
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Mech. Eng. , 26 July 2017
Sec. Mechatronics
Volume 3 - 2017 | https://doi.org/10.3389/fmech.2017.00007
 Arijit Ghosh1
Arijit Ghosh1 ChangKyu Yoon2
ChangKyu Yoon2 Federico Ongaro3
Federico Ongaro3 Stefano Scheggi3
Stefano Scheggi3 Florin M. Selaru4
Florin M. Selaru4 Sarthak Misra3,5
Sarthak Misra3,5 David H. Gracias1,2*
David H. Gracias1,2*
Untethered microtools that can be precisely navigated into deep in vivo locations are important for clinical procedures pertinent to minimally invasive surgery and targeted drug delivery. In this mini-review, untethered soft grippers are discussed, with an emphasis on a class of autonomous stimuli-responsive gripping soft tools that can be used to excise tissues and release drugs in a controlled manner. The grippers are composed of polymers and hydrogels and are thus compliant to soft tissues. They can be navigated using magnetic fields and controlled by robotic path-planning strategies to carry out tasks like pick-and-place of microspheres and biological materials either with user assistance, or in a fully autonomous manner. It is envisioned that the use of these untethered soft grippers will translate from laboratory experiments to clinical scenarios and the challenges that need to be overcome to make this transition are discussed.
Biomedical applications, such as minimally invasive surgery (MIS) (Dogangil et al., 2010) or controlled and sustained drug delivery (Traverso and Langer, 2015) require new approaches in materials synthesis, fabrication, and robotic control. Present day MIS procedures utilize laparoscopic or catheter-based technologies in which a variety of tethered probes with imaging, suctioning, cutting, cauterizing, or suturing modalities are inserted through small external or internal incisions. These methods have significantly reduced invasiveness and patient trauma in many surgical procedures. A classic example is the minimally invasive mitral valve repair, which previously could only be achieved using the significantly more invasive bypass heart surgery (Felger et al., 2001). However, MIS procedures like intracranial stenosis (Lazzaro and Chen, 2010), video-assisted robotic thoracic surgery (Cerfolio et al., 2016), or ureteral hysterectomy (Packiam et al., 2016) performed with tethered probes in deep and/or tortuous regions of the body still suffer from compromised dexterity and inaccessibility, or risk of injury. The use of the tether or connection to external controls may also cause injuries due to motion of highly deformable soft tissues (Dogangil et al., 2010). Further, it can be challenging, if not impossible, to access submillimeter tortuous regions, or a highly branched system such as the capillary network in the vascular system.
New MIS techniques in which an untethered robotic surgical tool can be inserted and guided to a specific location to perform a surgical task in the body are emerging (Nelson et al., 2010; Sitti et al., 2015). These untethered devices have a negligible footprint to perform tasks like drilling, biopsy, small tumor ablation, as well as delivery of small molecule drugs or biomolecules to locations in previously hard to access regions in the body. Recent demonstrations include biopsies of the biliary tree of a live pig (Gultepe et al., 2013), delivery of a drug-simulant Rhodamine-B to the posterior segment of the eye in a rabbit (Fusco et al., 2014b), and patching wounds in in vitro gastrointestinal models (Miyashita et al., 2016). In addition to untethered operation, the use of materials that are compliant to tissues reduces the possibility of collateral injury during in vivo use, especially in hard to reach places (Majidi, 2014).
A variety of soft robot and, in particular, gripper actuation mechanisms have been developed and many of them have been envisioned for alternate applications, such as deep sea exploration (Galloway et al., 2016). The reader is directed elsewhere (Rus and Tolley, 2015) for a more comprehensive overview on the recent developments of soft robotic actuators and their applications. This mini-review describes soft robotic and, in particular, gripping tools that are suitable for biomedical applications. A broad discussion of soft-gripping actuation mechanisms is presented in the context of in vivo and clinical applicability. The review then focuses on a class of potentially clinically relevant stimuli-responsive soft-grippers (Malachowski et al., 2014; Breger et al., 2015), which can be controlled in an untethered manner and navigated to specific locations. These physiological stimuli-responsive grippers are made of polymers and hydrogels having a modulus in the range of 100 kPa to 200 MPa (Breger et al., 2015) and thus have a rigidity similar to biomaterials such as tissue (Rus and Tolley, 2015). The navigation and imaging techniques that have been developed to robotically maneuver these grippers is described thereafter. The review ends with a discussion of the developments that are required to take such soft gripping tools to clinical environments.
Soft robotic actuation can provide enhanced capabilities for endoscope or catheter-based MIS procedures (Loeve et al., 2010; Mazzolai et al., 2012; Martinez et al., 2013; Cianchetti et al., 2014) by providing local and on-demand stiffening and better maneuverability. Also, due to their easily deformable shapes and larger degrees of freedom, the soft robots have the additional advantage of conforming to the shape and morphology of a surface in a passive manner resulting in a better contact (Hughes et al., 2016). This is evident in the universal gripper (Brown et al., 2010), which is based on the jamming transition of granular materials or the gecko feet-inspired gripper (Song and Sitti, 2014). A miniaturized soft gripper, which can conform to the shape of any soft material can lead to new ways to measure the stiffness of small tumors and more efficient drug delivery. Below, the most common soft gripper actuation mechanisms are classified. A comparative analysis of the different methods can be found in Table 1.
Electroactive polymers (EAPs) (Bar-cohen, 2004) have a sandwiched elastomer in between two metal electrodes. When a large voltage (~kilovolt to megavolt) is applied, the polymer can be bent by electrostatic interactions and has for example been used to fabricate a highly compliant soft gripper (Shintake et al., 2016) that can handle a wide variety of delicate materials like eggs and paper. Polymers like ionic polymer metal composites (Shahinpoor et al., 1998) or conjugated polymers (Smela, 2003), on the other hand, can operate at a significantly lower voltage (~volts) and still produce strains as large as EAPs. Macroscale gripping tools replicating the human hand (Polygerinos et al., 2015) have been used to assist limb movements of patients with compromised motor functions due to stroke or cerebral palsy.
More recently, pneumatic and fluidic actuators (Ching-Ping and Hannaford, 1996), which are based on utilizing a pressurized gas or liquid have been used to create a soft-robotic hand (Deimel and Brock, 2016). The robotic hand was able to grasp objects such as a telephone and a pair of chopsticks. A pneumatic network inside parallel inflatable elastomer chambers was used to generate complex motions of a gripper and grasp an anesthetized mouse (Ilievski et al., 2011).
Elastic materials like polyurethane and Sylgard have been mixed with powders of neodymium iron boride to make millimeter-sized magnetically actuated grippers that were actuated and navigated selectively with programmable and dynamic magnetization capabilities (Diller and Sitti, 2014; Zhang et al., 2017). Magnetic actuation has also been used to generate gripping actions in a miniaturized device made of highly elastic Nitinol alloy, which could be used to cut tissue from an ex vivo porcine liver (Ullrich et al., 2015).
The shape memory effect has been exploited in hard alloys of Ni–Ti to develop very thin catheters for natural orifice transluminal surgery (Chiang, 1988; Phee et al., 2002; Koh and Cho, 2013; Cianchetti et al., 2014), in which the heat-induced martensitic–austenitic phase transformation can result in peristaltic or inchworm like motion. Shape memory polymers (SMPs), on the other hand, can also transform shape to a predefined form when subjected to an appropriate stimulus such as temperature or light (Lendlein and Langer, 2002; Lendlein et al., 2005). They have been used to develop smart sutures or wound closures, for aneurysm treatment as well as to develop blood clot removal tools (Lendlein et al., 2010).
While the electrostatic/ionic and the fluidic actuation schemes require an electrical or fluidic connection to the outside world, the magnetic- or SMP-based actuators can be untethered. Another promising technique for generating untethered actuation at small length scales are stimuli-responsive hydrogels and polymers (de Las Heras Alarcon et al., 2005b; Andersen et al., 2009; Ionov, 2011, 2013; Gracias, 2013). For example, when two layers of polymers having different swelling ratios form a bilayer, then swelling can trigger bending (Hu et al., 1995; Kwag et al., 2016). Unlike SMPs, these stimuli-responsive polymers and hydrogels can be readily patterned and photocrosslinked using a variety of techniques including conventional photolithography and even 3D printing (Gladman et al., 2016) giving rise to a variety of shape changing structures that could be utilized in a range of clinical scenarios.
As noted above, stimuli-responsive materials are attractive for designing robots with untethered actuation. The use of temperature as the stimulus ensures proper functionality independent of the chemical composition of the environment. Though there are many thermoresponsive polymers (Gil and Hudson, 2004), in order to make the actuation of the soft robotic gripper fully autonomous at physiological temperatures, the thermally responsive polymer pNIPAm (Hirokawa et al., 1984; Schild, 1992; Schmaljohann, 2006) is an appropriate choice. The co-polymer system of pNIPAm and acrylic acid shows a sharp transition from hydrophilic to hydrophobic states in the tunable range of physiological relevance between 32 and 38°C. Breger et al. (2015) used this property to engineer soft microgrippers, which can be triggered to open and close autonomously, using physiological temperature as the stimulus (Figure 1A), with verified reversibility over 50 thermal cycles. However, pNIPAm is a very soft, deformable material and needed to be integrated with a significantly stiffer material like poly-propylene fumarate or SU8 photoresist to apply a secure grip. The inclusion of a stiff segment enabled applicability to excision of soft tissue as illustrated in Figures 1B,C, where a gripper excised a clump of cells from a cell culture mass. Breger et al. (2015) also implemented a finite element model to simulate the effect of various design parameters on the opening and closing of a soft gripper based on the balance between entropic and enthalpic interactions and the mechanical forces of swelling. Such models can be used to tune parameters such as layer thickness and swelling ratios needed for optimal performance.
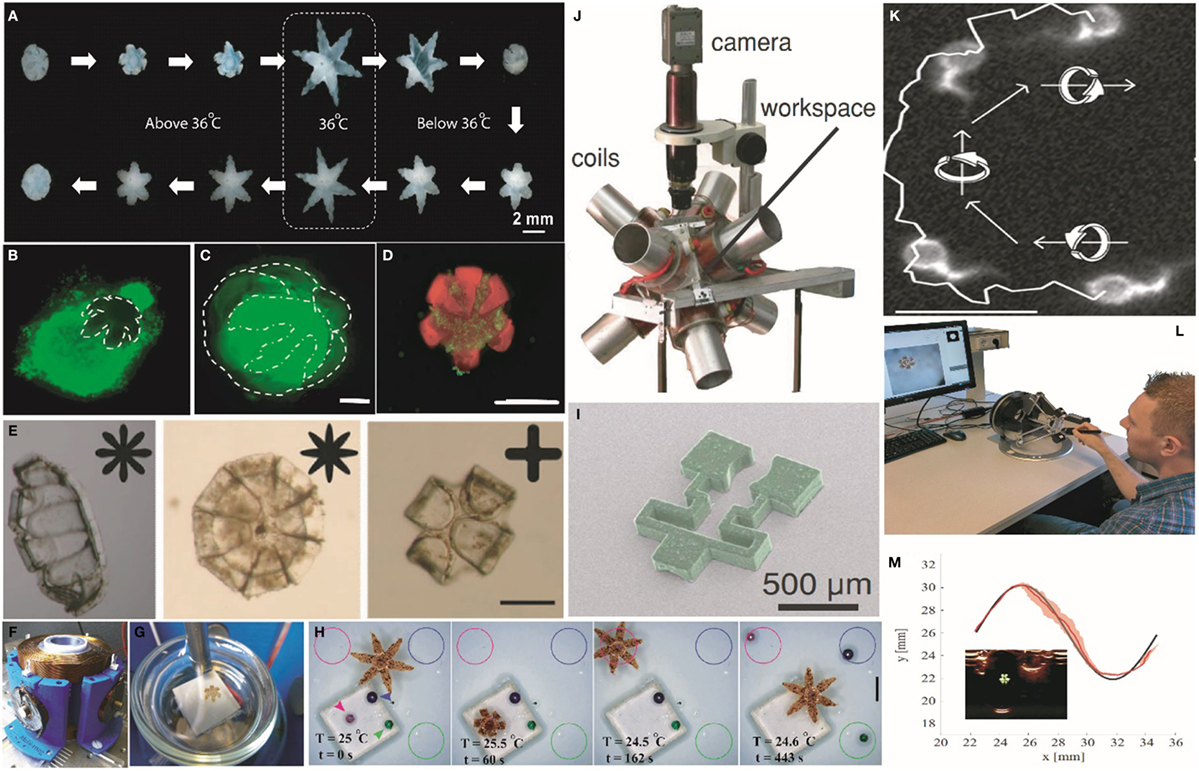
Figure 1. Actuation, navigation, and tracking of untethered stimuli-responsive soft grippers. (A) Experimental snapshots showing the actuation of a soft stimuli-responsive gripper in response to heating and cooling above and below physiological temperature. Reprinted with permission from Breger et al. (2015) ©ACS Publications. (B) Capture and excision of a lump of fibroblast cells (scale bar = 1 mm). Reprinted with permission from Breger et al. (2015) ©ACS Publications. (C) A gripper with the excised lump of fibroblast cells in its grasp (scale bar = 500 µm). Reprinted with permission from Breger et al. (2015) ©ACS Publications. (D) A soft gripper eluting chemotherapeutic drug doxorubicin while grabbing a clump of breast cancer cells (scale bar = 1 mm). Reprinted with permission from Malachowski et al. (2014) ©John Wiley and Sons. (E) IR-responsive self-folding soft grippers fabricated from PEGDA and a composite of graphene oxide and pNIPAm. Scale bars = 200 µm. Reprinted with permission from Fusco et al. (2014a) ©2013, WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim. (F) An electromagnetic coil setup used to navigate the soft grippers. Reprinted with permission from Ongaro et al. (2016b) ©2016, IEEE. (G) A soft gripper on a Petri dish as mounted in the magnetic coil. The white background is the Peltier element used to heat the gripper. Reprinted with permission from Ongaro et al. (2016b) ©2016, IEEE. (H) A fully autonomous object sorting task executed by a thermoresponsive magnetic gripper, in which differently colored beads were picked up and placed in the similarly colored circle. The second and the third images in the sequence show the detailed sorting of the pink colored bead. Scale bar = 2 mm. Reprinted with permission from Ongaro et al. (2017) ©2016. (I) A magnetic gripper containing two different materials, which can be both actuated and navigated using a (J) magnetic coil setup. Reprinted with permission from Diller and Sitti (2014) ©2014, WILEY-VCH Verlag GmbH and Co. KGaA, Weinheim. (K) The smallest soft microrobot till date that exploits bundle of assembled DNA for generating swimming-based propulsion using a rotating magnetic field. Scale bar = 10 µm. Reprinted with permission from Maier et al. (2016) ©2016, American Chemical Soceity. (L) The haptic interface used for controlled navigation of stimuli-responsive soft grippers by human users. Reprinted with permission from Pacchierotti et al. (2017) ©1969, IEEE. (M) Motion of a soft gripper in a sinusoidal path using ultrasound image feedback. The SD in tracking the gripper is denoted by the red shaded area. Inset, an ultrasound image of the gripper (Scheggi et al., 2017, ©2017, IEEE).
In addition, soft grippers, which are composed of polymer and hydrogel layers can be made porous, and thus can also be loaded with drugs (Gupta et al., 2002; Kikuchi and Okano, 2002; Xia and Pack, 2015). For example, Malachowski et al. (2014) loaded soft grippers with anti-inflammatory and chemotherapeutic drugs like mesalamine and doxorubicin (Figure 1D) using different methods to achieve drug release over different temporal periods. The therapeutic grippers or theragrippers were small enough to be deployed using endoscopic catheters under in vivo conditions, and they were used to elute a dye in the stomach of a live pig. The successful loading and release of drugs in a controlled manner from the theragrippers offers the possibility for the realization of self-gripping drug delivery patches that could potentially grab onto tissue and release drugs for an extended period of time. Such chemomechanical devices are attractive because they could augment patches that rely only on mucoadhesives (Andrews et al., 2009).
It should be noted that composites of pNIPAm can respond to alternate stimuli such as pH, light, and ionic strength. For example, devices composed of pNIPAm, graphene, and pEGDA composites (Fusco et al., 2014a) have been developed (Figure 1E) where the gripping action was triggered by near infrared radiation (NIR). The choice of material is not unique to pNIPAm and other soft materials could also be utilized. Thus, IR wavelength selective bending has also been achieved using liquid crystalline elastomers (Kohlmeyer and Chen, 2013). Instead of using physiological temperature as the stimulus, pH-responsive hydrogels can also be utilized. These include patterned bilayers of PMMA and poly (EGDMA) (Guan et al., 2005) or poly (HEMA-co-AA) and poly (HEMA) (Shim et al., 2012), which swell differently under acidic and basic conditions. For example, Li et al. (2016) demonstrated the fabrication of a gripper from a bilayer of pHEMA and pEGDA, in which the grippers were closed at higher pH and opened at lower pH releasing microbeads. It is noteworthy that care must be taken to passivate or coat the surfaces of the robots to avoid biofouling, clotting, or infection, especially when they are used over longer periods of time such as for drug delivery. Non-fouling agents such as polyethylene glycol, poly (2-hydroxyethyl methacrylate), as well as zwitterionic hydrogels have been developed (Castner and Ratner, 2002; Zhang et al., 2013) to reduce interactions between the soft-device and the body and minimize trauma, or side effects. In addition, polymers like pNIPAm has been shown to attach to cells and bacteria in a temperature-dependent manner (de Las Heras Alarcon et al., 2005a; Cooperstein and Canavan, 2010; Schmidt et al., 2010), which can lead to unwanted accumulation of cells on the robots.
Though autonomous and untethered actuation of the grippers allow them to be safely manipulated inside a live animal, it is important to navigate and control their spatial position to carry out tasks in specific locations. Untethered navigation of soft robots is an emerging field of research with only few demonstrations of successful locomotion. SMAs and dielectric elastomers have been used to generate caterpillar or inchworm like locomotion (Seok et al., 2010; Lin et al., 2011). Fluid pressurization and depressurization have led to a quadrupedal multigait robot capable of locomotion underneath obstacles (Shepherd et al., 2011). A centimeter scale battery-operated soft robotic fish was shown to exhibit swimming motion under water (Marchese et al., 2014).
The above examples of navigation that exploit the flexibility of the soft robotic body often require a surface to assist motion and require a tether/battery for power delivery. While these schemes might be useful to operate in larger spaces, the realization of tether less navigation in a millimeter or submillimeter scale is difficult because of the inherent complexity of the fabrication. In order to actuate a multifunctional miniaturized surgical gripper, alternate schemes need to be explored, so that they can be easily implemented in a clinical scenario. A variety of methods have been developed to achieve tetherless navigation of miniaturized agents. Chemical motors, where the surrounding environment provides the fuel, which reacts with the micromotor to generate thrust, have been used to propel cargo and achieve functional tasks (Paxton et al., 2004; Mano and Heller, 2005). Other sources of energy like electricity (Calvo-Marzal et al., 2010), light (Eelkema et al., 2006), or ultrasound (US) (Wang et al., 2012; Garcia-Gradilla et al., 2013) have also been used to transport microscale spheres and bacteria-like E. coli or S. aureus. In a particularly attractive demonstration, structured monochromatic light was used to achieve locomotion in a millimeter scale robot made of liquid crystal elastomers (Palagi et al., 2016). However, these schemes either depend on the composition of the surrounding media, cannot propagate through tissue, or can be difficult to implement in vivo because of high power requirements, three dimensionality, and the presence of obstacles.
In contrast, magnetic fields have been shown to be very suitable for generating propulsion (Zhang et al., 2009; Ghosh et al., 2012) or to provide directionality (Ghosh et al., 2014) in various materials ranging from biofluids like human blood (Lekshmy Venugopalan et al., 2014) to the peritoneal cavity of a live mouse (Servant et al., 2015). They pose very minimal risk of injury and can be transmitted through both opaque and transparent objects. Previously, a millimeter-sized particle of chrome-steel was shown to be safely manipulated inside the carotid artery of a live pig (Martel et al., 2007) using gradient magnetic fields generated by a magnetic resonance (MR) imaging machine. Untethered magnetic actuation of soft grippers can be achieved by impregnating magnetic particles in the body of the robot. Ongaro et al. (2016a,b, 2017) used gradient magnetic fields (Figures 1F,G) along with visual and control algorithms to navigate soft stimuli-responsive grippers on a piece of porcine tissue and avoid both static and dynamic obstacles. The closed loop system even allowed the grippers to perform autonomous pick and place tasks (Figure 1H) with squishy biological materials like egg yolk. Alternatively, human operator-assisted guidance could also be enabled (Ongaro et al., 2016a; Pacchierotti et al., 2017), such as with a haptic feedback control, which is envisioned in a surgeon-assisted intervention (Figure 1L). Similarly, millimeter scale soft magnetic grippers were loaded with neodymium iron boride particles to achieve navigation (Figures 1I,J) in a spatially selective manner (Diller and Sitti, 2014). Other examples of magnetic actuation of miniaturized soft robots include swimming bundles of DNA (Figure 1K) (Maier et al., 2016) and pNIPAm-based shape changing swimmers (Huang et al., 2016). In addition, magnetic fields were used to navigate the motion of small scale bio-hybrid robots like magnetosperms (Magdanz et al., 2013) or magnetotactic (Khalil et al., 2013) bacteria and were used to carry drugs to tumor hypoxic regions (Felfoul et al., 2016).
It is noteworthy that since the body is opaque to visible light, it can be challenging to enable vision-based feedback and tracking. Consequently, feedback based on US images has also been demonstrated (Scheggi et al., 2017), as US provides sufficient depth of imaging inside the body with millimeter scale resolution, apart from being inexpensive. Coupled with a magnetic motion control system, US imaging was used for automated pick and place with soft grippers (Figure 1M).
Biologically inspired robotics have led to the embodiment of different locomotion and sensing capabilities including robots with self-learning capabilities (Pfeifer et al., 2007; McEvoy and Correll, 2015). While exciting, many of these robots require an external power source; but, for tasks like targeted in vivo surgery, a tether can seriously limit the region of applicability. Integrating an adequately powered source of electrical/pneumatic energy on to the body of a miniaturized functional soft robot is still a challenge. As highlighted, the stimuli-responsive soft grippers described here overcome this limitation. However, several challenges need to be overcome to enable translation of these devices to the patient.
Innovative solutions are required in materials synthesis and soft-robot integration, to perform tasks in complex, cluttered, and dynamic in vivo environments. For example, the soft grippers, described here, actuate within a few minutes of being exposed to the body temperature when inserted from a cold state. But for some applications like targeted surgery or delivery, the triggering mechanism necessitates that the grippers close only when they reach the target site, which may occur at shorter or longer times. Hence, it is necessary to develop grippers that can respond to other physicochemical cues such as pH (Guan et al., 2005; Shim et al., 2012), enzymes, or other biomolecular triggers (Bassik et al., 2010), or external optical and magnetic stimuli (Zrinyi, 1997; Zhang et al., 2011).
In order to fully realize the vision of untethered surgery, other tool designs such as cutters, tweezers, or microscopic sutures also need to be developed. In order to successfully execute such tasks, material properties like stiffness or morphology of the shapes have to be taken into account so that sufficient forces are exerted to carry out the tasks (Hughes et al., 2016). Fabricating these complicated devices using 2D microfabrication schemes are challenging and will require new fabrication techniques like 3D printing or two photon polymerization (Maruo et al., 1997; Rengier et al., 2010).
An important focus area is patient safety; in the absence of a tether, there is always the risk that the robot could get lost or lodged within tissue and hence it is preferred if the tools are composed of biodegradable materials so that they can be broken down and cleared from the body. It should be noted that there are a number of soft biodegradable materials that have been developed, primarily for tissue engineering applications and these could be utilized in the synthesis of dissolvable soft robots (Hutmacher et al., 2001; Gunatillake and Adhikari, 2003).
In terms of tracking, further studies using US, MR, or NIR need to be done in animal models to demonstrate feasibility. Though MR shows very good penetration in the deep tissues, it suffers from poor speed (~minutes) to achieve a high enough resolution (≈400 µm) (Bodle et al., 2013; Van Veluw et al., 2013). These problems are partially solved with techniques like magnetic particle imaging (MPI) (Weizenecker et al., 2009), where a resolution of around 400 µm can be achieved with a temporal resolution as low as 21 ms. MPI has been shown to be able to image the beating heart of a live mouse. As described in the previous section, US imaging can provide almost real-time imaging but still suffers from limited spatial resolution (Wells, 2000; Nelson et al., 2010). NIR or radiolabeling using quantum dots or nanoparticles on the other hand, works well only very close to the surface of the skin as it quickly attenuates inside the body (Frangioni, 2003; Gao et al., 2004) and thus imaging very small robots deep inside the body is still a challenge and breakthroughs in imaging technology are needed.
As discussed above, soft robots have been used to perform drug delivery or surgery in the GI tract of large animals. However, to extend their use in more confined spaces like the vascular system, they need to be further miniaturized. To achieve navigation in blood vessels that are 20–30 µm in width or even smaller, stronger magnetic materials like Fe–Co or Ni–Co alloys need to be investigated (Pouponneau et al., 2009). Inspiration for more effective transport in vivo can also be obtained from nature where one/group of flexible flagellum/flagella can either be rotated in a helical fashion (Ghosh and Fischer, 2009) or waved in a single plane (Maier et al., 2016) to generate propulsive force in bacterial systems.
In conclusion, miniaturized untethered soft robotic structures that can be navigated through tortuous paths can deliver drugs or carry out surgical tasks in locations that are impossible to reach non-invasively using current tethered devices. The field is in its infancy and stimuli-responsive soft robots could benefit from recent advances in stretchable electronics and onboard energy harvesting (Bauer et al., 2014; Lu and Kim, 2014), to broaden applicability by enabling sensing and communication to outside world (McEvoy and Correll, 2015). With the collaborative effort of scientists, engineers, and doctors, smart in vivo communicable soft untethered robots that can perform on demand therapy might not be a distant dream.
AG and DG wrote the manuscript. FS and SM provided expert inputs to improve the manuscript. CY, FO, and SS provided data for the manuscript. All authors helped to edit the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by National Institutes of Health, grant 1R01EB017742-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research leading to these results has received funding from the Netherlands Organization for Scientific Research (NWO) Innovative Medical Devices Initiative (IMDI)—Project: Ultrasound Enhancement (USE) and from the European Union’s Horizon 2020 Research and Innovation Program—Project: ROBOTAR (Grant Agreement #638428).
Andersen, E. S., Dong, M., Nielsen, M. M., Jahn, K., Subramani, R., Mamdouh, W., et al. (2009). Self-assembly of a nanoscale DNA box with a controllable lid. Nature 459, 73–76. doi: 10.1038/nature07971
Andrews, G. P., Laverty, T. P., and Jones, D. S. (2009). Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 71, 505–518. doi:10.1016/j.ejpb.2008.09.028
Bassik, N., Brafman, A., Zarafshar, A. M., Jamal, M., Luvsanjav, D., Selaru, F. M., et al. (2010). Enzymatically triggered actuation of miniaturized tools. J. Am. Chem. Soc. 132, 16314–16317. doi:10.1021/ja106218s
Bauer, S., Bauer-Gogonea, S., Graz, I., Kaltenbrunner, M., Keplinger, C., and Schwodiauer, R. (2014). 25th anniversary article: a soft future: from robots and sensor skin to energy harvesters. Adv. Mater. Weinheim 26, 149–162. doi:10.1002/adma.201303349
Bodle, J. D., Feldmann, E., Swartz, R. H., Rumboldt, Z., Brown, T., and Turan, T. N. (2013). High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 44, 287–292. doi:10.1161/STROKEAHA.112.664680
Breger, J. C., Yoon, C., Xiao, R., Kwag, H. R., Wang, M. O., Fisher, J. P., et al. (2015). Self-folding thermo-magnetically responsive soft microgrippers. ACS Appl. Mater. Interfaces 7, 3398–3405. doi:10.1021/am508621s
Brown, E., Rodenberg, N., Amend, J., Mozeika, A., Steltz, E., Zakin, M. R., et al. (2010). Universal robotic gripper based on the jamming of granular material. Proc. Natl. Acad. Sci. U.S.A. 107, 18809–18814. doi:10.1073/pnas.1003250107
Calvo-Marzal, P., Sattayasamitsathit, S., Balasubramanian, S., Windmiller, J. R., Dao, C., and Wang, J. (2010). Propulsion of nanowire diodes. Chem. Commun. 46, 1623–1624. doi:10.1039/b925568k
Castner, D. G., and Ratner, B. D. (2002). Biomedical surface science: foundations to frontiers. Surf. Sci. 500, 28–60. doi:10.1016/S0039-6028(01)01587-4
Cerfolio, R. J., Bess, K. M., Wei, B., and Minnich, D. J. (2016). Incidence, results, and our current intraoperative technique to control major vascular injuries during minimally invasive robotic thoracic surgery. Ann. Thorac. Surg. 102, 394–399. doi:10.1016/j.athoracsur.2016.02.004
Chiang, T. H. (1988). Catheter Apparatus Employing Shape Memory Alloy Structures. U.S. Patent No 4919133 A. Washington, DC: U.S. Patent and Trademark Office.
Ching-Ping, C., and Hannaford, B. (1996). Measurement and modeling of McKibben pneumatic artificial muscles. IEEE Trans. Rob. Autom. 12, 90–102. doi:10.1109/70.481753
Cianchetti, M., Ranzani, T., Gerboni, G., Nanayakkara, T., Althoefer, K., Dasgupta, P., et al. (2014). Soft robotics technologies to address shortcomings in today’s minimally invasive surgery: the STIFF-FLOP approach. Soft Robot. 1, 122–131. doi:10.1089/soro.2014.0001
Cooperstein, M. A., and Canavan, H. E. (2010). Biological cell detachment from poly (N-isopropyl acrylamide) and its applications. Langmuir 26, 7695–7707. doi:10.1021/la902587p
de Las Heras Alarcon, C., Farhan, T., Osborne, V. L., Huck, W. T. S., and Alexander, C. (2005a). Bioadhesion at micro-patterned stimuli-responsive polymer brushes. J. Mater. Chem. 15, 2089–2094. doi:10.1039/b419142k
de Las Heras Alarcon, C., Pennadam, S., and Alexander, C. (2005b). Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 34, 276–285. doi:10.1039/B406727D
Deimel, R., and Brock, O. (2016). A novel type of compliant and underactuated robotic hand for dexterous grasping. Int. J. Rob. Res. 35, 161–185. doi:10.1177/0278364915592961
Diller, E., and Sitti, M. (2014). Three-dimensional programmable assembly by untethered magnetic robotic micro-grippers. Adv. Funct. Mater. 24, 4397–4404. doi:10.1002/adfm.201400275
Dogangil, G., Davies, B. L., and Rodriguez y Baena, F. (2010). A review of medical robotics for minimally invasive soft tissue surgery. Proc. Inst. Mech. Eng. H. 224, 653–679. doi:10.1243/09544119JEIM591
Eelkema, R., Pollard, M. M., Vicario, J., Katsonis, N., Ramon, B. S., Bastiaansen, C. W. M., et al. (2006). Molecular machines: nanomotor rotates microscale objects. Nature 440, 163. doi:10.1038/440163a
Felfoul, O., Mohammadi, M., Taherkhani, S., de Lanauze, D., Xu, Y. Z., Loghin, D., et al. (2016). Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol. 11, 1–5. doi:10.1038/nnano.2016.137
Felger, J. E., Chitwood, W. R., Nifong, L. W., and Holbert, D. (2001). Evolution of mitral valve surgery: toward a totally endoscopic approach. Ann. Thorac. Surg. 72, 1203–1209. doi:10.1016/S0003-4975(01)02978-2
Frangioni, J. V. (2003). In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 7, 626–634. doi:10.1016/j.cbpa.2003.08.007
Fusco, S., Sakar, M. S., Kennedy, S., Peters, C., Bottani, R., Starsich, F., et al. (2014a). An integrated microrobotic platform for on-demand, targeted therapeutic interventions. Adv. Mater. Weinheim 26, 952–957. doi:10.1002/adma.201304098
Fusco, S., Ullrich, F., Pokki, J., Chatzipirpiridis, G., Ozkale, B., Sivaraman, K. M., et al. (2014b). Microrobots: a new era in ocular drug delivery. Expert Opin. Drug Deliv. 5247, 1–12. doi:10.1517/17425247.2014.938633
Galloway, K. C., Becker, K. P., Phillips, B., Kirby, J., Licht, S., Tchernov, D., et al. (2016). Soft robotic grippers for biological sampling on deep reefs. Soft Robot. 3, 23–33. doi:10.1089/soro.2015.0019
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. K., and Nie, S. (2004). In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 22, 969–976. doi:10.1038/nbt994
Garcia-Gradilla, V., Orozco, J., Sattayasamitsathit, S., Soto, F., Kuralay, F., Pourazary, A., et al. (2013). Functionalized ultrasound-propelled magnetically guided nanomotors: toward practical biomedical applications. ACS Nano 7, 9232–9240. doi:10.1021/nn403851v
Ghosh, A., and Fischer, P. (2009). Controlled propulsion of artificial magnetic nanostructured propellers. Nano Lett. 9, 2243–2245. doi:10.1021/nl900186w
Ghosh, A., Paria, D., Rangarajan, G., and Ghosh, A. (2014). Velocity fluctuations in helical propulsion: how small can a propeller be. J. Phys. Chem. Lett. 5, 62–68. doi:10.1021/jz402186w
Ghosh, A., Paria, D., Singh, H. J., Venugopalan, P. L., and Ghosh, A. (2012). Dynamical configurations and bistability of helical nanostructures under external torque. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 86, 031401. doi:10.1103/PhysRevE.86.031401
Gil, E. S., and Hudson, S. M. (2004). Stimuli-responsive polymers and their bioconjugates. Prog. Polym. Sci. 29, 1173–1222. doi:10.1016/j.progpolymsci.2004.08.003
Gladman, A. S., Matsumoto, E. A., Nuzzo, R. G., Mahadevan, L., and Lewis, J. A. (2016). Biomimetic 4D printing. Nat. Mater. 15, 413–419. doi:10.1038/nmat4544
Gracias, D. H. (2013). Stimuli responsive self-folding using thin polymer films. Curr. Opin. Chem. Eng. 2, 112–119. doi:10.1016/j.coche.2012.10.003
Guan, J., He, H., Hansford, D. J., and Lee, L. J. (2005). Self-folding of three-dimensional hydrogel microstructures. J. Phys. Chem. B 109, 23134–23137. doi:10.1021/jp054341g
Gultepe, E., Randhawa, J. S., Kadam, S., Yamanaka, S., Selaru, F. M., Shin, E. J., et al. (2013). Biopsy with thermally-responsive untethered microtools. Adv. Mater. Weinheim 25, 514–519. doi:10.1002/adma.201203348
Gunatillake, P. A., and Adhikari, R. (2003). Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 5, 1–16. doi:10.22203/eCM.v005a01
Gupta, P., Vermani, K., and Garg, S. (2002). Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov. Today 7, 569–579. doi:10.1016/S1359-6446(02)02255-9
Hirokawa, Y., Tanaka, T., Johnson, D. L., and Sen, P. N. (1984). Volume phase transition in a non-ionic gel. AIP Conf. Proc. 107, 203–208. doi:10.1063/1.34300
Hu, Z., Zhang, X., and Li, Y. (1995). Synthesis and application of modulated polymer gels. Science 269, 525. doi:10.1126/science.269.5223.525
Huang, H., Sakar, M. S., Petruska, A. J., Pane, S., and Nelson, B. J. (2016). Soft micromachines with programmable motility and morphology. Nat. Commun. 7:12263. doi:10.1038/ncomms12263
Hughes, J., Culha, U., Giardina, F., Günther, F., and Rosendo, A. (2016). Soft manipulators and grippers: a review. Front. Rob. AI 3:1–12. doi:10.3389/frobt.2016.00069
Hutmacher, D. W., Goh, J. C., and Teoh, S. H. (2001). An introduction to biodegradable materials for tissue engineering applications. Ann. Acad. Med. Singapore 30, 183–191.
Ilievski, F., Mazzeo, A. D., Shepherd, R. F., Chen, X., and Whitesides, G. M. (2011). Soft robotics for chemists. Angew. Chem. Int. Ed. Engl. 50, 1890–1895. doi:10.1002/anie.201006464
Ionov, L. (2011). Soft microorigami: self-folding polymer films. Soft Matter 7, 6786–6791. doi:10.1039/c1sm05476g
Ionov, L. (2013). Biomimetic hydrogel-based actuating systems. Adv. Funct. Mater. 23, 4555–4570. doi:10.1002/adfm.201203692
Khalil, I. S. M., Pichel, M. P., Abelmann, L., and Misra, S. (2013). Closed-loop control of magnetotactic bacteria. Int. J. Rob. Res. 32, 637–649. doi:10.1177/0278364913479412
Kikuchi, A., and Okano, T. (2002). Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 54, 53–77. doi:10.1016/S0169-409X(01)00243-5
Koh, J.-S., and Cho, K.-J. (2013). Omega-shaped inchworm-inspired crawling robot with large-index-and-pitch (LIP) SMA spring actuators. IEEE/ASME Trans. Mechatron. 18, 419–429. doi:10.1109/TMECH.2012.2211033
Kohlmeyer, R. R., and Chen, J. (2013). Wavelength selective IR light driven hinges based on liquid crystalline elastomer composites. Angew. Chem. Int. Ed. 52, 9234–9237. doi:10.1002/anie.201210232
Kwag, H. R., Serbo, J. V., Korangath, P., Sukumar, S., Romer, L. H., and Gracias, D. H. (2016). A self-folding hydrogel in vitro model for ductal carcinoma. Tissue Eng. Part C Methods 22, 398–407. doi:10.1089/ten.TEC.2015.0442
Lazzaro, M. A., and Chen, M. (2010). Interventional management of intracranial stenosis. Open Atheroscler. Thromb. J. 3, 24–34. doi:10.2174/1876506801003010024
Lekshmy Venugopalan, P., Sai, R., Chandorkar, Y., Basu, B., Shivashankar, S., and Ghosh, A. (2014). Conformal cytocompatible ferrite coatings facilitate the realization of a nanovoyager in human blood. Nano Lett. 14, 1968–1975. doi:10.1021/nl404815q
Lendlein, A., Behl, M., Hiebl, B., and Wischke, C. (2010). Shape memory polymers as a technology platform for biomedical applications. Expert Rev. Med. Devices 7, 357–379. doi:10.1586/erd.10.8
Lendlein, A., Jiang, H., Junger, O., and Langer, R. (2005). Light-induced shape-memory polymers. Nature 434, 879–882. doi:10.1038/nature03496
Lendlein, A., and Langer, R. (2002). Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 296, 1673–1676. doi:10.1126/science.1066102
Li, H., Go, G., Ko, S. Y., Park, J., and Park, S. (2016). Magnetic actuated pH responsive hydrogel based soft microrobot for targeted drug delivery. Smart Mater. Struct. 25, 027001. doi:10.1088/0964-1726/25/2/027001
Lin, H., Leisk, G. G., and Trimmer, B. (2011). GoQBoT: a caterpillar inspired soft bodied rolling robot. Bioinspir. Biomim. 6, 026007. doi:10.1088/1748-3182/6/2/026007
Loeve, A., Breedveld, P., and Dankelman, J. (2010). Scopes too flexible… and too stiff. IEEE Pulse 1, 26–41. doi:10.1109/MPUL.2010.939176
Lu, N., and Kim, D.-H. (2014). Flexible and stretchable electronics paving the way for soft robotics. Soft Robot. 1, 53–61. doi:10.1089/soro.2013.0005
Magdanz, V., Sanchez, S., and Schmidt, O. G. (2013). Development of a sperm-flagella driven micro-bio-robot. Adv. Mater. Weinheim 25, 6581–6588. doi:10.1002/adma.201302544
Maier, A. M., Weig, C., Oswald, P., Frey, E., Fischer, P., and Liedl, T. (2016). Magnetic propulsion of microswimmers with DNA-based flagellar bundles. Nano Lett. 16, 906–910. doi:10.1021/acs.nanolett.5b03716
Majidi, C. (2014). Soft robotics: a perspective – current trends and prospects for the future. Soft Robot. 1, 5–11. doi:10.1089/soro.2013.0001
Malachowski, K., Breger, J., Kwag, H. R., Wang, M. O., Fisher, J. P., Selaru, F. M., et al. (2014). Stimuli-responsive theragrippers for chemomechanical controlled release. Angew. Chem. Int. Ed. Engl. 53, 8045–8049. doi:10.1002/anie.201311047
Mano, N., and Heller, A. (2005). Bioelectrochemical propulsion. J. Am. Chem. Soc. 127, 11574–11575. doi:10.1021/ja053937e
Marchese, A. D., Onal, C. D., and Rus, D. (2014). Autonomous soft robotic fish capable of escape maneuvers using fluidic elastomer actuators. Soft Robot. 1, 75–87. doi:10.1089/soro.2013.0009
Martel, S., Mathieu, J. B., Felfoul, O., Chanu, A., Aboussouan, E., Tamaz, S., et al. (2007). Automatic navigation of an untethered device in the artery of a living animal using a conventional clinical magnetic resonance imaging system. Appl. Phys. Lett. 90, 3–6. doi:10.1063/1.2713229
Martinez, R. V., Branch, J. L., Fish, C. R., Jin, L., Shepherd, R. F., Nunes, R. M. D., et al. (2013). Robotic tentacles with three-dimensional mobility based on flexible elastomers. Adv. Mater. Weinheim 25, 205–212. doi:10.1002/adma.201203002
Maruo, S., Nakamura, O., and Kawata, S. (1997). Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 22, 132–134. doi:10.1364/OL.22.000132
Mazzolai, B., Margheri, L., Cianchetti, M., Dario, P., and Laschi, C. (2012). Soft-robotic arm inspired by the octopus: II. From artificial requirements to innovative technological solutions. Bioinspir. Biomim. 7, 25005. doi:10.1088/1748-3182/7/2/025005
McEvoy, M. A., and Correll, N. (2015). Materials that couple sensing, actuation, computation, and communication. Science 347, 1261689. doi:10.1126/science.1261689
Miyashita, S., Guitron, S., Yoshida, K., Li, S., Damian, D. D., and Rus, D. (2016). “Ingestible, controllable, and degradable origami robot for patching stomach wounds,” in IEEE International Conference on Robotics and Automation (ICRA), Stockholm, 909–916.
Nelson, B. J., Kaliakatsos, I. K., and Abbott, J. J. (2010). Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 12, 55–85. doi:10.1146/annurev-bioeng-010510-103409
Ongaro, F., Pacchierotti, C., Yoon, C., Prattichizzo, D., Gracias, D. H., and Misra, S. (2016a). “Evaluation of an electromagnetic system with haptic feedback for control of untethered, soft grippers affected by disturbances,” in Proceedings of the IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics, Singapore, 900–905. doi:10.1109/BIOROB.2016.7523742
Ongaro, F., Yoon, C., Van Den Brink, F., Abayazid, M., Oh, S. H., Gracias, D. H., et al. (2016b). “Control of untethered soft grippers for pick-and-place tasks,” in Proceedings of the IEEE RAS and EMBS International Conference on Biomedical Robotics and Biomechatronics, Singapore, 299–304. doi:10.1109/BIOROB.2016.7523642
Ongaro, F., Scheggi, S., Yoon, C., van den Brink, F., Oh, S. H., Gracias, D. H., et al. (2017). Autonomous planning and control of soft untethered grippers in unstructured environments. J Micro-Bio Robot. 12, 45–52. doi:10.1007/s12213-016-0091-1
Pacchierotti, C., Ongaro, F., van den Brink, F., Yoon, C., Prattichizzo, D., Gracias, D. H., et al. (2017). Steering and control of miniaturized untethered soft magnetic grippers with haptic assistance. IEEE Trans. Autom. Sci. Eng. 1–17. doi:10.1109/TASE.2016.2635106
Packiam, V. T., Cohen, A. J., Pariser, J. J., Nottingham, C. U., Faris, S. F., and Bales, G. T. (2016). The impact of minimally invasive surgery on major iatrogenic ureteral injury and subsequent ureteral repair during hysterectomy: a national analysis of risk factors and outcomes. Urology 98, 183–188. doi:10.1016/j.urology.2016.06.041
Palagi, S., Mark, A. G., Reigh, S. Y., Melde, K., Qiu, T., Zeng, H., et al. (2016). Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 15, 647–654. doi:10.1038/nmat4569
Paxton, W. F., Kistler, K. C., Olmeda, C. C., Sen, A., St. Angelo, S. K., Cao, Y., et al. (2004). Catalytic nanomotors: autonomous movement of striped nanorods. J. Am. Chem. Soc. 126, 13424–13431. doi:10.1021/ja047697z
Pfeifer, R., Lungarella, M., and Iida, F. (2007). Self-organization, embodiment, and biologically inspired robotics. Science 318, 1088–1093. doi:10.1126/science.1145803
Phee, L., Accoto, D., Menciassi, A., Stefanini, C., Carrozza, M. C., and Dario, P. (2002). Analysis and development of locomotion devices for the gastrointestinal tract. IEEE Trans. Biomed. Eng. 49, 613–616. doi:10.1109/TBME.2002.1001976
Polygerinos, P., Wang, Z., Galloway, K. C., Wood, R. J., and Walsh, C. J. (2015). Soft robotic glove for combined assistance and at-home rehabilitation. Rob. Auton. Syst. 73, 135–143. doi:10.1016/j.robot.2014.08.014
Pouponneau, P., Leroux, J. C., and Martel, S. (2009). Magnetic nanoparticles encapsulated into biodegradable microparticles steered with an upgraded magnetic resonance imaging system for tumor chemoembolization. Biomaterials 30, 6327–6332. doi:10.1016/j.biomaterials.2009.08.005
Rengier, F., Mehndiratta, A., Von Tengg-Kobligk, H., Zechmann, C. M., Unterhinninghofen, R., Kauczor, H.-U., et al. (2010). 3D printing based on imaging data: review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 5, 335–341. doi:10.1007/s11548-010-0476-x
Rus, D., and Tolley, M. T. (2015). Design, fabrication and control of soft robots. Nature 521, 467–475. doi:10.1038/nature14543
Scheggi, S., Chandrasekar, K. K. T., Yoon, C., Sawaryn, B., van de Steeg, G., Gracias, D. H., et al. (2017). “Magnetic motion control and planning of untethered soft grippers using ultrasound image feedback,” in Proceedings of the IEEE International Conference on Robotics and Automation (ICRA), Singapore.
Schild, H. G. (1992). Poly (N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 17, 163–249. doi:10.1016/0079-6700(92)90023-R
Schmaljohann, D. (2006). Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 58, 1655–1670. doi:10.1016/j.addr.2006.09.020
Schmidt, S., Zeiser, M., Hellweg, T., Duschl, C., Fery, A., and Möhwald, H. (2010). Adhesion and mechanical properties of PNIPAM microgel films and their potential use as switchable cell culture substrates. Adv. Funct. Mater. 20, 3235–3243. doi:10.1002/adfm.201090084
Seok, S., Onal, C. D., Wood, R., Rus, D., and Kim, S. (2010). “Peristaltic locomotion with antagonistic actuators in soft robotics,” in IEEE International Conference on Robotics and Automation (ICRA), Anchorage, 1228–1233.
Servant, A., Qiu, F., Mazza, M., Kostarelos, K., and Nelson, B. J. (2015). Controlled in vivo swimming of a swarm of bacteria-like microrobotic flagella. Adv. Mater. Weinheim 27, 2981–2988. doi:10.1002/adma.201404444
Shahinpoor, M., Bar-Cohen, Y., Simpson, J. O., and Smith, J. (1998). Ionic polymer-metal composites (IPMCs) as biomimetic sensors, actuators and artificial muscles – a review. Smart Mater. Struct. 7, R15–R30. doi:10.1088/0964-1726/7/6/001
Shepherd, R. F., Ilievski, F., Choi, W., Morin, S. A., Stokes, A. A., Mazzeo, A. D., et al. (2011). Multigait soft robot. Proc. Natl. Acad. Sci. U.S.A. 108, 20400–20403. doi:10.1073/pnas.1116564108
Shim, T. S., Kim, S. H., Heo, C. J., Jeon, H. C., and Yang, S. M. (2012). Controlled origami folding of hydrogel bilayers with sustained reversibility for robust microcarriers. Angew. Chem. Int. Ed. Engl. 51, 1420–1423. doi:10.1002/anie.201106723
Shintake, J., Rosset, S., Schubert, B., Floreano, D., and Shea, H. (2016). Versatile soft grippers with intrinsic electroadhesion based on multifunctional polymer actuators. Adv. Mater. Weinheim 28, 231–238. doi:10.1002/adma.201504264
Sitti, M., Ceylan, H., Hu, W., Giltinan, J., Turan, M., Yim, S., et al. (2015). Biomedical applications of untethered mobile milli/microrobots. Proc. IEEE 103, 205–224. doi:10.1109/JPROC.2014.2385105
Smela, E. (2003). Conjugated polymer actuators for biomedical applications. Adv. Mater. Weinheim 15, 481–494. doi:10.1002/adma.200390113
Song, S., and Sitti, M. (2014). Soft grippers using micro-fibrillar adhesives for transfer printing. Adv. Mater. Weinheim 26, 4901–4906. doi:10.1002/adma.201400630
Traverso, G., and Langer, R. (2015). Perspective: special delivery for the gut. Nature 519, S19. doi:10.1038/519S19a
Ullrich, F., Dheman, K. S., Schuerle, S., and Nelson, B. J. (2015). “Magnetically actuated and guided milli-gripper for medical applications,” in IEEE International Conference on Robotics and Automation (ICRA) 2015, Seattle, 1751–1756.
Van Veluw, S. J., Zwanenburg, J. J., Engelen-Lee, J., Spliet, W. G., Hendrikse, J., Luijten, P. R., et al. (2013). In vivo detection of cerebral cortical microinfarcts with high-resolution 7T MRI. J. Cereb. Blood Flow Metab. 33, 322–329. doi:10.1038/jcbfm.2012.196
Wang, W., Castro, L. A., Hoyos, M., and Mallouk, T. E. (2012). Autonomous motion of metallic microrods propelled by ultrasound. ACS Nano 6, 6122–6132. doi:10.1021/nn301312z
Weizenecker, J., Gleich, B., Rahmer, J., Dahnke, H., and Borgert, J. (2009). Three-dimensional real-time in vivo magnetic particle imaging. Phys. Med. Biol. 54, L1–L10. doi:10.1088/0031-9155/54/5/L01
Wells, P. N. T. (2000). Current status and future technical advances of ultrasonic imaging. Eng. Med. Biol. Mag. IEEE 19, 14–20. doi:10.1109/51.870227
Xia, Y., and Pack, D. W. (2015). Uniform biodegradable microparticle systems for controlled release. Chem. Eng. Sci. 125, 129–143. doi:10.1016/j.ces.2014.06.049
Zhang, J., Onaizah, O., Middleton, K., You, L., and Diller, E. (2017). Reliable grasping of three-dimensional untethered mobile magnetic microgripper for autonomous pick and place. IEEE Rob. Autom. Lett. 2, 835–840. doi:10.1109/LRA.2017.2657879
Zhang, L., Abbott, J. J., Dong, L., Kratochvil, B. E., Bell, D., and Nelson, B. J. (2009). Artificial bacterial flagella: fabrication and magnetic control. Appl. Phys. Lett. 94:064107. doi:10.1063/1.3079655
Zhang, L., Cao, Z., Bai, T., Carr, L., Ella-Menye, J.-R., Irvin, C., et al. (2013). Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556. doi:10.1038/nbt.2580
Zhang, X., Pint, C. L., Lee, M. H., Schubert, B. E., Jamshidi, A., Takei, K., et al. (2011). Optically and thermally responsive programmable materials based on carbon nanotube-hydrogel polymer composites. Nano Lett. 11, 3239–3244. doi:10.1021/nl201503e
Keywords: robotics, surgery, computer-assisted, nanotechnology, microtechnology, polymers
Citation: Ghosh A, Yoon C, Ongaro F, Scheggi S, Selaru FM, Misra S and Gracias DH (2017) Stimuli-Responsive Soft Untethered Grippers for Drug Delivery and Robotic Surgery. Front. Mech. Eng. 3:7. doi: 10.3389/fmech.2017.00007
Received: 15 March 2017; Accepted: 05 July 2017;
Published: 26 July 2017
Edited by:
Thrishantha Nanayakkara, Imperial College London, United KingdomReviewed by:
Kohei Nakajima, University of Tokyo, JapanCopyright: © 2017 Ghosh, Yoon, Ongaro, Scheggi, Selaru, Misra and Gracias. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David H. Gracias, ZGdyYWNpYXNAamh1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.