
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mater., 13 March 2025
Sec. Environmental Degradation of Materials
Volume 12 - 2025 | https://doi.org/10.3389/fmats.2025.1545245
This article is part of the Research TopicMicrobial Corrosion and Material Challenges in Marine EnvironmentsView all 3 articles
 Arunagiri Santhosh Kumar1
Arunagiri Santhosh Kumar1 Lakshminarayanan Sivakumar1
Lakshminarayanan Sivakumar1 Suriyaprakash Rajadesingu2
Suriyaprakash Rajadesingu2 Sambath Sathish3
Sambath Sathish3 Tabarak Malik4,5*
Tabarak Malik4,5* Punniyakotti Parthipan1*
Punniyakotti Parthipan1*Microbiologically influenced corrosion (MIC) significantly affects the durability and integrity of different materials. In the MIC, corrosion on metals is induced by microbial activities and their metabolites, either directly or indirectly. Sulfate-reducing bacteria (SRB), acid-producing bacteria (APB), and iron-reducing bacteria (IRB) are particularly noteworthy to mention as the dominating group accounting for 70% of corrosion incidents due to the MIC. The metabolites produced by these microbial activities majorly influence the metal’s susceptibility or they accelerate to corrosion. MICs are prevalent in marine environments and also encountered in various sectors including oil fields, storage tanks, and cooling water systems, substantially contributing to the degradation of various mechanical materials. This degradation frequently leads to pipeline leakage and equipment failures directly attributed to MIC. Beyond the economic losses, MIC poses severe safety risks, including potential combustion and explosions. Researchers have developed various strategies to mitigate MIC, such as applying heterocyclic organic inhibitors, plant-based green inhibitors, biosurfactants, nanomaterial-based coatings, and inorganic inhibitors. Among these approaches, applying corrosion inhibitors is highly cost-effective, efficient, and practically possible for preventing MIC. These inhibitors are typically selected based on the corrosion type that needs to be mitigated, for MIC chosen inhibitors should act as biocides. Extensive research has been conducted to elucidate the mechanisms of the corrosion inhibition activity. This review evaluates the effectiveness of various types of inhibitors used to mitigate MIC with detailed insights into their prevention strategies and mechanisms.
The industrial sector utilizes different engineering materials such as carbon steel, mild steel, copper, and aluminum alloys for structural applications. These metals have attracted due to their abundance, excellent mechanical stability, and outstanding thermal conductivity under common conditions. With these advantages, there are few concerns associated with metal and alloys that are prone to corrosion problems. According to the National Association of Corrosion Engineers (NACE), Corrosion is a natural chemical process that gradually deteriorates materials, especially metals, through reactions with their surrounding environment (Akpan et al., 2024). This process poses a severe problem for various industries, including the gas and oil, bioprocessing industry, aviation, defense, nuclear power plants, and submarine systems. It may cause damage to specific parts and structures thus leading to system failure, posing a risk to public safety, major financial losses, and having detrimental effects on the ecosystems (Al-Amiery et al., 2023).
According to recent corrosion studies, the global cost of corrosion management was estimated at USD 2.5 trillion in 2016 (Kamaruzzaman et al., 2022). Furthermore, the maintaining of corrosion-related pipeline problems accounts for approximately 3.5% of the global gross domestic product (GDP) (Shehata and El-Shamy, 2023). According to recent estimates, the corrosion-related cost in the United States alone was USD 1.1 trillion for unforeseen expenses in 2016 (Umoren et al., 2019). Furthermore, accidental corrosion costs spent by China in 2015 were USD 300 billion (Kainuma et al., 2019). According to a recent report, corrosion causes a loss of 3%–5% of each country’s gross national product (GNP) (Abdel-Karim and El-Shamy, 2022; Gupta et al., 2023). There are different types of corrosion including pitting corrosion, localized corrosion, atmospheric corrosion, crevice corrosion, erosion corrosion, galvanic corrosion, and biocorrosion affecting the industrial process based on the material types, and environmental, and biological factors. Among these, the involvement of microbiologically influenced corrosion (MIC) or biocorrosion is a prevalent issue that can severely impact the reliability of engineering equipment (Yazdi et al., 2022). About 20%–30% of the overall corrosion is attributed to MIC only (Lu et al., 2024). For instance, it was strongly believed that microbial activity played a crucial role in the Prudhoe Bay oil spill that occurred on 2 March 2006, at Alaska’s north slope, which resulted in the release of many million barrels of crude oil, significantly caused environmental damage by affecting different marine aquatic ecosystems, and substantial economic losses due to post leakage repair managements (Little et al., 2020).
MIC can be classified into two major categories first one is metabolite microbiologically influenced corrosion (MMIC) because microorganisms influence corrosion through the creation of corrosive metabolites, such as sulfur species, organic acids, or protons, and the second category is electrical MIC (EMIC), when occurring, extracellular polymeric substances (EPS) secreted by microbial cells has many components with electrochemical activity and redox properties that play a very important role in corrosion and microbial respiration. The usage of water and metals in the different industrial sectors favours the existence of active corrosion-causing microorganisms in different industrial sectors where they propagate, develop, and contribute to accelerating corrosion reactions. Research findings indicate that biofilm development plays a crucial role in MIC (Sabel and Victor, 2017). The biofilm formation starts with the precipitation of inorganic minerals and the adsorption of small amounts of an organic compound from the surrounding medium, which facilitates microorganisms to adhere over the substrate surfaces, and form a slime-like film layer. Through the metabolic processes of microorganisms (quorum sensing) they synthesize various metabolites, which are helpful for the formation of biofilms. The growth and decay of the microorganisms within the biofilm are essential aspects of this highly dynamic and spontaneous process (Hossain et al., 2020). The following key points highlight how the microorganism’s metabolism can influence biofilm’s impact on the metallic corrosion process: (1) Impacting changes on the cathode or anode, thereby encouraging electrochemical corrosive material; (2) Altering the deterioration reaction, thereby accelerating the rate of corrosion; (3) Developing conditions that encourage microbiological corrosion; (4) Microbial metabolism which generates substances that either encourage (most often) or prevent metal corrosion.
MIC is strongly associated with biofilm development and metabolite production. Although mature biofilms have low-metabolically active cells and a protective matrix, they are tough to eliminate (Obłąk et al., 2014). Remarkably, bacterial growth and reproduction increase exponentially in the right circumstances. Consequently, it is imperative to prevent microbiological corrosion promptly. The current methods for preventing MIC are cathodic preservation, microbial control, the incorporation of antimicrobial metal elements, biocides, and organic/inorganics inhibitors. Certain traditional biocides, including isothiazolidinone, glutaraldehyde, and dodecyl dimethyl benzyl ammonium chloride exhibit significant antimicrobial effects. However, prolonged use of these biocides can lead to the development of resistance in microorganisms, thereby reducing their inhibitory effectiveness (Li et al., 2023). Implementing corrosion preventives must be quicker, effective, and cost-effective. These substances prevent biofilm formation, lower cell adhesion, and adsorb onto metallic surfaces to reduce corrosion. However, these synthetic inhibitors are highly hazardous to the environment and associated living organisms. Therefore, developing new environmental inhibitors that may effectively prevent metal corrosion is a primary research focus in corrosion prevention approaches. This systematic review provides insights into the various corrosion prevention methods for the prevention of MIC. In addition, recent advancements in each corrosion protection approach were analyzed.
Numerous microbial genera and species have been associated with biocorrosion (Ejileugha et al., 2021). The overview of the microbial groups involved in biocorrosion, detailing their modes of action, oxygen requirements, and corrosive effects is provided in Table 1. Bacteria can adhere to living and non-living surfaces, forming microbial communities (Akbarian et al., 2022). This attachment occurs when motility organs, such as flagella and pili, find a suitable surface. This type of microbial community matrix is referred to as a biofilm. The various stages and mechanisms involved in biofilm formation are depicted in Figure 1. The initial stage occurs when microorganisms find a suitable surface, typically rich in organic matter, using multiple motility techniques such as swarming, swimming, and twisting. Bacteria bind to the surface through van der Waals forces, either reversibly (non-biofilm) or irreversibly (biofilm). Once the bacteria have adhered irreversibly to the surface in a monolayer, quorum sensing initiates the formation of a multilayered biofilm and the production of EPS, which creates a 3D biofilm matrix. The mature biofilm contains irreversible and reversible cells, which can disperse to form biofilms on other surfaces. The age of a biofilm can alter the chemical composition, and its persistence makes the microorganisms more resistant to harmful substances. Although the schematic conceptual model of biofilm development is commonly used to explain biofilms, it may only partially capture the complexities of biofilms in real-world situations, such as industrial, natural, and clinical environments. Biofilm formation is a dynamic process, and corrosion begins when the chemical interaction between the metal and the biofilm changes. Understanding microbial biofilms and developing countermeasures for certain microenvironments are essential because biofilms significantly influence microbial corrosion processes and mitigation techniques (Li H. et al., 2021). Once the biofilm forms, various mechanisms influence the corrosion reactions: (1) the electrochemical process produces enzymes that can accelerate cathodic reactions, and (2) substances produced by microorganisms can either accelerate or decelerate corrosion. Additionally, biofilm formation on a metal surface induces changes in their thermodynamic state. Therefore, the dynamic interactions among various compartments lead to the emergence of MIC. Several factors influence this process, including the metal surface characteristics, chemical composition, hydrophobicity, roughness, and environmental factors such as static magnetic fields, shear stress, pH, and ionic strength (Pal and Lavanya, 2022).
Numerous studies on the structure and composition of microbial biofilms have revealed various factors that may affect their development and stability. These factors include environmental conditions such as pH, temperature, and nutrient availability, as well as the presence of other microorganisms that may interact with microbes and influence biofilm development. Moreover, recent research has underscored the importance of quorum sensing in forming biofilms by SRB. Quorum sensing is a mechanism through which bacteria exchange information and synchronize their actions by producing and detecting signaling molecules (Dobretsov et al., 2009). Understanding these processes is crucial for minimizing and preventing SRB-induced corrosion. The accumulation of SRB within a biofilm can initiate or accelerate the corrosion process by providing favorable circumstances for corrosion (Welikala et al., 2024). SRBs impact the corrosion on metallic surfaces by altering the chemical environment beneath the biofilm through the metabolites they produce. This leads to increased inherent diversity and severe localized deterioration of the metal (Eduok et al., 2019). SRB accelerates corrosion by utilizing the released molecular hydrogen and promoting their dissociation from the metal. As a result, SRB forms colonies and draws in additional molecules to adhere to the metallic surfaces, increasing the corrosion rate (Dordević et al., 2021).
Current studies on MIC have less explored the impacts of biofilm formation in biocorrosion. Specifically, lab experiments simulating biocorrosion primarily focus on the effects of their toxic products (H2S, strong and weak acid) as the corrosion product, neglecting biofilm. Although other organic sources can also facilitate corrosion, H2S undoubtedly significantly produces corrosion products through biofilm (AuthorAnonymous and Mulky, 2023). Numerous studies have focused on iron, but less research has explored using other sources of electrons better suited to natural environments (Anandkumar et al., 2016). Expanding research to consider alternative electron donors, such as organic carbon sources, can provide insights into MIC mechanisms in nutrient-limited environments. Understanding MIC is crucial when SRBs are present in environments with limited carbon sources, such as oil fields. In a study, Usher et al. (2014) utilized an organic source instead of the traditional iron source as the electron donor. Further, they summarized that a 100% carbon intake leads to diminished biofilm formation and reduced production of corrosive substances. MIC pitting on the material surface beneath microbial biofilms is also possible (Bairi et al., 2023). Often, corrosion research on biofilms overlooks that different species can detach from the biofilm. While most studies focus on mono-species biofilms or individual bacterial strains, they frequently ignore the interactions between different species. These interspecies interactions, which may be synergistic or antagonistic, significantly impact corrosion dynamics and deserve more complete investigation. Despite significant progress in understanding biofilms and biocorrosion, critical gaps remain in modeling multi-species interactions and exploring alternative electron sources, essential for real-time monitoring of microbial-induced corrosion. Advanced experimental setups, such as microfluidic devices and high-resolution imaging techniques, including confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM), facilitate detailed visualization of biofilm structures and changes on metal surfaces. Additionally, computational models are increasingly utilized to predict biofilm behavior and corrosion outcomes under varying environmental conditions. Therefore, adopting advanced techniques and instruments for real-time monitoring biofilms and their effects on metal surfaces is crucial for advancing future biocorrosion research.
Microorganisms essentially facilitate the electrochemical phenomenon in microbiologically influenced corrosion (MIC). The diverse range of microorganisms contributed to the sophistication of the underlying deterioration mechanisms. Microorganisms can exhibit different corrosive behaviours when they encounter metals. Additionally, within an attached biofilm, microorganisms exist in a distinct micro-environment compared to their independent condition. Cell-cell interaction among the same groups or with different groups assisted the biofilm formation with mutual benefits. For example, the organic material (acetate and organic acids like formate, succinate, and pyruvate) produced by algae and cyanobacteria through fermentation have served as a carbon source for development and propagation of fungi (Aspergillus niger). In certain instances, fungi produced micro growth factors that played a crucial role in photosynthesis and assisted in the transfer of inorganic nutrients (Bhantana et al., 2021).
Additionally, antibiotic-producing microorganisms can impede or eliminate other microorganisms. For example, Streptomyces sp produces nystatin, which suppresses yeasts and molds, while penicillin produced by Penicillium notatum, suppresses most of the Gram-positive bacteria. Microorganisms can not only transform water components into insoluble biological substances that may be deposited on the surface but also transport materials that do not initially deposit themselves on the surface to develop biofilms. The biofilm development can alter the distribution of anions and cations, which influences the electrochemical corrosion process and affects the anodic and cathodic reactions on the material’s surface. Additionally, microbial metabolism can lead to localized corrosion on metal surfaces by altering the local environment (e.g., pH, and concentration). A comprehensive understanding of microbial corrosion mechanisms is essential for the prevention and control. This basic understanding could be very helpful for the researchers to develop more effective strategies for mitigation of corrosion impact. Biofilms have been found to harbor a diverse range of bacterial species, including sulfate-reducing bacteria (SRB), nitrate-reducing bacteria (NRB), iron-oxidizing bacteria (IOB), acid-producing bacteria (APB), Manganese oxidizing bacteria (MOB), Sulfur-oxidizing bacteria (SOB), slime-producing bacteria (SPB), and various heterotrophic microorganisms Figure 2.

Figure 2. Schematic representation of biofilm formation with diverse microbial groups involved in the microbiologically influenced corrosion on metallic surface. Note: NRB-Nitrate reducing bacteria; IOB-Iron oxidizing bacteria; APB-Acid producing bacteria; MOB-Manganese oxidizing bacteria; SOB-Sulfur-oxidizing bacteria; SPB-Slime producing bacteria; SRB-Sulfate reducing bacteria.
SRB is a strictly anaerobic, chemotrophic bacterium that can survive in extreme environments, which makes it a predominant microorganism responsible for causing MIC (Sun et al., 2024). In nature, SRB comprises three major genera: Desulfovibrio, Desulfotomaculum, and Desulfomonas. Most of the SRBs in natural environments belong to the Desulfovibrio genus, consisting of gram-negative with non-spore-forming bacteria that typically thrive in low-to-medium-temperature environments and become non-viable if temperatures exceed 28°C. In contrast, Desulfotomaculum can produce spores and survive at higher temperatures, while Desulfomonas is considered for their rod-type structure with curving, and non-helical axis. SRBs are highly versatile and can utilize various organic compounds as electron donors, contributing to their abundance in diverse environments such as soil, rivers, and coastal waters (Demin et al., 2024). Additionally, SRBs have been observed in extreme habitats, including ocean floors at depths of 3,000 m and clay deposits located 21 m below the surface (Feng et al., 2022). The sulfate plays a crucial role in the metabolic process of SRBs by serving as a terminal electron acceptor, which is then reduced to hydrogen sulfide (H2S) (Bagheri Novair et al., 2024). This reduction, coupled with the decay of organic material for energy, which produces H2S, significantly impacts the surrounding environment by causing substantial corrosion to nearby metal structures as illustrated in Figure 3.
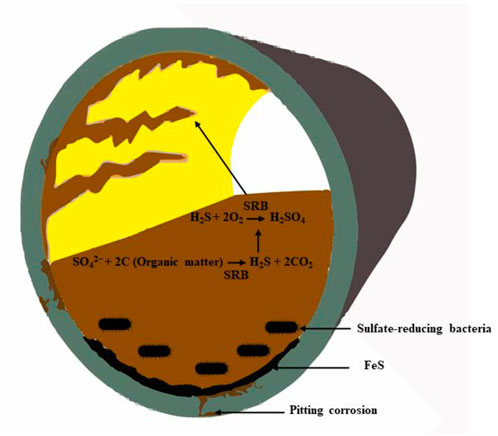
Figure 3. Schematic illustration of sulfate-reducing bacteria involvement in the microbial corrosion on internal pipeline failure.
SRBs are the primary groups of microorganisms causing MIC in various industrial surroundings, including petrochemical facilities, oilfield production sites, and subterranean transportation pipelines (Wang et al., 2024). About 1% of water content and a pH of 7.0–7.8 are sufficient to support SRB growth for several weeks (Zhang et al., 2022). Despite the relatively low nutrient content in water due to the lesser solubility of petroleum components, SRBs can meet their nutrient requirements by reducing other substances. In secondary oil recovery processes, where water displaces subsurface oil, microbial proliferation, and reproduction are significantly enhanced, complicating predicting and managing resulting corrosion problems (Permeh and Lau, 2023). Reports indicate that SRBs cause oil pipeline failures through various mechanisms, including cracking, holes, and corrosive stress. The corrosive reactions facilitated by SRBs lead to the accumulation of insoluble iron sulfide (FeS) residues, which can cause blockages (He et al., 2024). Furthermore, SRBs continuously form biofilms, producing substantial amounts of EPS, primarily contributing to corrosion (Belkaid et al., 2024). Moreover, in a recent study (Venzlaff et al., 2013), proposed that SRB is associated with the mechanisms of electron transfer onto metallic surfaces with interconnected electrochemical reactions. Numerous studies have been conducted on the corrosion caused by SRB and their underlying mechanisms, resulting in diverse theoretical perspectives. Researchers have proposed primary mechanisms to explain SRB-induced microbial corrosion based on the forms of growth and biochemical attributes of SRB while also employing electrochemical methods.
The corrosion reaction on the metallic substrates tends to end anaerobically, along with cathodic depolarizers. Nevertheless, in the presence of SRB, the corrosion reaction may persist. In oxygen-depleted marine water, the primary cathodic response to metallic corrosion is the hydrogen evolution process. Turning H into H2 through cathodic reactions on metal substrates takes more energy. Hydrogenase in the SRB enables the utilization of hydrogen produced on the cathode region to facilitate sulfate reduction (Equation 1). This process enhances the cathodic reaction and accelerates metallic corrosion. The depolarising concept is a significant aspect of the corrosion process when SRB is involved. This concept posits that SRB depletes cathodic hydrogen while reducing sulfate.
The process of secondary corrosive substances formation:
The total reaction:
The fundamental of this method is that the SRB depletes the atomic hydrogen on the metallic iron surface (cathode) causing the cathode to depolarise and initiate other reactions. This facilitates the conversion of Fe into Fe2+, which subsequently interacts with S2- and OH− to produce corrosive substances such as FeS and Fe(OH)₂ (Equations 2, 3) (Núñez et al., 2023). Secondary substances from corrosion can generate flexible corrosion patches on the surface of iron, leading to the development of a concentration gradient battery between the interior and exterior, which accelerates the corrosion reaction (Equation 4).
The biofilms found on metallic substrates generally comprise sessile cells, planktonic cells, and extracellular polymeric substances, all of these contribute to the corrosion phenomenon (Shineh et al., 2023). The progression of MIC is predominantly governed by biofilms, as there are no established connections underlying phytoplankton and MIC. In biofilms, immobile microorganisms play a crucial role in the rusting of metal. When there is an adequate supply of organic carbon, a certain group of bacteria (Desulfovibrio sp, Shewanella sp, Pseudomonas sp) within the biofilm act as electron donors. The transfer of electrons across the cell wall is not necessary for this process, as both the release of organic carbon electrons and the reduction of SO₄2⁻ occur within the SRB’s cytoplasm. Iron is an electron acceptor when organic carbon is deficient in SRB biofilms. Iron is released extracellularly, while sulfate (SO₄2⁻) reduction occurs intracellularly, which is facilitated by cytoplasmic enzymes in SRB. At the movement, SRB biofilms are required to facilitate the transfer of electrons over the cell wall, also known as extracellular electron transfer (EET) (Pu et al., 2023). Electron migration, or EET, transports electrons across cell walls in microorganisms. Two primary mechanisms facilitate EET from the metallic substrate to the cell wall: the first one is mediated electron transfer (MET) and the second one is direct electron transfer (DET), as illustrated in Figure 4 (Černoušek et al., 2020). Both DET and MET are involved in the transfer of electrons from the outer surface of the metal to cytochrome-c, which subsequently attaches to the cell wall. In DET, the cell wall directly contacts the metallic substrate, whereas in MET, electron transfer is facilitated by mediators within the medium. Two commonly used types of electron transfer mediators are phenazines produced by Pseudomonas and flavins produced by Shewanella (Hernandez et al., 2004). According to (Zhang et al., 2015) riboflavin facilitates electron transfer and accelerates the corrosion process on metallic surfaces.
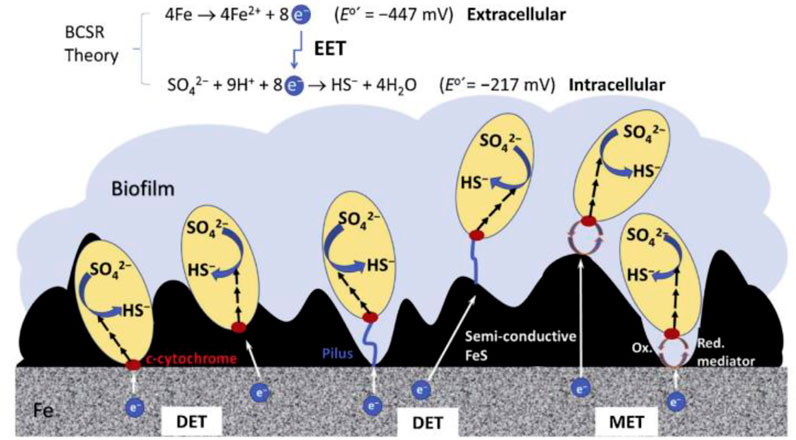
Figure 4. Schematic illustration of Direct Electron Transfer (DET) and Mediated Electron Transfer (MET) in Extracellular Electron Transfer (EET) mechanisms of MIC. (Reprinted from the study by T. Gu et al., 2019 with permission from Elsevier).
The Nitrate-reducing bacteria (NRB) can convert nitrate (NO3−) into nitrite (NO2−) and then further change nitrite into gaseous nitrogen molecules and transfer it as ammonium (NH4+) through a process called denitrification. In the production of oil, wells are filled with nitrate-containing water to keep pressure high, which makes it easier for the transportation of oil to the production wells. Wan et al. (2018) have introduced the concept of biocatalytic cathodic nitrate reduction through their studies on the corrosion process involving NRB and X80 metal in soil environments (Wan et al., 2018). According to their theory, NRB catalyzes the reduction of NO3− or NO2− to nitrogen gas (N2) and ammonium (NH4+). In a similar study Etique et al. (2014), investigated the metabolic capabilities of Klebsiella mobilis on the production of green rust, composed of iron (II) and iron (III) hydroxides, under nitrate-enriched conditions featuring ferrous ions (Fe2+). Their experimental observations revealed that K. mobilis can oxidize Fe2+ to Fe3+, concurrently reducing nitrate. In this biogeochemical process, the involvement of oxidation due to nitrate reduction has been demonstrated with a thermodynamic perspective. Furthermore, the presence of NRB has significantly enhanced the redox reactions, suggesting a substantial microbial influence on metal corrosion dynamics. In a study Xu et al. (2013), revealed that NRB can develop biofilms and induce a pitting type of corrosion on the outermost layer of C1018 carbon steel with a depth of 14.5 μm. Under the biofilm and corrosive product film, NRB takes electrons from the metal to reduce NO3−, which leads to metal pitting corrosion as depicted in Figure 5.
The iron-oxidizing bacteria (IOB) is a consortium of bacteria that obtain energy by converting Fe2⁺ into Fe³⁺ with the releasing of electrons in the process. They produce Fe³⁺ compounds in the form of iron hydroxides, which are present both inside and outside the bacterial membrane. The IOB bacteria are generally classified into anaerobic and aerobic types based on oxygen requirements. IOB obtains energy through iron oxidation, which enhances the rate of metal corrosion. This process converts trivalent iron complexes that deposit on the metal surface, such as ferric hydroxide, into soluble ferrous compounds. Significant quantities of sludge and bacteria have the potential to create nodules, obstruct pipelines, and encourage localized pitting corrosion. While the energy produced by the oxidation process of IOB is modest, the rate at which IOB oxidizes Fe2⁺ is significantly higher than that of conventional chemical oxidation processes. Consequently, as IOB grows, a substantial quantity of Fe2⁺ will undergo oxidation, accelerating the deterioration of metal and leading to severe localized corrosion. Simultaneously, large amounts of iron hydroxides develop and gather, which leads to the formation of rusty deposits. As a result, IOB-caused corrosion in pipelines can degrade the quality of the solution and result in significant blockages (Yang et al., 2022). In a study, Diercks et al. (1991) studied the effects of IOB on galvanized metal and stainless metal. Their research revealed that IOB induces corrosion in these materials and expedites the production of iron and zinc precipitates. Nevertheless, the corrosive effect of IOB on metals consistently leads to significant corrosion. The metal substrate can be partially shielded when the corrosion product film and biofilm combine to produce a thick protective layer.
The acid-producing bacteria (APB) produces different inorganic and organic acids as metabolites, which reduce the pH of the environment and lead to severe pore erosion and pore leakage (Etim et al., 2023). For instance, bacteria that oxidize ammonia and nitrite to produce HNO2 and HNO3, can corrode metallic surfaces. Thiobacillus species can oxidize reduced sulfur compounds, such as elemental sulfur, sulfites, and thiosulfates, into metabolites like H₂SO₃ or H₂SO₄, can cause steel corrosion (Sowards and Mansfield, 2014). Additionally, when acetic acid dissolves in water, it can also corrode metals (Sriphochanart et al., 2023). In a recent study, Zhong Li. (2024) investigated severe corrosion of 316L SS caused by Acidithiobacillus ferrooxidans under acidic bioleaching conditions. The corrosion current density increased from 118 ± 10 nA cm−2 to 2.55 ± 0.21 μA cm−2 due to pyrite leaching and Fe³⁺ ions. A non-contact corrosion mechanism was proposed, offering insights into metal protection in bioleaching. Liu et al. (2024) investigated the impact of electron transfer through organic acids from Acetobacter aceti on 2205 duplex stainless steel (DSS) corrosion (Liu et al., 2024). They found that A. aceti formed a thick biofilm with lower pH and dissolved oxygen, leading to severe corrosion. The absence of a carbon source intensified corrosion, indicating that A. aceti uses the metal as an electron donor and produces acidic by-products. APB has been increasingly recognized for their significant role in causing corrosion in recent years.
The manganese-oxidizing bacteria (MOB) facilitate the conversion of divalent, soluble Mn (II) ions into insoluble manganese oxide (MnO). This process leads to readily observable extracellular accumulations of brown-black manganese oxides. Microorganisms can catalyze manganese oxidation, including algae, bacteria, and fungi (Zhou and Fu, 2020). Certain species from the genera Cyanobacteria, Leptothrix, Hypomicrobium, Pseudomonas, and Metallogenium can oxidize manganese (Cai et al., 2023). The conversion of Mn (II) to Mn (IV) through oxidation is energetically beneficial in the presence of oxygen, with a free energy change of around −16 kcal/mol (Nealson et al., 1988). The energy barrier for aeration can be overcome by increasing the pH or adding manganese-binding compounds. The distribution and chemical-based specification of manganese is governed by kinetic factors, which enable the incorporation of microorganisms and their byproducts into the system, as illustrated in Figure 6. Certain microorganisms employ manganese oxides as an energy source through reduction processes. The reduction of manganese dioxide in this process is described as a rivalry among biological and electrochemical processes. The significance of biological reduction depends on the composition of bacteria in the biofilm, particularly on the metallic substrate. The effect of manganese dioxide on the deterioration of metals is influenced by the type of anodic reaction that causes the metal to dissolve.
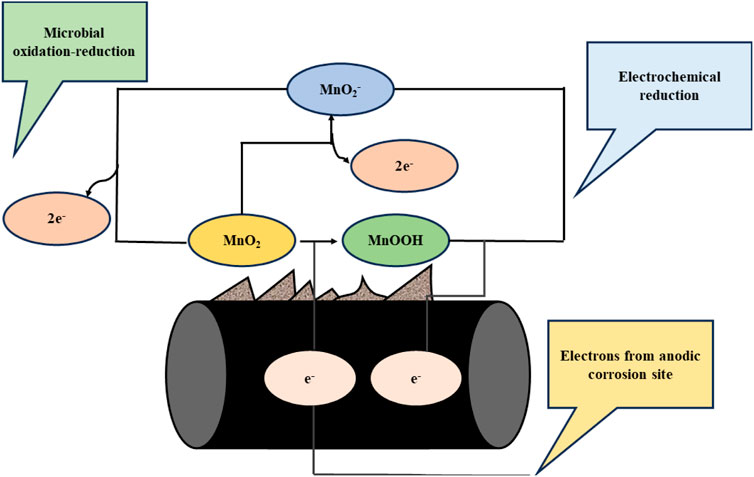
Figure 6. Manganese cycling on the steel surface drives the cathodic reaction in the corrosion process (Olesen et al., 2000).
Sulfur-oxidizing bacteria (SOB) are typically found in sulfur-rich environments, including sulfur springs, acid mine drainage, and marine habitats (Bhandari and Choudhary, 2022). They are rod-shaped cells, typically measuring about 0.5 µm in diameter and 1.0–4.0 µm in length, with some species being motile due to the presence of flagella. These gram-negative obligate aerobes derive energy by oxidizing reduced sulfur compounds, such as sulfides, elemental sulfur, thiosulfates, polythionates, and sulfites, with sulfate as the final oxidation product (Nyamath et al., 2024). The bacteria can release metabolites with significant effectiveness, including various organic acids (e.g., acetic, succinic, and isobutyric acids) and inorganic acids (e.g., sulfuric acid). The SOB, primarily from the genus Thiobacillus, produces sulfuric acid, a highly corrosive substance. This acid is especially harmful to steel structures, as it accelerates the degradation of steel and the dissolution of carbonates. The Thiobacillus species are aerobic, chemo-lithotrophic autotrophs that derive energy by fixing CO2 through a series of reactions that oxidize sulfur, hydrogen sulfide, and other reduced sulfur compounds into sulfuric acid (Robertson and Kuenen, 2006). Many bacteria produce iron sulfide crystals on metal surfaces, contributing to localized rust under the sulfide film and biofilm formation. While biofilm formation reduces the splitting of iron sulfide deposits compared to the abiotic environment, the risk of localized rusting remains significant (Rajala et al., 2022). Thiobacillus species perform a sequence of processes (equations that lead to the production of sulfuric acid) (Equations 5–7) (Coetser and Cloete, 2005).
Strains T. thiooxidans and T. concretivourus can thrive in an acidic environment at a pH of 0.7. The presence of sulfuric acid at such levels has a substantial effect on metals (Coetser and Cloete, 2005). Strain T. ferrooxidans is particularly significant because of their ability to oxidize both ferrous iron and sulfur compounds (Rawlings, 2001).
The Slime-forming bacteria (SFB) also referred to as saprophytic bacteria, are commonly found in marine seawater environments. They are particularly notable for producing substantial EPS. Common examples of SFBs include Pseudomonas, Bacillus, Clostridium, Flavobacterium, Desulfotomaculum, and Desulfovibrio. In the marine environment, Pseudomonas aeruginosa a type of SFB secretes slimy EPS, primarily consisting of the exopolysaccharide especially alginic acid which can interact with metal ions to form complexes (Ma et al., 2022). In addition, the EPS adheres to the surface of the material, and the bacteria merged, resulting in an uneven biofilm and a concentration gradient, leading to localized corrosion.
Introducing inhibitors represents one of the easiest and most common approaches to preventing metallics from corrosion. Various types of organic, inorganic, and biological compounds have been applied as corrosion inhibitors. Surfactants generally demonstrate significant efficiency in inhibiting MIC (Zhou and Wang, 2020). In seawater containing bacteria, negatively charged chloride ions and SRB attach to the positively charged surface of stainless steel, leading to the formation of a negatively charged region. Subsequently, the positively charged heterocyclic quaternary ammonium salt surfactant adheres to the carbon steel surface via electrostatic attraction, such as physical adsorption, forming a protective barrier. This film effectively inhibits the corrosion of stainless steel by preventing anodic disintegration. Simultaneously, the negatively charged surface of the SRB biofilm can preferentially adsorb the positively charged heterocyclic quaternary ammonium salt surfactant, allowing it to penetrate the cell membrane. This interaction affects several biological functions of the cell membrane, including electron transport, selective material permeability, and overall membrane integrity. The disruption of the cell membrane’s selective permeability and genetic mechanisms suppresses or halts SRB activity, leading to cellular death. This alteration significantly impedes the localized corrosion typically driven by the electrochemical coupling and electron transfer between the SRB and carbon steel interfaces.
Concurrently, the molecular structure of heterocyclic quaternary ammonium salt surfactants includes a hydrophilic head group, a triazine ring, and a heterocyclic ring, all characterized by numerous heteroatoms (N, S) and shared π-electrons, which enhance their functional properties. The lone pair electrons of these structures can form coordination bonds with the unoccupied d orbitals of iron, promoting chemical adsorption. Furthermore, the hydrophobic alkyl chains of the molecule extend into the simulated seawater, preventing the penetration of corrosive ions such as chloride. Furthermore, organic substances enriched with heteroatoms such as nitrogen, oxygen, and sulfur exhibit notable corrosion inhibition and antimicrobial effects. These organic corrosion inhibitors possess active sites that can readily donate or accept electrons, enabling interactions with atoms on the metal surface and promoting their adsorption. The inhibitor adsorption layer isolates the metallic surfaces from the corrosion medium, effectively inhibiting corrosion. However, organic compounds’ inherent volatility and toxicity detract from their environmental friendliness, considerably restricting their practical use. Researchers have recently investigated numerous plant extracts as environmentally friendly inhibitors, exhibiting substantial antimicrobial properties. The discussion that follows provides an overview of different types of inhibitors used to minimize MIC.
Surfactants are considered effective corrosion inhibitors, particularly cationic surfactants, due to their specific adsorption behavior. Cationic surfactants include gemini surfactants (GS), and polyquaternary ammonium salt surfactants. The cationic gemini surfactants demonstrated excellent efficacy in preventing biocorrosion and corrosion on metallic steel, achieving over 95% inhibition even at low concentrations (Lavanya and Machado, 2024). The GS has a distinct molecular structure comprising two hydrophilic head groups connected by a spacer chain, enhancing surface activity. These substances can improve their capacity for adhesion to metallic surfaces. Their cationic properties enhance their antibacterial efficacy by affecting the cellular membranes of microorganisms. In addition, GS demonstrates significant efficacy in inhibiting (Devi et al., 2024).
A study was conducted by Deyab et al. (2018) to assess the efficacy of dodecyl dimethyl ammonium chloride (DDAC) for reducing microbial degradation caused by SRB and their potential use in waste remediation in the oil sector. This study discovered that the application of DDAC resulted in a considerable reduction in the corrosion rates of metal samples, with an electrochemical impedance spectroscopy (EIS) based inhibition efficiency of 93.7%. Additionally, DDAC disrupted the formation of SRB biofilms, reduced microbial activity, and lowered hydrogen sulfide production. These findings demonstrate the efficiency of DDAC in preventing MIC in industrial environments. Inhibition efficiencies above 75% are effective for moderately corrosive environments, while efficiencies exceeding 90% are critical for harsh industrial conditions requiring long-term protection. Furthermore, Shaban et al. (2016) evaluated the antibacterial properties, corrosion inhibition, and surface activity of the three cationic surfactants. The results indicated that chemical surfactants effectively inhibit bacterial activity. Additionally, the surfactant with a hydrophobic carbon chain length of 12 was the most effective in removing SRB bacteria from oilfield waste. The GS exhibits superior antibacterial properties due to their increased contact with metal surfaces and ability to draw the phospholipid bilayer of the barrier closer together (Zhou et al., 2019).
In a study, Pakiet et al. (2019) assessed the efficacy of GS as a biocorrosion inhibitor against SRB. Their study determined that the GS exhibited an inhibitory MIC at 0.018 mM, effectively limiting SRB growth. Additionally, open-circuit potential measurements demonstrated that the surfactant significantly decreased corrosion rates of metal surfaces, with an EIS inhibition efficiency of 95%, showcasing their dual effectiveness as both a biocide and a corrosion inhibitor. The inhibition efficiency depends upon the incubation time due to the bacterial resistance mechanism. In this study, Aiad et al. (2013) investigated the anti-biocorrosion properties of imidazolium compounds based on GS against SRB (Aiad et al., 2013). The study demonstrated that these compounds effectively suppress SRB growth. The GS compound substituted with dodecyl groups exhibited the most potent antibacterial activity, significantly inhibiting bacterial growth and corrosion. Conversely, the GS compound with hexadecyl substituents showed decreased effectiveness against microorganisms. These results emphasize the potential of imidazolium-based GS compounds in reducing MIC by inhibiting SRB activity.
In a study, Fu et al. (2016) synthesized and investigated gemini quaternary ammonium salts (GQASs) with a rigid spacer to examine their physicochemical properties and antibacterial efficacy. The results demonstrated that these GQASs had reduced surface tension and exhibited entropy-driven micellization, indicating stable physicochemical properties. The GQAS with dodecyl substituents showed the most potent antibacterial action, significantly inhibiting the growth of both Gram-positive and Gram-negative bacteria. These findings underscore the potential of these compounds for antibacterial applications. Asadov et al. (2020) studied GC with mono- and di-(2-hydroxypropyl) ammonium head groups, examining the effect of spacer length on their properties (Asadov et al., 2020). The study found that shorter spacers decreased the critical micelle concentration (CMC) and enhanced surface activity. The surfactants demonstrated strong antibacterial properties, especially those with di-(2-hydroxypropyl) ammonium head groups, which significantly inhibited the growth of both Gram-positive and Gram-negative bacteria. Additionally, these surfactants provided efficient corrosion inhibition by forming protective layers on metal surfaces exposed to SRB. In a similar study, Labena et al. (2014) developed a novel cationic GS and evaluated their effectiveness in high-salinity environments, specifically in water tanks used in oil fields. The surfactant demonstrated antibacterial solid activity, significantly reducing the viability of SPB. At a concentration of 0.1 mM, it effectively inhibited biofilm formation, and at 1 mM, it achieved a corrosion EIS-based inhibition efficiency of 95% by forming protective coatings on metal surfaces. Additionally, GS exhibit lower environmental toxicity in their hydrophilic form or when they contain shorter alkyl side chains. During biodegradation in aquatic and other ecological systems, GS can be transformed into non-toxic compounds, thereby minimizing their impact on organisms. These findings highlight the potential of GS as an effective and environmentally sustainable MIC inhibitor for industrial applications.
Heterocyclic organic compounds containing electron-rich heteroatoms (N, O, S) or functional groups (i.g., aromatic rings, triple bonds, double bonds) in their structures have been traditionally used as effective corrosion inhibitors (Feng et al., 2019). These electron-rich systems can interact with metal atoms through physical adsorption (i.g., van der Waals forces) or chemical adsorption (i.g., coordination covalent bonds), forming protective films on metal surfaces. This interaction offers both bacteriostatic effects and corrosion inhibition. Such organic inhibitors are advantageous due to their high efficiency and strong chemical compatibility. However, it also exhibits drawbacks, such as limited solubility, susceptibility to adsorption loss, and higher production costs pose challenges. Recent research has been focused on the inhibitory effects of various heterocyclic compounds on MIC. Heterocycles can be categorized into monocyclic and polycyclic structures based on their composition. Monocyclic heterocycles are further classified into five-membered and six-membered rings, while polycyclic heterocycles consist of aromatic structures with five to six-ring components.
In a study, Wang et al. (2019) conducted experimental and quantum chemical evaluations to assess the antibacterial efficacy and anti-corrosion properties of six-membered heterocyclic thionine pyridine sodium (SPT). This study demonstrated that at a concentration of 80 mg/L, SPT effectively reduced both planktonic and SRB on X80 carbon aluminum alloys. Furthermore, SPT significantly suppressed MIC in stainless steel, achieving a reduction of over 80% inhibition efficiency as determined by weight loss. Molecular dynamics modeling further substantiated the adsorption of SPT molecules onto the surface of the X80 carbon aluminum alloy. In a similar study, Obot and Edouk. (2017) investigated the mechanisms of corrosion inhibitions by benzimidazole derivatives on metal-organic systems under acidic, alkaline, and nearly neutral environments. They proposed the application of these compounds for the inhibition of MIC achieving an -based inhibition efficiency of 93.7%. Additionally, substantial research was conducted on thiazole and thiadiazole derivatives, examining their corrosion-inhibiting and antibacterial properties. Furthermore, thiazole and thiadiazole derivatives exhibited promising corrosion inhibition and antibacterial activity results. Recently, Eduok et al. (2018) investigated the impact of 2-mercaptobenzothiazole (MBT) and SRB on hot-dip galvanized steel. The results indicated that the addition of MBT effectively inhibited SRB growth and prevented pitting corrosion on the metal surface. At an MBT concentration of 100 ppm in the SRB medium, the inhibition efficiency was observed to reach 79.54% based on weight loss. Maruschak et al. (2020) investigated SRB-induced biocorrosion in 17G1S-U steel pipes, assessing two organic inhibitors using profilometry and fracture mechanics. Their findings demonstrated that SRB activity significantly increased stress concentration and micro defect formation, while the proposed diagnostic method improved corrosion assessment. Another study by Rochdi et al. (2014) conducted electrochemical tests to evaluate the effectiveness of 2,5-bis (N-methylphenyl)-1,3,4-oxadiazole in preventing metal corrosion in a simulated cooling water system. At a concentration of 20 ppm, the combination of oxadiazole and the studied inhibitor effectively protected brass from microbiological corrosion, achieving an EIS-based inhibition efficiency of 94% and enhanced the resistance to pitting corrosion. Further, Poupin et al. (1998) found that a single heterocycle of morpholine (C4H9NO) significantly influenced microbial degradation. Oil-soluble corrosion inhibitors (OSCI) like Baker NC351 may benefit oil pipelines. These inhibitors consist of amine-based mono-carboxylic acid compounds, which prevent microorganisms from degrading the corrosion inhibitors. Figure 7 illustrates the acceleration of corrosion due to the presence of microorganisms and the mechanism by which inhibitor chemicals work to inhibit MIC. Despite the promising antibacterial activity of numerous synthetic compounds, their widespread application remains limited due to concerns about their potential toxicity to human health and the environment. While these compounds have demonstrated encouraging results, their adverse impacts on ecological systems and human health pose significant barriers to broader adoption (Martelli and Giacomini, 2018). To address these challenges, future research should focus on developing safer and more environmentally sustainable inhibitors that retain high efficiency and compatibility. Advancing these technologies would enable the long-term and sustainable use of heterocyclic compounds for corrosion prevention and microbial growth control across a wide range of industrial sectors.
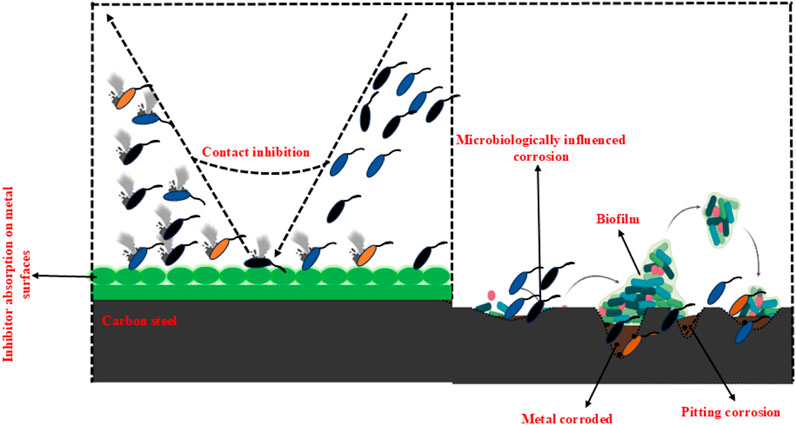
Figure 7. A schematic illustration of microbiologically influenced corrosion formation and the mechanism behind inhibition by inhibitors.
The field of green chemistry emphasizes environmental protection, human life preservation, efficiency enhancement, and loss reduction. Traditional inhibitors have high costs, toxicity, and adverse environmental impacts (Verma et al., 2024). Developing natural green inhibitors for environmentally friendly protection has become the dominant trend. Green inhibitors offer several advantages, including ready availability, biodegradability, ease of production and extraction, cost-effectiveness, and renewable nature as a material source (Zhang X. et al., 2020). Researchers have determined that natural inhibitors derived from plants contain various secondary metabolites, such as flavonoids, heteroatoms, alkaloids, tannins, proteins, and carbohydrates, which are present in different portions of a plant, for instance, in seeds, flowers, leaves, roots, and stems (Varvara et al., 2020). A natural inhibitor for biocorrosion control should be selected based on essential criteria, such as demonstrating antibacterial activity against various microorganisms (both gram-positive and gram-negative). However, it is important to note that some natural inhibitors may act as nutrients, potentially enhancing biofilm growth on metal surfaces rather than preventing it (Keasler et al., 2013). Therefore, it is crucial to evaluate the antibacterial efficacy of green inhibitors before their application. A green inhibitor containing heteroatoms (N, S, P, and O) should demonstrate significant inhibitory effects against both chemical corrosion and biocorrosion (Mehta et al., 2021).
Researchers have used plant-based natural inhibitors to prevent biofilm formation and microbial corrosion, following specified criteria. A study by Parthipan et al. (2018a) found that applying garlic extract significantly mitigates the corrosion of carbon steel and stainless steel under oilfield conditions. The study also demonstrated that garlic extract effectively kills Bacillus subtilis A1 and Streptomyces parvus B7, which causes microbial corrosion. Furthermore, empirical evidence has shown that garlic extract can effectively inhibit chemical and microbial corrosion, with an inhibition efficiency of 81% based on weight loss. Therefore, garlic extract could be used as a dual-function inhibitor, potentially eliminating the need for a separate chemical inhibitor for MIC. Fascinatingly, Packiavathy et al. (2019) conducted a study highlighting the effectiveness of the antimicrobial compound methyl eugenol, derived from Cuminum cyminum, in inhibiting biofilm formation. In this study, researchers found that this bioactive compound protects stainless steel from biocorrosion in natural pond water, with an inhibition efficiency of 60% based on weight loss. Additionally, studies have shown that catechin hydrate (CH) can damage P. aeruginosa cellular membranes, hindering the bacterium’s ability to form biofilms and reducing the number of fully developed biofilms. This damage can cause the release of cellular components, resulting in an apparent inhibitory effectiveness of 99.80% (Lekbach et al., 2019).
Recently, discovered that Azadirachta indica leaf extract, as an environmentally friendly inhibitor, can adhere over copper surfaces to inhibit bacterial growth, thereby preventing corrosion (Swaroop et al., 2016). The research also suggests that Glycyrrhiza glabra leaves may effectively prevent biofilm formation and microbial corrosion while being environmentally safe, achieving a weight loss-based inhibition efficiency of 71%. Narenkumar et al. (2017) utilized ginger root extract to inhibit MIC caused by Bacillus thuringiensis EN2 on mild steel. At a concentration of 20 ppm, the extract achieved approximately 80% of inhibition (Narenkumar et al., 2017). Similarly, Abdelaziz et al. (2021) studied the anti-corrosion properties of Arbutus unedo leaf extracts. The addition of the plant extract, even at low concentrations, significantly improved the inhibition efficiency. At a temperature of 24.85°C, a concentration of 0.5% leaf extract achieved the highest level of inhibition with 91.7%. Another study by (Berrissoul et al., 2020) adapted Lavandula Maier Humbert extract as a novel environmentally friendly corrosion inhibitor for soft iron in 1 M HCl. They found that the extract exhibited a maximum inhibitory efficiency of 92% at a temperature of 30°C at a concentration of 0.4 g/L. Wang et al. (2022) conducted experiments to evaluate the effectiveness of Artemisia argyi leaf extract (ALE) as a corrosion inhibitor for carbon steel in hydrochloric acid (HCl) solution (Wang et al., 2022). The results indicated that ALE achieved a maximum inhibition efficiency of 96.4% at 298 K. These studies emphasize the increasing interest in green corrosion inhibitors derived from natural sources and recognized for their lower environmental toxicity than chemical inhibitors, such as synthetic organic compounds. While chemical inhibitors generally exhibit higher efficiency in corrosion prevention mechanisms, green inhibitors are preferred for their sustainability and reduced toxicity. The phytochemical extraction method is essential in determining the toxicity of green corrosion inhibitors. For instance, plant extracts obtained through aqueous and methanolic extraction methods are widely applied in biocorrosion prevention studies. However, methanolic extracts may result in higher toxicity due to the hazardous nature of methanol. This solvent-related toxicity is a key consideration when assessing the environmental advantages of green corrosion inhibitors.
After selecting plant parts, drying often involves grinding and sieving the material into a powder. Typically, all portions of a plant need to undergo an initial drying process. Conventional drying methods usually require a lengthy duration and operate at room temperature. For instance, the darkness takes between 20 and 30 days to dry (Marsoul et al., 2020; Salleh et al., 2021). This approach can be performed either in direct sunlight or under shade conditions. After drying the plant extracts, we can separate and extract them using various techniques. The extraction method entails using solvent extraction, compression, sublimation, and distillation procedures to extract bioactive compounds (Luksta and Spalvins, 2023). To put it briefly, the extraction processes involve heat, subsequent cooling, and separation of the active components while the solvent is present. Solvent extraction is the most popular technique for extracting plants. The standard solvent extraction techniques facilitate the removal of various bioactive compounds from plant parts. In solvent extraction, a solvent is necessary to permeate the plant tissue, dissolve the molecules (phytochemicals), and ultimately extract them (Alaiya and Odeniyi, 2023). Figure 8 illustrates preparation methods for green inhibitors and the associated corrosion inhibition processes. In addition, Tables 2, 3 present recent studies on plant extract-based green inhibitors for biocorrosion and acid corrosion. Inhibition efficiencies above 75% are effective for moderately corrosive environments, while efficiencies exceeding 90% are critical for harsh industrial conditions requiring long-term protection. Efficiency thresholds are influenced by temperature, salinity, pH, and the inhibitor’s long-term durability, while cost-effectiveness and environmental sustainability remain critical considerations in selecting suitable inhibitors for specific applications. Among solvent options, aqueous extract is considered the most environmentally friendly solvent due to its non-toxic, cost-effective, and sustainable nature, posing minimal risk to aquatic ecosystems (Konwar et al., 2020). In contrast, organic solvents such as methanol, ethanol, and acetone are volatile and contribute to air pollution through Volatile organic compounds (VOC) emissions, with improper disposal increasing toxicity risks and using water as a solvent supports safer and more sustainable extraction methods for green inhibitors (David and Niculescu, 2021).
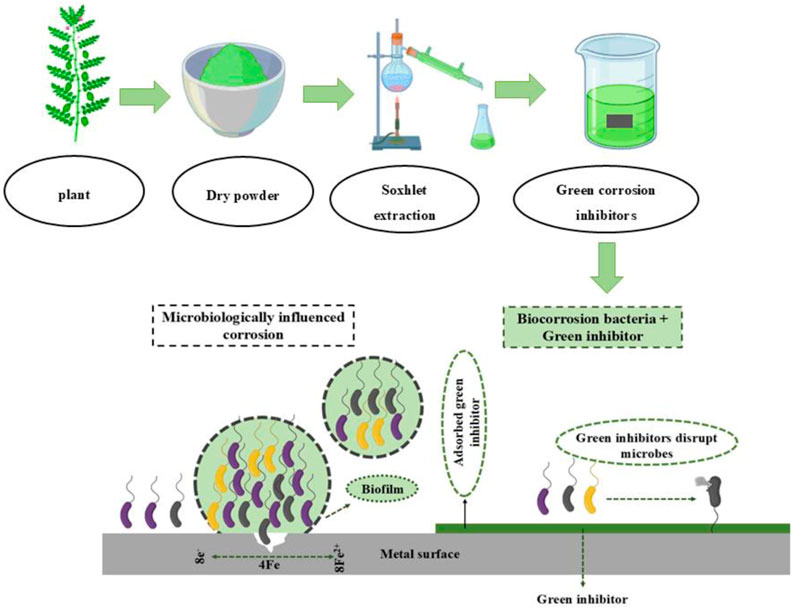
Figure 8. A schematic illustration of microbiologically influenced corrosion and the prevention mechanism by green corrosion inhibitors on metal surfaces.
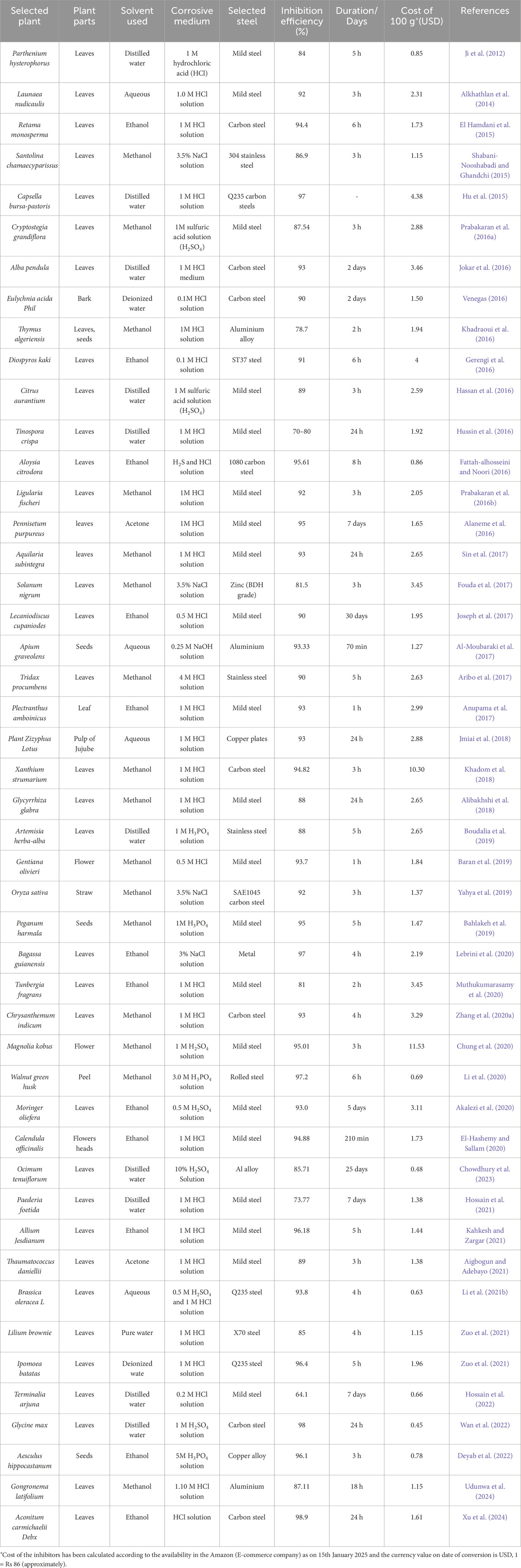
Table 3. The recent reports of plant extract-based green inhibitors for chemical corrosion (both acidic and alkaline) control.
Nanoparticles are highly effective due to their broad applications across various fields. Many nanomaterials possess environmentally favorable properties, making them suitable for diverse uses, including drug delivery and biological activities such as antibacterial and cytotoxic effects. Researchers are exploring various nanomaterials, including zinc, copper, and silver, as potential protective materials to enhance metal efficiency (Nizami et al., 2021). Several techniques are utilized for coating metal nanoparticles, such as electrodeposition, the sol-gel method, plasma interface, chemical vapor deposition, spin coating, and dip coating. Among these methods, electrodeposition is the most widely used due to their ability to cover large surface areas, ease of parameter adjustment, and low cost. Recently, researchers have synthesized various nanoparticles and nanocomposite materials using natural resources and less hazardous precursors or reducing agents.
Devadoss et al. (2023) have synthesized copper oxide (CuO) nanoparticles using a green method with Murraya koenigii (Devadoss et al., 2023). These nanoparticles were characterized using various analytical techniques and subsequently employed as an anti-corrosive material. The study demonstrated that the synthesized CuO nanoparticles exhibited strong anticorrosion properties, with an inhibition efficiency of 58.15%, indicating their potential as an environmentally friendly corrosion-protecting solution. A recent study by Ituen et al. (2021) synthesized silver nanoparticles (AgNPs) using an eco-friendly method using Citrus reticulata peels. These corrosion-resistant nanoparticles demonstrated potent inhibitory properties against microbial and acid-induced corrosion, with an inhibition efficiency of 90%. Recently, there has been a growing trend in synthesizing nanoparticles and nanocomposite materials using natural resources or less toxic precursors and reducing agents. In a research investigation (Hu et al., 2022), synthesized an Ag/Cu nanocomposite using ginger extract. This nanocomposite was applied as an anti-corrosive compound and biocide for microbial corrosion caused by SRB Desulfovibrio sp. The adsorption of the nanocomposite on the steel surface inhibits corrosion by providing film resistance, with an inhibition efficiency of 65% during the application period. Rasheed et al. (2019) conducted a study with ZnO/chitosan nanocomposite as a corrosion-inhibiting nanocomposite for SRB biofilm (Rasheed et al., 2019). The nanocomposite exhibited a high SRB inhibition efficiency of 74% at a concentration of 250 μg/mL on carbon steel. These findings highlighted the potential of the nanocomposite as an effective biocorrosion inhibitor. A study by Aung et al. (2020) investigated ZnO as a filler in an epoxy-polyamide polymer nanocomposite coating applied to a mild steel substrate. The study demonstrated that the coating achieved about 99.91% corrosion inhibition after 792 h of immersion with 5% ZnO. Further, EIS was employed to quantify the protection efficiency in a solution containing 3.5% sodium chloride (NaCl). These findings underscore the effectiveness of ZnO-enhanced polymer nanocomposite coatings in providing long-term corrosion resistance. Nanoparticles and nanocomposites synthesized through eco-friendly techniques have shown considerable potential in improving corrosion resistance while providing sustainable solutions for various industrial applications. However, nanoparticles synthesized using chemical-reducing agents can generate toxic byproducts detrimental to the environment and non-target organisms. Green and bio-based nanoparticles have been developed to mitigate these challenges as eco-friendly and less harmful alternatives. Nevertheless, even green synthesis methods may result in nanoparticles, such as copper, exhibiting toxicity toward certain organisms. This study demonstrates the effectiveness of eco-friendly nanomaterials and nanocomposites in protecting metal surfaces and inhibiting microbial growth.
Microorganisms exist in various forms and exhibit complicated life processes. The corrosion process is a highly complex phenomenon involving biological and chemical interactions across multiple disciplines, including environmental science, microbiology, physics, chemistry, electrochemistry, and materials science. MIC’s primary cause is the biofilm’s presence and interaction with the metal surface. The formation and establishment of microbial membranes should be regarded as a dynamic process that evolves and is influenced by the surrounding environment. Furthermore, the species, abundance, distribution, structure, composition, and chemical properties of the microorganisms within biofilms exhibit variability, which has not yet been fully explained or understood. Understanding the process behind microbial corrosion requires developing an approach to control the microbial barrier and prevent microbial corrosion in real engineering metals. Furthermore, during the corrosion process, it is frequently the case that several microorganisms combine in different ways. Research on collaborative corrosion in the presence of microorganisms aids in understanding how MIC functions in real life and developing theories to support ways to control and prevent it. The primary focus of corrosion inhibitor development is the creation of a multi-functional, environmentally friendly, and highly efficient corrosion inhibitor.
The MIC inhibitors are highly selective in targeting corrosion-causing bacterial strains and prevent their development. However, many corrosion inhibitors have adverse effects on the environment and human health. Consequently, researchers have focused on developing environmentally friendly MIC inhibitors, such as bioactive compounds with potential antimicrobial agents derived from plants, microbial metabolites, and nanomaterials. Despite this, the approach has drawbacks, including the potential for bacteria to develop resistance to antibacterial agents, which can lead to the resurgence of biofilm over time. Increasing inhibitor dosage may prevent the MIC significantly but that could increase the production cost. Many organic inhibitors promote microbial growth instead of inhibiting their growth, which could cause negative impacts. Also, many of these green inhibitors could pose antimicrobial activity against specific groups of tested organisms, but green inhibitors applied to control MIC should have a broad range of antimicrobial activity against all the specified groups that could cause MIC. In addition, green inhibitors should be prepared with less toxic solvents like aqueous extract would be more sustainable inhibitors for the water and oil transporting pipelines and cooling water tower applications. Because, inhibitors prepared with toxic solvents such as methanol, chloroform, and ethyl acetate are may effective but their suitability for the broader range is doubtful and harmful. Also, there are several international standards available for the testing of corrosion inhibitors but not followed in many of the reports that should be considered to confirm their effectiveness. In addition, many studies have focused on the narrow objectives of obtaining corrosion inhibition against specific controlled conditions and did not consider extended studies nor performed any field studies. To assess the effectiveness of the inhibitors on a broader scale it’s very important to conduct field study. Also, the stability and durability of the inhibitors need to be assessed in the field conditions for longer applications.
AS: Resources, Validation, Writing–original draft, Writing–review and editing. LS: Data curation, Investigation, Methodology, Writing–review and editing. SR: Data curation, Formal Analysis, Validation, Writing–review and editing. SS: Data curation, Formal Analysis, Visualization, Writing–review and editing. TM: Conceptualization, Resources, Supervision, Validation, Writing–original draft, Writing–review and editing. PP: Conceptualization, Resources, Supervision, Validation, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelaziz, S., Benamira, M., Messaadia, L., Boughoues, Y., Lahmar, H., and Boudjerda, A. (2021). Green corrosion inhibition of mild steel in HCl medium using leaves extract of Arbutus unedo L. plant: an experimental and computational approach. Colloids Surf. A Physicochem Eng. Asp. 619, 126496. doi:10.1016/j.colsurfa.2021.126496
Abdel-Karim, A. M., and El-Shamy, A. M. (2022). A review on green corrosion inhibitors for protection of archeological metal artifacts. J. Bio Tribocorros 8, 35. doi:10.1007/s40735-022-00636-6
Aiad, I., Emam, D., El-Deeb, A., and Abd-Alrahman, E. (2013). Retracted: novel imidazolium-based gemini surfactants: synthesis, surface properties, corrosion inhibition and biocidal activity against sulfate-reducing bacteria. J. Surfactants Deterg. 16, 927–935. doi:10.1007/s11743-013-1491-z
Aigbogun, J. A., and Adebayo, M. A. (2021). Green inhibitor from Thaumatococcus daniellii for corrosion mitigation of mild steel in 1M HCl. Curr. Res. Green Sustain. Chem. 4, 100201. doi:10.1016/j.crgsc.2021.100201
Akalezi, C. O., Maduabuchi, A. C., Enenebeaku, C. K., and Oguzie, E. E. (2020). Experimental and DFT evaluation of adsorption and inhibitive properties of Moringa oliefera extract on mild steel corrosion in acidic media. Arabian J. Chem. 13, 9270–9282. doi:10.1016/j.arabjc.2020.11.010
Akbarian, M., Chen, S.-H., Kianpour, M., Farjadian, F., Tayebi, L., and Uversky, V. N. (2022). A review on biofilms and the currently available antibiofilm approaches: matrix-destabilizing hydrolases and anti-bacterial peptides as promising candidates for the food industries. Int. J. Biol. Macromol. 219, 1163–1179. doi:10.1016/j.ijbiomac.2022.08.192
Akpan, E. D., Kumar Singh, A., Lgaz, H., Quadri, T. W., Kumar Shukla, S., Mangla, B., et al. (2024). Coordination compounds as corrosion inhibitors of metals: a review. Coord. Chem. Rev. 499, 215503. doi:10.1016/j.ccr.2023.215503
Alaiya, M. A., and Odeniyi, M. A. (2023). Utilisation of Mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: a review. Futur J. Pharm. Sci. 9, 29. doi:10.1186/s43094-023-00479-z
Al-Amiery, A. A., Isahak, W. N. R. W., and Al-Azzawi, W. K. (2023). Corrosion inhibitors: natural and synthetic organic inhibitors. Lubricants 11, 174. doi:10.3390/lubricants11040174
Alaneme, K. K., Olusegun, S. J., and Alo, A. W. (2016). Corrosion inhibitory properties of elephant grass (Pennisetum purpureum) extract: effect on mild steel corrosion in 1M HCl solution. Alexandria Eng. J. 55, 1069–1076. doi:10.1016/j.aej.2016.03.012
Alibakhshi, E., Ramezanzadeh, M., Bahlakeh, G., Ramezanzadeh, B., Mahdavian, M., and Motamedi, M. (2018). Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Mol. Liq. 255, 185–198. doi:10.1016/j.molliq.2018.01.144
Alkhathlan, H. Z., Khan, M., Abdullah, M. M. S., Al-Mayouf, A. M., Mousa, A. A., and Al-Othman, Z. A. M. (2014). Launaea nudicaulis as a source of new and efficient green corrosion inhibitor for mild steel in acidic medium: a comparative study of two solvent extracts. Int. J. Electrochem Sci. 9, 870–889. doi:10.1016/S1452-3981(23)07763-5
Al-Moubaraki, A. H., Al-Howiti, A. A., Al-Dailami, M. M., and Al-Ghamdi, E. A. (2017). Role of aqueous extract of celery (Apium graveolens L.) seeds against the corrosion of aluminium/sodium hydroxide systems. J. Environ. Chem. Eng. 5, 4194–4205. doi:10.1016/j.jece.2017.08.015
AlSalhi, M. S., Devanesan, S., Rajasekar, A., and Kokilaramani, S. (2023). Characterization of plants and seaweeds based corrosion inhibitors against microbially influenced corrosion in a cooling tower water environment. Arabian J. Chem. 16, 104513. doi:10.1016/j.arabjc.2022.104513
Anandkumar, B., George, R. P., Maruthamuthu, S., Parvathavarthini, N., and Mudali, U. K. (2016). Corrosion characteristics of sulfate-reducing bacteria (SRB) and the role of molecular biology in SRB studies: an overview. Corros. Rev. 34, 41–63. doi:10.1515/corrrev-2015-0055
Anupama, K. K., Ramya, K., and Joseph, A. (2017). Electrochemical measurements and theoretical calculations on the inhibitive interaction of Plectranthus amboinicus leaf extract with mild steel in hydrochloric acid. Measurement 95, 297–305. doi:10.1016/j.measurement.2016.10.030
Aribo, S., Olusegun, S. J., Ibhadiyi, L. J., Oyetunji, A., and Folorunso, D. O. (2017). Green inhibitors for corrosion protection in acidizing oilfield environment. J. Assoc. Arab Univ. Basic Appl. Sci. 24, 34–38. doi:10.1016/j.jaubas.2016.08.001
Asadov, Z. H., Ahmadova, G. A., Rahimov, R. A., Hashimzade, S.-Z. F., Abdullayev, Y., Ismailov, E. H., et al. (2020). Aggregation and antimicrobial properties of gemini surfactants with mono- and di-(2-hydroxypropyl)ammonium head-groups: effect of the spacer length and computational studies. J. Mol. Liq. 302, 112579. doi:10.1016/j.molliq.2020.112579
Aung, M. M., Li, W. J., and Lim, H. N. (2020). Improvement of anticorrosion coating properties in bio-based polymer epoxy acrylate incorporated with nano zinc oxide particles. Ind. Eng. Chem. Res. 59, 1753–1763. doi:10.1021/acs.iecr.9b05639
Bagheri Novair, S., Biglari Quchan Atigh, Z., Asgari Lajayer, B., Shu, W., and Price, G. W. (2024). The role of sulphate-reducing bacteria (SRB) in bioremediation of sulphate-rich wastewater: focus on the source of electron donors. Process Saf. Environ. Prot. 184, 190–207. doi:10.1016/j.psep.2024.01.103
Bahlakeh, G., Ramezanzadeh, B., Dehghani, A., and Ramezanzadeh, M. (2019). Novel cost-effective and high-performance green inhibitor based on aqueous Peganum harmala seed extract for mild steel corrosion in HCl solution: detailed experimental and electronic/atomic level computational explorations. J. Mol. Liq. 283, 174–195. doi:10.1016/j.molliq.2019.03.086
Bairi, L. R., Bhuyan, P., Ghosh, A., Narang, M., and Mandal, S. (2023). Microbially induced corrosion issues in the underground buried crude oil and natural gas bearing pipelines: a review. Mater. Corros. 75, 197–211. doi:10.1002/maco.202313950
Baran, E., Cakir, A., and Yazici, B. (2019). Inhibitory effect of Gentiana olivieri extracts on the corrosion of mild steel in 0.5 M HCl: electrochemical and phytochemical evaluation. Arabian J. Chem. 12, 4303–4319. doi:10.1016/j.arabjc.2016.06.008
Belkaid, S., Mansour, D., Salah, F. L., and Amrane, A. (2024). Cuprous ions influence on the biocorrosion of a carbon steel in the presence of sulphate reducing bacteria marine biofilm. Reg. Stud. Mar. Sci. 69, 103307. doi:10.1016/j.rsma.2023.103307
Berrissoul, A., Ouarhach, A., Benhiba, F., Romane, A., Zarrouk, A., Guenbour, A., et al. (2020). Evaluation of Lavandula mairei extract as green inhibitor for mild steel corrosion in 1 M HCl solution. Experimental and theoretical approach. J. Mol. Liq. 313, 113493. doi:10.1016/j.molliq.2020.113493
Bhandari, P., and Choudhary, S. (2022). Insights on the role of sulfur oxidizing bacteria in acid mine drainage biogeochemistry. Geomicrobiol. J. 39, 270–281. doi:10.1080/01490451.2021.1985190
Bhantana, P., Rana, M. S., Sun, X., Moussa, M. G., Saleem, M. H., Syaifudin, M., et al. (2021). Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 84, 19–37. doi:10.1007/s13199-021-00756-6
Boudalia, M., Fernández-Domene, R. M., Tabyaoui, M., Bellaouchou, A., Guenbour, A., and García-Antón, J. (2019). Green approach to corrosion inhibition of stainless steel in phosphoric acid of Artemesia herba albamedium using plant extract. J. Mater. Res. Technol. 8, 5763–5773. doi:10.1016/j.jmrt.2019.09.045
Cai, Y., Yang, K., Qiu, C., Bi, Y., Tian, B., and Bi, X. (2023). A review of manganese-oxidizing bacteria (MnOB): applications, future concerns, and challenges. Int. J. Environ. Res. Public Health 20, 1272. doi:10.3390/ijerph20021272
Černoušek, T., Shrestha, R., Kovářová, H., Špánek, R., Ševců, A., Sihelská, K., et al. (2020). Microbially influenced corrosion of carbon steel in the presence of anaerobic sulphate-reducing bacteria. Corros. Eng. Sci. Technol. 55, 127–137. doi:10.1080/1478422X.2019.1700642
Chowdhury, M. A., Ahmed, M. M. S., Hossain, N., Islam, M. A., Islam, S., and Rana, M. M. (2023). Tulsi and green tea extracts as efficient green corrosion inhibitor for the corrosion of aluminum alloy in acidic medium. Results Eng. 17, 100996. doi:10.1016/j.rineng.2023.100996
Chung, I.-M., Malathy, R., Priyadharshini, R., Hemapriya, V., Kim, S.-H., and Prabakaran, M. (2020). Inhibition of mild steel corrosion using Magnolia kobus extract in sulphuric acid medium. Mater Today Commun. 25, 101687. doi:10.1016/j.mtcomm.2020.101687
Coetser, S. E., and Cloete, T. E. (2005). Biofouling and biocorrosion in industrial water systems. Crit. Rev. Microbiol. 31, 213–232. doi:10.1080/10408410500304074
David, E., and Niculescu, V.-C. (2021). Volatile organic compounds (VOCs) as environmental pollutants: occurrence and mitigation using nanomaterials. Int. J. Environ. Res. Public Health 18, 13147. doi:10.3390/ijerph182413147
Demin, K. A., Prazdnova, E. V., Minkina, T. M., and Gorovtsov, A. V. (2024). Sulfate-reducing bacteria unearthed: ecological functions of the diverse prokaryotic group in terrestrial environments. Appl. Environ. Microbiol. 90, e0139023. doi:10.1128/aem.01390-23
Devadoss, D., Asirvatham, A., Kujur, A., Saaron, G., Devi, N., and John Mary, S. (2023). Green synthesis of copper oxide nanoparticles from Murraya koenigii and its corrosion resistivity on Ti-6Al-4V dental alloy. J. Mech. Behav. Biomed. Mater 146, 106080. doi:10.1016/j.jmbbm.2023.106080
Devi, Y. G., Adhikari, S., Pulikkal, A. K., and Rajaraman, P. V. (2024). Impacts of pyridinium gemini surfactants on corrosion inhibition of carbon steel. Surfaces Interfaces 45, 103796. doi:10.1016/j.surfin.2023.103796
Deyab, M. A. (2018). Efficiency of cationic surfactant as microbial corrosion inhibitor for carbon steel in oilfield saline water. J. Mol. Liq. 255, 550–555. doi:10.1016/j.molliq.2018.02.019
Deyab, M. A., Mohsen, Q., and Guo, L. (2022). Aesculus hippocastanum seeds extract as eco-friendly corrosion inhibitor for desalination plants: experimental and theoretical studies. J. Mol. Liq. 361, 119594. doi:10.1016/j.molliq.2022.119594
Diercks, M., Sand, W., and Bock, E. (1991). Microbial corrosion of concrete. Experientia 47, 514–516. doi:10.1007/BF01949869
Dobretsov, S., Teplitski, M., and Paul, V. (2009). Mini-review: quorum sensing in the marine environment and its relationship to biofouling. Biofouling 25, 413–427. doi:10.1080/08927010902853516
Dordević, D., Jančíková, S., Vítězová, M., and Kushkevych, I. (2021). Hydrogen sulfide toxicity in the gut environment: meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 27, 55–69. doi:10.1016/j.jare.2020.03.003
Eduok, U., Faye, O., and Szpunar, J. (2018). Effect of benzothiazole biocide on SRB-induced biocorrosion of hot-dip galvanized steel. Eng. Fail Anal. 93, 111–121. doi:10.1016/j.engfailanal.2018.07.008
Eduok, U., Ohaeri, E., and Szpunar, J. (2019). Accelerated corrosion of pipeline steel in the presence of Desulfovibrio desulfuricans biofilm due to carbon source deprivation in CO2 saturated medium. Mater. Sci. Eng. C 105, 110095. doi:10.1016/j.msec.2019.110095
Ejileugha, C., Ezealisiji, K. M., Ezejiofor, A. N., and Orisakwe, O. E. (2021). Microbiologically influenced corrosion: uncovering mechanisms and discovering inhibitor—metal and metal oxide nanoparticles as promising biocorrosion inhibitors. J. Bio Tribocorros 7, 109. doi:10.1007/s40735-021-00545-0
El Hamdani, N., Fdil, R., Tourabi, M., Jama, C., and Bentiss, F. (2015). Alkaloids extract of Retama monosperma (L.) Boiss. seeds used as novel eco-friendly inhibitor for carbon steel corrosion in 1 M HCl solution: electrochemical and surface studies. Appl. Surf. Sci. 357, 1294–1305. doi:10.1016/j.apsusc.2015.09.159
El-Hashemy, M. A., and Sallam, A. (2020). The inhibitive action of Calendula officinalis flower heads extract for mild steel corrosion in 1 M HCl solution. J. Mater. Res. Technol. 9, 13509–13523. doi:10.1016/j.jmrt.2020.09.078
Etim, I.-I. N., Njoku, D. I., Uzoma, P. C., Kolawole, S. K., Olanrele, O. S., Ekarenem, O. O., et al. (2023). Microbiologically influenced corrosion: a concern for oil and gas sector in africa. Chem. Afr. 6, 779–804. doi:10.1007/s42250-022-00550-x
Etique, M., Jorand, F. P. A., Zegeye, A., Grégoire, B., Despas, C., and Ruby, C. (2014). Abiotic process for Fe(II) oxidation and green rust mineralization driven by a heterotrophic nitrate reducing bacteria (Klebsiella mobilis). Environ. Sci. Technol. 48, 3742–3751. doi:10.1021/es403358v
Fattah-alhosseini, A., and Noori, M. (2016). Corrosion inhibition of SAE 1018 carbon steel in H2S and HCl solutions by lemon verbena leaves extract. Measurement 94, 787–793. doi:10.1016/j.measurement.2016.09.029
Feng, L., Zhang, S., Xu, Y., Qiang, Y., and Chen, S. (2019). The electron donating effect of novel pyrazolo-pyrimidine inhibitors on anticorrosion of Q235 steel in picking solution. J. Mol. Liq. 286, 110893. doi:10.1016/j.molliq.2019.110893
Feng, L., Zhu, H., Ma, X., Hu, Z., and Zomorodian, A. (2022). “Inhibitors for microbiologically influenced corrosion (MIC),” in Eco-friendly corrosion inhibitors (Elsevier), 137–154. doi:10.1016/B978-0-323-91176-4.00001-5
Fouda, A. E.-A. S., El-Katori, E. E., and Al-Mhyawi, S. (2017). Methanol extract of slanum nigrum as eco-friendly corrosion inhibitor for zinc in sodium chloride polluted solutions. Int. J. Electrochem Sci. 12, 9104–9120. doi:10.20964/2017.10.64
Fu, S. Q., Guo, J. W., Zhong, X., Yang, Z., and Lai, X. F. (2016). Synthesis, physiochemical property and antibacterial activity of gemini quaternary ammonium salts with a rigid spacer. RSC Adv. 6, 16507–16515. doi:10.1039/C5RA22368G
Gerengi, H., Uygur, I., Solomon, M., Yildiz, M., and Goksu, H. (2016). Evaluation of the inhibitive effect of Diospyros kaki (Persimmon) leaves extract on St37 steel corrosion in acid medium. Sustain Chem. Pharm. 4, 57–66. doi:10.1016/j.scp.2016.10.003
Gupta, S. K., Mehta, R. K., Yadav, M., Dagdag, O., Mehmeti, V., Berisha, A., et al. (2023). Diazenyl derivatives as efficient corrosion inhibitors for mild steel in HCl medium: gravimetric, electrochemical and computational approach. J. Mol. Liq. 382, 121976. doi:10.1016/j.molliq.2023.121976
Hassan, K. H., Khadom, A. A., and Kurshed, N. H. (2016). Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S Afr. J. Chem. Eng. 22, 1–5. doi:10.1016/j.sajce.2016.07.002
He, J.-Y., Xie, F., Wang, D., Liu, G.-X., Wu, M., and Qin, Y. (2024). Stress corrosion cracking behavior of buried oil and gas pipeline steel under the coexistence of magnetic field and sulfate-reducing bacteria. Pet. Sci. 21, 1320–1332. doi:10.1016/j.petsci.2023.10.013
Hernandez, M. E., Kappler, A., and Newman, D. K. (2004). Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol. 70, 921–928. doi:10.1128/AEM.70.2.921-928.2004
Hossain, N., Chowdhury, M. A., Iqbal, A. K. M. P., Islam, M. S., Sheikh Omar, N. Y., and Saifullah, A. Z. A. (2021). Paederia Foetid leaves extract as a green corrosion inhibitor for mild steel in hydrochloric acid solution. Curr. Res. Green Sustain. Chem. 4, 100191. doi:10.1016/j.crgsc.2021.100191
Hossain, N., Chowdhury, M. A., Rana, M., Hassan, M., and Islam, S. (2022). Terminalia arjuna leaves extract as green corrosion inhibitor for mild steel in HCl solution. Results Eng. 14, 100438. doi:10.1016/j.rineng.2022.100438
Hossain, S. M. Z., Razzak, S. A., and Hossain, M. M. (2020). Application of essential oils as green corrosion inhibitors. Arab. J. Sci. Eng. 45, 7137–7159. doi:10.1007/s13369-019-04305-8
Hu, Q., Qiu, Y., Zhang, G., and Guo, X. (2015). Capsella bursa-pastoris extract as an eco-friendly inhibitor on the corrosion of Q235 carbon steels in 1mol·L−1 hydrochloric acid. Chin. J. Chem. Eng. 23, 1408–1415. doi:10.1016/j.cjche.2015.05.002
Hu, Y., Feng, Q., Zeng, H., Banat, I. M., Si, Y., Huang, P., et al. (2022). Corrosion inhibition of sulphate-reducing bacterial by Ag/Cu bimetallic nanoparticles synthesised from ginger extract. J. Clean. Prod. 377, 134204. doi:10.1016/j.jclepro.2022.134204
Hussin, M. H., Jain Kassim, M., Razali, N. N., Dahon, N. H., and Nasshorudin, D. (2016). The effect of Tinospora crispa extracts as a natural mild steel corrosion inhibitor in 1M HCl solution. Arabian J. Chem. 9, S616–S624. doi:10.1016/j.arabjc.2011.07.002
Ituen, E., Singh, A., Yuanhua, L., and Akaranta, O. (2021). Green synthesis and anticorrosion effect of Allium cepa peels extract-silver nanoparticles composite in simulated oilfield pickling solution. SN Appl. Sci. 3, 679. doi:10.1007/s42452-021-04670-w
Ji, G., Shukla, S. K., Dwivedi, P., Sundaram, S., Ebenso, E. E., and Prakash, R. (2012). Parthenium hysterophorus plant extract as an efficient green corrosion inhibitor for mild steel in acidic environment. Int. J. Electrochem Sci. 7, 9933–9945. doi:10.1016/S1452-3981(23)16249-3
Jmiai, A., El Ibrahimi, B., Tara, A., Chadili, M., El Issami, S., Jbara, O., et al. (2018). Application of Zizyphus Lotuse - pulp of Jujube extract as green and promising corrosion inhibitor for copper in acidic medium. J. Mol. Liq. 268, 102–113. doi:10.1016/j.molliq.2018.06.091
Jokar, M., Farahani, T. S., and Ramezanzadeh, B. (2016). Electrochemical and surface characterizations of morus alba pendula leaves extract (MAPLE) as a green corrosion inhibitor for steel in 1M HCl. J. Taiwan Inst. Chem. Eng. 63, 436–452. doi:10.1016/j.jtice.2016.02.027
Joseph, O. O., Fayomi, O. S. I., Joseph, O. O., and Adenigba, O. A. (2017). Effect of lecaniodiscus cupaniodes extract in corrosion inhibition of normalized and annealed mild steels in 0.5 M HCl. Energy Procedia 119, 845–851. doi:10.1016/j.egypro.2017.07.136
Kahkesh, H., and Zargar, B. (2021). Corrosion protection evaluation of Allium Jesdianum as a novel and green source inhibitor for mild steel in 1M HCl solution. J. Mol. Liq. 344, 117768. doi:10.1016/j.molliq.2021.117768
Kainuma, S., Yang, M., Ishihara, S., Kaneko, A., and Yamauchi, T. (2019). Corrosion protection of steel members using an Al-Zn base sacrificial anode and fiber sheet in an atmospheric environment. Constr. Build. Mater 224, 880–893. doi:10.1016/j.conbuildmat.2019.07.067
Kamaruzzaman, W. M. I. W. M., Nasir, N. A. M., Hamidi, N. A. S. M., Yusof, N., Shaifudin, M. S., Suhaimi, A. M. A. A. M., et al. (2022). 25 years of progress on plants as corrosion inhibitors through a bibliometric analysis using the Scopus database (1995–2020). Arabian J. Chem. 15, 103655. doi:10.1016/j.arabjc.2021.103655
Keasler, V., Bennett, B., Keller, C., Whalen, P., Cairns, J., and De Paula, R. M. (2013). Expanding the microbial monitoring toolkit: evaluation of traditional and molecular monitoring methods. Int. Biodeterior. Biodegrad. 81, 51–56. doi:10.1016/j.ibiod.2012.07.002
Khadom, A. A., Abd, A. N., and Ahmed, N. A. (2018). Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: kinetics and mathematical studies. S Afr. J. Chem. Eng. 25, 13–21. doi:10.1016/j.sajce.2017.11.002
Khadraoui, A., Khelifa, A., Hachama, K., and Mehdaoui, R. (2016). Thymus algeriensis extract as a new eco-friendly corrosion inhibitor for 2024 aluminium alloy in 1M HCl medium. J. Mol. Liq. 214, 293–297. doi:10.1016/j.molliq.2015.12.064
Kokilaramani, S., Narenkumar, J., AlSalhi, M. S., Devanesan, S., Obulisamy, P. K., Balagurunathan, R., et al. (2022). Evaluation of crude methanolic mangrove leaves extract for antibiofilm efficacy against biofilm-forming bacteria on a cooling tower wastewater system. Arabian J. Chem. 15, 103948. doi:10.1016/j.arabjc.2022.103948
Konwar, M., Dutta, A., and Sarma, D. (2020). “Biomass-derived aqueous extracts as green solvents for organic transformations,” in Green sustainable process for chemical and environmental engineering and science (Elsevier), 123–154. doi:10.1016/B978-0-12-819539-0.00006-3
Labena, A., Hegazy, M. A., Horn, H., and Müller, E. (2014). Cationic gemini surfactant as a corrosion inhibitor and a biocide for high salinity sulfidogenic bacteria originating from an oil-field water tank. J. Surfactants Deterg. 17, 419–431. doi:10.1007/s11743-013-1551-4
Lavanya, M., and Machado, A. A. (2024). Surfactants as biodegradable sustainable inhibitors for corrosion control in diverse media and conditions: a comprehensive review. Sci. Total Environ. 908, 168407. doi:10.1016/j.scitotenv.2023.168407
Lebrini, M., Suedile, F., Salvin, P., Roos, C., Zarrouk, A., Jama, C., et al. (2020). Bagassa guianensis ethanol extract used as sustainable eco-friendly inhibitor for zinc corrosion in 3% NaCl: electrochemical and XPS studies. Surfaces Interfaces 20, 100588. doi:10.1016/j.surfin.2020.100588
Lekbach, Y., Dong, Y., Li, Z., Xu, D., El Abed, S., Yi, Y., et al. (2019). Catechin hydrate as an eco-friendly biocorrosion inhibitor for 304L stainless steel with dual-action antibacterial properties against Pseudomonas aeruginosa biofilm. Corros. Sci. 157, 98–108. doi:10.1016/j.corsci.2019.05.021
Lekbach, Y., Xu, D., El Abed, S., Dong, Y., Liu, D., Khan, M. S., et al. (2018). Mitigation of microbiologically influenced corrosion of 304L stainless steel in the presence of Pseudomonas aeruginosa by Cistus ladanifer leaves extract. Int. Biodeterior. Biodegrad. 133, 159–169. doi:10.1016/j.ibiod.2018.07.003
Li, H., Qiang, Y., Zhao, W., and Zhang, S. (2021a). A green Brassica oleracea L extract as a novel corrosion inhibitor for Q235 steel in two typical acid media. Colloids Surf. A Physicochem Eng. Asp. 616, 126077. doi:10.1016/j.colsurfa.2020.126077
Li, H., Zhong, X., Hu, J., Wang, J., and Yu, J. (2023). The inhibition of sulfate reducing bacteria adhesion and corrosion on the carbon steel surface using ZnO particles. J. Adhes. Sci. Technol. 37, 270–283. doi:10.1080/01694243.2022.2028075
Li, X., Deng, S., Du, G., and Xie, X. (2020). Synergistic inhibition effect of walnut green husk extract and sodium lignosulfonate on the corrosion of cold rolled steel in phosphoric acid solution. J. Taiwan Inst. Chem. Eng. 114, 263–283. doi:10.1016/j.jtice.2020.09.010
Li, Z., Chang, W., Cui, T., Xu, D., Zhang, D., Lou, Y., et al. (2021b). Adaptive bidirectional extracellular electron transfer during accelerated microbiologically influenced corrosion of stainless steel. Commun. Mater 2, 67. doi:10.1038/s43246-021-00173-8
Little, B. J., Blackwood, D. J., Hinks, J., Lauro, F. M., Marsili, E., Okamoto, A., et al. (2020). Microbially influenced corrosion—any progress? Corros. Sci. 170, 108641. doi:10.1016/j.corsci.2020.108641
Liu, D., Liang, Y., Wei, H., Liu, P., Jin, D., Yassir, L., et al. (2024). Enhanced corrosion of 2205 duplex stainless steel by Acetobacter aceti through synergistic electron transfer and organic acids acceleration. Bioelectrochemistry 157, 108665. doi:10.1016/j.bioelechem.2024.108665
Lu, S., Sun, J., Xue, N., Gu, T., Xia, M., Chu, W., et al. (2024). Study of copper corrosion via extracellular electron transfer by nitrate reducing Halomonas titanicae. Corros. Sci. 231, 111996. doi:10.1016/j.corsci.2024.111996
Luksta, I., and Spalvins, K. (2023). Methods for extraction of bioactive compounds from products: a review. Environ. Clim. Technol. 27, 422–437. doi:10.2478/rtuect-2023-0031
Ma, L. Z., Wang, D., Liu, Y., Zhang, Z., and Wozniak, D. J. (2022). Regulation of biofilm exopolysaccharide biosynthesis and degradation in Pseudomonas aeruginosa. Annu. Rev. Microbiol. 76, 413–433. doi:10.1146/annurev-micro-041320-111355
Manu, S. K., Victoria, S. N., and R, M. (2022). Investigation of bio-corrosion in galvanized steel by Desulfovibrio desulfuricans and its mitigation by Butea monosperma leaf extract as green inhibitor. J. Indian Chem. Soc. 99, 100652. doi:10.1016/j.jics.2022.100652
Marsoul, A., Ijjaali, M., Elhajjaji, F., Taleb, M., Salim, R., and Boukir, A. (2020). Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L): evaluation of the inhibitory effect in acidic medium 1 M HCl. Mater Today Proc. 27, 3193–3198. doi:10.1016/j.matpr.2020.04.202
Martelli, G., and Giacomini, D. (2018). Antibacterial and antioxidant activities for natural and synthetic dual-active compounds. Eur. J. Med. Chem. 158, 91–105. doi:10.1016/j.ejmech.2018.09.009
Maruschak, P. O., Lytvynenko, Ya. V., Dzyura, V. O., Bishchak, R. T., and Polutrenko, M. S. (2020). Detection of microdefects on the surfaces of corroded steel pipes. Mater. Sci. 56, 400–409. doi:10.1007/s11003-020-00443-9
Mehta, H., Kaur, G., Chaudhary, G. R., and Prabhakar, N. (2021). Assessment of bio-corrosion inhibition ability of Hafnium based cationic metallosurfactant on iron surface. Corros. Sci. 179, 109101. doi:10.1016/j.corsci.2020.109101
Muthukumarasamy, K., Pitchai, S., Devarayan, K., and Nallathambi, L. (2020). Adsorption and corrosion inhibition performance of Thunbergia fragrans extract on mild steel in acid medium. Mater Today Proc. 33, 4054–4058. doi:10.1016/j.matpr.2020.06.533
Narenkumar, J., Ananthaselvam, A., Alsalhi, M. S., Devanesan, S., Kadier, A., Kannan, M. M., et al. (2021). Effect of crude methanolic extract of Lawsonia inermis for anti-biofilm on mild steel 1010 and its effect on corrosion in a re-circulating wastewater system. J. King Saud. Univ. Sci. 33, 101611. doi:10.1016/j.jksus.2021.101611
Narenkumar, J., Parthipan, P., Usha Raja Nanthini, A., Benelli, G., Murugan, K., and Rajasekar, A. (2017). Ginger extract as green biocide to control microbial corrosion of mild steel. 3 Biotech. 7, 133. doi:10.1007/s13205-017-0783-9
Nealson, K. H., Tebo, B. M., and Rosson, R. A. (1988). Occurrence and mechanisms of microbial oxidation of manganese. Adv. Appl. Microbiol., 279–318. doi:10.1016/S0065-2164(08)70209-0
Nizami, M. Z. I., Xu, V. W., Yin, I. X., Yu, O. Y., and Chu, C.-H. (2021). Metal and metal oxide nanoparticles in caries prevention: a review. Nanomaterials 11, 3446. doi:10.3390/nano11123446
Núñez, A., García, A. M., Ranninger, C., and Moreno, D. A. (2023). Microbiologically influenced corrosion on naval carbon steel inside the hull of tugboats: a case study of prevention and control. Biofouling 39, 257–270. doi:10.1080/08927014.2023.2209013
Nyamath, S., Subburamu, K., Kalyanasundaram, G. T., Balachandar, D., Suresh, M., and Anandham, R. (2024). Multifarious characteristics of sulfur-oxidizing bacteria residing in rice rhizosphere. Folia Microbiol. (Praha) 69, 395–405. doi:10.1007/s12223-023-01080-w
Obłąk, E., Piecuch, A., Guz-Regner, K., and Dworniczek, E. (2014). Antibacterial activity of gemini quaternary ammonium salts. FEMS Microbiol. Lett. 350, 190–198. doi:10.1111/1574-6968.12331
Obot, I. B., and Edouk, U. M. (2017). Benzimidazole: small planar molecule with diverse anti-corrosion potentials. J. Mol. Liq. 246, 66–90. doi:10.1016/j.molliq.2017.09.041
Olesen, B. H., Avci, R., and Lewandowski, Z. (2000). Manganese dioxide as a potential cathodic reactant in corrosion of stainless steels. Corros. Sci. 42, 211–227. doi:10.1016/S0010-938X(99)00071-2
Packiavathy, I. A. S., Maruthamuthu, S., Gnanaselvan, G., Manoharan, S., Paul, J. B. J., Annapoorani, A., et al. (2019). The control of microbially induced corrosion by methyl eugenol – a dietary phytochemical with quorum sensing inhibitory potential. Bioelectrochemistry 128, 186–192. doi:10.1016/j.bioelechem.2019.04.010
Pakiet, M., Kowalczyk, I., Leiva Garcia, R., Moorcroft, R., Nichol, T., Smith, T., et al. (2019). Gemini surfactant as multifunctional corrosion and biocorrosion inhibitors for mild steel. Bioelectrochemistry 128, 252–262. doi:10.1016/j.bioelechem.2019.04.005
Pal, M. K., and Lavanya, M. (2022). Microbial influenced corrosion: understanding bioadhesion and biofilm formation. J. Bio Tribocorros 8, 76. doi:10.1007/s40735-022-00677-x
Parthipan, P., Elumalai, P., Narenkumar, J., Machuca, L. L., Murugan, K., Karthikeyan, O. P., et al. (2018a). Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int. Biodeterior. Biodegrad. 132, 66–73. doi:10.1016/j.ibiod.2018.05.005
Parthipan, P., Elumalai, P., Narenkumar, J., Machuca, L. L., Murugan, K., Karthikeyan, O. P., et al. (2018b). Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int. Biodeterior. Biodegrad. 132, 66–73. doi:10.1016/j.ibiod.2018.05.005
Parthipan, P., Narenkumar, J., Elumalai, P., Preethi, P. S., Usha Raja Nanthini, A., Agrawal, A., et al. (2017). Neem extract as a green inhibitor for microbiologically influenced corrosion of carbon steel API 5LX in a hypersaline environments. J. Mol. Liq. 240, 121–127. doi:10.1016/j.molliq.2017.05.059
Permeh, S., and Lau, K. (2023). Identification of steel corrosion associated with sulfate-reducing bacteria by electrochemical noise technique. Mater. Corros. 74, 20–32. doi:10.1002/maco.202213238
Poupin, P., Truffaut, N., Combourieu, B., Besse, P., Sancelme, M., Veschambre, H., et al. (1998). Degradation of morpholine by an environmental Mycobacterium strain involves a cytochrome P-450. Appl. Environ. Microbiol. 64, 159–165. doi:10.1128/AEM.64.1.159-165.1998
Prabakaran, M., Kim, S.-H., Hemapriya, V., and Chung, I.-M. (2016a). Evaluation of polyphenol composition and anti-corrosion properties of Cryptostegia grandiflora plant extract on mild steel in acidic medium. J. Industrial Eng. Chem. 37, 47–56. doi:10.1016/j.jiec.2016.03.006
Prabakaran, M., Kim, S.-H., Kalaiselvi, K., Hemapriya, V., and Chung, I.-M. (2016b). Highly efficient Ligularia fischeri green extract for the protection against corrosion of mild steel in acidic medium: electrochemical and spectroscopic investigations. J. Taiwan Inst. Chem. Eng. 59, 553–562. doi:10.1016/j.jtice.2015.08.023
Pu, Y., Dou, W., Cheng, Y. F., Chen, S., Xu, Z., and Chen, Z. (2023). Biogenic H2S and extracellular electron transfer resulted in two-coexisting mechanisms in 90/10 Cu-Ni alloy corrosion by a sulfate-reducing bacteria. Corros. Sci. 211, 110911. doi:10.1016/j.corsci.2022.110911
Rajala, P., Cheng, D.-Q., Rice, S. A., and Lauro, F. M. (2022). Sulfate-dependant microbially induced corrosion of mild steel in the deep sea: a 10-year microbiome study. Microbiome 10, 4. doi:10.1186/s40168-021-01196-6
Rasheed, P. A., Jabbar, K. A., Rasool, K., Pandey, R. P., Sliem, M. H., Helal, M., et al. (2019). Controlling the biocorrosion of sulfate-reducing bacteria (SRB) on carbon steel using ZnO/chitosan nanocomposite as an eco-friendly biocide. Corros. Sci. 148, 397–406. doi:10.1016/j.corsci.2018.12.028
Rawlings, D. E. (2001). The molecular genetics of Thiobacillus ferrooxidans and other mesophilic, acidophilic, chemolithotrophic, iron- or sulfur-oxidizing bacteria. Hydrometallurgy 59, 187–201. doi:10.1016/S0304-386X(00)00182-1
Robertson, L. A., and Kuenen, J. G. (2006). “The genus Thiobacillus,” in The prokaryotes, (New York, NY: Springer), 812–827. doi:10.1007/0-387-30745-1_37
Rochdi, A., Kassou, O., Dkhireche, N., Touir, R., El Bakri, M., Ebn Touhami, M., et al. (2014). Inhibitive properties of 2,5-bis(n-methylphenyl)-1,3,4-oxadiazole and biocide on corrosion, biocorrosion and scaling controls of brass in simulated cooling water. Corros. Sci. 80, 442–452. doi:10.1016/j.corsci.2013.11.067
Sabel, C. F., and Victor, D. G. (2017). Governing global problems under uncertainty: making bottom-up climate policy work. Clim. Change 144, 15–27. doi:10.1007/s10584-015-1507-y
Salleh, S. Z., Yusoff, A. H., Zakaria, S. K., Taib, M. A. A., Abu Seman, A., Masri, M. N., et al. (2021). Plant extracts as green corrosion inhibitor for ferrous metal alloys: a review. J. Clean. Prod. 304, 127030. doi:10.1016/j.jclepro.2021.127030
Shaban, S. M., Fouda, A. S., Rashwan, S. M., Ibrahim, H. E., and El-Bhrawy, M. F. (2016). Synthesis and characterization of newly cationic surfactants based on 2-(2-(dimethylamino)ethoxy)ethanol: physiochemical, thermodynamic and evaluation as biocide. J. Mol. Liq. 221, 224–234. doi:10.1016/j.molliq.2016.05.088
Shabani-Nooshabadi, M., and Ghandchi, M. S. (2015). Santolina chamaecyparissus extract as a natural source inhibitor for 304 stainless steel corrosion in 3.5% NaCl. J. Industrial Eng. Chem. 31, 231–237. doi:10.1016/j.jiec.2015.06.028
Shehata, M. F., and El-Shamy, A. M. (2023). Hydrogen-based failure in oil and gas pipelines a review. Gas Sci. Eng. 115, 204994. doi:10.1016/j.jgsce.2023.204994
Shineh, G., Mobaraki, M., Perves Bappy, M. J., and Mills, D. K. (2023). Biofilm Formation, and related impacts on healthcare, food processing and packaging, industrial manufacturing, marine industries, and sanitation–A review. Appl. Microbiol. 3, 629–665. doi:10.3390/applmicrobiol3030044
Sin, H. L. Y., Abdul Rahim, A., Gan, C. Y., Saad, B., Salleh, M. I., and Umeda, M. (2017). Aquilaria subintergra leaves extracts as sustainable mild steel corrosion inhibitors in HCl. Measurement 109, 334–345. doi:10.1016/j.measurement.2017.05.045
Sowards, J. W., and Mansfield, E. (2014). Corrosion of copper and steel alloys in a simulated underground storage-tank sump environment containing acid-producing bacteria. Corros. Sci. 87, 460–471. doi:10.1016/j.corsci.2014.07.009
Sriphochanart, W., Krusong, W., Mekkerdchoo, O., Suwapanich, R., Sriprom, P., and Tantratian, S. (2023). Impact of temperature on gluconic acid production during acetification by Acetobacter aceti. Biotechnol. Appl. Biochem. 70, 992–1000. doi:10.1002/bab.2414
Sun, M. Q., Yang, J., Wang, Z. B., and Zheng, Y. G. (2024). Effect of coexistence of sulfate reducing bacteria and nitrate reducing bacteria on the under-deposit corrosion of carbon steel. Corros. Sci. 231, 111958. doi:10.1016/j.corsci.2024.111958
Swaroop, B. S., Victoria, S. N., and Manivannan, R. (2016). Azadirachta indica leaves extract as inhibitor for microbial corrosion of copper by Arthrobacter sulfureus in neutral pH conditions—a remedy to blue green water problem. J. Taiwan Inst. Chem. Eng. 64, 269–278. doi:10.1016/j.jtice.2016.04.007
Udunwa, D. I., Onukwuli, O. D., Menkiti, M. C., Nwanonenyi, S. C., Ezekannagha, C. B., and Aniagor, C. O. (2024). Experimental, computational, and theoretical studies on the corrosion inhibition potential of green Gongronema latifolium extract as corrosion inhibitor for aluminum alloy in HCl solutions. J. Mol. Struct. 1302, 137508. doi:10.1016/j.molstruc.2024.137508
Umoren, S. A., Solomon, M. M., Obot, I. B., and Suleiman, R. K. (2019). A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Industrial Eng. Chem. 76, 91–115. doi:10.1016/j.jiec.2019.03.057
Usher, K. M., Kaksonen, A. H., Cole, I., and Marney, D. (2014). Critical review: microbially influenced corrosion of buried carbon steel pipes. Int. Biodeterior. Biodegrad. 93, 84–106. doi:10.1016/j.ibiod.2014.05.007
Varvara, S., Caniglia, G., Izquierdo, J., Bostan, R., Găină, L., Bobis, O., et al. (2020). Multiscale electrochemical analysis of the corrosion control of bronze in simulated acid rain by horse-chestnut (Aesculus hippocastanum L.) extract as green inhibitor. Corros. Sci. 165, 108381. doi:10.1016/j.corsci.2019.108381
Venegas, R. (2016). Evaluation of Eulychnia acida phil. (Cactaceae) extracts as corrosion inhibitors for carbon steel in acidic media. Int. J. Electrochem Sci., 3651–3663. doi:10.20964/110449
Venzlaff, H., Enning, D., Srinivasan, J., Mayrhofer, K. J. J., Hassel, A. W., Widdel, F., et al. (2013). Accelerated cathodic reaction in microbial corrosion of iron due to direct electron uptake by sulfate-reducing bacteria. Corros. Sci. 66, 88–96. doi:10.1016/j.corsci.2012.09.006
Verma, C., Chauhan, D. S., Aslam, R., Banerjee, P., Aslam, J., Quadri, T. W., et al. (2024). Principles and theories of green chemistry for corrosion science and engineering: design and application. Green Chem. 26, 4270–4357. doi:10.1039/D3GC05207A
Wan, H., Song, D., Zhang, D., Du, C., Xu, D., Liu, Z., et al. (2018). Corrosion effect of Bacillus cereus on X80 pipeline steel in a Beijing soil environment. Bioelectrochemistry 121, 18–26. doi:10.1016/j.bioelechem.2017.12.011
Wan, S., Wei, H., Quan, R., Luo, Z., Wang, H., Liao, B., et al. (2022). Soybean extract firstly used as a green corrosion inhibitor with high efficacy and yield for carbon steel in acidic medium. Ind. Crops Prod. 187, 115354. doi:10.1016/j.indcrop.2022.115354
Wang, J., Hou, B., Xiang, J., Chen, X., Gu, T., and Liu, H. (2019). The performance and mechanism of bifunctional biocide sodium pyrithione against sulfate reducing bacteria in X80 carbon steel corrosion. Corros. Sci. 150, 296–308. doi:10.1016/j.corsci.2019.01.037
Wang, Q., Liu, L., Zhang, Q., Wu, X., Zheng, H., Gao, P., et al. (2022). Insight into the anti–corrosion performance of Artemisia argyi leaves extract as eco–friendly corrosion inhibitor for carbon steel in HCl medium. Sustain Chem. Pharm. 27, 100710. doi:10.1016/j.scp.2022.100710
Wang, Y., Wang, G., Xie, F., Wu, M., Zhou, Y., Liu, F., et al. (2024). Corrosion mechanism and research progress of metal pipeline corrosion under magnetic field and SRB conditions: a review. Corros. Rev. 42, 203–223. doi:10.1515/corrrev-2023-0028
Welikala, S., Al-Saadi, S., Gates, W. P., Panter, C., and Singh Raman, R. K. (2024). Sulphate reducing bacteria (SRB) biofilm development and its role in microbial corrosion of carbon steel. Front. Mater 11. doi:10.3389/fmats.2024.1360869
Xu, D., Li, Y., Song, F., and Gu, T. (2013). Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros. Sci. 77, 385–390. doi:10.1016/j.corsci.2013.07.044
Xu, N., Yang, X. B., and Zhang, Q. H. (2024). Insight into interfacial adsorption and inhibition mechanism of Aconitum carmichaelii Debx extract as high-efficient corrosion inhibitor for carbon steel in acidic solution. J. Mol. Liq. 393, 123602. doi:10.1016/j.molliq.2023.123602
Yahya, S., Othman, N. K., and Ismail, M. C. (2019). Corrosion inhibition of steel in multiple flow loop under 3.5% NaCl in the presence of rice straw extracts, lignin and ethylene glycol. Eng. Fail Anal. 100, 365–380. doi:10.1016/j.engfailanal.2019.02.036
Yang, G., Gong, M., Zheng, X., Lin, L., Fan, J., Liu, F., et al. (2022). A review of microbial corrosion in reclaimed water pipelines: challenges and mitigation strategies. Water Pract. Technol. 17, 731–748. doi:10.2166/wpt.2022.007
Yazdi, M., Khan, F., Abbassi, R., Quddus, N., and Castaneda-Lopez, H. (2022). A review of risk-based decision-making models for microbiologically influenced corrosion (MIC) in offshore pipelines. Reliab Eng. Syst. Saf. 223, 108474. doi:10.1016/j.ress.2022.108474
Y, G. A., and Mulky, L. (2023). Biofilms and beyond: a comprehensive review of the impact of Sulphate Reducing Bacteria on steel corrosion. Biofouling 39, 897–915. doi:10.1080/08927014.2023.2284316
Zhang, H., Ni, Z., Wu, H., Xu, P., Li, Z., Zhang, W., et al. (2020a). Corrosion inhibition of carbon steel in hydrochloric acid by Chrysanthemum indicum extract. Int. J. Electrochem Sci. 15, 5487–5499. doi:10.20964/2020.06.22
Zhang, P., Xu, D., Li, Y., Yang, K., and Gu, T. (2015). Electron mediators accelerate the microbiologically influenced corrosion of 304 stainless steel by the Desulfovibrio vulgaris biofilm. Bioelectrochemistry 101, 14–21. doi:10.1016/j.bioelechem.2014.06.010
Zhang, X., Li, W., Yu, G., Zuo, X., Luo, W., Zhang, J., et al. (2020b). Evaluation of Idesia polycarpa Maxim fruits extract as a natural green corrosion inhibitor for copper in 0.5 M sulfuric acid solution. J. Mol. Liq. 318, 114080. doi:10.1016/j.molliq.2020.114080
Zhang, Z., Zhang, C., Yang, Y., Zhang, Z., Tang, Y., Su, P., et al. (2022). A review of sulfate-reducing bacteria: metabolism, influencing factors and application in wastewater treatment. J. Clean. Prod. 376, 134109. doi:10.1016/j.jclepro.2022.134109
Zhou, C., and Wang, Y. (2020). Structure–activity relationship of cationic surfactants as antimicrobial agents. Curr. Opin. Colloid Interface Sci. 45, 28–43. doi:10.1016/j.cocis.2019.11.009
Zhou, H., and Fu, C. (2020). Manganese-oxidizing microbes and biogenic manganese oxides: characterization, Mn(II) oxidation mechanism and environmental relevance. Rev. Environ. Sci. Biotechnol. 19, 489–507. doi:10.1007/s11157-020-09541-1
Zhou, T., Yuan, J., Zhang, Z., Xin, X., and Xu, G. (2019). The comparison of imidazolium Gemini surfactant [C14-4-C14im]Br2 and its corresponding monomer as corrosion inhibitors for A3 carbon steel in hydrochloric acid solutions: Experimental and quantum chemical studies. Colloids Surf. A Physicochem Eng. Asp. 575, 57–65. doi:10.1016/j.colsurfa.2019.05.004
Zlatić, G., Martinović, I., Pilić, Z., Kodranov, I., Ciganović, J., and Sokol, V. (2023). The effect of Artemisia annua L. extract on microbiologically influenced corrosion of A36 steel caused by Pseudomonas aeruginosa. Bioelectrochemistry 152, 108447. doi:10.1016/j.bioelechem.2023.108447
Keywords: biocorrosion, biofilm, corrosion inhibitors, biocides, surface protection
Citation: Santhosh Kumar A, Sivakumar L, Rajadesingu S, Sathish S, Malik T and Parthipan P (2025) Sustainable corrosion inhibition approaches for the mitigation of microbiologically influenced corrosion -a systematic review. Front. Mater. 12:1545245. doi: 10.3389/fmats.2025.1545245
Received: 14 December 2024; Accepted: 11 February 2025;
Published: 13 March 2025.
Edited by:
Ruiyong Zhang, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Pavlo Maruschak, Ternopil Ivan Pului National Technical University, UkraineCopyright © 2025 Santhosh Kumar, Sivakumar, Rajadesingu, Sathish, Malik and Parthipan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tabarak Malik, bWFsaWtpdHJjQGdtYWlsLmNvbQ==; Punniyakotti Parthipan, cGFydGhpcHBAc3JtaXN0LmVkdS5pbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.