/ef300747n
Perry, B. A., Mueller, M. E., and Masri, A. R. (2017). A two mixture fraction flamelet model for large eddy simulation of turbulent flames with inhomogeneous inlets. Proc. Combust. Inst. 36 (2), 1767–1775. doi:10.1016/j.proci.2016.07.029
Peters, N. (2001). Turbulent combustion. Meas. Sci. Technol. 12 (11), 2022. doi:10.1088/0957-0233/12/11/708
Philip, S. M. (2019). Modelling of spray combustion with doubly conditional moment closure. Flow, Turbulence and Combustion. 96, 933.
Philip Sitte, M., and Mastorakos, E. (2019). Large eddy simulation of a spray jet flame using doubly conditional moment closure. Combust. flame 199, 309–323. doi:10.1016/j.combustflame.2018.08.026
Poinsot, T., and Veynante, D. (2005). Theoretical and numerical combustion. Philadeplhia: RT Edwards Inc.
Ramanathan, A., Dharmalingam, B., and Thangarasu, V. (2023). Advances in clean energy. Boca Raton: CRC Press. 1. doi:10.1201/9781003055686
Simon, B., Mason, J., Broderick, J., and Larkin, A. (2020). Shipping and the paris climate agreement: a focus on committed emissions. BMC Energy 2 (1), 5. doi:10.1186/s42500-020-00015-2
Thorsen, L. S., Jensen, M. S. T., Pullich, M. S., Christensen, J. M., Hashemi, H., Glarborg, P., et al. (2023). High pressure oxidation of nh3/n-heptane mixtures. Combust. Flame 254, 112785. doi:10.1016/j.combustflame.2023.112785
Tolga Kurumus, A., Bhattacharya, A., Tamadonfar, P., and Kaario, O. (2024). A skeletal mechanism for n-dodecane/ammonia combustion and an open-source reaction scheme optimization tool. Fuel 373, 132168. doi:10.1016/j.fuel.2024.132168
United Nations (2018). Review of Maritime transport 2018, in United Nations Conference on Trade and Development (UNCTAD), Geneva, 30 December 1964.
UNFCCC (2024). United Nations Framework Convention on Climate Change (UNFCCC). The paris agreement. Available at: https://en.wikipedia.org/wiki/United_Nations_Framework_Convention_on_Climate_Change (Accessed: 2024-January-23).
Valera-Medina, A., Xiao, H., Owen-Jones, M., David, W. I. F., and Bowen, P. J. (2018). Ammonia for power. Prog. Energy Combust. Sci. 69, 63–102. doi:10.1016/j.pecs.2018.07.001
Wong, A. Y. H., Selin, N. E., Eastham, S. D., Mounaïm-Rousselle, C., Zhang, Y., and Allroggen, F. (2024). Climate and air quality impact of using ammonia as an alternative shipping fuel. Environ. Res. Lett. 19, 084002. doi:10.1088/1748-9326/ad5d07
Yu, P., and Watanabe, H. (2023). A simplified two-mixture-fraction-based flamelet modelling and its validation on a non-premixed staged combustion system. Combust. Theory Model. 27 (1), 37–56. doi:10.1080/13647830.2022.2144460
Keywords: ammonia, conditional moment closure, non-premixed, decane, dual-fuel
Citation: Kylikas A and Mastorakos E (2025) Simulations of decane-ammonia autoignition in two mixture fractions. Front. Mech. Eng. 10:1498820. doi: 10.3389/fmech.2024.1498820
Received: 19 September 2024; Accepted: 12 December 2024;
Published: 30 January 2025.
Edited by:
Georgios Bikas, Georg Simon Ohm University of Applied Sciences Nuremberg, GermanyReviewed by:
Jinlong Liu, Zhejiang University, ChinaGiancarlo Sorrentino, Istituto di Scienze e Tecnologie per l’Energia e la Mobilità Sostenibili (STEMS) - CNR, Italy
Copyright © 2025 Kylikas and Mastorakos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Epaminondas Mastorakos, ZW0yNTdAZW5nLmNhbS5hYy51aw==
 Angelos Kylikas
Angelos Kylikas Epaminondas Mastorakos
Epaminondas Mastorakos