- 1Chemistry Department, Yildiz Technical University, Istanbul, Türkiye
- 2Graduate School of Engineering, Chiba University, Chiba, Japan
2-(Carboxymethoxy) thioxanthone (TX-OCH₂COOH), a thioxanthone derivative, was utilized for the esterification of polyvinyl alcohol (PVA) to synthesize a macromolecular photoinitiator, both in the absence and presence of Cysteine (Cys). The covalent attachment of the thioxanthone (TX) group to PVA through esterification enabled the exploration of the photophysical properties of the resulting macromolecular photoinitiators (TXOCH₂COO-PVA and TXOCH₂COO-PVA-Cys) via UV-Vis and fluorescence studies. UV-Vis absorption spectrum of TXOCH₂COO-PVA confirmed the covalent bonding of TX, marked by a characteristic absorption peak at 397 nm corresponding to the thioxanthone chromophore. Fluorescence lifetimes were recorded as TXOCH₂COO-PVA was cast into a mold and air dried, resulting in a flexible form of PVA esterified with TXOCH₂COOH. In-situ synthesis of both silver and selenium nanoparticles was carried out using both TXOCH₂COO-PVA and TXOCH₂COO-PVA-Cys macromolecular initiators, successfully yielding nanocomposite flexible polymeric films.
Highlights
• PVA-based macromolecular photoinitiators were prepared with and without cysteine.
•Photophysical characterizations of macro photoinitiators were performed.
•Nanocomposite thin films consisting of AgNPs and SeNPs were synthesized by situ photochemical method
1 Introduction
Polyvinyl alcohol (PVA) is an environmentally friendly polymer (Maruoka et al., 2006), and its functionalization can enhance its performance in various applications, including adhesives, coatings (Nassar et al., 2023; Nishiyabu et al., 2020; Madhusudhana et al., 2022) and biomedical materials (Rafique et al., 2016; Chong et al., 2013). This process involves chemical modifications such as esterification (Ko et al., 2020; Salavagione et al., 2009; Schanuel et al., 2015), acrylation (Mühlebach et al., 1997; Crispim et al., 2006) and grafting (Alexandre et al., 2014). By attaching different molecules to PVA, its properties can be enhanced, improving characteristics like flexibility (Dicharry et al., 2006), biodegradability (Acik and Karatavuk, 2020) and thermal stability (Singh et al., 2020; Chiou et al., 2013). Photoinitiators absorb light and generate reactive species that initiate the photopolymerization reaction (Dietliker et al., 2004; Yagci et al., 2010; Keskin Dogruyol et al., 2023). Radical photoinitiators are classified as Type I and Type II based on their photoinitiation mechanisms (Aydin et al., 2003). Type I photoinitiators, such as benzoin and amino acetophenones, produce radicals through homolytic bond cleavage (Hammoud et al., 2022). On the other hand, Type II photoinitiators require co-initiators such as amines or thiols to initiate the photopolymerization (Balta et al., 2008). One-component type II photoinitiators feature both light-absorbing and hydrogen-donating regions within a single structure (Koyuncu et al., 2021; Metin et al., 2020) (see Scheme 1).
A macromolecular photoinitiator can be synthesized by reacting a polymer with a photoinitiator containing carboxyl groups (Kork et al., 2015). These macromolecular photoinitiators offer many advantages, such as high molar absorption, low migration, and good dissolution in polymerizable formulations due to their unique structures (Deng et al., 2020). Therefore, they are strong candidates for photopolymerization processes. Photopolymerization is an environmentally friendly process (Tehfe et al., 2013) that consumes less energy, minimizes waste (Pierau et al., 2022), and operates at low reaction temperatures. These processes, which have long been integral to chemical applications, are currently being explored in areas such as coatings (He et al., 2023), adhesives, 3D printing (Bagheri and Jin, 2019; Voet et al., 2021), self-healing materials (Guo, 2022), and metal nanocomposites (Riad et al., 2020; Ozcelik Kazancioglu et al., 2021). In this study, we present the synthesis of an environmentally friendly PVA-based macromolecular photoinitiator, incorporating TX alone as well as both TX and cysteine derivatives via an esterification reaction. Characterization and photophysical properties are also presented to highlight the impact of cysteine on the macromolecular chain of PVA. Furthermore, nanocomposites containing Ag and Se nanoparticles were fabricated.
2 Experimental section
2.1 Materials
Silver nitrate (AgNO3, Sigma-Aldrich, 99%), Polyvinyl alcohol (PVA, Alfa Aesar, Mn: 12,500 g/mol, 80% hydrolyzed), Acrylamide (AA, Fluka, ≥98%), L-cysteine (Sigma-Aldrich, C-7755) Thiosalicylic acid (Merck, 97%), Phenoxyacetic acid (Merck, 98%), dimethyl sulfoxide (DMSO, ISOLAB, 99.9%), hydrochloric acid (HCl, Merck, 37%), sulfuric acid (H2SO4, Merck, 98%), ethanol (Merck, 99%), Hexane (Merck, 99%), and distilled water were used without further purification.
2.2 Instrumentation
1H NMR spectra were measured with the JEOL ECS 400 MHz NMR spectrometer using DMSO-D6 as solvents. FT-IR spectra were measured with a Nicolet 6700 FT-IR spectrometer. Hellma Analytics quartz cuvettes were used for spectroscopic measurements. UV-Vis absorption studies were performed with a Varian Cary 50 Conc Spectrophotometer. Fluorescence emission-excitation spectra were analyzed with a Horiba Fluoromax-3P fluorescence spectrophotometer. Fluorescence emission decay measurements were performed at room temperature using a Hamamatsu Quantaurus-Tau C11367 spectrometer. Transmission electron microscopy (TEM) images were recorded using a Hitachi H-7650 TEM electron microscope.
2.3 Synthesis of TXOCH2COOH
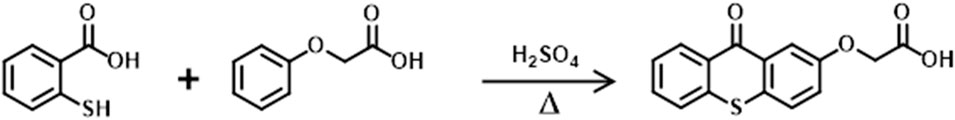
The previously reported method in the literature was used for the synthesis of the photoinitiator (Aydin et al., 2003) TXOCH2COOH as given in Scheme 2.
2.4 Esterification reaction of PVA with TXOCH2COOH in the presence and absence of cysteine
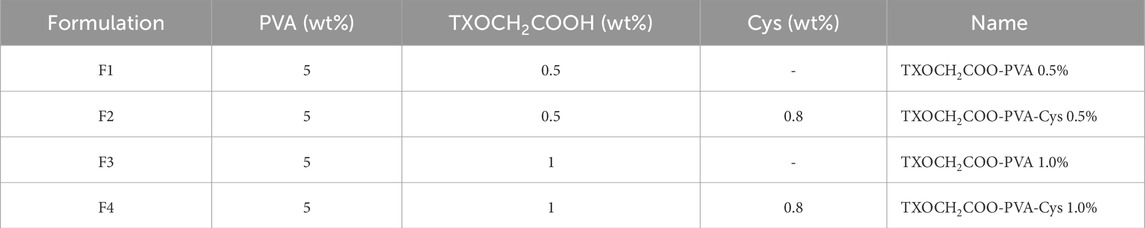
A 10% wt. PVA solution was prepared in 0.1 M hydrochloric acid (HCl). In a separate beaker, TXOCH₂COOH and cysteine were dissolved in 0.1 M HCl at the ratios specified in Table 1, with a volume equal to that of the PVA solution. The two solutions were mixed until a homogeneous mixture was obtained, resulting in a PVA concentration of 5% wt., which was then transferred to a reaction flask. The reaction was carried out under a nitrogen atmosphere for 3 h at 100°C. After the reaction, excess water was removed using a rotary evaporator. The resulting formulations were poured into a mold, wrapped in foil, and left to dry in a dark cabinet at room temperature.
1H NMR (400 MHz, DMSO-D6, 25°C): d = 8.47–7.30 (m, aromatic), 4.82 (s, O-CH-O), 2.47 (s, CH3-C=O), 1.60–1.28 (m, aliphatic backbone).
2.5 In-situ photochemical preparation of silver nanoparticles (AgNPs) and selenium nanoparticles (SeNPs)
Each film consisting of TXOCH₂COO-PVA and TXOCH₂COO-PVA-Cys was dissolved in pure water, and the corresponding metal salts, such as AgNO₃ and Na₂SeO₃·5H₂O, were added to the formulation at 4% wt. The formulations were irradiated using a medium-pressure mercury lamp for 5–400 s. After irradiation, the formulations were placed in molds and allowed to dry at room temperature. Additionally, a separate formulation containing both metal salts (AgNO₃ and Na₂SeO₃·5H₂O) was also prepared.
3 Results and discussion
The esterification reactions are illustrated in Scheme 3. The covalent attachment of the TX group to PVA through an esterification reaction produced a flexible polymeric film, as shown in Figure 1. Interestingly, the covalent linkage of the photoinitiator TXOCH2COOH increased the solubility of PVA in water.
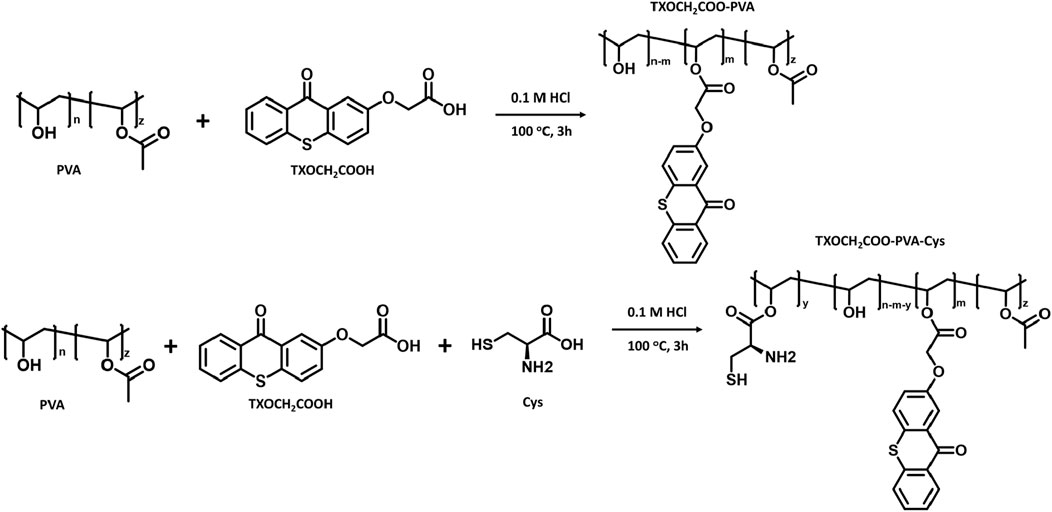
Scheme 3. Schematic representation of the esterification reactions between PVA and TXOCH2COOH, both in the presence and absence of Cysteine.
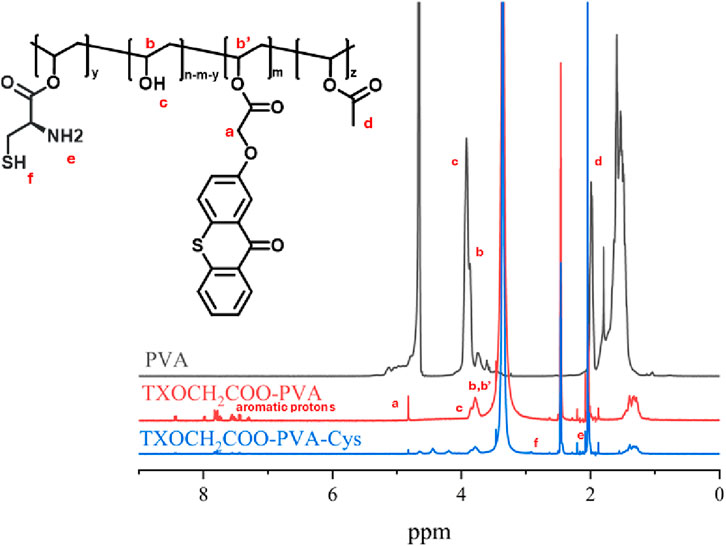
The characterization was performed using 1H-NMR and FT-IR spectroscopy. The 1H-NMR spectra confirmed the successful esterification reaction between TXOCH2COOH and PVA. The spectra of the esterification products, recorded in DMSO-D6, displayed characteristic signals for TXOCH2COOH and PVA, as shown in Figure 2. Aromatic proton signals appeared between 8.47 and 7.30 ppm, with additional signals at 4.82 ppm for the CH₂ group. Aliphatic protons and CH₃ signals from polyvinyl acetate were observed at 2.47 ppm and in the range of 1.60 to 1.28. The H proton of the SH group in cysteine incorporated into PVA was observed at 2.9, and the NH2 peaks around 2.08 in the NMR spectrum. For the comparison PVA 1H-NMR spectrum was recorded in deuterium oxide (D2O).
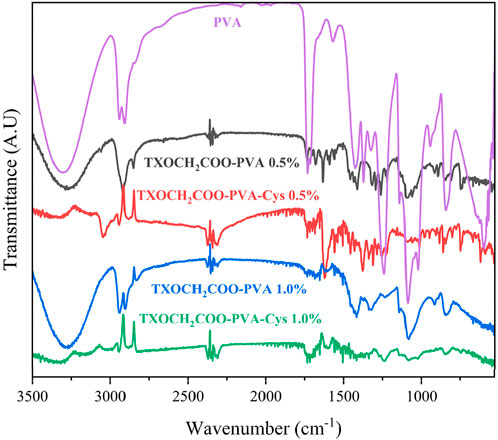
The FTIR spectra indicates that all samples obtained from esterification reaction display similar characteristic peaks, as shown in Figure 3. The broad band around 3,400 cm⁻1 indicates the presence of hydroxyl (-OH) groups in polyvinyl alcohol (PVA). Peaks around 2,900 cm⁻1 correspond to aliphatic C-H stretching vibrations. The strong band near 1700 cm⁻1 represents the carbonyl (C=O) stretching vibration resulting from the esterification reaction between thioxanthone (TX) and PVA.
In the spectra of cysteine-containing samples (PVA- TXOCH₂COO-Cys), a weak band around 2,500 cm⁻1 is observed, indicating the presence of the thiol (-SH) group in the cysteine molecule. This observation confirms the successful incorporation of cysteine into the polymer structure.
The films were analyzed for their steady-state and excited-state properties. The steady-state properties of PVA-TX and PVA-TX-Cys were examined using UV-Vis spectroscopy, as shown in Figure 4. It was observed that the absorption band broadened upon cysteine binding to the PVA-TX.
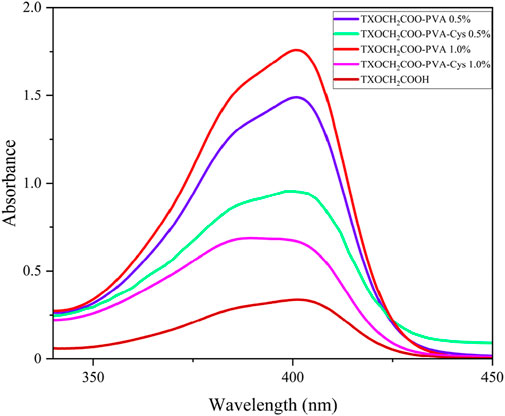
Figure 4. UV-Vis absorbance spectra of synthesized films (TXOCH2COO-PVA [1 mg mL−1] and TXOCH2COO-PVA-Cys [1 mg mL−1]) and TXOCH2COOH [1 × 10−4 M] show a maximum absorbance at 400 nm in DMSO.
Photolysis studies of PVA films were carried out to investigate the effect of the covalently bound photoinitiator amount in esterification reactions, using two different initiator concentrations and incorporating the cysteine molecule (see Figure 5).
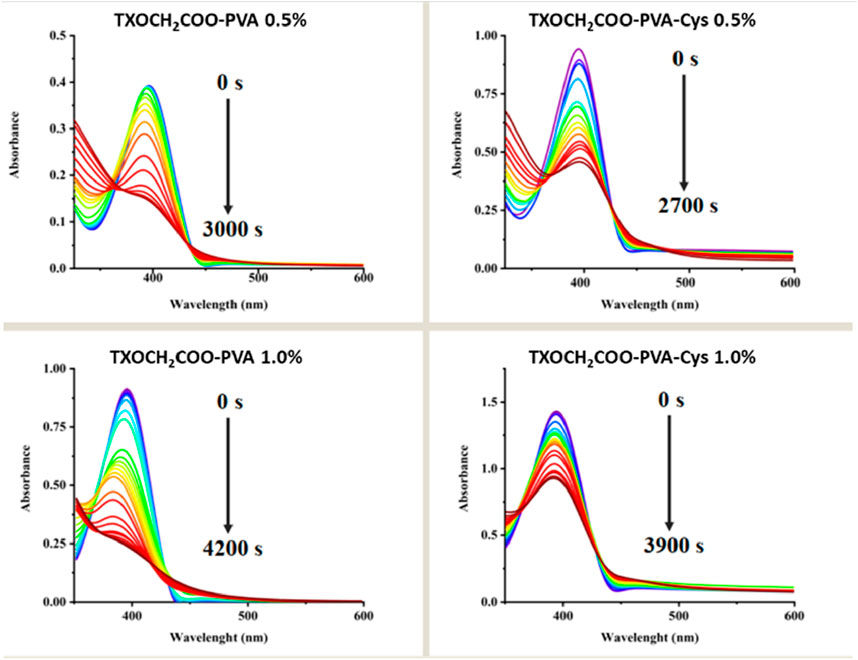
Figure 5. Change in UV-Vis spectrum during the photolysis of TXOCH₂COO-PVA and TXOCH₂COO-PVA-Cys at different concentrations.
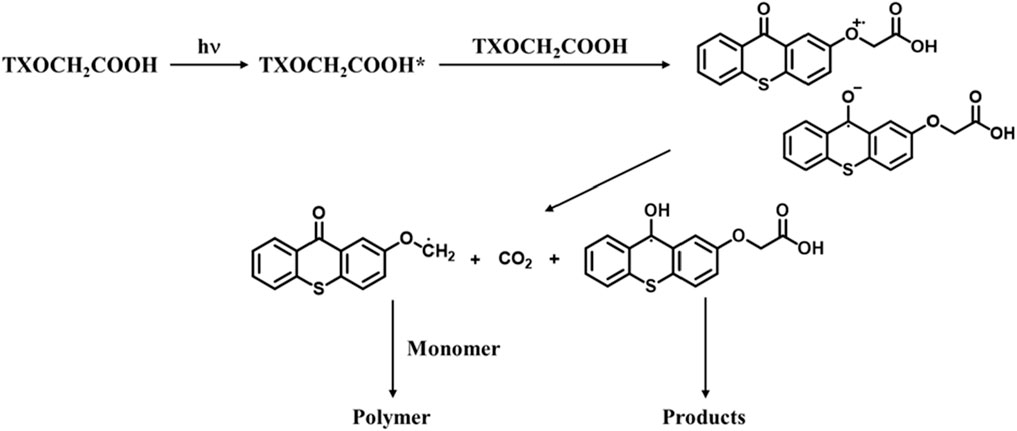
It was observed that the photolysis of PVA-TX films with higher photoinitiator concentrations required longer time. However, when cysteine molecules were incorporated into the PVA-TX films, the bleaching occurred more rapidly. This finding suggests that reactive thiyl radicals may be generated through hydrogen abstraction from the -SH group of cysteine by TX. These results indicate that both photoinitiator concentration and the covalent attachment of cysteine play a significant role in influencing the photolysis kinetics.
The fluorescence emission spectra of TXOCH₂COOH, TXOCH₂COO-PVA and TXOCH₂COO-PVA-Cys films were recorded after dissolving the films in DMSO and exciting them at 390 nm. The excitation and emission spectra are shown in Figures 6, 7. All emissions from the films closely resembled those of the TX photoinitiator.
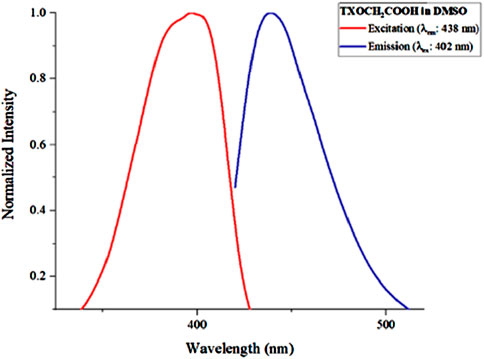
Figure 6. Fluorescence emission-excitation spectra TXOCH2COOH in DMSO. Excitation wavelength for emission spectra was 402 nm, and monitoring wavelength for excitation spectra was 438 nm.
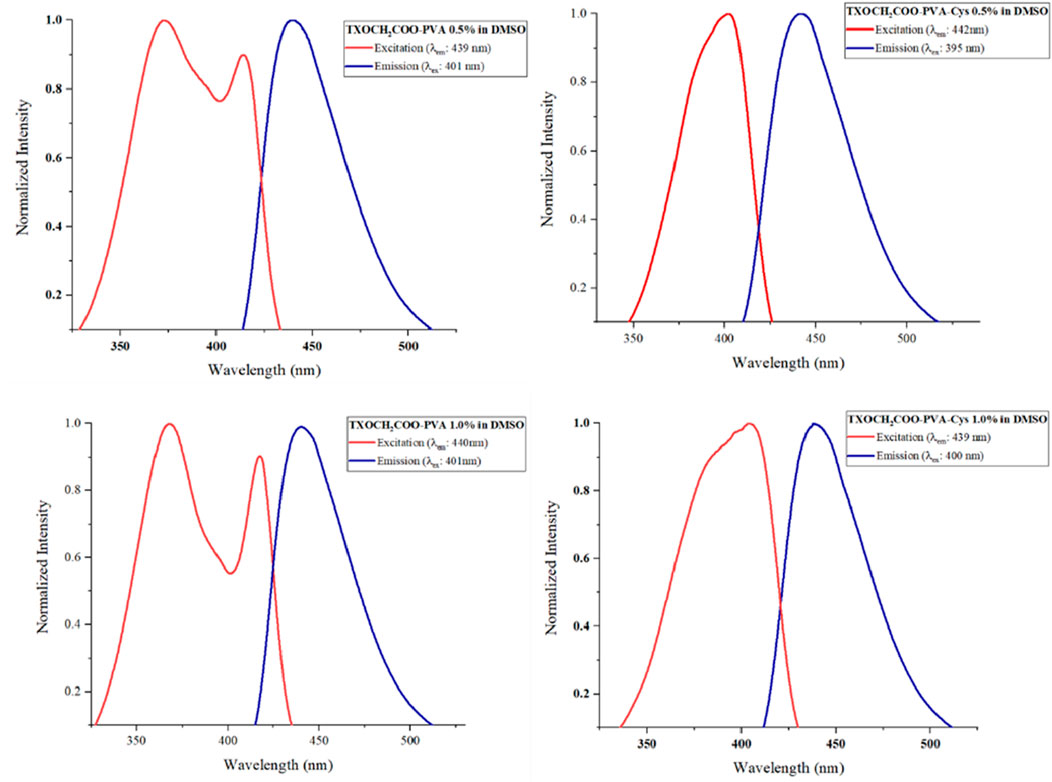
Figure 7. Florescence emission-excitation spectrums of TXOCH₂COO-PVA and TXOCH₂COO-PVA-Cys at different concentrations. Excitation wavelength (λex) and monitoring wavelength (λem) were noted in the spectra.
Figure 7 displays the fluorescence spectra of TXOCH₂COOH in DMSO, showing an excitation band at 400 nm and an emission band at 438 nm.
The presence of cysteine did not notably alter the spectral properties. These results suggest that the fluorescence properties of PVA films are primarily governed by the TX-based photoinitiators, with cysteine molecules having a minimal effect on these properties.
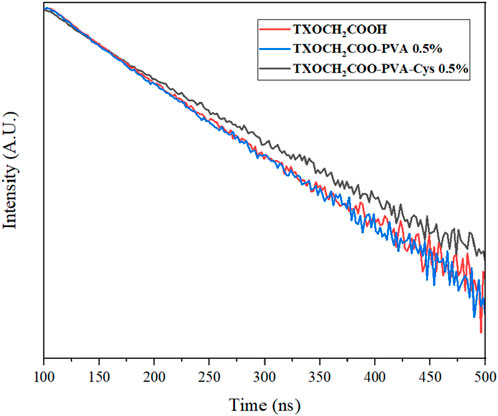
Fluorescence lifetime refers to the duration of a molecule spends transitioning from the excited state to the ground state. The fluorescence lifetimes of TXOCH2COOH, TXOCH₂COO-PVA and TXOCH₂COO-PVA-Cys were compared and analyzed, as shown in (Figure 8). The polymers and photoinitiators were dissolved in DMSO and placed in a quartz cell with a 1 mm optical path length for lifetime measurements. The excitation wavelength was set to 402 nm, and the monitored wavelength for luminescence was 440 nm. τ1, the shorter lifetime about 3.3 ns observed for nearly all molecules, including the bare compound TXOCH2COOH, likely represents the molecule’s primary excited state. τ2, the longer duration, may suggest presence of different luminescence conditions of photoinitiators by interacting with surrounding molecules such as DMSO molecule or polymer. Indeed, PVA- TXOCH2COOH -Cys exhibited a slightly longer lifetime than other samples. The prolonged excited lifetime of the photoinitiator may enable promotion of intersystem crossing to a triplet state and interactions with nearby molecules (see Table 2).
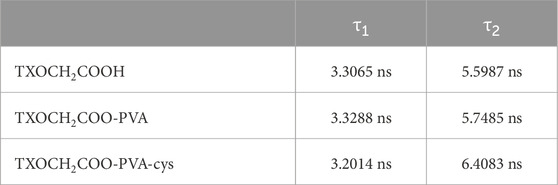
Table 2. Fluorescence lifetimes (τ1 and τ2) of TXOCH2COOH, TXOCH2COO-PVA, and TXOCH2COO-PVA-Cys systems measured at room temperature.
3.1 The in-situ photochemical synthesis of silver nanoparticles (AgNPs) and selenium nanoparticles (SeNPs)
Nanocomposite thin films containing either silver nanoparticles (AgNPs), selenium nanoparticles (SeNPs), or both were synthesized using an in-situ photochemical method, as illustrated in Figure 9.
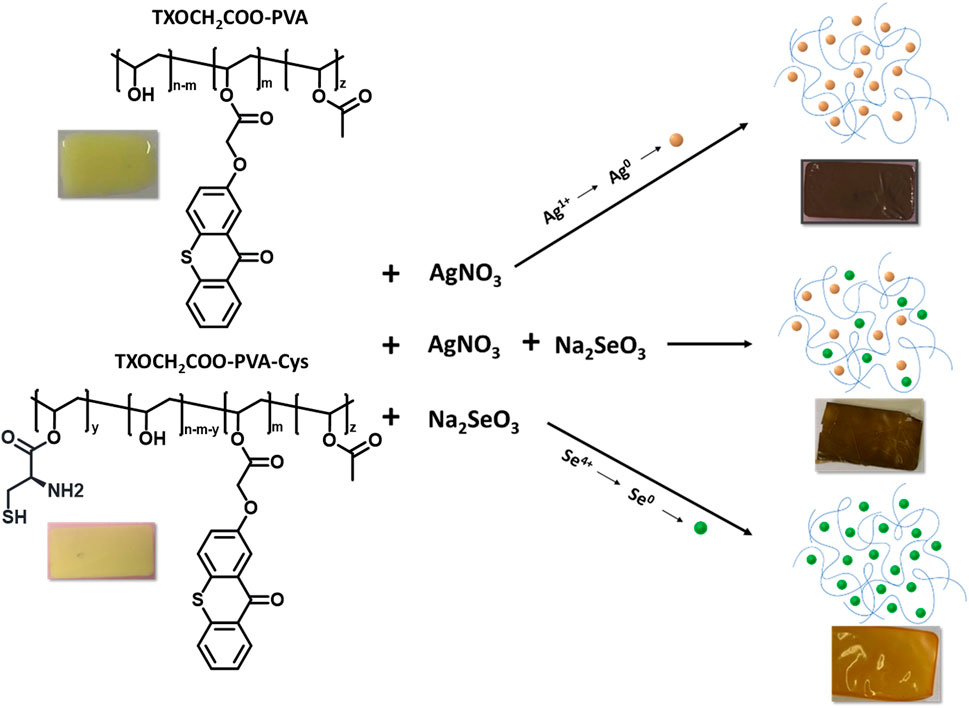
Figure 9. In-situ synthesized nanocomposites consisting of Ag NPs and SeNPs were incorporated into PVA-TX and PVA-TX-Cys polymeric matrices, resulting in noticeable color changes in the flexible films.
TXOCH₂COO-PVA and TXOCH₂COO-PVA-Cys were utilized as one-component macromolecular photoinitiators. They were dissolved in water, and ionic salts were added to the formulations before being irradiated by a medium-pressure mercury lamp for 200–300 s. As described in the experimental section, the color change of the cured formulations was observed, offering a visual method to confirm the formation of nanoparticles within the PVA polymer, as shown in Figure 9. The mechanism behind the in-situ formed nanocomposites likely involves intramolecular hydrogen abstraction, resulting in the formation of ketyl radicals that act as photoreducing agents for ionic species such as silver and selenium salts. In the case of TXOCH₂COO-PVA-Cys, the excited state of TX abstracts hydrogen from the thiol group of cysteine, resulting in the formation of thiyl radicals. Both thiyl and ketyl radicals are essential in reducing ionic species, facilitating the nucleation and growth of nanoparticles. Transmission electron microscopy (TEM) was employed to analyze the morphology and size distribution of silver (Ag) and selenium (Se) nanoparticles within PVA-based polymeric films. The polymeric films were dissolved in ethanol, and the nanoparticles were isolated from the polymer matrix through centrifugation. After drying, the separated nanoparticles were placed on a TEM grid for imaging.
TEM images of the synthesized Ag and Se nanoparticles are shown in Figure 10. The images indicate that silver nanoparticles have a spherical morphology, whereas selenium nanoparticles display a needle-like shape. The average size of the Ag nanoparticles is approximately 10–15 nm, while the Se nanoparticles measure around 20–25 nm.
4 Conclusion
In this study, polyvinyl alcohol (PVA), an environmentally friendly polymer, was esterified using 2-(Carboxymethoxy) thioxanthone and cysteine as a single-component secondary photoinitiator. The photophysical properties of the PVA-TX and PVA-TX-Cys films were investigated after their preparation, focusing on UV-Vis absorption, fluorescence emission spectra, and fluorescence lifetimes. Furthermore, PVA nanocomposites embedded with silver and selenium nanoparticles were synthesized via the in-situ photoreduction of silver and selenium salts in an aqueous medium, followed by their nucleation and growth. The findings indicate that the synthesized polymeric films hold potential as promising materials for optical, electronic, and biomedical applications. Future studies could aim to further optimize their properties and evaluate their performance in specific applications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MK: Investigation, Methodology, Validation, Visualization, Writing–review and editing, Data curation, Formal Analysis. KN: Investigation, Methodology, Validation, Supervision, Writing–review and editing. NA: Investigation, Methodology, Supervision, Validation, Writing–review and editing, Conceptualization, Visualization, Writing–original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Melisa Konar was partly supported by JPS (Japan Society for the Promotion of Science, Core to Core Program) for the project Formation of a Strategic Base in Asia: Creating and Developing Global-Minded Imaging Science (BAGIS).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2025.1544162/full#supplementary-material
References
Acik, G., and Karatavuk, A. O. (2020). Synthesis, properties and biodegradability of cross-linked amphiphilic poly (vinyl acrylate)-poly (tert-butyl Acrylate)s by photo-initiated radical polymerization. Eur. Polym. J. 127, 109602. doi:10.1016/j.eurpolymj.2020.109602
Alexandre, N., Ribeiro, J., Gärtner, A., Pereira, T., Amorim, I., Fragoso, J., et al. (2014). Biocompatibility and hemocompatibility of polyvinyl alcohol hydrogel used for vascular grafting- in vitro and in vivo studies: biocompatibility and hemocompatibility of PVA hydrogel. J. Biomed. Mat. Res. A. doi:10.1002/jbm.a.35098
Aydin, M., Arsu, N., and Yagci, Y. (2003). One-component bimolecular photoinitiating systems, 2. Macromol. Rapid Commun. 24 (12), 718–723. doi:10.1002/marc.200300019
Bagheri, A., and Jin, J. (2019). Photopolymerization in 3D printing. ACS Appl. Polym. Mat. 1 (4), 593–611. doi:10.1021/acsapm.8b00165
Balta, D. K., Cetiner, N., Temel, G., Turgut, Z., and Arsu, N. (2008). An annelated thioxanthone as a new type II initiator. J. Photochem. Photobiol. Chem. 199 (2–3), 316–321. doi:10.1016/j.jphotochem.2008.06.008
Chiou, K., Hollanger, E., Agag, T., and Ishida, H. (2013). Highly improved thermal properties of hydroxyl-containing polymers via modification by benzoxazine groups. Macromol. Chem. Phys. 214 (14), 1629–1635. doi:10.1002/macp.201300032
Chong, S., Smith, A. A. A., and Zelikin, A. N. M. (2013). Microstructured, functional PVA hydrogels through bioconjugation with oligopeptides under physiological conditions. Small 9 (6), 942–950. doi:10.1002/smll.201201774
Crispim, E. G., Piai, J. F., Schüquel, I. T. A., Rubira, A. F., and Muniz, E. C. (2006). Functionalization of poly (vinyl alcohol) by addition of methacryloyl groups: characterization by FTIR and NMR and optimization of reaction conditions by RSM. E-Polym. 6 (1). doi:10.1515/epoly.2006.6.1.793
Deng, L., Tang, L., and Qu, J. (2020). Synthesis and photopolymerization of novel UV-curable macro-photoinitiators. Prog. Org. Coat. 141, 105546. doi:10.1016/j.porgcoat.2020.105546
Dicharry, R. M., Ye, P., Saha, G., Waxman, E., Asandei, A. D., and Parnas, R. S. (2006). Wheat Gluten−Thiolated poly (vinyl alcohol) blends with improved mechanical properties. Biomacromolecules 7 (10), 2837–2844. doi:10.1021/bm060432n
Dietliker, K., Jung, T., Benkhoff, J., Kura, H., Matsumoto, A., Oka, H., et al. (2004). New developments in photoinitiators. Macromol. Symp. 217 (1), 77–98. doi:10.1002/masy.200451307
Guo, Z. (2022). Research advances in UV-curable self-healing coatings. RSC Adv. 12 (50), 32429–32439. doi:10.1039/D2RA06089B
Hammoud, F., Pavlou, A., Petropoulos, A., Graff, B., Siskos, M. G., Hijazi, A., et al. (2022). Naphthoquinone-based imidazolyl esters as blue-light-sensitive type I photoinitiators. Polym. Chem. 13 (33), 4817–4831. doi:10.1039/D2PY00753C
He, X., Zang, L., Xin, Y., and Zou, Y. (2023). An overview of photopolymerization and its diverse applications. Appl. Res. 2 (6), e202300030. doi:10.1002/appl.202300030
Keskin Dogruyol, S., Dogruyol, Z., Ozcelik Kazancioglu, E., Gokcek, H., and Arsu, N. (2023). Thioxanthonation of 2-naphthalene-thioacetic acid as a visible-light photoinitiator: the investigation of photophysical and photochemical properties. Eur. Polym. J. 198, 112440. doi:10.1016/j.eurpolymj.2023.112440
Ko, H., Kim, J. W., Kim, H. C., Zhai, L., and Kim, J. (2020). Esterified PVA-lignin resin by maleic acid applicable for natural fiber reinforced composites. J. Appl. Polym. Sci. 137 (26), 48836. doi:10.1002/app.48836
Kork, S., Yilmaz, G., and Yagci, Y. (2015). Poly (Vinyl alcohol)-thioxanthone as one-component type II photoinitiator for free radical polymerization in organic and aqueous media. Macromol. Rapid Commun. 36 (10), 923–928. doi:10.1002/marc.201500043
Koyuncu, U., Metin, E., Ocal, N., and Arsu, N. (2021). Synthesis of one-component type II dithioxanthone-disulfide photoinitiator and investigation of photophysical and photochemical properties. Eur. Polym. J. 153, 110510. doi:10.1016/j.eurpolymj.2021.110510
Madhusudhana, A. M., Mohana, K. N. S., Hegde, M. B., Nayak, S. R., Rajitha, K., and Sunil Kumar, M. C. (2022). Functionalized graphene oxide dispersed polyvinyl alcohol-epoxidized linseed oil composite: an eco-friendly and promising anticorrosion coating material. Colloids Surf. Physicochem. Eng. Asp. 650, 129382. doi:10.1016/j.colsurfa.2022.129382
Maruoka, S., Matsuura, T., Kawasaki, K., Okamoto, M., Yoshiaki, H., Kodama, M., et al. (2006). Biocompatibility of polyvinylalcohol gel as a vitreous substitute. Curr. Eye Res. 31 (7–8), 599–606. doi:10.1080/02713680600813854
Metin, E., Arsu, N., Catak, S., and Aviyente, V. (2020). Photophysical, kinetic and thermodynamic study of one-component type II thioxanthone acetic acid photoinitiators. Eur. Polym. J. 136, 109909. doi:10.1016/j.eurpolymj.2020.109909
Mühlebach, A., Müller, B., Pharisa, C., Hofmann, M., Seiferling, B., and Guerry, D. (1997). New water-soluble photo crosslinkable polymers based on modified poly (vinyl alcohol). J. Polym. Sci. Part Polym. Chem. 35 (16), 3603–3611. doi:10.1002/(SICI)1099-0518(19971130)35:16<3603::AID-POLA28>3.0.CO;2-I
Nassar, M. M. A., Alzebdeh, K. I., Awad, S. A., and Khalaf, E. M. (2023). New strategies for surface modification of poly (vinyl alcohol) toward click chemistry applications. Polym. Eng. Sci. 63 (4), 1195–1205. doi:10.1002/pen.26275
Nishiyabu, R., Takahashi, Y., Yabuki, T., Gommori, S., Yamamoto, Y., Kitagishi, H., et al. (2020). Boronate sol–gel method for one-step fabrication of polyvinyl alcohol hydrogel coatings by simple cast- and dip-coating techniques. RSC Adv. 10 (1), 86–94. doi:10.1039/C9RA08208E
Ozcelik Kazancioglu, E., Aydin, M., and Arsu, N. (2021). Photochemical synthesis of bimetallic gold/silver nanoparticles in polymer matrix with tunable absorption properties: superior photocatalytic activity for degradation of methylene blue. Mat. Chem. Phys. 269, 124734. doi:10.1016/j.matchemphys.2021.124734
Pierau, L., Elian, C., Akimoto, J., Ito, Y., Caillol, S., and Versace, D.-L. (2022). Bio-sourced monomers and cationic photopolymerization–the green combination towards eco-friendly and non-toxic materials. Prog. Polym. Sci. 127, 101517. doi:10.1016/j.progpolymsci.2022.101517
Rafique, A., Mahmood Zia, K., Zuber, M., Tabasum, S., and Rehman, S. (2016). Chitosan functionalized poly (vinyl alcohol) for prospects biomedical and industrial applications: a review. Int. J. Biol. Macromol. 87, 141–154. doi:10.1016/j.ijbiomac.2016.02.035
Riad, K. B., Arnold, A. A., Claverie, J. P., Hoa, S. V., and Wood-Adams, P. M. (2020). Photopolymerization using metal oxide semiconducting nanoparticles for epoxy-based coatings and patterned films. ACS Appl. Nano Mat. 3 (3), 2875–2880. doi:10.1021/acsanm.0c00147
Salavagione, H. J., Gómez, M. A., and Martínez, G. (2009). Polymeric modification of graphene through esterification of graphite oxide and poly (vinyl alcohol). Macromolecules 42 (17), 6331–6334. doi:10.1021/ma900845w
Schanuel, F. S., Raggio Santos, K. S., Monte-Alto-Costa, A., and De Oliveira, M. G. (2015). Combined nitric oxide-releasing poly (vinyl alcohol) film/F127 hydrogel for accelerating wound healing. Colloids Surf. B Biointerfaces 130, 182–191. doi:10.1016/j.colsurfb.2015.04.007
Singh, K., Jain, N., Verma, A., Singh, V. K., and Chauhan, S. (2020). Functionalized graphite–reinforced cross-linked poly (vinyl alcohol) nanocomposites for vibration isolator application: morphology, mechanical, and thermal assessment. Mat. Perform. Charact. 9 (1), 215–230. doi:10.1520/MPC20190254
Tehfe, M., Louradour, F., Lalevée, J., and Fouassier, J.-P. (2013). Photopolymerization reactions: on the way to a green and sustainable chemistry. Appl. Sci. 3 (2), 490–514. doi:10.3390/app3020490
Voet, V. S. D., Guit, J., and Loos, K. (2021). Sustainable photopolymers in 3D printing: a review on biobased, biodegradable, and recyclable alternatives. Macromol. Rapid Commun. 42 (3), 2000475. doi:10.1002/marc.202000475
Keywords: photoinitiator, flexible polymeric films, thioxanthones (TX), polyvinyl alcohol (PVA), esterification reaction, silver nanoparticles (AgNPs)
Citation: Konar M, Nakamura K and Arsu N (2025) Synthesis of PVA-based macromolecular photoinitiators through esterification with TXOCH₂COOH and cysteine, and investigation of their photophysical properties: in situ fabrication of flexible nanocomposite films. Front. Mater. 12:1544162. doi: 10.3389/fmats.2025.1544162
Received: 12 December 2024; Accepted: 07 January 2025;
Published: 10 February 2025.
Edited by:
Kerem Kaya, Istanbul Technical University, TürkiyeReviewed by:
Azra Kocaarslan, Karlsruhe Institute of Technology (KIT), GermanyHuseyin Kiliclar, Istanbul Technical University, Türkiye
Copyright © 2025 Konar, Nakamura and Arsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nergis Arsu, bmFyc3VAeWlsZGl6LmVkdS50cg==
 Melisa Konar
Melisa Konar Kazuki Nakamura2
Kazuki Nakamura2 Nergis Arsu
Nergis Arsu