- STEMS-CNR, Institute of Science and Technology for Sustainable Energy and Mobility, Italian National Research Council, Napoli, Italy
Ammonia (NH3) is among the largest-volume chemicals produced and distributed in the world and is mainly known for its use as a fertilizer in the agricultural sector. In recent years, it has sparked interest in the possibility of working as a high-quality energy carrier and as a carbon-free fuel in internal combustion engines (ICEs). This review aimed to provide an overview of the research on the use of green ammonia as an alternative fuel for ICEs with a look to the future on possible applications and practical solutions to related problems. First of all, the ammonia production process is discussed. Present ammonia production is not a “green” process; the synthesis occurs starting from gaseous hydrogen currently produced from hydrocarbons. Some ways to produce green ammonia are reviewed and discussed. Then, the chemical and physical properties of ammonia as a fuel are described and explained in order to identify the main pros and cons of its use in combustion systems. Then, the most viable solutions for fueling internal combustion engines with ammonia are discussed. When using pure ammonia, high boost pressure and compression ratio are required to compensate for the low ammonia flame speed. In spark-ignition engines, adding hydrogen to ammonia helps in speeding up the flame front propagation and stabilizing the combustion. In compression-ignition engines, ammonia can be successfully used in dual-fuel mode with diesel. On the contrary, an increase in NOx and the unburned NH3 at the exhaust require the installation of apposite aftertreatment systems. Therefore, the use of ammonia seems to be more practicable for marine or stationary engine application where space constraints are not a problem. In conclusion, this review points out that ammonia has excellent potential to play a significant role as a sustainable fuel for the future in both retrofitted and new engines. However, significant further research and development activities are required before being able to consider large-scale industrial production of green ammonia. Moreover, uncertainties remain about ammonia safe and effective use and some technical issues need to be addressed to overcome poor combustion properties for utilization as a direct substitute for standard fuels.
1 Introduction
The increase in the overall temperature on and above Earth’s surface represents one of the key issues we need to face in the next future. Our planet’s climate is significantly changing mainly due to human activities, and the transportation sector plays a significant role in this global warming. Therefore, at the moment, actions to mitigate the effects of transport on the Earth’s atmosphere are extensively studied and analyzed. Along with the electrification of the sector, manufacturers and researchers are exploring alternative and advanced fuels that may represent an efficient way to reduce global CO2 emissions when assessed on a well-to-wheels basis.
Moreover, the very unstable equilibrium of the world political situation makes it increasingly necessary to untie the transport sector from fossil fuels.
In this scenario, new carbon-free fuels attainable from renewable sources are of increasing strategic relevance.
Ammonia is a versatile chemical, composed of nitrogen and hydrogen (NH3). It is mainly known for its use as a fertilizer in the agricultural sector, but it is also widely adopted as a base for chemical synthesis or involved as a molecule in many processes in different fields. For example, it is an intermediate in the synthesis of sodium bicarbonate, explosives, nylon and synthetic fibers, plastics, and polymers; a component for paints, hair dyes, and household cleaner; a refrigerant; a solvent; a whitener in the paper industry; a stabilizer in the rubber industry; a reducing agent in metallurgy; and a reagent for the control of nitrogen oxides (NOx) in the exhaust of diesel engines in aqueous solutions. For this reason, ammonia is among the largest-volume chemicals produced in the world (USGS (2021) Nitrogen Statistics and Information).
In recent years, ammonia has attracted interest because of the possibility of working as a hydrogen carrier and a carbon-free fuel. It can be defined as a hydrogen carrier due to its remarkably high hydrogen density. Indeed, it contains 1.5 mol of molecular hydrogen for each mole. It is well known about the importance of hydrogen in the transition toward the decarbonization of the transport sector (Capurso et al., 2022; Zhang et al., 2022). However, hydrogen storage remains a major challenge, limiting its direct application to vehicles: It must be stored at −253°C as a liquid or at pressures of about 700 bar as a gas. Liquid ammonia, on the other side, can be stored at a reasonable temperature of -33°C at standard pressure and +20°C at 9 bar. This makes the storage and transport of this energy carrier much easier. Hydrogen production from ammonia has been widely studied. It can be obtained through thermal decomposition or catalytic cracking of ammonia into nitrogen and hydrogen and electrolysis or electro-oxidation. Ammonia decomposition is a slow reaction with a very high energy requirement, and metal catalysts are often used to speed up hydrogen production. Much research has been devoted to developing suitable catalysts for this purpose. It is widely agreed that low-available ruthenium is the best catalyst for decomposition at 400°C, since it is highly active (Ashik et al., 2018). The widely available less expensive Ni-based catalysts perform comparably at 600°C. Further cost reductions and optimization of the catalyst and reaction processes will be required to ensure that the energy losses from the ammonia decomposition reaction are close to the theoretical minimum value of approximately 7% of the energy stored in the ammonia molecule (Lamb et al., 2018).
In addition, ammonia can be directly used as a fuel in combustion systems and, in particular, in internal combustion engines. This study examines precisely the potential of ammonia as an engine fuel. First of all, the ammonia production process is briefly discussed with more emphasis on new ways to produce green ammonia. The chemical and physical properties of ammonia as a fuel are described and explained in order to identify the main pros and cons of its use in internal combustion engines. Then, the most viable solutions for powering internal combustion engines with ammonia are discussed.
2 Ammonia Production Process
The synthesis of ammonia occurs starting from gaseous hydrogen and nitrogen according to the direct reaction in the gaseous phase:
3H2 + N2 → 2 NH3.
The reaction is reversible and exothermic with ΔH = −92.4 kJ/mol.
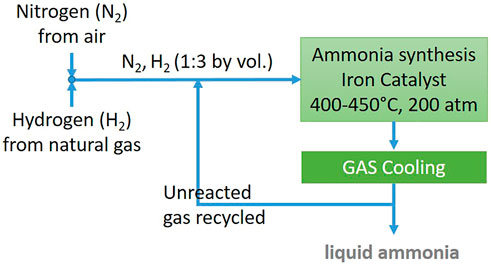
The main process for ammonia production is the Haber–Bosch (H-B) one in which the reaction takes place in the presence of catalysts, at a pressure of 200 atm and a temperature of 400–450°C. Figure 1 shows a simplified scheme of a typical Haber–Bosch process. Suitable catalysts are based on osmium, ruthenium, uranium, or iron, even though the latter is generally used, preparing the catalyst starting from magnetite. The H-B process is a closed-loop process, where the ammonia is separated from the product stream by cooling and further condensation. Next, the unreacted synthesis gas is mixed with the fresh feed and sent back to the ammonia synthesis reactor (Rossetti, 2020).
The Haber–Bosch process has been optimized over the past century, starting with an energy consumption of about 100 GJ/tNH₃ in the 1930s down to about 26 GJ/tNH₃ today (Rouwenhorst et al., 2021).
There are also alternative processes (Fauser, Casale, Claude, NEC, Mont-Cenis, etc.), which differ in the pressure at which the reaction takes place and therefore in the synthesis apparatus.
For all these processes, nitrogen (large-scale production) is obtained by rectification of liquid air produced with “Linde” or with “Claude” processes (Agrawal and Woodward, 1991). Moreover, technologies such as pressure swing adsorption or membranes can be used for smaller production capacities (Sánchez and Martín, 2018).
Hydrogen used as a reagent is currently mainly produced from natural gas, which can be subjected to steam reforming or alternatively to an autothermal process; the latter is a cheaper method from a plant engineering point of view and involves partial oxidation of the hydrocarbon into carbon monoxide and hydrogen using an appropriate amount of air, in a muffled oven above 1,000°C in the presence of an oxide-supported nickel catalyst of magnesium. A lower percentage of hydrogen is produced through coal gasification, which is significantly more emission-intensive than natural gas-based production. Most of the ammonia production from coal takes place in China. About 85% of the country’s ammonia production occurs through coal gasification. Much smaller amounts of ammonia are produced through coal gasification in the United States, South Africa, and Indonesia. In 2020, of the 185 Mt of ammonia produced, 72% relied on natural gas–based steam reforming, 26% on coal gasification, and about 1% on oil products. These numbers indicate that currently, ammonia is primarily produced from fossil fuels (called “gray ammonia”): This kind of production requires high energy (∼28–33 GJ/tNH3) and produces high CO2 emissions [∼1.6 tCO2/tNH3, about 1.8% of global carbon dioxide emissions (The Royal Society, 2020)] mainly due to the energy- and carbon-intensive reforming process to produce hydrogen (Tock et al., 2015).
Ammonia is defined as “blue” when carbon capture and storage (CCS) systems are included in its production. CCS technologies are expected to increasingly contribute to cleaner energy transitions by significantly reducing CO2 emissions from point sources on a large scale, including power generation plants and industrial plants using fossil fuels or biomass. Globally, this can mitigate the increase in CO2 emissions, but these technologies are at an early stage of research and development, so they are not ready for commercialization and are not cost-effective (Mallouppas et al., 2022).
When ammonia is synthesized only from renewable energy sources, it is named “green” ammonia. It is predicted that the global green ammonia market will rapidly grow, at a compound annual growth rate of 7.8%, from 2021 to 2027 (Han et al., 2022), which could reduce the dependence of ammonia production on fossil fuels.
2.1 Production of Green Ammonia
There are two different routes to produce green ammonia: The first one, of considerable long-term scope, is based on the Haber–Bosch process fed with hydrogen from renewable sources (green hydrogen), and the entire process is powered by fully renewable electricity. The second path is based on the electrochemical ammonia synthesis; in this case, the H-B process is no longer required.
2.1.1 First Route: Renewable Sourced Hydrogen + Haber–Bosch Process
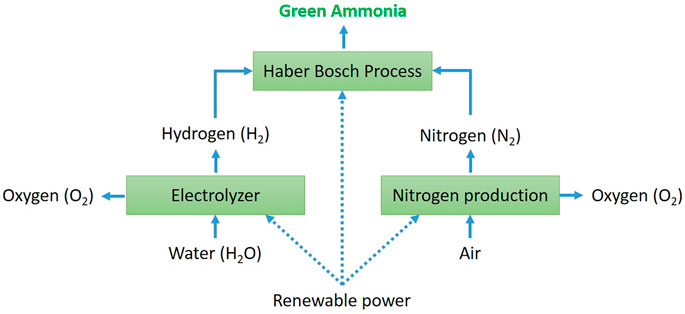
One way to synthetize green ammonia is to use hydrogen produced by renewable sources and nitrogen produced in the air separation unit (ASU). These are then fed into the Haber–Bosch process, all powered by sustainable electricity (Valera-Medina et al., 2018).
The most established technology to produce hydrogen from renewable sources is water electrolysis even if, at present, only 4% of hydrogen can be obtained in this way mainly due to economic issues (Shiva Kumar and Himabindu, 2019). Figure 2 shows a scheme of green ammonia production through electrolysis and H-B process. Electrolysis consists of splitting water molecules to produce hydrogen and oxygen as a by-product using an electric current. A water electrolysis cell consists of two electrodes separated by an electrolyte that is the medium responsible for transporting the generated chemical charges [anions (-) or cations (+)] from one electrode to the other. Water electrolysis can be classified into four types based on the type of electrolyte:
• Alkaline water electrolysis (AWE),
• Proton exchange membrane (PEM) water electrolysis,
• Solid oxide electrolysis (SOE), and
• Microbial electrolysis cells (MEC).
The electrolyzer is composed of the stack (where the actual splitting of water into hydrogen and oxygen takes place) and the rest of the plant, which comprises power supply, water supply and purification, compression, possibly electricity and hydrogen buffers, and hydrogen processing (Yue et al., 2021).
The most common and commercially viable electrolyzers are based on PEM and AWE, classified as low-temperature electrolyzers since they operate between 30°C and 80°C. SOE cells perform a high-temperature electrolysis ranging between 800 and 1,000°C. They are also under development but have yet to be deployed at scale.
Microbial electrolysis cells (MEC) are an emerging bioelectrochemical technology to produce H2 from organic matter. MEC technology is very suitable and has the potential for effective energy production in waste biorefineries (Mallick et al., 2022).
To take electrolysis from niche to mainstream, from potential to reality, research is focusing on overcoming current challenges. The first goal is to reduce the cost of green hydrogen produced in this way: The European Commission (European Commission, 2021) recently indicated that our target should be €1.8/kg by 2030, while currently (in 2020), the cost of hydrogen produced through water electrolysis powered by renewable electricity is estimated to be €6/kg. This drastic reduction can be achieved through a better understanding of the trade-offs in performance, cost, and durability of electrolyzer systems in predicted future dynamic modes of operation using CO2-free electricity such as the one generated by solar, wind, and hydropower resources. At the same time, the capital cost of the electrolyzers unit (stack) and of the rest of the plant should be reduced. The greatest potential for short-term cost reduction is in the rest of the plant, while in a long-term perspective, it is mandatory to reduce the capital cost of the stack. Furthermore, RD&D is called to show the way to improve energy efficiency for the conversion of electricity to hydrogen in a wide range of operating conditions, to deepen the understanding of the degradation processes of electrolytic cells and the stacks, and to develop mitigation strategies to increase stack operational life (IRENA—International Renewable Energy Agency, 2020).
An alternative way to produce green hydrogen that can be coupled to the H-B process for ammonia production is biomass gasification, a thermochemical process that converts biomass into hydrogen and other products (CO, CO2, CH4, and N2) without combustion. This process is carried out under substoichiometric conditions, by causing a gasification agent to flow into the reactor, which reacts with the biomass. The most commonly used gasification agent is air; however, to increase the H2 content and avoid dilution of N2, other gases can be used, namely O2, steam, or a combination thereof (Piazzi et al., 2022). Today, no plant is operated using this technology due to its high expected costs and low technological maturity. Framework conditions are yet missing for biomass gasification-based hydrogen production to be economically competitive with fossil-based hydrogen production (IEA Bioenergy et al., 2018).
In the first route of green ammonia production, it is necessary to consider that the Haber–Bosch process integrated with water electrolysis and powered by renewable electricity will need a new optimization. To enable electrically operated H-B systems to cope with the geographically isolated and intermittent nature of renewable energy, small-scale processes with low capital costs and simple operation and control, capable of agile and adjustable operation, could be designed. Developing more active H-B catalysts would facilitate operation under milder conditions and reduce energy demand, making the process more amenable to variable and smaller-scale operations (Smith and Torrente-Murciano, 2021). Moreover, ideally the H-B process runs continuously, a feature that contrasts with the intermittent supply of solar or wind electricity: This could be addressed through the creation of an intermediate energy storage solution (MacFarlane et al., 2020).
2.1.2 Second Route: Electrochemical Ammonia Synthesis
The electrochemical ammonia synthesis (EAS) has attracted a lot of attention in the present century as it does not involve carbon emissions and the optimal operating conditions require atmospheric temperature and pressure (Shen et al., 2021). The very unsustainable H-B process is not required, and the EAS can be driven by renewable electrical energy generated by solar, wind, or hydropower. EAS mainly consists of electrochemical N2 reduction reaction (NRR) to produce NH3; H-source is ultimately water.
There are different modes of this process being actively researched:
(1) the electrochemical nitrogen reduction reaction (eNRR) in which an electrocatalyst enables direct electron and proton addition to the N2 molecule,
(2) indirect or mediated NRR mechanisms in which a redox mediator is first reduced and then, via a series of reactions, ammonia is produced and the mediator is regenerated. Recently, lithium-mediated electrochemical NRR has received renewed attention due to its reproducibility. Lithium-mediated NRR begins with electrochemical deposition of lithium, followed by two chemical processes of dinitrogen splitting and protonation to ammonia (Cai et al., 2021).
Significant and encouraging progress has been made in the electrochemical nitrogen reduction reaction (NRR) under ambient conditions over the past 5 years. This process would be ideal for distributed generation and more amenable to intermittent power supplies. However, to date, only low rates of ammonia production have been demonstrated in laboratory studies. This process is currently at TRLs 1–2; key challenges remain that need to be addressed. Although thermodynamically feasible, high overpotentials are generally required to overcome the kinetic barrier of the strong N–N triple bond. Both the yield rate and the Faradaic efficiency (FE) toward NH3 formation are low. The large overpotential and low NH3 selectivity intensify the problem of energy efficiency. Another fundamental issue is the durability of the NRR process: Stability tests reported in the scientific literature are commonly below 20 h, too short a duration for the industrial implementation where thousands of hours of stable operation at high current density are expected. Therefore, future protocols for catalyst durability need to aim for much longer test durations. It is also necessary to work toward understanding catalyst degradation mechanisms. To promote electrochemical synthesis, catalyst and electrolyte should always be considered and optimized together (Shen et al., 2021).
There are several other ongoing studies on the scientific community for green ammonia synthesis such as electrochemical synthesis from nitric oxide (Long et al., 2020), electrochemical production through photoelectrochemically produced hydrogen under concentrated sunlight (Yusuf and Ibrahim, 2017), photo-electrochemical synthesis (Boucher et al., 1995; Martín et al., 2019), ammonia synthesis using nitrogenase organisms and biomimetic catalysts to create a biotechnological route (Chen et al., 2020), and electromagnetic-induced (Ghassan and Ibrahim, 2020) ammonia synthesis (Chehade and Dincer, 2021). All these processes are currently at TRL 1; further research and development would be required before large-scale industrial production could be considered.
3 Chemical and Physical Properties of Ammonia as a Fuel
3.1 NH3 Chemical and Physical Properties
Ammonia has been identified as a high-density and safely transportable hydrogen carrier to use in energy production and transportation systems, which may promptly respond to environmental policies conceived to decarbonize energy production chains. In contrast to hydrogen, the main advantage consists of available storage/transportation infrastructures, developed over the time to deliver ammonia as a chemical. Albeit with these advantages, some crucial drawbacks exist, for the combustion-based system, due to NH3 chemical/physical properties.
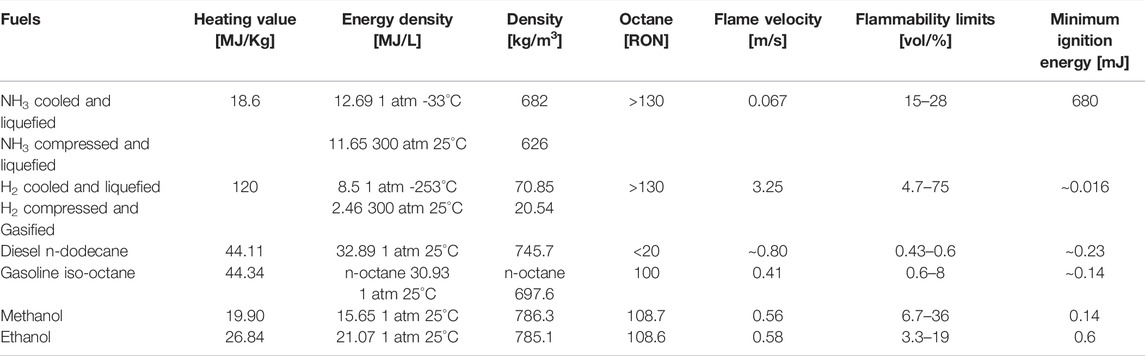
Ammonia oxidation properties, along with the comparison of common fuels for ICEs, congruently reported to highlight its problematic issues, are reported in Table 1 (readapted from https://www.iea-amf.org/content/fuel_information/ammonia).
Table 1 reports parameters relevant to ICEs, in terms of fuel energy content as lower heating value (LHV), in both the gravimetric and the volumetric form (MJ/Kg and MJ/L, respectively), fuel density (Kg/m3), octane number (RON), flame velocity (m/s), flammability limits (vol%), and minimum ignition energy (MIE, mJ). NH3 is considered in the liquified form, cooled at atmospheric pressure and T = −33°C, or pressurized at typical vessel conditions (300 bar) and at 25°C, to easily compare with liquefied or compressed hydrogen, at the same reference pressure. For comparison, diesel, such as n-dodecane, and gasoline, such as iso- or n-octane, are considered as conventional fuels in ICEs. The analysis is then extended to alcohols, such as methanol and ethanol, as relevant low-carbon fuels in ICEs, producible from renewable resources.
Among the considered fuels, NH3 has the lowest LHV in terms of MJ/Kg, comparable only with alcohols, as partially oxidized species, while H2, on a weight basis, has an energy content of 120 KJ/Kg, almost three times greater than gasoline and diesel (∼44 MJ/Kg). If the energy content is then expressed in terms of volume (MJ/L), H2 has the lowest energy density, both in pressurized (2.46 MJ/L) or in liquefied (8.5 MJ/L) forms, highlighting main concerns related to H2 storage and transportation. Apart from safety reasons, the compression work of H2 is extremely high, about 1.05 kWh/kg H2 from 20 to 300 atm, while H2 cryogenic liquefaction at −253°C requires 2.88 kWh/Kg (Gardiner and Satyapal, 2009). In turn, NH3 has an energy content equal to 12.69 MJ/L in the cooled liquified form, and 11.65 MJ/L if liquefied by compression. The conversion from gravimetric to volumetric energy contents passes through the fuel density, thus highlighting the very low hydrogen density, while conferring to NH3 the property of a high H2-density vector.
The main practical issues related to NH3 as a fuel in combustion systems are related to its poor ignition quality (RON>130), with a high autoignition temperature (924 K), low flame speed (∼7 cm/s for a stoichiometric NH3/air mixture, P = 1 atm, T = 298 K), about one order of magnitude lower with respect to conventional fuels and alcohols, and 3 with respect to H2. Then, its narrow flammability limits (15%–28% by volume in air) and a very exceptional high minimum ignition energy (MIE = 680 mJ), with respect to the other considered fuels (see Table.1), complete the picture of NH3 combustion properties.
In addition, it is worth mentioning NH3 has a high latent heat of vaporization (1370 KJ/Kg). For instance, ethanol, liquid H2, and gasoline have a latent heat of vaporization equal to 840, 445.6, and 305 KJ/Kg, respectively. This implies that if NH3 is injected into engines, combustion temperature may drastically decrease, causing incomplete combustion and loss of engine efficiency (Okafor et al., 2021).
The high NH3 RON value may be conceived as an advantage for SI engines, as its high anti-knocking tendency can result in higher compression ratios (CR) and thus in an engine efficiency increase (Cornelius et al., 1966).
Regarding NOx emissions, the high tendency to fuel NOx formation from NH3 oxidation has to be properly chamfered, through the implementation of primary (air-fuel staging, flue gas recirculation, and humidification) or post-combustion techniques [selective non-catalytic reduction (SNCR) and selective catalytic reduction (SCR)] (Skalska et al., 2010).
From a chemical point of view, many of these characteristics derive from its molecular structure. NH3 has a trigonal pyramidal shape, similar to methane, with a central nitrogen covalently bonded with three hydrogen atoms and an unshared pair of electrons. The covalent N-H bond nature implies a high dissociation energy, which reflects in NH3 lower reactivity with respect to the standard fuels in combustion systems.
The structure with the lone pair, which easily accepts a proton, gives an alkaline characteristic to ammonia. On the contrary, the trigonal pyramidal asymmetrical shape, with nitrogen much more electronegative than the three H atoms, makes ammonia a molecule with a strong polarity, even higher than water. This yields to a highly hygroscopic characteristic that causes the formation of moistures, possibly very corrosive on metals, especially on copper and brass, commonly used in ICEs as gaskets.
The strong polarity of ammonia also significantly affects its oxidation kinetics, as it will be discussed in the following. In addition, NH3 under oxidative conditions may lead to conspicuous emissions of fuel NOx; thus, the comprehension of NH3 ammonia chemistry and NOx formation routes is of paramount relevance to opportunely tune system operating conditions to ensure ammonia full conversion while reducing the risk of NOx emissions.
From an organoleptic point of view, ammonia is a colorless gas, with a sharp smell. This is an advantage with respect to its toxicity. Indeed, a possible leakage is easily perceived (at about 5 ppm), well before reaching the safety levels, fixed at 25 and 35 ppm, respectively, as short-term and time-weighted average exposure limits (New Jersey Department of Health, 2016).
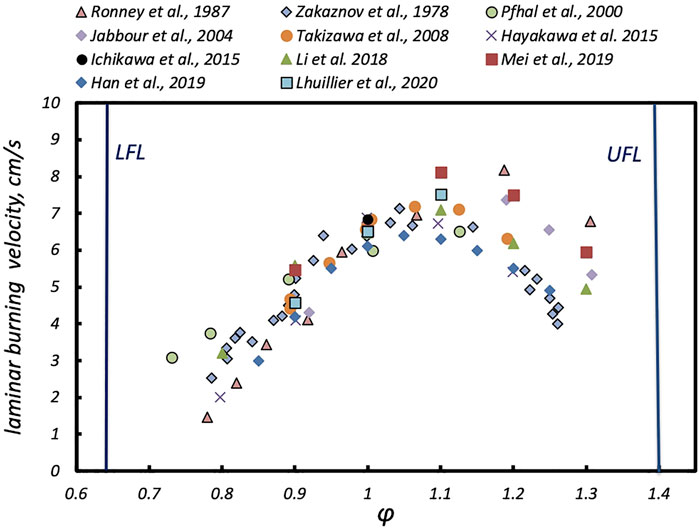
Laminar burning speed (SL) and ignition delay times are target parameters for ICEs. Restricting the analysis to SL, as a comprehensive parameter representative of characteristic chemical timescales, some consideration on the NH3 ammonia utilization can be drawn up. As a relevant parameter to combustion application, ICE and gas turbines, the laminar flame speed (SL) of NH3 has been experimentally characterized over the years with respect to mixture equivalence ratio (φ) (Figure 3) (Zakaznov et al., 1978; Ronney and Wachman, 1985; Pfahl et al., 2000; Jabbour and Clodic, 2004; Takizawa et al., 2008; Li et al., 2014; Hayakawa et al., 2015; Han et al., 2019; Mei et al., 2019; Lhuillier et al., 2020a; Lubrano Lavadera et al., 2020).
The NH3/air laminar flame speed versus φ at 298 K and 1 atm are in-between 1.4 and 8.23 cm/s for φ in the range 0.7–1.3. SL increases with φ from fuel-lean to fuel-rich conditions and peaks at around φ = 1.1, where the maximum values vary from 6.3 to 8.2 cm/s. As expected, it decreases with a further increase in φ. Small differences are identifiable for fuel-lean mixtures, while the discrepancy among experimental data increases for fuel-rich conditions, with a maximum difference of about 2 cm/s.
Hayakawa et al. (2015) demonstrated that for NH3/air mixture, an increase in the pressure from 1 to 5 atm decreases the laminar flame speed, almost linearly with P. For instance, for φ = 1, it decreases from 7 cm/s down to about 5 cm/s.
Such a low laminar flame speed may hinder the direct use of NH3 in ICEs; thus, several strategies have been proposed to increase this crucial parameter. First, the SL has been experimentally characterized for preheated NH3/air mixture. Han et al. (2019) and Lhuillier et al. (2020b) reported data with preheating the mixture up to 448 and 473 K, respectively. Restricting the analysis to the stoichiometric NH3/air mixture, SL increases almost linearly with T, up to 14 cm/s for T = 448 K.
Common strategies to improve NH3 oxidation properties rely on the use of fuel “enhancers,” such as H2, CH4, syngas, and other conventional or e-fuels (Pfahl et al., 2000; Okafor et al., 2018; Han et al., 2019; Lubrano Lavadera et al., 2020; Wang et al., 2020; Wang et al., 2021; Ariemma et al., 2022), in compliance with energy production decarbonization strategies, or modifying the environmental atmosphere toward O2-enriched conditions. H2 can also be produced directly through NH3 partial catalysis (Li et al., 2014) techniques, prior to the injection in combustion systems. Further options to improve NH3 oxidation features could come through the implementation of plasma-assisted systems (Choe et al., 2021).
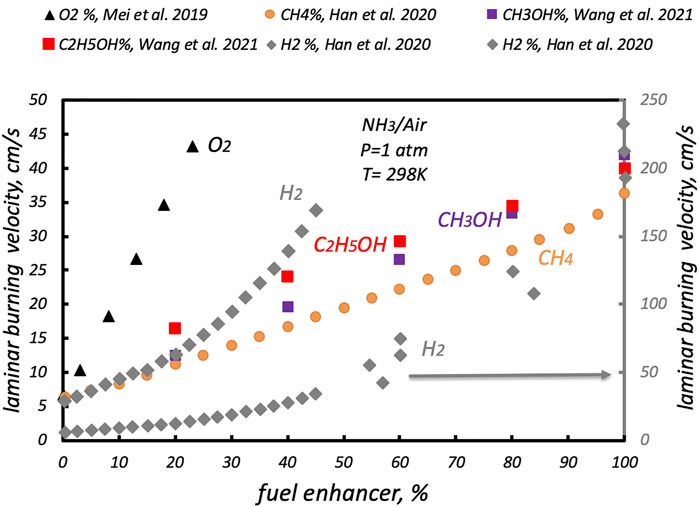
Limiting the analysis to H2, CH4, and alcohols as fuel enhancers from recent studies, in Figure 4 the ammonia laminar flame speed is reported as a function of the “fuel enhancer” concentration (reported as %) or for O2-enriched environments for a stoichiometric NH3/O2 flame. For the O2-enriched environment, the O2 concentration has to be read as a surplus with respect to the “air” condition.
The case of H2 as a “fuel enhancer” is reported on both the primary axis, up to a H2 concentration of 45%, to highlight some peculiar features, and the secondary axis, as the pure H2 laminar flame speed is 1 order of magnitude greater than the other pure “fuel” enhancers.
It is possible to note that the addition of H2 or CH4 (Han et al., 2019), and CH3OH or C2H5OH (Wang et al., 2021) notably increases the NH3 laminar burning velocity. For instance, H2 (rhombus), CH4 (dot), and alcohols (squares) increase the ammonia SL almost equally up to a relative concentration of 20%. For higher concentrations, CH4 and alcohols increase the NH3 laminar flame speed linearly with their concentration, while H2 increases that with an exponential law. A particular efficient way to increase the NH3 laminar flame speed is as follows: Under O2-enriched environments, SL is promptly increased with O2 concentration (Mei et al., 2019), starting from the “air” condition (triangles).
3.2 NH3 Oxidation Chemical Kinetic Issues
As mentioned before, the understanding of NH3 oxidation chemistry and the identification of the fuel NOx route are crucial to define the a priori system operating conditions. With this aim, over the years, many advancements in the comprehension of NH3 oxidation chemistry have been reached based on experimental and theoretical studies, albeit the development of reliable and robust kinetic schemes, especially in engine-relevant conditions, still needs some efforts. This is mainly due to the reduced availability of experimental data under high-pressure/high-temperature conditions. Recently, several experimental pieces of evidence has been proposed by different research groups, while sustaining the development of numerical studies toward more comprehensive schemes.
All the mechanisms are explained in the general description of the NH3 oxidation chemistry: In Figure 5 a general scheme of NH3 oxidation chemistry is provided.
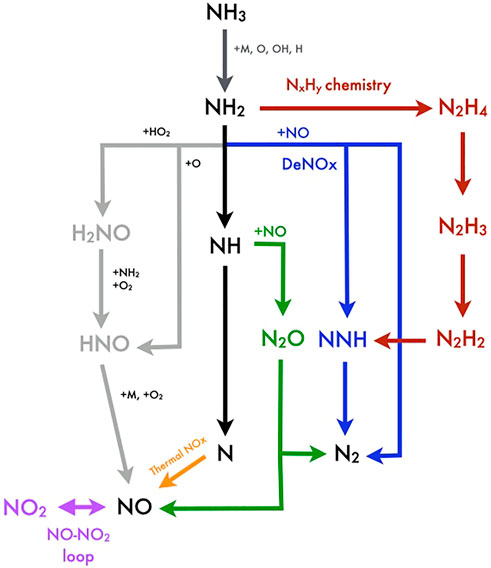
FIGURE 5. Generic scheme for NH3 oxidation chemistry, NOx formation routes, DeNOx, NxHy, and N2O chemistry, and the NO-NO2 loop.
NH3 may undergo dehydrogenation reactions to NH2 through OH, H, and O radicals or thermal decomposition (dark gray pathway). NH2 radicals may recombine to N2Hx species (red pathway), especially for fuel-rich/pyrolytic conditions and under high-pressure conditions. Konnov and de Ruyck, 2000, have introduced the N2Hx route, from N2H4 decomposition experiments in shock tubes, relevant to fuel-rich conditions, defining later also a new NOx formation route through N2H3 radicals (Konnov and de Ruyck, 2001). The importance of this pathway for the prediction of NH3 laminar flame velocities, especially for fuel-rich conditions, has been highlighted by many authors (Nakamura and Hasegawa, 2017; Okafor et al., 2018; Otomo et al., 2018; Shrestha et al., 2018; Stagni et al., 2020).
Alternatively, NH2 radicals can react with HO2 radicals at low temperatures or high pressures, forming H2NO (light-gray pathway). Song et al. (2016) and Stagni et al. (2020) have recently updated the rate constants of H2NO + O2 = HNO + HO2, for high-pressure conditions (up to 300 bar) and low–intermediate temperatures (450–925 K). Then, HNO radicals form NO, outlining an important fuel NO formation route.
Alternatively, NH2 can also react with NO (DeNOx chemistry) through a termination and a branching reaction, respectively, NH2+ NO = N2+H2O and NH2+NO = NNH + OH (blue pathway), active between 1,100 and 1400 K (Glarborg et al., 1986; Miller and Bowman, 1989; Miller and Glarborg, 1999). Laminar flame speed and ignition delay time strongly depend on the ratio between rate constants of the DeNOx chemistry.
In the following step, NNH decomposes via reaction NNH = N2+H. This reaction is very important as it releases H radicals to sustain H2/O2 radical branching mechanisms, while its tunneling nature and the NNH short lifetime make modeling activities very complex (Klippenstein et al., 2011). The concurrent reaction NNH + O=NO + NH has then received a lot of attention as a further important NO formation route (Konnov and de Ruyck, 2001).
For higher temperatures (>1400 K), the NH2 radicals are quickly dehydrogenated to NH ones (black pathway), which can consume NO, while forming N2O (via reaction NH + NO = N2O + H, recently revised by Klippenstein et al., 2011), or release N radicals, available for the classical Zeldovich mechanism (orange pathway) (O + N2 = N + NO, N + O2 = O + NO, N + OH = H + NO) (Drake and Blint, 1991) at high temperatures (>1600 K). Under high pressures/high temperatures, it has to be remarked that the NOx formation tends to reduce as third-body reactions (H + OH + M = H2O, H + O2+M = HO2+M) decrease radical concentration, necessary to sustain the Zeldovich mechanism (Kobayashi et al., 2019; Valera-Medina et al., 2019).
N2O (green pathway) acts as a reservoir of O radicals as it can decompose via reaction N2O + M = N2+O + M under low pressures, enhancing the reaction rate of H2+O=H + OH, while for high-pressure or fuel-ultra-lean conditions, this reaction reverts defining an important N2O formation route. Alternatively, N2O is consumed through N2O + H=N2+OH, releasing OH radicals for temperatures above 1650 K, while decreasing characteristic fuel ignition delay times (Mathieu et al., 2012).
The NO-NO2 loop (purple pathway) boosts the conversion of unreactive HO2 radicals into reactive OH radicals through the reaction NO + HO2 = NO2+OH, thus enhancing the mixture reactivity, while recycling back NO via NO2+H=NO + OH, together with a further release of OH radicals.
The N2O and NO-NO2 effects on fuel characteristic chemical times should be carefully evaluated for EGR engines (Mathieu et al., 2012; Ahmed et al., 2016), where flue gases are recirculated back to the engine to limit the adiabatic flame temperature, while constraining NOx emissions to threshold values.
Mathieu and Petersen (2015) measured NH3 ignition delay time for high pressures (30 bar) and high temperatures in a shock tube and highlighted the importance of N2O and NO2 routes. Ahmed et al. (2016) and Zhang et al. (2017) clarified the NO-NO2 interconversion comes through a more complex pathway, involving intermediate species such as HONO, HNO2, HONO2, and HNO3.
Beyond the relatively simple description of NH3 oxidation chemistry, any routes here described have been and are actually constantly discussed and updated on the basis of new experimental evidence and theoretical studies.
Given the large number of kinetic schemes developed in the last decade (Glarborg, et al., 1986; Konnov and de Ruyck, 2000; Skreiberg et al., 2004; Dagaut et al., 2008; Mével et al., 2009; Tian et al., 2009; Duynslaegher et al., 2012; Mathieu and Petersen, 2015; Song et al., 2016; Nakamura and Hasegawa, 2017; Zhang et al., 2017; Otomo et al., 2018; Shrestha et al., 2018; Stagni et al., 2020), Rocha et al. (2021) performed a numerical study to compare available detailed models against ignition delay times from Mathieu and Petersen (2015) and laminar flame data from Hayakawa et al. (2015), Pfahl et al. (2000), and Duynslaegher et al. (2012) (up to 5 atm). The claimed main differences among mechanisms are related to NOx formation routes, recognizing the reaction HNO + O2 = HO2+NO and the ammonia pyrolysis for fuel-rich conditions (such as 2NH2 = NH3+NH) as fundamental steps to predict NO emissions. Nakamura and Hasegawa (2017) and Zhang et al. (2017) have also demonstrated the importance of thermochemical properties to predict NH3 weak flames and ignition delay times at high pressures. Kovács et al. (2020) compared 8 detailed kinetic mechanisms for NH3 pyrolysis and oxidation with respect to experimental data. The authors noticed a large discrepancy among model predictions, while warning about the necessity to extent the validation procedure against a larger NH3 database.
From this brief overview of kinetic scheme development over the recent years, it is clear that NH3 oxidation chemistry along with NO formation routes still presents many open issues: Large rate constant uncertainties, different thermochemical parameters, the significant non-Arrhenius behavior of NH3 dehydrogenation reactions, rate pressure dependence description, especially relevant to high-pressure conditions, the NH3 pyrolysis chemistry along with the NxHy route, the tunneling nature of NNH reactions, the strong sensitivity of NO to the DeNOx chemistry, and the kinetics related to the NO-NO2 loop, to N2O, and to H2NO/HONO species still have to be properly addressed.
Recently, experimental investigations relevant to engines have been realized also for NH3/H2 (He et al., 2019; Chen et al., 2021), NH3/CH4 (Dai et al., 2020; Xiao et al., 2020; Arunthanayothin et al., 2021; Shu et al., 2021), NH3/diesel (Feng et al., 2020), NH3/n-heptane (Yu et al., 2020), NH3/DEE (Issayev et al., 2021), and NH3/CH3OH and C2H5OH (Wang et al., 2021) blends as strategies to improve the laminar flame speed/ignition delay times of NH3.
The oxidation chemistry of NH3 and fuel “enhancer” has to be carefully addressed, since many authors reported a strong chemical interaction. In addition, for complex molecules, detailed kinetic mechanisms had to be constructed ad hoc by simply merging kinetic models; thus, NH3–fuel interaction may suffer in the absence of chemistry subsets.
For instance, Chen et al. (2021) have characterized H2-NH3 ignition delay times up to 10 atm, demonstrating that the reaction NH3+H=NH2+H2 reconverts back NH2 radicals to NH3, releasing H radicals to sustain the high-temperature branching reaction H + O2 = OH + O, thus accelerating ignition delay times. Sabia et al. (2020) and Manna et al. (2022) have performed experiments in a JSFR for NH3/H2 mixtures, providing for evidence of a mutual inhibiting interaction among H2 and NH3, with a plausible role of NH3 as a strong collider in third-body reactions, due to NH3 strong polarity, like water (Michael et al., 2002). This aspect should be carefully addressed for high-pressure systems, where reaction rate pressure dependence, involving also the NxHy species formation route with many third-body reactions, covers an important role (Glarborg et al., 2021).
Rasmussen et al. (2008) and then Dai et al. (2020) demonstrated that fuel NO can have a strong sensitizing effect on CH4 ammonia chemistry through the interaction with NO-NO2 species via NO2+CH3 = NO + CH3O and NO + CH3OO = NO2+CH3O, as well as through the NO-NO2 loop, while CH4+NH2 = CH3+NH3 contributes substantially to the decomposition of CH4 under fuel-rich conditions.
4 Ammonia as Fuel in Internal Combustion Engines
Nowadays, ammonia is considered the most promising solution for abating GHG emission in large-bore internal combustion engines for marine transportation and power generation sector (Kurien and Mittal, 2022), where the low energy density characteristic of the current battery technologies makes electric propulsion unfeasible. Some modifications are necessary to allow internal combustion engines running with NH3. First, when working with port fuel injection, an 8-bar fuel tank and a supply system similar to LPG have to be introduced, generally equipped with a heated vaporizer before the injector (Frigo and Gentili, 2013). Due to the growing interest in ammonia, methanol, and other low-viscosity fuels, more recent solutions have been developed allowing high-pressure common rail direct injection (Willmann et al., 2021). In case of spark-ignition engines, an increase in compression ratio is provided to exploit the high octane number of ammonia and compensate for the low laminar flame speed (Garabedian and Johnson, 1966). In order to accelerate the ammonia burning rate, few percentages of H2 are generally added and a dissociation catalyst is often employed to generate H2 onboard by ammonia cracking (Ryu et al., 2014a). In this case, in spark-ignition engines the spark plug material is changed to avoid hydrogen backfiring phenomena (Dimitriou and Javaid, 2020).
Even though great attention is paid by the scientific community and industry, practical applications on internal combustion engines fueled with ammonia are still limited. This section aims at giving an insight into ammonia combustion in both spark- and compression-ignition engines, highlighting the strengths and weaknesses of each investigated solution.
4.1 Ammonia as Fuel in Spark-Ignition Engines
First attempts of fueling spark-ignition engines with NH3 date back to the end of the 60s for military application with the aim of making vehicles independent of hydrocarbon fuels (Cornelius et al., 1966). In the previous study (Garabedian and Johnson, 1966), the authors experimentally investigated the effect of anhydrous ammonia on the performance of a 48 kW maximum rated power, 4,000 rpm maximum engine speed, and 4-cylinder spark-ignition engine. First tests without any modification except an LPG carburetor for ammonia supply highlighted a reduction in maximum rated power and engine speed to 7 kW and 2000 rpm, respectively. By adding a few quantities of hydrogen (between 2% and 4%), an increase in power output of up to about 20 kW was found, while the speed range was extended up to 3,200 rpm. Further improvements were achieved by increasing the compression ratio thanks to the high octane number of NH3, allowing 40 kW power output at 4,000 rpm. More recent applications on spark-ignition engines mainly concern the use of ammonia in a blend with a secondary fuel to increase the burning rate, generally H2, CH4 (Chai et al., 2021), or H2/CH4 (Mashruk et al., 2022). In Lhuillier et al.’s (2019a) study, the authors tested the performance of a GDI single-cylinder engine fueled with gaseous ammonia/air mixture at a fixed engine speed of 1,500 rpm by varying the intake pressure. They demonstrated the feasibility of pure ammonia combustion at wide-open throttle conditions. Further improvements in stability were achieved with boosted pressure, while at partial load, the addition of hydrogen was needed to keep the cycle-to-cycle variability under acceptable values. At full load, the engine performance with pure ammonia was comparable with the one obtained with methane used as a reference, with an indicated efficiency of about 36%. Based on the literature, the need for a combustion enhancer in blend with the ammonia is apparent to operate the engine over the whole load range. With this aim, hydrogen is the best solution when thinking about the engine performance; on the contrary, the issues related to its storage and transportation make this solution unreliable in a dual-fuel configuration. As argued by first studies on the NH3 combustion (Garabedian and Johnson, 1966; Starkman et al., 1967), an NH3 dissociation catalyst can provide the necessary amount of hydrogen, avoiding to stocking it in a separate tank. Interestingly, the heat required by ammonia cracking endothermic reactions can be recovered by engine gas exhaust (Comotti and Frigo, 2015). In Lhuillier et al.’s (2019b) study, the authors investigated the influence of hydrogen addition on the performance and emissions of an ammonia-fueled SI engine. The H2 volume content varied from 5% to 15% at different equivalent ratios and intake pressures. The use of hydrogen as a combustion enhancer allowed acceptable combustion stability over the whole engine map. 10% H2 concentration was found as the optimum to increase combustion efficiency without compromising exhaust emissions. The best efficiency results were achieved at lean near stoichiometric conditions. Further charge dilution results in a significant increase in NOx and NH3 at the exhaust without combustion efficiency improvements. Similar findings were highlighted in Frigo and Gentili (2013).
Lhuillier et al. (2020a) performed an extensive investigation on the use of NH3/H2 blends with different equivalence ratios and H2 volume concentrations up to 60% in a single-cylinder spark-ignition engine. Figure 6 shows the indicated efficiency as a function of equivalence ratio, ϕ, for different H2 volume fractions. In agreement with aforementioned works, the optimal indicated efficiency was reached with low concentrations of hydrogen, between 5% and 20%, and lean near stochiometric condition. A maximum of about 39% was achieved with H2 20%v at ϕ 0.9. Too high hydrogen concentrations result in efficiency detriment due to an increase in wall heat losses, as a consequence of higher flame temperatures.
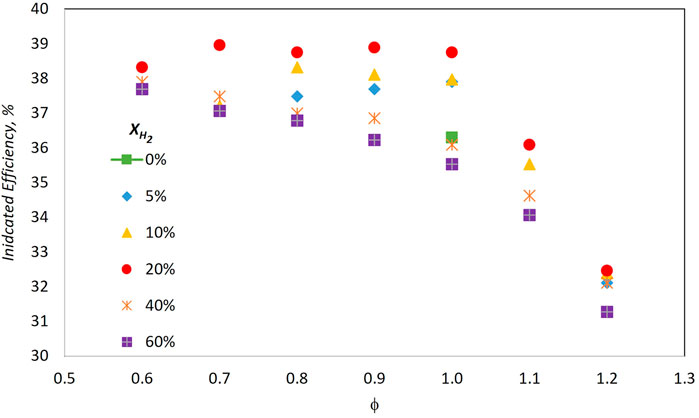
FIGURE 6. Indicated efficiency as a function of equivalence ratio ϕ under different hydrogen concentrations (Lhuillier et al., 2020b).
Alternative or in combination with hydrogen, natural gas is considered a viable way to sustain the NH3 combustion as it was widely used in the recent past as a primary fuel in the large-bore engine. Therefore, using a blend of natural gas and ammonia represents a step toward the decarbonization path. Oh et al. (2021) tested the influence of ammonia–natural gas blends on the performance and exhaust emissions of a heavy-duty, 6-cylinder spark-ignition engine designed for compressed natural gas (CNG) fueling. Experiments were focused on partial load, low-speed conditions: The engine was fueled with 50% NH3-50% CNG. The substitution of CNG with ammonia resulted in a CO2 reduction of about 28%, while the charge dilution was limited by the detriment in combustion stability involved with low NH3 burning velocity allowing a maximum equivalence ratio of 1.5. Controversial results were obtained by Lhuillier et al. (2021), concerning the effect of methane on the combustion duration of ammonia-fueled spark-ignition engines. CH4 volume concentration was varied between 5 and 15% at different equivalence ratios, to investigate the potential of CH4 as a combustion promoter. Methane slightly sped up the combustion at the rich mixture condition (ϕ = 1.1), which did not significantly influence the combustion duration at the stoichiometric condition, while it reduced burning velocity at the lean condition (ϕ = 0.9). With the aim of reducing CO2 emissions, ammonia was also used as secondary fuel in binary/ternary blends with gasoline and alcohol fuels. Due to its high octane number, ammonia allows advancing spark timing and boosting intake pressure; in this way, it is possible to compensate for the loss in power output linked to the low energy density and burning velocity. In Ryu et al.’s (2014b) study, the potential of NH3 direct injection was explored in a dual-fuel gasoline port fuel–injected spark-ignition engine. When working in a dual-fuel mode, the power output ascribable to gasoline was kept constant, while the total power was increased by adjusting the ammonia injection. Results were compared with pure gasoline operation at the same load, highlighting similar overall brake-specific energy consumption. On the contrary, NOx emissions were strongly penalized. Haputhanthri et al. (2014) explored the potential of ethanol and methanol to increase the ammonia solubility in gasoline and hence improve its content in ternary blends. They found an increase in ammonia content from 3.7%v in the case of binary NH3/gasoline blends to 30%v in ternary blends with 30%v of methanol. Independent of percentage composition, at high engine speed, introducing ammonia in alcohol/gasoline blends resulted in a torque increase.
4.2 Ammonia as Fuel in Compression-Ignition Engines
Combustion of ammonia in compression-ignition engines is challenging due to its very low reactivity, which makes it more attractive for positive ignition. On the contrary, the recent interest in ammonia as fuel is mainly pushed by the marine shipping industry, which prominently runs with compression-ignition large-bore engines. In light of this, several solutions are under investigation to burn ammonia, either in pure or in dual-fuel mode, in compression-ignition engines. First studies on the use of ammonia in diesel engines date back to the end of the 60s on behalf of the US Army Energy Depot program. In 1967, Gray et al. (1967) burned pure ammonia in a Cooperative Fuel Research (CFR) engine, increasing the compression ratio up to 35:1 and intake temperature to 423 K. The low reactivity of ammonia combined with the high compression ratio resulted in a pressure peak of about 150 bar, likely due to a homogeneous charge combustion process. Introducing diesel fuel pilot as an ignition trigger allowed a reduction in compression to 15.2:1. Similar results can be found in the literature of the same age (Pearsall and Garabedian, 1968), suggesting the use of dual-fuel mode combustion. Therefore, more recent studies aimed to optimize the performance and exhaust emissions of NH3/diesel-fueled engines to reduce GHG emissions without penalizing the performance. Yousefi et al. (2022) investigated the effect of ammonia/diesel ratio and diesel injection strategy on the behavior of a single-cylinder, heavy-duty common rail dual-fuel engine. Ammonia energy fraction was regulated through a port fuel injection. The reduction in the diffusive diesel combustion stage due to the introduction of premixed air/NH3 charge results in a lower in-cylinder pressure peak, prolonging the combustion duration. Consequently, a reduction in NOx up to 58% is achieved with the maximum NH3 energy fraction of 40%. Diesel injection strategy optimization allows keeping ITE almost constant with a reduction in GHG of about 12%. As demonstrated by Reiter and Kong (2008), NH3 can be used as a primary fuel in turbocharged diesel engines with few modifications to the intake port. Tests were performed at different engine loads and speeds. An ammonia energy fraction of 95% was achieved with engine torque close to maximum. Further improvements in combustion efficiency can be achieved by directly injecting ammonia in a combustion chamber at high pressure. Li et al. (2022) compared the combustion characteristics and exhaust emissions of a dual-fuel diesel engine with those of two different ammonia injection systems: indirect low-pressure and direct high-pressure one. Results highlighted an increase in maximum energy replacement when switching from the low- to high-pressure injection system from 80% up to 97%. The low-pressure dual-fuel mode provided a higher indicated thermal efficiency, while high-pressure dual-fuel mode limited the unburned NH3 exhaust emissions.
Several applications have been explored in the use of NH3 in combination with alternative fuels in compression-ignition engines. Reiter and Kong (2008) investigated the effect of soybean oil methyl ester as a combustion trigger for ammonia. Replacing biodiesel with diesel did not result in significant performance and emission variations. Tay et al. (2017) performed a numerical study on the use of kerosene in the mixture or as an alternative to diesel for triggering the combustion of NH3. They found an advance in the start of ignition when switching from diesel to kerosene, resulting in a more complete combustion of NH3 in the regions close to cylinder liner and crevices. Some attempts have been performed to inject dimethyl ether (DME)/NH3 mixtures as NH3 is miscible with DME. In Ryu et al.’s (2014c) study, pure DME and two different DME/NH3 mixtures (40% and 60%w of NH3) were directly injected into a compression-ignition engine. The authors found a worsening of performance with higher cyclic variability by increasing NH3%. Moreover, the combustion prolongation and lower temperature of NH3 combustion resulted in higher CO and HC emissions.
NH3 can be successfully used as a hydrogen carrier thanks to the reliability of onboard systems for hydrogen production by means of NH3 cracking or dissociation (Kurien and Mittal, 2022). Gill et al. (2012) performed an experimental analysis of the influence of dissociated NH3 on the combustion and emission of the direct-injection diesel engine. With this aim, the 3%v of intake air was replaced with gaseous ammonia, dissociated ammonia, and pure hydrogen. Figure 7 shows the effect of the dual-fuel operation on the brake thermal efficiency and CO2 emissions. Both ammonia and hydrogen provide a reduction in combustion efficiency compared with pure diesel fueling. This trend can be explained by combustion mechanisms of H2 and NH3 compared with diesel. In fact, the dilute mixture conditions, typical of compression-ignition engines, penalize the premixed flame propagation worsening the combustion efficiency. Replacing 3%v of intake air with carbon-free NH3 or H2 allows reducing diesel amount by about 15% with benefits on CO2 emission up to about 12%.
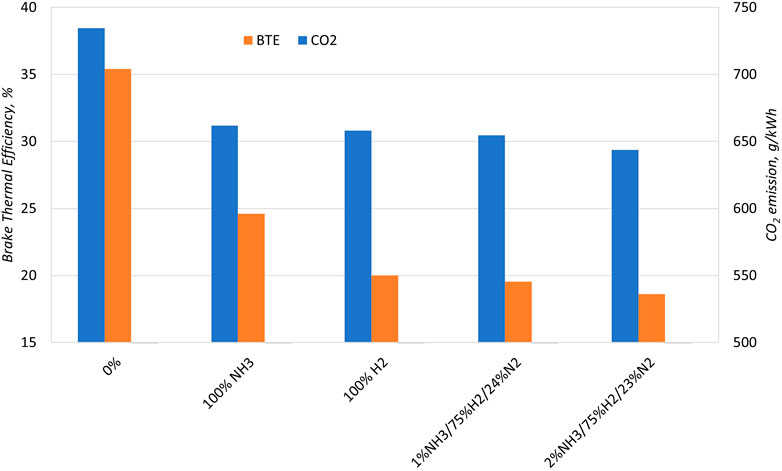
FIGURE 7. Brake thermal efficiency for different intake air additives: gaseous ammonia, pure hydrogen, and dissociated ammonia (Gill et al., 2012).
5 Conclusion
The need for immediate solutions to reduce greenhouse gas emissions represents one of the most serious challenges for the transport sector since the internal combustion engine invention. Despite the run to the electrification, the route from combustion to electric propulsion is long and several issues have to be addressed before reaching this goal. Furthermore, electric propulsion cannot replace internal combustion engines, even though they are competing with each other. In this scenario, green ammonia is considered a promising fuel as it is carbon-free, it has a relatively high-volume energy density, and it is easy to store and transport.
There are several drawbacks, in terms of safety and efficiency, that have limited the deployment of ammonia on a large scale in the past due to its chemical–physical characteristics. Indeed, toxicity and poor combustion properties limit ammonia utilization as a direct substitute for standard fuels in the available conversion systems, with main concerns in its application in the internal combustion engine.
Nevertheless, the potential impact of its use as a energy carrier on decarbonization has catalyzed the attention of academic and industrial research on studying and testing possible solutions to overcome disadvantages, from its green production to its effective use.
Ammonia will only make sense as a green fuel if the routes for its production turn green. Currently, at an industrial level, ammonia is produced using nitrogen and hydrogen in the gas phase through the Haber–Bosch (H-B) process. From an environmental point of view, this path is too energy-intensive and unsustainable: The H-B process is mainly based on fossil fuels as an energy source and as a raw material since almost all of the hydrogen gases used for ammonia synthesis are produced by steam reforming of natural gas. In this review, two different pathways for producing green ammonia were evaluated: The first, of considerable long-term scope, is based on the H-B process fueled with hydrogen from renewable sources (green hydrogen), and the whole process is powered by electricity from completely renewable sources. The conversion of traditional synthesis plants from steam reforming feed to green hydrogen feed (e.g., by integrating water electrolysis into the process) requires plant design flexibility and optimal allocation of energy sources; and the second path is based on the electrochemical synthesis of ammonia; in this case, the H-B process is no longer necessary: The processes connected to this second path are currently at TRL 1, and further research and development activities would be required before being able to consider large-scale industrial production.
Much attention has been dedicated to NH3 as fuel, with the development and optimization of many detailed kinetic schemes. Their availability is mandatory for designing and optimizing thermal conversion systems where ammonia is used, as well as for setting up their digital twins. Most of these schemes were derived from mechanisms conceived for NOx formation/reduction, at low pressures (through prompt, fuel, and thermal NO routes), and thermal De-NOx, and have been updated on the basis of recent experimental evidence, while at only recently high-pressure/high-temperature conditions, relevant to ICEs, experimental data have been proposed, thus boosting a further development in an attempt to provide for reliable and robust kinetic schemes. However, NH3 oxidation chemistry presents still many open issues, mainly related to large rate constant uncertainties, different thermochemical parameters, and rate pressure dependence description, relevant to high-pressure conditions. In addition, some critical steps, such as the NH3 pyrolysis chemistry along with the NxHy route, the tunneling nature of NNH reactions, and the strong sensitivity of NH3 to the DeNOx chemistry, to the NO-NO2 loop, and to N2O and H2NO/HONO species, still have to be properly addressed. These issues are stressed in case of addition of fuel enhancers, where a direct coupling between ammonia–ammonia radicals and fuel oxidation chemistry has to be taken into account. Recently, many efforts have been done in virtue of the availability of new data at high pressure/high temperature, in the form of laminar flame speeds and autoignition delay times as fundamental parameters to predict for ICE conditions and a set of detailed kinetic models to be used for the evaluation of combustion parameters are available, along with reduced schemes for CFD purposes.
Practical applications of ammonia in internal combustion engines are attracting the interest from shipping stakeholders who look at ammonia since it may be burnt by existing engine architecture with retrofitting modifications. The high autoignition temperature and the low burning velocity impose the use of combustion promoters in order to limit flame quenching and misfiring. The most convincing solution to this issue is considered onboard production of H2 from NH3 cracking, recovering engine out heat to support endothermic reactions. Experimental tests on spark-ignition engines demonstrate that H2-added NH3 combustion can replace CH4 without efficiency losses. Further improvements are expected with the development of high-pressure direct-injection systems, which avoid NH3 slip and increase combustion stability. Interest is also paid to the combustion of ammonia in compression-ignition engines since it should limit modifications to existing ship engine architectures. On the contrary, this solution results in a too high detriment of efficiency since the low reactivity of NH3 implies a change in the combustion mechanism, which is predominantly premixed, compromising the thermal efficiency. The increase in injection pressure of modern common rail systems for low-viscosity fuels could lead the way to a new generation of compression-ignition engines allowing diesel similar to the combustion of ammonia.
Author Contributions
CT and MJ conceptualized the study, investigated the study, wrote the original draft, and wrote, reviewed, and edited the manuscript. LM and PS conceptualized the study, investigated the study, and wrote the original draft.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agrawal, R., and Woodward, D. W. (1991). Efficient Cryogenic Nitrogen Generators: An Exergy Analysis. Gas Sep. Purif. 5 (3), 139–150. doi:10.1016/0950-4214(91)80012-t
Ahmed, S. F., Santner, J., Dryer, F. L., Padak, B., and Farouk, T. I. (2016). Computational Study of NOx Formation at Conditions Relevant to Gas Turbine Operation, Part 2: NOx in High Hydrogen Content Fuel Combustion at Elevated Pressure. Energy fuels. 30 (9), 7691–7703. doi:10.1021/acs.energyfuels.6b00421
Ariemma, G. B., Sorrentino, G., Ragucci, R., de Joannon, M., and Sabia, P. (2022). Ammonia/Methane Combustion: Stability and NOx Emissions. Combust. Flame 241, 112071. doi:10.1016/j.combustflame.2022.112071
Arunthanayothin, S., Stagni, A., Song, Y., Herbinet, O., Faravelli, T., and Battin-Leclerc, F. (2021). Ammonia-methane Interaction in Jet-Stirred and Flow Reactors: An Experimental and Kinetic Modeling Study. Proc. Combust. Inst. 38 (1), 345–353. doi:10.1016/j.proci.2020.07.061
Ashik, U. P. M., Viswan, A., Kudo, S., and Hayashi, J.-i. (2018). “Nanomaterials as Catalysts,” in Micro and Nano Technologies, Applications of Nanomaterials. Editors S. M. Bhagyaraj, O. S. Oluwafemi, N. Kalarikkal, and S. Thomas, 45–82. doi:10.1016/B978-0-08-101971-9.00003-X
Bicer, Y., and Dincer, I. (2017). Assessment of a Sustainable Electrochemical Ammonia Production System Using Photoelectrochemically Produced Hydrogen under Concentrated Sunlight. ACS Sustain. Chem. Eng. 5 (9), 8035–8043. doi:10.1021/acssuschemeng.7b01638
IEA Bioenergy Binder, M., Kraussler, M., Kuba, M., and Luisser, M. (2018). Hydrogen from Biomass Gasification. https://www.ieabioenergy.com/wp-content/uploads/2019/01/Wasserstoffstudie_IEA-final.pdf (Accessed May 15, 2022).
Boucher, D. L., Davies, J. A., Edwards, J. G., and Mennad, A. (1995). An Investigation of the Putative Photosynthesis of Ammonia on Iron-Doped Titania and Other Metal Oxides. J. Photochem. Photobiol. A Chem. 88 (1), 53–64. doi:10.1016/1010-6030(94)03994-6
Cai, X., Fu, C., Iriawan, H., Yang, F., Wu, A., Luo, L., et al. (2021). Lithium-mediated Electrochemical Nitrogen Reduction: Mechanistic Insights to Enhance Performance. iScience 24 (10), 103105. doi:10.1016/j.isci.2021.103105
Capurso, T., Stefanizzi, M., Torresi, M., and Camporeale, S. M. (2022). Perspective of the Role of Hydrogen in the 21st Century Energy Transition. Energy Convers. Manag. 251, 114898. doi:10.1016/j.enconman.2021.114898
Chai, W. S., Bao, Y., Jin, P., Tang, G., and Zhou, L. (2021). A Review on Ammonia, Ammonia-Hydrogen and Ammonia-Methane Fuels. Renew. Sustain. Energy Rev. 147, 111254. doi:10.1016/j.rser.2021.111254
Chehade, G., and Dincer, I. (2021). Progress in Green Ammonia Production as Potential Carbon-free Fuel. Fuel 299, 120845. doi:10.1016/j.fuel.2021.120845
Chen, H., Dong, F., and Minteer, S. D. (2020). The Progress and Outlook of Bioelectrocatalysis for the Production of Chemicals, Fuels and Materials. Nat. Catal. 3, 225–244. doi:10.1038/s41929-019-0408-2
Chen, J., Jiang, X., Qin, X., and Huang, Z. (2021). Effect of Hydrogen Blending on the High Temperature Auto-Ignition of Ammonia at Elevated Pressure. Fuel 287, 119563. doi:10.1016/j.fuel.2020.119563
Choe, J., Sun, W., Ombrello, T., and Carter, C. (2021). Plasma Assisted Ammonia Combustion: Simultaneous NOx Reduction and Flame Enhancement. Combust. Flame 228, 430–432. doi:10.1016/j.combustflame.2021.02.016
Comotti, M., and Frigo, S. (2015). Hydrogen Generation System for Ammonia-Hydrogen Fuelled Internal Combustion Engines. Int. J. Hydrogen Energy 40 (33), 10673–10686. doi:10.1016/j.ijhydene.2015.06.080
Cornelius, W., Huellmantel, L. W., and Mitchell, H. R. (1966). Ammonia as an Engine Fuel. SAE Trans. 74, 300–326.
Dagaut, P., Glarborg, P., and Alzueta, M. (2008). The Oxidation of Hydrogen Cyanide and Related Chemistry. Prog. Energy Combust. Sci. 34 (1), 1–46. doi:10.1016/j.pecs.2007.02.004
Dai, L., Gersen, S., Glarborg, P., Mokhov, A., and Levinsky, H. (2020). Autoignition Studies of NH3/CH4 Mixtures at High Pressure. Combust. Flame 218, 19–26. doi:10.1016/j.combustflame.2020.04.020
Dimitriou, P., and Javaid, R. (2020). A Review of Ammonia as a Compression Ignition Engine Fuel. Int. J. Hydrogen Energy 45 (11), 7098–7118. doi:10.1016/j.ijhydene.2019.12.209
Drake, M. C., and Blint, R. J. (1991). Calculations of NOx Formation Pathways in Propagating Laminar, High Pressure Premixed CH4/air Flames. Combust. Sci. Technol. 75 (4-6), 261–285. doi:10.1080/00102209108924092
Duynslaegher, C., Contino, F., Vandooren, J., and Jeanmart, H. (2012). Modeling of Ammonia Combustion at Low Pressure. Combust. Flame 159 (9), 2799–2805. doi:10.1016/j.combustflame.2012.06.003
European Commission (2021). Opening Keynote by the President: European Hydrogen Week. Available at https://ec.europa.eu/commission/presscorner/detail/en/speech_21_6421 (Accessed May 15, 2022).
Feng, Y., Zhu, J., Mao, Y., Raza, M., Qian, Y., Yu, L., et al. (2020). Low-temperature Auto-Ignition Characteristics of NH3/diesel Binary Fuel: Ignition Delay Time Measurement and Kinetic Analysis. Fuel 281, 118761. doi:10.1016/j.fuel.2020.118761
Frigo, S., and Gentili, R. (2013). Analysis of the Behaviour of a 4-stroke Si Engine Fuelled with Ammonia and Hydrogen. Int. J. Hydrogen Energy 38 (3), 1607–1615. doi:10.1016/j.ijhydene.2012.10.114
Garabedian, C. G., and Johnson, J. H. (1966). The Theory of Operation of an Ammonia-Burning Internal Combustion Engine. USCFSTI, AD Rep. (United States) 41 (15).
Gardiner, M., and Satyapal, S. (2009). Energy Requirements for Hydrogen Gas Compression and Liquefaction as Related to Vehicle Storage Needs DOE Hydrogen and Fuel Cells Program Record. Available online: https://www.hydrogen.energy.gov/pdfs/9013_energy_requirements_for_hydrogen_gas_compression.pdf (accessed on June 13, 2019).
Ghassan, C., and Ibrahim, D. (2020). A Novel Method for a New Electromagnetic-Induced Ammonia Synthesizer. Int. J. Energy Res. 44 (9), 7183–7197. doi:10.1002/er.v44.910.1002/er.5355
Gill, S. S., Chatha, G. S., Tsolakis, A., Golunski, S. E., and York, A. P. E. (2012). Assessing the Effects of Partially Decarbonising a Diesel Engine by Co-fuelling with Dissociated Ammonia. Int. J. hydrogen energy 37 (7), 6074–6083. doi:10.1016/j.ijhydene.2011.12.137
Glarborg, P., Hashemi, H., Cheskis, S., and Jasper, A. W. (2021). On the Rate Constant for NH2+HO2 and Third-Body Collision Efficiencies for NH2+H(+M) and NH2+NH2(+M). J. Phys. Chem. A 125 (7), 1505–1516. doi:10.1021/acs.jpca.0c11011
Glarborg, P., Miller, J. A., and Kee, R. J. (1986). Kinetic Modeling and Sensitivity Analysis of Nitrogen Oxide Formation in Well-Stirred Reactors. Combust. flame 65 (2), 177–202. doi:10.1016/0010-2180(86)90018-0
Gray, J. T., Dimitroff, E., Meckel, N. T., and Quillian, R. D. (1967). Ammonia Fuel—Engine Compatibility and Combustion. SAE Trans. 75, 785–807.
Han, D., Liu, Y., and Huang, Z. (2022). “The Use of Ammonia as a Fuel for Combustion Engines,” in Engines and Fuels for Future Transport. Energy, Environment, and Sustainability. Editors G. Kalghatgi, A. K. Agarwal, F. Leach, and K. Senecal (Singapore: Springer), 233–256. doi:10.1007/978-981-16-8717-4_10
Han, X., Wang, Z., Costa, M., Sun, Z., He, Y., and Cen, K. (2019). Experimental and Kinetic Modeling Study of Laminar Burning Velocities of NH3/air, NH3/H2/air, NH3/CO/air and NH3/CH4/air Premixed Flames. Combust. Flame 206, 214–226. doi:10.1016/j.combustflame.2019.05.003
Haputhanthri, S. O., Maxwell, T. T., Fleming, J., and Austin, C. (2014). Ammonia Gasoline-Ethanol/methanol Tertiary Fuel Blends as an Alternate Automotive Fuel. ASME Int. Mech. Eng. Congr. Expo. 46514, V06AT07A071. doi:10.1115/imece2014-38026
Hayakawa, A., Goto, T., Mimoto, R., Arakawa, Y., Kudo, T., and Kobayashi, H. (2015). Laminar Burning Velocity and Markstein Length of Ammonia/air Premixed Flames at Various Pressures. Fuel 159, 98–106. doi:10.1016/j.fuel.2015.06.070
He, X., Shu, B., Nascimento, D., Moshammer, K., Costa, M., and Fernandes, R. X. (2019). Auto-ignition Kinetics of Ammonia and Ammonia/hydrogen Mixtures at Intermediate Temperatures and High Pressures. Combust. Flame 206, 189–200. doi:10.1016/j.combustflame.2019.04.050
IRENA - International Renewable Energy Agency (2020). Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5⁰C Climate Goal. https://irena.org/-/media/Files/IRENA/Agency/Publication/2020/Dec/IRENA_Green_hydrogen_cost_2020.pdf (Accessed May 15, 2022).
Issayev, G., Giri, B. R., Elbaz, A. M., Shrestha, K. P., Mauss, F., Roberts, W. L., et al. (2021). Combustion Behavior of Ammonia Blended with Diethyl Ether. Proc. Combust. Inst. 38 (1), 499–506. doi:10.1016/j.proci.2020.06.337
Jabbour, T., and Clodic, D. F. (2004). Burning Velocity and Refrigerant Flammability Classification/DISCUSSION. ASHRAE Trans. 110, 522.
Klippenstein, S. J., Harding, L. B., Glarborg, P., and Miller, J. A. (2011). The Role of NNH in NO Formation and Control. Combust. Flame 158 (4), 774–789. doi:10.1016/j.combustflame.2010.12.013
Kobayashi, H., Hayakawa, A., Somarathne, K. D. K. A., and Okafor, E. C. (2019). Science and Technology of Ammonia Combustion. Proc. Combust. Inst. 37 (1), 109–133. doi:10.1016/j.proci.2018.09.029
Konnov, A. A., and de Ruyck, J. (2001). Temperature-dependent Rate Constant for the Reaction NNH + O → NH + NO. Combust. Flame 125 (4), 1258–1264. doi:10.1016/s0010-2180(01)00250-4
Konnov, A. A., and Ruyck, J. D. (2001). A Possible New Route for No Formation Vian2h3. Combust. Sci. Technol. 168 (1), 1–46. doi:10.1080/00102200108907830
Konnov, A. A., and Ruyck, J. D. (2000). Kinetic Modeling of the Thermal Decomposition of Ammonia. Combust. Sci. Technol. 152 (1), 23–37. doi:10.1080/00102200008952125
Kovács, M., Papp, M., Zsély, I. G., and Turányi, T. (2020). Determination of Rate Parameters of Key N/H/O Elementary Reactions Based on H2/O2/NOx Combustion Experiments. Fuel 264, 116720.
Kurien, C., and Mittal, M. (2022). Review on the Production and Utilization of Green Ammonia as an Alternate Fuel in Dual-Fuel Compression Ignition Engines. Energy Convers. Manag. 251, 114990. doi:10.1016/j.enconman.2021.114990
Lamb, K. E., Viano, D. M., Langley, M. J., Hla, S. S., and Dolan, M. D. (2018). High-Purity H2 Produced from NH3 via a Ruthenium-Based Decomposition Catalyst and Vanadium-Based Membrane. Ind. Eng. Chem. Res. 57 (23), 7811–7816. doi:10.1021/acs.iecr.8b01476
Lhuillier, C., Brequigny, P., Contino, F., and Mounaïm-Rousselle, C. (2019a). “Combustion Characteristics of Ammonia in a Modern Spark-Ignition Engine,” in Conference on Sustainable Mobility. doi:10.4271/2019-24-0237
Lhuillier, C., Brequigny, P., Contino, F., and Mounaïm-Rousselle, C. (2021). Experimental Investigation on Ammonia Combustion Behavior in a Spark-Ignition Engine by Means of Laminar and Turbulent Expanding Flames. Proc. Combust. Inst. 38 (4), 5859–5868. doi:10.1016/j.proci.2020.08.058
Lhuillier, C., Brequigny, P., Contino, F., and Mounaïm-Rousselle, C. (2020a). Experimental Study on Ammonia/hydrogen/air Combustion in Spark Ignition Engine Conditions. Fuel 269, 117448. doi:10.1016/j.fuel.2020.117448
Lhuillier, C., Brequigny, P., Contino, F., and Mounaïm-Rousselle, C. (2019b). “Performance and Emissions of an Ammonia-Fueled SI Engine with Hydrogen Enrichment,” in 14th International Conference on Engines & Vehicles. doi:10.4271/2019-24-0137
Lhuillier, C., Brequigny, P., Lamoureux, N., Contino, F., and Mounaïm-Rousselle, C. (2020b). Experimental Investigation on Laminar Burning Velocities of Ammonia/hydrogen/air Mixtures at Elevated Temperatures. Fuel 263, 116653. doi:10.1016/j.fuel.2019.116653
Li, J., Huang, H., Kobayashi, N., He, Z., and Nagai, Y. (2014). Study on Using Hydrogen and Ammonia as Fuels: Combustion Characteristics and NOxformation. Int. J. Energy Res. 38 (9), 1214–1223. doi:10.1002/er.3141
Li, T., Zhou, X., Wang, N., Wang, X., Chen, R., Li, S., et al. (2022). A Comparison between Low- and High-Pressure Injection Dual-Fuel Modes of Diesel-Pilot-Ignition Ammonia Combustion Engines. J. Energy Inst. 102, 362–373. doi:10.1016/j.joei.2022.04.009
Long, J., Chen, S., Zhang, Y., Guo, C., Fu, X., Deng, D., et al. (2020). Direct Electrochemical Ammonia Synthesis from Nitric Oxide. Angew. Chem. Int. Ed. 59 (24), 9711–9718. doi:10.1002/anie.202002337
Lubrano Lavadera, M., Han, X., and Konnov, A. A. (2020). Comparative Effect of Ammonia Addition on the Laminar Burning Velocities of Methane, N-Heptane, and Iso-Octane. Energy fuels. 35 (9), 7156–7168. doi:10.1021/acs.energyfuels.0c03424
MacFarlane, D. R., Cherepanov, P. V., Choi, J., Suryanto, B. H. R., Hodgetts, R. Y., Bakker, J. M., et al. (2020). A Roadmap to the Ammonia Economy. Joule 4 (6), 1186–1205. doi:10.1016/j.joule.2020.04.004
Mallick, D., Sharma, S. D., Kushwaha, A., Brahma, H. S., Nath, R., and Bhowmik, R. (2022). “Emerging Commercial Opportunities for Conversion of Waste to Energy: Aspect of Gasification Technology,” in Waste-to-Energy Approaches towards Zero Waste. Editors C. M. Hussain, S. Singh, and L. Goswami, 105–127. doi:10.1016/B978-0-323-85387-3.00012-4
Mallouppas, G., Ioannou, C., and Yfantis, E. A. (2022). A Review of the Latest Trends in the Use of Green Ammonia as an Energy Carrier in Maritime Industry. Energies 15, 1453. doi:10.3390/en15041453
Manna, M. V., Sabia, P., Sorrentino, G., Viola, T., Ragucci, R., and de Joannon, M. (2022). New Insight into NH-H2 Mutual Inhibiting Effects and Dynamic Regimes at Low-Intermediate Temperatures. Combust. Flame, 111957. doi:10.1016/j.combustflame.2021.111957
Martín, A. J., Shinagawa, T., and Pérez-Ramírez, J. (2019). Electrocatalytic Reduction of Nitrogen: from Haber-Bosch to Ammonia Artificial Leaf. Chem 5 (2), 263–283. doi:10.1016/j.chempr.2018.10.010
Mashruk, S., Vigueras-Zuniga, M. O., Tejeda-del-Cueto, M. E., Xiao, H., Yu, C., Maas, U., et al. (2022). Combustion Features of CH4/NH3/H2 Ternary Blends. Int. J. Hydrogen Energy. doi:10.1016/j.ijhydene.2022.03.254
Mathieu, O., Levacque, A., and Petersen, E. L. (2012). Effects of N2O Addition on the Ignition of H2-O2 Mixtures: Experimental and Detailed Kinetic Modeling Study. Int. J. hydrogen energy 37 (20), 15393–15405. doi:10.1016/j.ijhydene.2012.07.071
Mathieu, O., and Petersen, E. L. (2015). Experimental and Modeling Study on the High-Temperature Oxidation of Ammonia and Related NOx Chemistry. Combust. Flame 162 (3), 554–570. doi:10.1016/j.combustflame.2014.08.022
Mei, B., Zhang, X., Ma, S., Cui, M., Guo, H., Cao, Z., et al. (2019). Experimental and Kinetic Modeling Investigation on the Laminar Flame Propagation of Ammonia under Oxygen Enrichment and Elevated Pressure Conditions. Combust. Flame 210, 236–246. doi:10.1016/j.combustflame.2019.08.033
Mével, R., Javoy, S., Lafosse, F., Chaumeix, N., Dupré, G., and Paillard, C. E. (2009). Hydrogen–nitrous Oxide Delay Times: Shock Tube Experimental Study and Kinetic Modelling. Proc. Combust. Inst. 32 (1), 359–366.
Michael, J. V., Su, M.-C., Sutherland, J. W., Carroll, J. J., and Wagner, A. F. (2002). Rate Constants for H + O2 + M → HO2 + M in Seven Bath Gases. J. Phys. Chem. A 106 (21), 5297–5313. doi:10.1021/jp020229w
Miller, J. A., and Bowman, C. T. (1989). Mechanism and Modeling of Nitrogen Chemistry in Combustion. Prog. energy Combust. Sci. 15 (4), 287–338. doi:10.1016/0360-1285(89)90017-8
Miller, J. A., and Glarborg, P. (1999). Modeling the Thermal De-NOx Process: Closing in on a Final Solution. Int. J. Chem. Kinet. 31 (11), 757–765. doi:10.1002/(sici)1097-4601(1999)31:11<757::aid-jck1>3.0.co;2-v
Nakamura, H., and Hasegawa, S. (2017). Combustion and Ignition Characteristics of Ammonia/air Mixtures in a Micro Flow Reactor with a Controlled Temperature Profile. Proc. Combust. Inst. 36 (3), 4217–4226. doi:10.1016/j.proci.2016.06.153
New Jersey Department of Health (2016). Hazardous Substance Fact Sheet – Ammonia. https://nj.gov/health/eoh/rtkweb/documents/fs/0084.pdf (Accessed May 15, 2022).
Oh, S., Park, C., Kim, S., Kim, Y., Choi, Y., and Kim, C. (2021). Natural Gas-Ammonia Dual-Fuel Combustion in Spark-Ignited Engine with Various Air-Fuel Ratios and Split Ratios of Ammonia under Part Load Condition. Fuel 290, 120095. doi:10.1016/j.fuel.2020.120095
Okafor, E. C., Kurata, O., Yamashita, H., Inoue, T., Tsujimura, T., Iki, N., Hayakawa, A., Ito, S., Uchida, M., and Kobayashi, H. (2021). Liquid Ammonia Spray Combustion in Two-Stage Micro Gas Turbine Combustors at 0.25 MPa; Relevance of Combustion Enhancement to Flame Stability and NOx Control. Appl. Energy Combust. Sci. 7, 100038. doi:10.1016/j.jaecs.2021.100038
Okafor, E. C., Naito, Y., Colson, S., Ichikawa, A., Kudo, T., Hayakawa, A., et al. (2018). Experimental and Numerical Study of the Laminar Burning Velocity of CH4-NH3-air Premixed Flames. Combust. flame 187, 185–198. doi:10.1016/j.combustflame.2017.09.002
Otomo, J., Koshi, M., Mitsumori, T., Iwasaki, H., and Yamada, K. (2018). Chemical Kinetic Modeling of Ammonia Oxidation with Improved Reaction Mechanism for Ammonia/air and Ammonia/hydrogen/air Combustion. Int. J. Hydrogen Energy 43 (5), 3004–3014. doi:10.1016/j.ijhydene.2017.12.066
Pearsall, T. J., and Garabedian, C. G. (1968). Combustion of Anhydrous Ammonia in Diesel Engines. SAE Trans. 76, 3213–3221.
Pfahl, U. J., Ross, M. C., Shepherd, J. E., Pasamehmetoglu, K. O., and Unal, C. (2000). Flammability Limits, Ignition Energy, and Flame Speeds in H2–CH4–NH3–N2O–O2–N2 Mixtures. Combust. flame 123 (1-2), 140–158. doi:10.1016/s0010-2180(00)00152-8
Piazzi, S., Patuzzi, F., and Baratieri, M. (2022). Energy and Exergy Analysis of Different Biomass Gasification Coupled to Fischer-Tropsch Synthesis Configurations. Energy 249, 123642. doi:10.1016/j.energy.2022.123642
Rasmussen, C. L., Rasmussen, A. E., and Glarborg, P. (2008). Sensitizing Effects of NOx on CH4 Oxidation at High Pressure. Combust. Flame 154 (3), 529–545. doi:10.1016/j.combustflame.2008.01.012
Reiter, A. J., and Kong, S.-C. (2008). Demonstration of Compression-Ignition Engine Combustion Using Ammonia in Reducing Greenhouse Gas Emissions. Energy fuels. 22 (5), 2963–2971. doi:10.1021/ef800140f
Rocha, R. C., Costa, M., and Bai, X.-S. (2021). Combustion and Emission Characteristics of Ammonia under Conditions Relevant to Modern Gas Turbines. Combust. Sci. Technol. 193 (14), 2514–2533. doi:10.1080/00102202.2020.1748018
Ronney, P. D., and Wachman, H. Y. (1985). Effect of Gravity on Laminar Premixed Gas Combustion I: Flammability Limits and Burning Velocities. Combust. Flame 62 (2), 107–119. doi:10.1016/0010-2180(85)90139-7
Rossetti (2020). “Reactor Design, Modelling and Process Intensification for Ammonia Synthesis,” in Sustainable Ammonia Production. Green Energy and Technology. Editors Inamuddin, R. Boddula, and A. Asiri (Cham: Springer), 17–48. doi:10.1007/978-3-030-35106-9_2
Rouwenhorst, K. H. R., Krzywda, P. M., Benes, N. E., Mul, G., and Lefferts, L. (2021). “Ammonia Production Technologies,” in Techno-Economic Challenges of Green Ammonia as an Energy Vector. Editors A. Valera-Medina, and R. Banares-Alcantara (Academic Press), 41–83. doi:10.1016/B978-0-12-820560-0.00004-7
Ryu, K., Zacharakis-Jutz, G. E., and Kong, S.-C. (2014a). Effects of Gaseous Ammonia Direct Injection on Performance Characteristics of a Spark-Ignition Engine. Appl. energy 116, 206–215. doi:10.1016/j.apenergy.2013.11.067
Ryu, K., Zacharakis-Jutz, G. E., and Kong, S.-C. (2014c). Performance Characteristics of Compression-Ignition Engine Using High Concentration of Ammonia Mixed with Dimethyl Ether. Appl. energy 113, 488–499. doi:10.1016/j.apenergy.2013.07.065
Ryu, K., Zacharakis-Jutz, G. E., and Kong, S.-C. (2014b). Performance Enhancement of Ammonia-Fueled Engine by Using Dissociation Catalyst for Hydrogen Generation. Int. J. hydrogen energy 39 (5), 2390–2398. doi:10.1016/j.ijhydene.2013.11.098
Sabia, P., Manna, M. V., Ragucci, R., and de Joannon, M. (2020). Mutual Inhibition Effect of Hydrogen and Ammonia in Oxidation Processes and the Role of Ammonia as "strong" Collider in Third-Molecular Reactions. Int. J. Hydrogen Energy 45 (56), 32113–32127. doi:10.1016/j.ijhydene.2020.08.218
Sánchez, A., and Martín, M. (2018). Scale up and Scale Down Issues of Renewable Ammonia Plants: Towards Modular Design. Sustain. Prod. Consum. 16, 176–192. doi:10.1016/j.spc.2018.08.001
Shen, H., Choi, C., Masa, J., Li, X., Qiu, J., Jung, Y., et al. (2021). Electrochemical Ammonia Synthesis: Mechanistic Understanding and Catalyst Design. Chem 7 (7), 1708–1754. doi:10.1016/j.chempr.2021.01.009
Shiva Kumar, S., and Himabindu, V. (2019). Hydrogen Production by PEM Water Electrolysis - A Review. Mater. Sci. Energy Technol. 2 (3), 442–454. doi:10.1016/j.mset.2019.03.002
Shrestha, K. P., Seidel, L., Zeuch, T., and Mauss, F. (2018). Detailed Kinetic Mechanism for the Oxidation of Ammonia Including the Formation and Reduction of Nitrogen Oxides. Energy fuels. 32 (10), 10202–10217. doi:10.1021/acs.energyfuels.8b01056
Shu, B., He, X., Ramos, C. F., Fernandes, R. X., and Costa, M. (2021). Experimental and Modeling Study on the Auto-Ignition Properties of Ammonia/methane Mixtures at Elevated Pressures. Proc. Combust. Inst. 38 (1), 261–268. doi:10.1016/j.proci.2020.06.291
Skalska, K., Miller, J. S., and Ledakowicz, S. (2010). Trends in NO Abatement: A Review. Sci. total Environ. 408 (19), 3976–3989. doi:10.1016/j.scitotenv.2010.06.001
Skreiberg, Ø., Kilpinen, P., and Glarborg, P. (2004). Ammonia Chemistry below 1400 K under Fuel-Rich Conditions in a Flow Reactor. Combust. Flame 136 (4), 501–518. doi:10.1016/j.combustflame.2003.12.008
Smith, C., and Torrente-Murciano, L. (2021). Guidance for Targeted Development of Ammonia Synthesis Catalysts from a Holistic Process Approach. Chem. Catal. 1 (6), 1163–1172. doi:10.1016/j.checat.2021.09.015
Song, Y., Hashemi, H., Christensen, J. M., Zou, C., Marshall, P., and Glarborg, P. (2016). Ammonia Oxidation at High Pressure and Intermediate Temperatures. Fuel 181, 358–365. doi:10.1016/j.fuel.2016.04.100
Stagni, A., Cavallotti, C., Arunthanayothin, S., Song, Y., Herbinet, O., Battin-Leclerc, F., et al. (2020). An Experimental, Theoretical and Kinetic-Modeling Study of the Gas-phase Oxidation of Ammonia. React. Chem. Eng. 5 (4), 696–711. doi:10.1039/c9re00429g
Starkman, E. S., Newhall, H. K., Sutton, R., Maguire, T., and Farbar, L. (1967). Ammonia as a Spark Ignition Engine Fuel: Theory and Application. Sae Trans. 75, 765–784.
Takizawa, K., Takahashi, A., Tokuhashi, K., Kondo, S., and Sekiya, A. (2008). Burning Velocity Measurements of Nitrogen-Containing Compounds. J. Hazard Mater 155 (1-2), 144–152. doi:10.1016/j.jhazmat.2007.11.089
Tay, K. L., Yang, W., Chou, S. K., Zhou, D., Li, J., Yu, W., et al. (2017). Effects of Injection Timing and Pilot Fuel on the Combustion of a Kerosene-Diesel/ammonia Dual Fuel Engine: A Numerical Study. Energy Procedia 105, 4621–4626. doi:10.1016/j.egypro.2017.03.1002
The Royal Society (2020). Ammonia: Zero-Carbon Fertiliser, Fuel and Energy. https://royalsociety.org/-/media/policy/projects/green-ammonia/green-ammonia-policy-briefing.pdf (Accessed May 15, 2022).
Tian, Z., Li, Y., Zhang, L., Glarborg, P., and Qi, F. (2009). An Experimental and Kinetic Modeling Study of Premixed NH3/CH4/O2/Ar Flames at Low Pressure. Combust. Flame 156 (7), 1413–1426. doi:10.1016/j.combustflame.2009.03.005
Tock, L., Maréchal, F., and Perrenoud, M. (2015). Thermo-environomic Evaluation of the Ammonia Production. Can. J. Chem. Eng. 93 (2), 356–362. doi:10.1002/cjce.22126
USGS (2021). Nitrogen Statistics and Information. https://www.usgs.gov/centers/national-minerals-information-center/nitrogen-statistics-and-information (Accessed May 15, 2022).
Valera-Medina, A., Gutesa, M., Xiao, H., Pugh, D., Giles, A., Goktepe, B., Marsh, R., and Bowen, P. (2019). Premixed Ammonia/hydrogen Swirl Combustion under Rich Fuel Conditions for Gas Turbines Operation. Int. J. Hydrogen Energy 44 (16), 8615–8626. doi:10.1016/j.ijhydene.2019.02.041
Valera-Medina, A., Xiao, H., Owen-Jones, M., David, W. I. F., and Bowen, P. J. (2018). Ammonia for Power. Prog. Energy Combust. Sci. 69, 63–102. doi:10.1016/j.pecs.2018.07.001
Wang, S., Wang, Z., Elbaz, A. M., Han, X., He, Y., Costa, M., Konnov, A. A., and Roberts, W. L. (2020). Experimental Study and Kinetic Analysis of the Laminar Burning Velocity of NH3/syngas/air, NH3/CO/air and NH3/H2/air Premixed Flames at Elevated Pressures. Combust. Flame 221, 270–287. doi:10.1016/j.combustflame.2020.08.004
Wang, Z., Han, X., He, Y., Zhu, R., Zhu, Y., Zhou, Z., et al. (2021). Experimental and Kinetic Study on the Laminar Burning Velocities of NH3 Mixing with CH3OH and C2H5OH in Premixed Flames. Combust. Flame 229, 111392. doi:10.1016/j.combustflame.2021.02.038
Willmann, M., Berger, I., and Bärow, E. (2021). “Woodward L'Orange's New Injector Generation - an Ideal Platform for the Combustion of E-Fuels in Large Engines,” in Heavy-Duty-, On- und Off-Highway-Motoren 2020. Editor J. Liebl (Wiesbaden: Springer Vieweg Press), 223–240. doi:10.1007/978-3-658-34362-0_13
Xiao, H., Lai, S., Valera‐Medina, A., Li, J., Liu, J., and Fu, H. (2020). Experimental and Modeling Study on Ignition Delay of Ammonia/methane Fuels. Int. J. Energy Res. 44 (8), 6939–6949. doi:10.1002/er.5460
Yousefi, A., Guo, H., Dev, S., Liko, B., and Lafrance, S. (2022). Effects of Ammonia Energy Fraction and Diesel Injection Timing on Combustion and Emissions of an Ammonia/diesel Dual-Fuel Engine. Fuel 314, 122723. doi:10.1016/j.fuel.2021.122723
Yu, L., Zhou, W., Feng, Y., Wang, W., Zhu, J., Qian, Y., et al. (2020). The Effect of Ammonia Addition on the Low-Temperature Autoignition of N-Heptane: An Experimental and Modeling Study. Combust. Flame 217, 4–11. doi:10.1016/j.combustflame.2020.03.019
Yue, M., Lambert, H., Pahon, E., Roche, R., Jemei, S., and Hissel, D. (2021). Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 146, 111180. doi:10.1016/j.rser.2021.111180
Zakaznov, V. F., Kursheva, L. A., and Fedina, Z. I. (1978). Determination of Normal Flame Velocity and Critical Diameter of Flame Extinction in Ammonia-Air Mixture. Combust. Explos. Shock Waves 14 (6), 710–713. doi:10.1007/bf00786097
Zhang, Y., Davis, D., and Brear, M. J. (2022). The Role of Hydrogen in Decarbonizing a Coupled Energy System. J. Clean. Prod. 346, 131082. doi:10.1016/j.jclepro.2022.131082
Keywords: ammonia, green fuel, internal combustion engine, decarbonization, combustion
Citation: Tornatore C, Marchitto L, Sabia P and De Joannon M (2022) Ammonia as Green Fuel in Internal Combustion Engines: State-of-the-Art and Future Perspectives. Front. Mech. Eng 8:944201. doi: 10.3389/fmech.2022.944201
Received: 14 May 2022; Accepted: 17 June 2022;
Published: 22 July 2022.
Edited by:
Evangelos G. Giakoumis, National Technical University of Athens, GreeceReviewed by:
José V. Pastor, Universitat Politècnica de València, SpainJinlong Liu, Zhejiang University, China
Qinglong Tang, King Abdullah University of Science and Technology, Saudi Arabia
Copyright © 2022 Tornatore, Marchitto, Sabia and De Joannon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Marchitto, bHVjYS5tYXJjaGl0dG9Ac3RlbXMuY25yLml0
 Cinzia Tornatore
Cinzia Tornatore Luca Marchitto
Luca Marchitto Pino Sabia
Pino Sabia Mara De Joannon
Mara De Joannon