
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mater., 26 March 2025
Sec. Polymeric and Composite Materials
Volume 12 - 2025 | https://doi.org/10.3389/fmats.2025.1535825
 Zahraa Sabah Ghnim1
Zahraa Sabah Ghnim1 Ayat Hussein Adhab2
Ayat Hussein Adhab2 Farag M. A. Altalbawy3,4
Farag M. A. Altalbawy3,4 Morug Salih Mahdi5
Morug Salih Mahdi5 Aseel Salah Mansoor6
Aseel Salah Mansoor6 Usama Kadem Radi7
Usama Kadem Radi7 Nasr Saadoun Abd8
Nasr Saadoun Abd8 Uday Abdul-Reda Hussein9
Uday Abdul-Reda Hussein9 Hadil Hussain Hamza10
Hadil Hussain Hamza10 Khursheed Muzammil11
Khursheed Muzammil11 Ahmad Alkhayyat12,13,14*
Ahmad Alkhayyat12,13,14*This study includes the synthesis of new hydrogel using pectin, chitosan, and Mo-MOF (pectin/chitosan Mo-MOF hydrogel). After confirming the structure of the synthetic hydrogel by Elemental Analysis (EA), Energy-Dispersive X-ray Spectroscopy (EDS), EDS mapping, Fourier-transform infrared spectroscopy (FT-IR), X-Ray Diffraction (XRD), Brunauer-Emmett-Teller (BET), Scanning Electron Microscopy (SEM), and Transmission Electron Microscopy (TEM), its application in wastewater treatment, including the absorption of Congo red and the inhibition of pathogenic bacterial strains in wastewater, was evaluated. The factors affecting the adsorption of Congo red, such as pH, temperature, and contact time, were studied. The highest adsorption rate was determined to be 93% using 0.06 g/L of pectin/chitosan Mo-MOF hydrogel under conditions including pH 8, temperature of 25°C, and contact time of 90 min. The microbiology evaluations of the pectin/chitosan Mo-MOF hydrogel, which were performed against the known strains of wastewater such as Campylobacter jejuni, Shigella dysenteriae, Vibrio cholerae, Yersinia enterocolitica, and Salmonella enterica, indicated its high antibacterial properties, so, Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values were observed between 4 and 32 μg/mL and 8–64 μg/mL. The diverse characteristics of the pectin/chitosan Mo-MOF hydrogel can be attributed to its physical and chemical properties, such as its constituent compounds, specific surface area, and porosity. Finally, the pectin/chitosan Mo-MOF hydrogel can be introduced as a functional composition with unique capabilities in controlling pathogenic bacterial strains of wastewater and absorbing dangerous chemical compounds of wastewater for environmental purposes.
The global demand for freshwater is projected to outstrip supply by 40% by 2030, with an estimated 1.6 billion people lacking access to safely managed drinking water (Du Plessis, 2022; Zohud and Alam, 2022). Currently, approximately 4 billion people live in water-scarce areas, and one in four cities faces water insecurity (He et al., 2021). This crisis is exacerbated by climate change, which disrupts the water cycle and leads to more frequent and severe droughts and floods (He et al., 2021). The situation is dire; by 2050, the urban population facing water scarcity could double from current levels (Alemu and Dioha, 2020; Zulqarnain and Khan, 2024). Wastewater is a significant contributor to the global water crisis, containing various pollutants, including pathogenic bacteria (Hube and Wu, 2021; Kesari et al., 2021), heavy metals (Qasem et al., 2021), and hazardous chemicals like Congo red (Oladoye et al., 2022). Congo red is a carcinogenic dye primarily used in textiles (Khan et al., 2022) but also found in industrial applications such as diagnostic use (Oladoye et al., 2022), including staining in amyloidosis (Shehabeldin et al., 2023). Its presence in wastewater poses severe risks to human health and aquatic ecosystems (Tkaczyk et al., 2020). Including in humans, it can lead to cancer due to the presence of an azo group in its structure (Khan et al., 2022; Punnakkal and Anila, 2023). Numerous techniques have been developed to remove Congo red from wastewater. Including, Adsorption Techniques (Harja et al., 2022): Utilizing materials such as activated carbon (Mandal et al., 2021; Aftab et al., 2023) or biochar (Yu et al., 2021). Chemical Oxidation (Luo et al., 2020): Employing agents like ozone (Mugaishudeen and Harish, 2024) or hydrogen peroxide (Starko et al., 2024). Biological Treatment (Manzoor et al., 2024): Utilizing microorganisms capable of degrading dyes (Skanda et al., 2023).
Recent advancements in nanotechnology, particularly the use of Metal-Organic Frameworks (MOFs), have shown promise in effectively removing pollutants (Bhatt et al., 2022; Li et al., 2023b) like Congo red (Uddin et al., 2021). MOFs are characterized by their high porosity and specific surface area (Zhang et al., 2020; Behera et al., 2022; Suksatan et al., 2022), making them suitable for adsorption applications (Liu et al., 2022) and other biological applications such as theragnostics and drug delivery (Lawson et al., 2021; Osterrieth and Fairen-Jimenez, 2021), anticancer (Fatima et al., 2023; Yang et al., 2023) and antimicrobial activity (Nong et al., 2021; Hubab and Al-Ghouti, 2024). The integration of MOFs with hydrogels presents a novel approach to wastewater treatment (Chai et al., 2022; Laddha et al., 2023; Zheng et al., 2023). Hydrogels can be designed to encapsulate MOFs, enhancing their effectiveness in antimicrobial properties (Ahmadi et al., 2024; Yao et al., 2024). For instance, Zn-MOF hydrogel have shown efficacy in inhibiting bacterial growth (Li et al., 2023a). However, there have been reports of Molybdenum-based MOFs (Mo-MOFs) with antimicrobial properties (Abdelkhalek et al., 2024) and absorbing Congo red (Jayaraj et al., 2024).
Pectin and chitosan which are mainly used for hydrogel synthesis (Robert et al., 2022; Morello et al., 2023) can demonstrated antimicrobial activity (Chanmontri et al., 2023; Zhao et al., 2023) and potential for dye absorption (Ilgin, 2022). Research has focused on synthesizing new nanostructures that combine the benefits of MOFs and hydrogels (Miao et al., 2022). These materials can be engineered to target specific pathogens found in wastewater while simultaneously removing hazardous chemicals (Kodoth and Badalamoole, 2020) like Congo red, such as CuI/CuII-MOF-incorporated hydrogel (Jin et al., 2023). Preliminary evaluations indicate that such materials can effectively inhibit bacterial strains commonly present in wastewater (Al-dolaimy et al., 2023) and adsorb Congo red efficiently (Jin et al., 2023).
Addressing the global water crisis requires urgent action across multiple fronts, including innovative wastewater treatment solutions. Developing new materials such as MOF-hydrogel composites represents a promising avenue for enhancing water security through effective pollutant removal. Moreover, by investing in these technologies, we can help ensure that future generations have access to safe and clean water resources. Therefore, a new pectin/chitosan Mo-MOF hydrogel was synthesized using molybdenum and a specific ligand. Microbial evaluations were conducted on several specialized bacteria present in wastewater, as well as assessments of Congo red adsorption. The newly synthesized pectin/chitosan Mo-MOF hydrogel demonstrated significant potential to inhibit bacterial strains found in wastewater while effectively adsorbing Congo red.
The most important advantage of the synthesized hydrogel in this study over activated carbon and biochar, which are known as traditional compounds in the wastewater treatment process, is both chemical and microbiological effectiveness. The novelties of the synthesized pectin/chitosan MOFs can be attributed to their high functional and application capabilities, both in terms of antimicrobial properties and dye adsorption properties, compared to other similar compounds (Al-dolaimy et al., 2023; Alkhatami et al., 2023). That is, by using one compound, two goals can be considered and it is very important from an economic and environmental point of view. In addition, the reusability of the synthesized compound in this study is also important.
In this study, detailed information regarding the chemicals, culture media, and bacterial strains used for synthesizing the pectin/chitosan Mo-MOF hydrogel and conducting antimicrobial evaluations is provided.
Pectin/Sigma-Aldrich (impurities ≤10% moisture), Sodium periodate/Thermo Scientific, Chemicals (99.8%), Molybdenum (V) chloride/Thermo Scientific Chemicals (95%), 2,2′-Bipyridine-4,4′-dicarboxylic acid/Tokyo chemical industry-TCI (96%), Chitosan/Sigma-Aldrich (10 mg/mL acetic acid: water) were used to synthesize the products.
Mueller-Hinton broth/Titan biotech limited (TM media) and Mueller-Hinton agar/Titan biotech limited (TM media) were used in antimicrobial assays. The bacterial strains, obtained from the American Type Culture Collection (ATCC), included: Campylobacter jejuni, Shigella dysenteriae, Vibrio cholera, Yersinia enterocolitica, Salmonella enterica.
Congo red/Sigma-Aldrich (97%) was used to investigate the absorption properties of the pectin/chitosan Mo-MOF hydrogel.
The synthesis of the pectin/chitosan Mo-MOF hydrogel was carried out using a Backer vCLEAN1-L9. Unico S-2150 Ultraviolet-visible spectroscopy was used to prepare the required concentration of bacterial suspension and measure the absorptions of Congo red activity.
Fourier Transform Infrared Spectroscopy (FT-IR) of the pectin/chitosan Mo-MOF hydrogel was performed using a PerkinElmer RX1 spectrometer. The specific active surface area and porosity of the pectin/chitosan Mo-MOF hydrogel were determined using the Brunauer-Emmett-Teller (BET) technique and Barrett-Joyner-Halenda (BJH) technique, respectively. Nitrogen adsorption/desorption was measured using a Micromeritics ASAP 2020 PLUS. X-ray Diffraction (XRD) patterns were obtained using a Shimadzu XRD 7000. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) images were prepared using a Hitachi S4800 and Hitachi H9500, respectively. For BET, a temperature of 77 K is required. For TEM, SEM, and XRD, set the temperature to 95°C with the aim of removing impurities and contaminants.
Oxidized pectin was synthesized from the oxidation reaction of pectin in the presence of sodium periodate, according to previous studies (Nejati et al., 2020).
5.5 g of Mo (V) and 4.9 g of 2,2′-bipyridine-4,4′-dicarboxylic acid were added to 100 mL of deionized water and stirred (800 rpm) for 10 min at 60°C. 1 g of oxidized pectin was added to the created homogenous mixture and placed under ultrasonication (250 W) for 30 min at ambient temperature. The obtained product was washed with water and ethanol and dried at 100°C for 4 h under vacuum. Using ultrasonication, 3.8 g of chitosan was dispersed in 100 mL of deionized water, and then 7.1 g of the substance produced in the previous step was added to it. It was stirred for 1 h at a temperature of 100°C. Then, 8.4 g of chitosan was added to the mixture and stirred (800 rpm) for 30 min at 30°C. The synthesized pectin/chitosan Mo-MOF hydrogel was placed in a water bath at 37°C for 4 h (Salama and Aziz, 2020).
The Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of pectin/chitosan Mo-MOF hydrogel were tested. “These tests were conducted using hydrogel concentrations ranging from 1 to 1,024 μg/mL against a suspension of common strains found in wastewater, with a density of 1 × 105 CFU/mL. The testing method followed the guidelines set by the Clinical and Laboratory Standards Institute (CLSI) (Al-Khafaji et al., 2023; Ali et al., 2024).
Mueller-Hinton broth (90 μL), 10 μL of the bacterial strain under study, and 100 μL of V/BP-MOF (prepared at each concentration separately in each well) were added to each well of a 96-well microplate. The microplate was placed in a shaker incubator at 37°C for 36 h. After incubation, the wells were examined, and for each bacterial strain tested, the lowest concentration at which the contents were clear was recorded as the Minimum Inhibitory Concentration (MIC) (Al-Khafaji et al., 2023; Ali et al., 2024).
For each bacterial strain studied, the contents of the clear wells from the previous step were cultured in Mueller-Hinton agar. The cultures were then incubated at 37°C for 72 h. Finally, for each strain, the concentration at which no growth occurred was recorded as the Minimum Bactericidal Concentration (MBC) (Al-Khafaji et al., 2023; Ali et al., 2024).
To measure the Congo red absorption percentage, a specific volume of V/BP-MOF was added to 0.1 L of Congo red solution in deionized water and stirred (200 rpm) thoroughly. The absorbance was then measured at 497 nm using a spectrophotometer, following the procedure outlined in Equation 1 (Moghaddam-Manesh et al., 2024).
The new pectin/chitosan Mo-MOF hydrogel was synthesized using oxidized pectin, chitosan, molybdenum (V) chloride, and 2,2′-bipyridine-4,4′-dicarboxylic acid. The proposed structure, presented in Figure 1A, is based on the spectral data that will be discussed and confirmed in the following sections. Oxidized pectin was synthesized from the oxidation reaction of pectin in the presence of sodium periodate (Nejati et al., 2020). A review of previous literature proves that oxidized pectin/chitosan hydrogel can be created from the Schiff reaction of the amine group of chitosan with the aldehyde of oxidized pectin/Figure 1B (Alkhatami et al., 2023).
Therefore, first 2,2′-bipyridine-4,4′-dicarboxylic acid and oxidized pectin are added to the molybdenum (V) chloride, and after creating a complex through the oxygen bonds of the carboxylic groups of 2,2′-bipyridine-4,4′-dicarboxylic acid and oxidized pectin with Mo, chitosan is added to it and reacts with its aldehyde groups of oxidized pectin and the proposed product presented in Figure 1A was synthesized.
Referring to the FT-IR spectrum of the pectin/chitosan Mo-MOF hydrogel/Figure 2A, peaks in regions 965 cm−1, 2,940 cm−1, 1,715 cm−1, 1,530 cm−1, 1,410 cm−1 and 1,150 cm−1, was due to bonds Mo-O, C-H, C=O, C=N, C=C, and C-O which is a proof of the proposed structure of Figure 1A. It can be stated that the O-H peak appeared in 3,200 cm–1 (Abdtawfeeq et al., 2022).
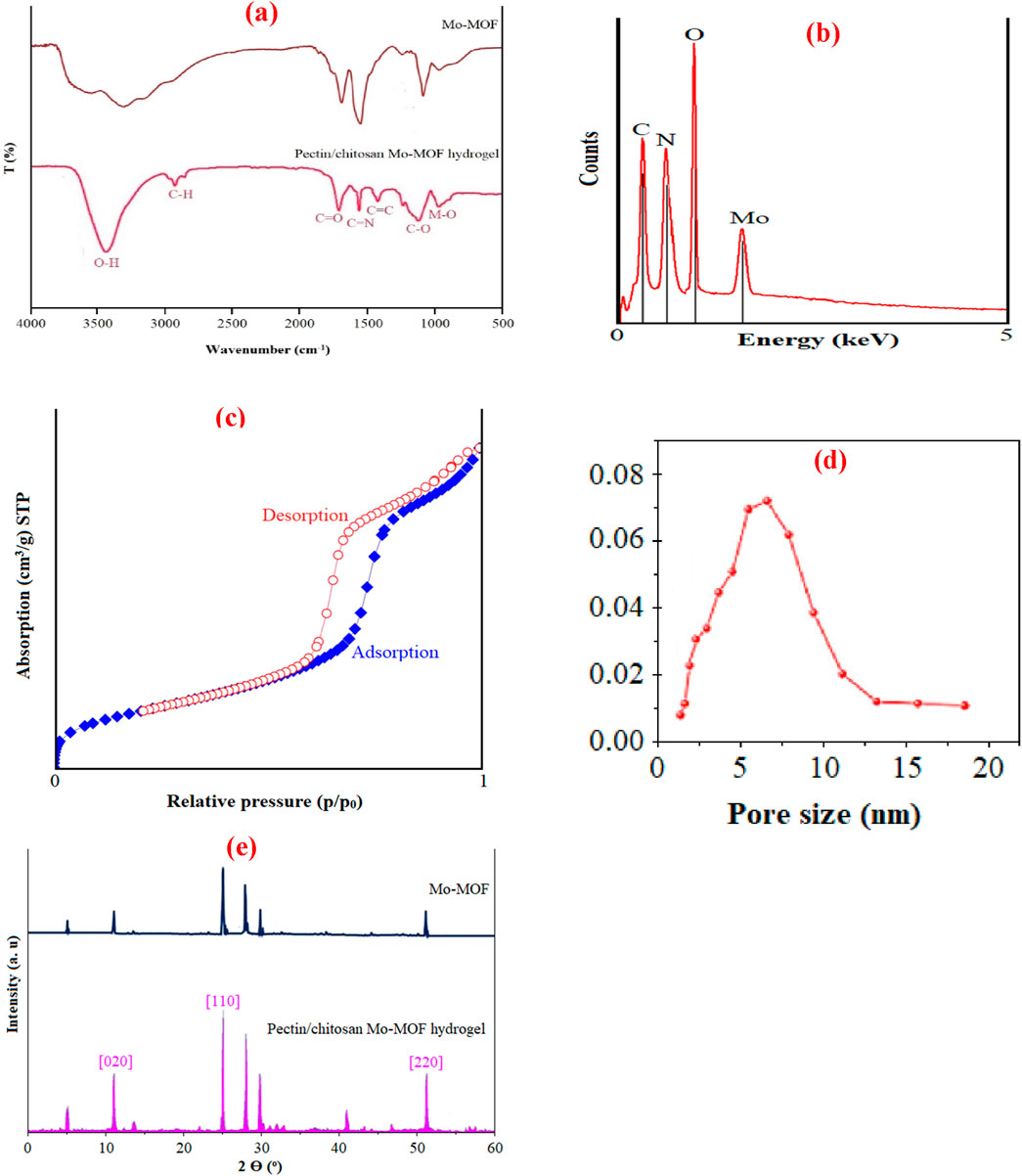
Figure 2. FT-IR (a), EDAX (b), nitrogen absorption/desorption (c), pore size distribution curve (d) and XRD (e) of pectin/chitosan Mo-MOF hydrogel.
Elemental analysis and EDAX of the pectin/chitosan Mo-MOF hydrogel, as shown in Figure 2B, provide proof of the presence of carbon, oxygen, nitrogen, hydrogen, and molybdenum. Thus, in the elemental analysis of the pectin/chitosan Mo-MOF hydrogel, carbon, hydrogen, nitrogen, and oxygen elements were observed as 45.17%, 4.65%, 6.32%, and 30.12%, respectively, and in EDAX, molybdenum metal was also observed.
The nitrogen absorption/desorption curve of the pectin/chitosan Mo-MOF hydrogel, which proves its high specific surface area and porosity, is shown in Figure 2C. Based on these curves, Brunauer-Emmett-Teller (BET) technique and Barrett-Joyner-Halenda (BJH) technique, the specific surface area of the pectin/chitosan Mo-MOF hydrogel and the volume pore were obtained as 2,349 m2/g and 0.62 cm3/g, respectively. The absorption and desorption curve of this compound is similar to type IV isotherms, which are unique to mesoporous compounds (Al-Ghouti and Da’ana, 2020). The pore size distribution curve of the pectin/chitosan Mo-MOF hydrogel shown in Figure 2D.
The crystal structure of molybdenum (JCPDS No. 05-0508) in the XRD pattern of the pectin/chitosan Mo-MOF hydrogel/Figure 2E was proved by using its peaks in areas 11°, 25°, and 52° corresponding to planes 020, 110, 220, (Illyaskutty et al., 2014; Abdtawfeeq et al., 2022). The peaks in regions 5°, 28°, and 30° can be attributed to 2,2′-bipyridine-4,4′-dicarboxylic acid (Saadh et al., 2024). According to the Scherer equation, the crystallite size of the pectin/chitosan Mo-MOF hydrogel was calculated to be 82 nm. Of course, the histogram curve, based on the TEM image, (Figure 3A), of the pectin/chitosan Mo-MOF hydrogel and its SEM (Figure 3B) and TEM (Figure 3C) images proved its nanosize and identical same morphology, as shown in Figure 3, and based on the histogram curve, the particle size was 76 nm.
As the characterization and confirmation of the structure proved, the pectin/chitosan Mo-MOF hydrogel has a porous crystalline nanostructure with high thermal stability and a highly specific active surface. These capabilities, especially the high specific active surface and its porosity, give it the ability to be used in various applications. These properties, which depend on the synthesis method of nanostructures, indicate the use of the appropriate method in synthesizing pectin/chitosan Mo-MOF hydrogel. In addition, the presence of compounds with biological properties such as pectin (Devasvaran and Lim, 2021), molybdenum (Jomova et al., 2022), 2,2′-bipyridine-4,4′-dicarboxylic acid (Mahadevi et al., 2022), and chitosan (Wang et al., 2020) in the structure of the pectin/chitosan Mo-MOF hydrogel can lead to the biological properties.
Therefore, considering the important capabilities of the pectin/chitosan Mo-MOF hydrogel, it was used as an absorbent of Congo red, which is one of the dangerous compounds of wastewater, and to remove pathogenic strains in wastewater.
As mentioned, the synthesized pectin/chitosan Mo-MOF hydrogel contains oxidized pectin, chitosan, molybdenum (V) chloride, and 2,2′-bipyridine-4,4′-dicarboxylic acid. A review of past literature showed that these compounds have biological properties, especially antimicrobial properties (Confederat et al., 2021; Liu et al., 2021; Al-Khafaji et al., 2023; Saadh et al., 2024).
Therefore, its antimicrobial activity against common pathogenic strains found in wastewater, including C. jejuni/ATCC 33291 (Figure 4A), S. dysenteriae/ATCC 13313 (Figure 4B), Vibrio cholerae/ATCC 14033 (Figure 4C), Y. enterocolitica/ATCC 27729 (Figure 4D), and S. enterica/ATCC 14028 (Figure 4E) were investigated and results are shown in Figure 4. To evaluate the effectiveness of the pectin/chitosan Mo-MOF hydrogel, common antibiotic drugs such as azithromycin, ampicillin, and ceftriaxone were used for comparison.
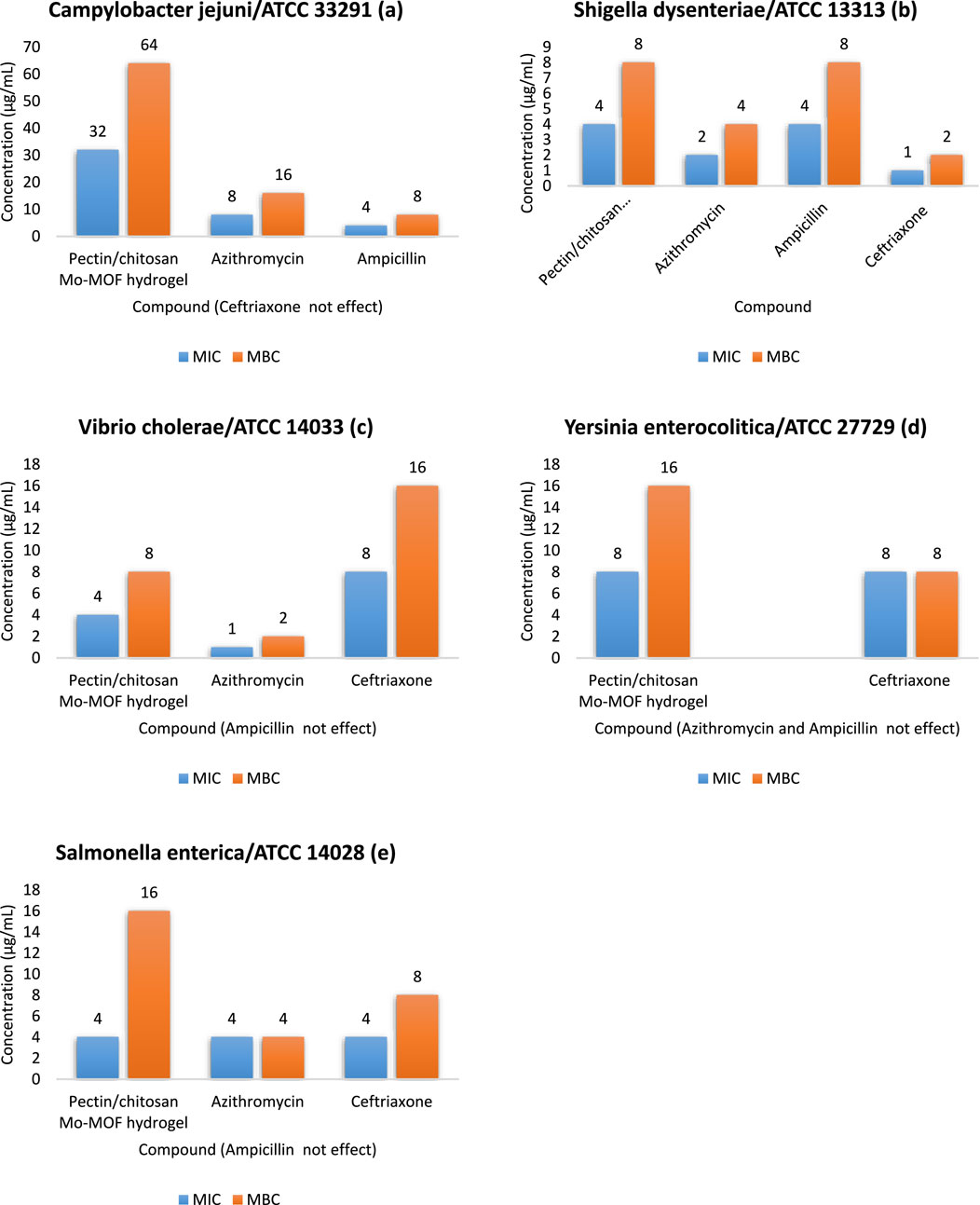
Figure 4. Antimicrobial activity results (a) Campylobacter jejuni/ATCC 33291. (b) Shigella dysenteriae/ATCC. (c) Vibrio cholerae/ATCC 14033. (d) Yersinia enterocolitica/ATCC 27729. (e) Salmonella enterica/ATCC 14028.
The highest effectiveness of the pectin/chitosan Mo-MOF hydrogel was observed against strain S. dysenteriae, V. cholerae. Thus, it’s MIC and MBC values were 4 μg/mL and 8 μg/mL, respectively. The effectiveness of ampicillin against S. dysenteriae was the same as pectin/chitosan Mo-MOF hydrogel. Vibrio cholerae was resistant to ampicillin, and ampicillin was not effective. In addition, ampicillin was not effective against Y. enterocolitica, and S. enterica, but the pectin/chitosan Mo-MOF hydrogel showed acceptable results against the mentioned strains. As shown in Figure 4, the MICs of 8 μg/mL and 4 μg/mL were obtained against Y. enterocolitica and S. enterica, respectively. Also, Y. enterocolitica showed resistance to azithromycin. The resistance of C. jejuni to ceftriaxone is notable. Therefore, it can be concluded that the synthesized pectin/chitosan Mo-MOF hydrogel has significant antibacterial and inhibitory properties against common pathogenic bacterial strains in wastewater.
Past studies indicate that the effectiveness of MOF compounds against bacterial strains is achieved in two ways, the first through contact of the MOF surface with the strains, and the second through trapping within MOF cavities (Cao et al., 2020). Therefore, compounds with biological activity play an important role in this field. In addition, the role of the special surface area and MOF cavities is undeniable (Al-Khafaji et al., 2023; Ali et al., 2024).
The synthesized pectin/chitosan Mo-MOF hydrogel was very effective in this field. As the results proved, factors such as high specific surface area, high porosity, as well as compounds with antimicrobial properties in its structure, such as oxidized pectin, chitosan, molybdenum (V) chloride, and 2,2′-bipyridine-4,4′-dicarboxylic acid, caused these results and its high ability to inhibit and destroy bacterial strains.
As can be seen in the structure of the pectin/chitosan Mo-MOF hydrogel (Figure 1A), the significant sites including oxygen and nitrogen are capable of hydrogen bonding. Of course, here also, the role of active special surface and porosity is undeniable. The removal of Congo red is facilitated by the formation of hydrogen bonds between the hydrogen atoms attached to the nitrogen of Congo red and the oxygen and nitrogen groups in the hydrogel structure (Figure 5).
It has been demonstrated by comparing the absorption percentages of Congo red by the pectin/chitosan Mo-MOF hydrogel and the pectin/chitosan hydrogel, as discussed in the following sections. To achieve maximum absorption of Congo red by the pectin/chitosan Mo-MOF hydrogel, various effective factors were investigated, including adsorbent dose, pH, temperature, and contact time. The results obtained from these investigations are discussed below. It can be stated that all tests performed on the pectin/chitosan Mo-MOF hydrogel were conducted under the same conditions as those for the pectin/chitosan hydrogel.
Different concentrations of Congo red were prepared, and their absorption was investigated using a constant amount of the pectin/chitosan Mo-MOF hydrogel.
For this purpose, solutions of Congo red at concentrations of 100, 150, 300, 600, 900, and 1,200 mg/L were prepared in double-distilled water, and their absorption was evaluated using 0.01 g of the pectin/chitosan Mo-MOF hydrogel. In these evaluations, the pH value was 7, the temperature was 25°C, and the absorption time was 120 min. The results obtained were as Figure 6A. As can be seen, with the increase in the concentration of Cogo Red, its absorption percentage has decreased with a constant amount of pectin/chitosan Mo-MOF hydrogel. The reason is entirely logical and acceptable. As the concentration of Congo red increases, the absorbable active sites of pectin/chitosan Mo-MOF hydrogel are saturated, so with the rise in the concentration of Congo red, its absorption percentage decreases (Salama and Aziz, 2020).
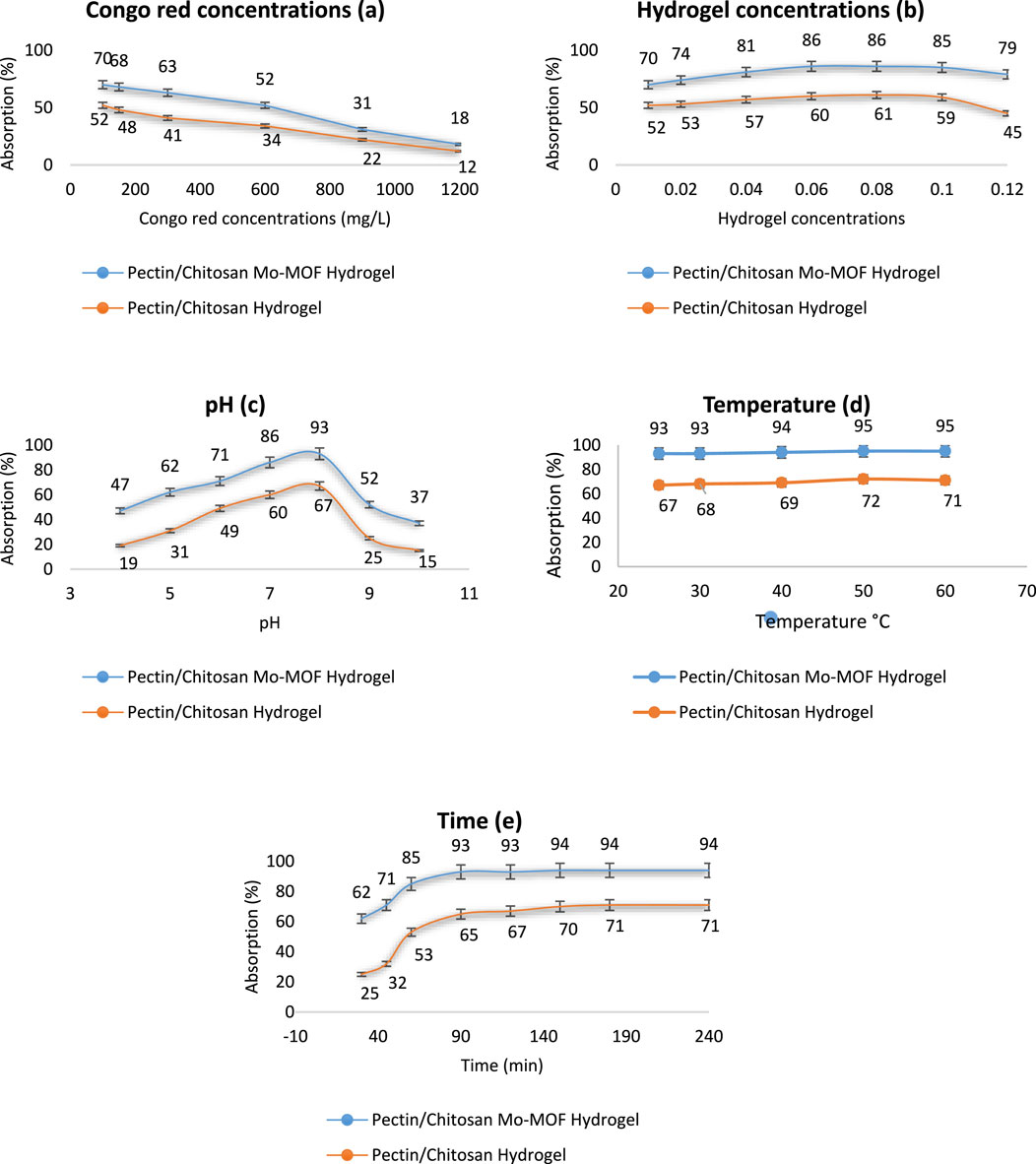
Figure 6. Examining different conditions in the absorption of Congo Red [(a) Congo red concentrations, (b) Hydrogel concentrations, (c) pH, (d) Temperature, (e) Time].
The 600 mg/L Congo red solution is used as a fixed concentration to evaluation of other factors.
Obtaining the optimal amount of pectin/chitosan Mo-MOF hydrogel is the next factor in evaluating the adsorption capacity of Congo red. Amounts of 0.01, 0.02, 0.04, 0.06, 0.08, 0.10, and 0.12 g/L pectin/chitosan Mo-MOF hydrogel were added to the concentration of 600 mg/L Congo red solution and the absorption percentage was obtained. It should be mentioned that pH 7, temperature of 25°C, and time of 120 min were considered constant conditions. The results obtained were as Figure 6B. The results showed that with the increase in the amount of pectin/chitosan Mo-MOF hydrogel from 0.1 to 0.06 g/L, absorption increased significantly and at values of 0.08 g/L, and 0.10 g/L the absorption percentage can be considered almost constant and even less absorption was observed in 0.12 g/L. The reason for the fixed and decreasing percentage of absorption at concentrations higher than 0.6 pectin/chitosan Mo-MOF hydrogel can be attributed to the accumulation of nanoparticles and the inactivation of the active absorbable sites of Congo red due to the increase in its concentration and agglomeration (Salama and Aziz, 2020). Therefore, the concentration of 0.06 g/L was chosen as the optimal amount of pectin/chitosan Mo-MOF hydrogel in other studies.
The pH of 4, 5, 6, 7, 8, 9, and 10 was another study in which the absorption percentage of 600 mg/L Congo red solution by 0.06 g/L pectin/chitosan Mo-MOF hydrogel was tested. The temperature and time of the tests in these studies were considered constant at 25°C and 120 min. The results obtained were as Figure 6C. The highest absorption percentage was observed at pH 8. Due to the hydrolysis and destruction of metal-oxygen bonds at both high and low pH levels, a substantial decrease in absorption was observed at pH values of 10, 9, 4, and 5. The reason can be justified by studying past texts. At a balanced alkaline pH that does not lead to hydrolysis, the density of negative charges on oxygen increases. The reason is the reduction of negative charge absorption towards the carbonyl group. At a balanced acidic pH that does not lead to hydrolysis, increasing pH results in protonated oxygen and nitrogen groups leading to a decrease in absorption. In an alkaline environment, hydroxyl (OH) groups interact with carbonyl groups, leading to a decline in the absorption capacity of negatively charged OH groups. This interaction ultimately increases the formation of hydrogen bonds between oxygen groups (Salama and Aziz, 2020). Therefore, a pH equal to 8 was chosen as the appropriate pH factor in the absorption process.
The evaluation of the absorption percentage of 600 mg/L Congo red solution using 0.06 g/L pectin/chitosan Mo-MOF hydrogel at pH 8 for 120 min at temperatures of 25, 30, 40, 50, and 60 C was another test that was done. The results obtained were as Figure 6D. Due to the high absorption of Congo red by pectin/chitosan Mo-MOF hydrogel at ambient temperature and since a high difference in efficiency is not observed at ambient temperature up to 70°C (approximately 2%), with regard to saving energy, ambient temperature is therefore used as the optimal temperature.
In the last step in obtaining optimal conditions, absorption time was investigated. In the optimal conditions obtained, including 600 mg/L Congo red solution, pH 8, 25°C, the amount of concentrated absorption of 0.06 g/L pectin/chitosan Mo-MOF hydrogel in the investigated times of 30, 45, 60, 90, 120, 150, 180, and 240 min was done. The results obtained were as Figure 6E. The results showed that up to 90 min, the process of increasing absorption is incremental, while beyond this time, the absorption activity remains relatively constant. Therefore, for up to 90 min, all the active sites of pectin/chitosan Mo-MOF hydrogel absorption are saturated, so that it can be reported as the optimal absorption time (Salama and Aziz, 2020).
As mentioned in the evaluations, the tests on the hydrogel were also performed as a comparison. In all the tests, it was observed that the absorption of pectin/chitosan Mo-MOF hydrogel is significantly higher than hydrogel. The reason for this, as mentioned at the beginning (Figure 5), is the large amount of absorption by the MOF cavities, which the hydrogel lacks.
After the adsorption process, the pectin/chitosan Mo-MOF hydrogel were washed with a mixture of water and ethanol, and after drying in an oven, their specific surface area was measured and obtained 2054 m2/g as shown in Figure 7.

Figure 7. Nitrogen absorption/desorption of pectin/chitosan Mo-MOF hydrogel after adsorption process.
The synthesis of a new hydrogel containing pectin, chitosan, Mo-MOF (pectin/chitosan Mo-MOF hydrogel), and its application in wastewater treatment was the subject of this study. After confirming the structure, it was found that the pectin/chitosan Mo-MOF hydrogel has a high specific surface area and porosity. Therefore, these properties could be utilized in the adsorption process of Congo red, which is known as one of the most important chemical pollutants in wastewater. The presence of compounds with antimicrobial activity in the structure of the pectin/chitosan Mo-MOF hydrogel was another factor investigated as an antimicrobial agent in the control of pathogenic bacterial strains present in wastewater. The evaluations proved that the synthesized final product can be used to eliminate pathogenic strains present in wastewater, such as C. jejuni, S. dysenteriae, V. cholerae, Y. enterocolitica, and S. enterica. The synthesized pectin/chitosan Mo-MOF hydrogel demonstrated superior efficacy compared to some commercial antibiotics, such as antibiotics azithromycin, ampicillin, and ceftriaxone. In adsorption studies, a concentration of 0.06 g/L of nanoparticles achieved 93% adsorption at pH of 8 within 90 min. Ultimately, this synthesized hydrogel can be introduced as a novel material with multiple functionalities.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
ZG: Methodology, Writing–original draft. AA: Formal Analysis, Writing–original draft. FA: Validation, Writing–review and editing. MS: Data curation, Writing–original draft. AS: Resources, Writing–original draft. UR: Formal Analysis, Writing–original draft. NS: Visualization, Writing–review and editing. UA-R: Investigation, Writing–review and editing. HH: Methodology, Writing–original draft. KM: Conceptualization, Writing–review and editing. AA: Funding acquisition, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. The authors thank the Deanship of Scientific Research and Graduate Studies at King Khalid University, Abha, KSA, for funding this work through a research group program under grant number RGP.2/584/46.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdelkhalek, M. M., Mohamed, A. M., Abdallah, R. Z., Khedr, G. E., Siam, R., and Allam, N. K. (2024). Zeolitic imidazolate framework-8 encapsulated with Mo-based polyoxometalates as surfaces with antibacterial activity against Escherichia coli. Nanoscale Adv. 6, 3355–3366. doi:10.1039/d4na00142g
Abdtawfeeq, T. H., Farhan, Z. A., Al-Majdi, K., Jawad, M. A., Zabibah, R. S., Riadi, Y., et al. (2022). Ultrasound-Assisted and one-pot synthesis of new Fe3O4/Mo-MOF magnetic nano polymer as a strong antimicrobial agent and efficient nanocatalyst in the multicomponent synthesis of novel pyrano [2, 3-d] pyrimidines derivatives. J. Inorg. Organomet. Polym. Mater. 33, 472–483. doi:10.1007/s10904-022-02514-7
Aftab, R. A., Zaidi, S., Khan, A. a.P., Usman, M. A., Khan, A. Y., Chani, M. T. S., et al. (2023). Removal of Congo red from water by adsorption onto activated carbon derived from waste black cardamom peels and machine learning modeling. Alexandria Eng. J. 71, 355–369. doi:10.1016/j.aej.2023.03.055
Ahmadi, S., Pourebrahimi, S., Malloum, A., Pirooz, M., Osagie, C., Ghosh, S., et al. (2024). Hydrogel-based materials as antibacterial agents and super adsorbents for the remediation of emerging pollutants: a comprehensive review. Emerg. Contam. 10, 100336. doi:10.1016/j.emcon.2024.100336
Al-Dolaimy, F., Altimari, U. S., Abdulwahid, A. S., Mohammed, Z. I., Hameed, S. M., Dawood, A. H., et al. (2023). Hydrogel assisted synthesis of polymeric materials based on chitosan, oxidized pectin, and tantalum MOF nanostructures as potent antibiotic agents against common pathogenic strains between humans and aquatic. J. Inorg. Organomet. Polym. Mater. 34, 874–884. doi:10.1007/s10904-023-02863-x
Alemu, Z. A., and Dioha, M. O. (2020). Modelling scenarios for sustainable water supply and demand in Addis Ababa city, Ethiopia. Environ. Syst. Res. 9, 7–14. doi:10.1186/s40068-020-00168-3
Al-Ghouti, M. A., and Da'ana, D. A. (2020). Guidelines for the use and interpretation of adsorption isotherm models: a review. J. Hazard. Mater. 393, 122383. doi:10.1016/j.jhazmat.2020.122383
Ali, E., Al-Saedi, H. F. S., Hussein, S. A., Mustafa, N. K., Abdulridui, H. A., Al-Abdeen, S. H. Z., et al. (2024). Fabrication and characterization of novel nanocomposite containing Zn-MOF/Gentamicin/Oxidized chitosan as a highly effective antimicrobial agent. J. Inorg. Organomet. Polym. Mater. 34, 4321–4331. doi:10.1007/s10904-024-03078-4
Al-Khafaji, H. H. J., Alsalamy, A., Jawad, M. A., Nasser, H. A., Dawood, A. H., Hasan, S. Y., et al. (2023). Synthesis of a novel Cu/DPA-MOF/OP/CS hydrogel with high capability in antimicrobial studies. Front. Chem. 11, 1236580. doi:10.3389/fchem.2023.1236580
Alkhatami, A. G., Khaled Younis Albahadly, W., Jawad, M. A., Ramadan, M. F., Alsaraf, K. M., Riyad Muedii, Z. a.-H., et al. (2023). Hydrogel assistant synthesis of new Ti-MOF cross-linked oxidized pectin and chitosan with anti-breast cancer properties. Front. Mater. 10, 1264529. doi:10.3389/fmats.2023.1264529
Behera, P., Subudhi, S., Tripathy, S. P., and Parida, K. (2022). MOF derived nano-materials: a recent progress in strategic fabrication, characterization and mechanistic insight towards divergent photocatalytic applications. Coord. Chem. Rev. 456, 214392. doi:10.1016/j.ccr.2021.214392
Bhatt, P., Pandey, S. C., Joshi, S., Chaudhary, P., Pathak, V. M., Huang, Y., et al. (2022). Nanobioremediation: a sustainable approach for the removal of toxic pollutants from the environment. J. Hazard. Mater. 427, 128033. doi:10.1016/j.jhazmat.2021.128033
Cao, P., Wu, X., Zhang, W., Zhao, L., Sun, W., and Tang, Z. (2020). Killing oral bacteria using metal–organic frameworks. Industrial and Eng. Chem. Res. 59, 1559–1567. doi:10.1021/acs.iecr.9b05659
Chai, Y., Zhang, Y., Wang, L., Du, Y., Wang, B., Li, N., et al. (2022). In situ one-pot construction of MOF/hydrogel composite beads with enhanced wastewater treatment performance. Sep. Purif. Technol. 295, 121225. doi:10.1016/j.seppur.2022.121225
Chanmontri, M., Swilem, A. E., Mutch, A. L., Grøndahl, L., and Suwantong, O. (2023). Physicochemical and in vitro biological evaluation of an injectable self-healing quaternized chitosan/oxidized pectin hydrogel for potential use as a wound dressing material. Int. J. Biol. Macromol. 242, 124984. doi:10.1016/j.ijbiomac.2023.124984
Confederat, L. G., Tuchilus, C. G., Dragan, M., Sha’at, M., and Dragostin, O. M. (2021). Preparation and antimicrobial activity of chitosan and its derivatives: a concise review. Molecules 26, 3694. doi:10.3390/molecules26123694
Devasvaran, K., and Lim, V. (2021). Green synthesis of metallic nanoparticles using pectin as a reducing agent: a systematic review of the biological activities. Pharm. Biol. 59, 492–501. doi:10.1080/13880209.2021.1910716
Fatima, S. F., Sabouni, R., Garg, R., and Gomaa, H. (2023). Recent advances in Metal-Organic Frameworks as nanocarriers for triggered release of anticancer drugs: brief history, biomedical applications, challenges and future perspective. Colloids Surfaces B Biointerfaces 225, 113266. doi:10.1016/j.colsurfb.2023.113266
Harja, M., Buema, G., and Bucur, D. (2022). Recent advances in removal of Congo Red dye by adsorption using an industrial waste. Sci. Rep. 12, 6087. doi:10.1038/s41598-022-10093-3
He, C., Liu, Z., Wu, J., Pan, X., Fang, Z., Li, J., et al. (2021). Future global urban water scarcity and potential solutions. Nat. Commun. 12, 4667. doi:10.1038/s41467-021-25026-3
Hubab, M., and Al-Ghouti, M. A. (2024). Recent advances and potential applications for metal-organic framework (MOFs) and MOFs-derived materials: characterizations and antimicrobial activities. Biotechnol. Rep. 42, e00837. doi:10.1016/j.btre.2024.e00837
Hube, S., and Wu, B. (2021). Mitigation of emerging pollutants and pathogens in decentralized wastewater treatment processes: a review. Sci. Total Environ. 779, 146545. doi:10.1016/j.scitotenv.2021.146545
Ilgin, P. (2022). High removal of methylene blue dye from aqueous solution by using a novel pectin-based hydrogel. Int. J. Environ. Anal. Chem. 102, 5413–5431. doi:10.1080/03067319.2020.1796995
Illyaskutty, N., Sreedhar, S., Kumar, G. S., Kohler, H., Schwotzer, M., Natzeck, C., et al. (2014). Alteration of architecture of MoO 3 nanostructures on arbitrary substrates: growth kinetics, spectroscopic and gas sensing properties. Nanoscale 6, 13882–13894. doi:10.1039/c4nr04529g
Jayaraj, S. K., Karthik, G., Antony, M., Panneerselvam, P., Paramasivam, T., H. Jadhav, A., et al. (2024). Ligand-engineered structural and physiochemical properties of 1D molybdenum-MOFs: a seldom explored system for photocatalytic applications. Inorg. Chem. 63, 15270–15282. doi:10.1021/acs.inorgchem.4c01829
Jin, Y., Li, Y., Du, Q., Chen, B., Chen, K., Zhang, Y., et al. (2023). Efficient adsorption of Congo red by MIL-53 (Fe)/chitosan composite hydrogel spheres. Microporous Mesoporous Mater. 348, 112404. doi:10.1016/j.micromeso.2022.112404
Jomova, K., Makova, M., Alomar, S. Y., Alwasel, S. H., Nepovimova, E., Kuca, K., et al. (2022). Essential metals in health and disease. Chemico-biological Interact. 367, 110173. doi:10.1016/j.cbi.2022.110173
Kesari, K. K., Soni, R., Jamal, Q. M. S., Tripathi, P., Lal, J. A., Jha, N. K., et al. (2021). Wastewater treatment and reuse: a review of its applications and health implications. Water, Air, and Soil Pollut. 232, 208–228. doi:10.1007/s11270-021-05154-8
Khan, R. R. M., Qamar, H., Hameed, A., Rehman, A. U., Pervaiz, M., Saeed, Z., et al. (2022). Biological and photocatalytic degradation of Congo red, a diazo sulfonated substituted dye: a review. Water, Air, and Soil Pollut. 233, 468. doi:10.1007/s11270-022-05935-9
Kodoth, A. K., and Badalamoole, V. (2020). Silver nanoparticle-embedded pectin-based hydrogel for adsorptive removal of dyes and metal ions. Polym. Bull. 77, 541–564. doi:10.1007/s00289-019-02757-4
Laddha, H., Jadhav, N. B., Agarwal, M., and Gupta, R. (2023). Enumeration of research journey of MOF@ hydrogel composite beads as potential adsorbents for adsorptive elimination of toxic contaminants. J. Environ. Chem. Eng. 11, 110642. doi:10.1016/j.jece.2023.110642
Lawson, H. D., Walton, S. P., and Chan, C. (2021). Metal–organic frameworks for drug delivery: a design perspective. ACS Appl. Mater. and interfaces 13, 7004–7020. doi:10.1021/acsami.1c01089
Li, J., Yan, Y., Chen, Y., Fang, Q., Hussain, M. I., and Wang, L.-N. (2023a). Flexible curcumin-loaded Zn-MOF hydrogel for long-term drug release and antibacterial activities. Int. J. Mol. Sci. 24, 11439. doi:10.3390/ijms241411439
Li, L., Han, J., Huang, X., Qiu, S., Liu, X., Liu, L., et al. (2023b). Organic pollutants removal from aqueous solutions using metal-organic frameworks (MOFs) as adsorbents: a review. J. Environ. Chem. Eng. 11, 111217. doi:10.1016/j.jece.2023.111217
Liu, D., Gu, W., Zhou, L., Wang, L., Zhang, J., Liu, Y., et al. (2022). Recent advances in MOF-derived carbon-based nanomaterials for environmental applications in adsorption and catalytic degradation. Chem. Eng. J. 427, 131503. doi:10.1016/j.cej.2021.131503
Liu, J., Wang, T., Huang, B., Zhuang, Y., Hu, Y., and Fei, P. (2021). Pectin modified with phenolic acids: evaluation of their emulsification properties, antioxidation activities, and antibacterial activities. Int. J. Biol. Macromol. 174, 485–493. doi:10.1016/j.ijbiomac.2021.01.190
Luo, C., Wu, D., Gan, L., Cheng, X., Ma, Q., Tan, F., et al. (2020). Oxidation of Congo red by thermally activated persulfate process: kinetics and transformation pathway. Sep. Purif. Technol. 244, 116839. doi:10.1016/j.seppur.2020.116839
Mahadevi, P., Sumathi, S., Metha, A., and Singh, J. (2022). Synthesis, spectral, antioxidant, in vitro cytotoxicity activity and thermal analysis of Schiff base metal complexes with 2, 2′-Bipyridine-4, 4′-dicarboxylic acid as co-ligand. J. Mol. Struct. 1268, 133669. doi:10.1016/j.molstruc.2022.133669
Mandal, S., Calderon, J., Marpu, S. B., Omary, M. A., and Shi, S. Q. (2021). Mesoporous activated carbon as a green adsorbent for the removal of heavy metals and Congo red: characterization, adsorption kinetics, and isotherm studies. J. Contam. Hydrology 243, 103869. doi:10.1016/j.jconhyd.2021.103869
Manzoor, K., Batool, M., Naz, F., Nazar, M. F., Hameed, B. H., and Zafar, M. N. (2024). A comprehensive review on application of plant-based bioadsorbents for Congo red removal. Biomass Convers. Biorefinery 14, 4511–4537. doi:10.1007/s13399-022-02741-5
Miao, Q., Jiang, L., Yang, J., Hu, T., Shan, S., Su, H., et al. (2022). MOF/hydrogel composite-based adsorbents for water treatment: a review. J. Water Process Eng. 50, 103348. doi:10.1016/j.jwpe.2022.103348
Moghaddam-Manesh, M., Darvishi, R., and Moshkriz, A. (2024). Innovative high-performance antimicrobial agent and dye adsorbent based on magnetic/copper nanoparticles. J. Polym. Environ. 32, 5231–5253. doi:10.1007/s10924-024-03289-3
Morello, G., De Iaco, G., Gigli, G., Polini, A., and Gervaso, F. (2023). Chitosan and pectin hydrogels for tissue engineering and in vitro modeling. Gels 9, 132. doi:10.3390/gels9020132
Mugaishudeen, G., Harish, M., and Singaraja gopal, R. (2024). Removal of Methylene blue and Congo red from the wastewater in a jet loop reactor using ozone and activated carbon. Desalination Water Treat. 319, 100471. doi:10.1016/j.dwt.2024.100471
Nejati, S., Soflou, R. K., Khorshidi, S., and Karkhaneh, A. (2020). Development of an oxygen-releasing electroconductive in-situ crosslinkable hydrogel based on oxidized pectin and grafted gelatin for tissue engineering applications. Colloids Surfaces B Biointerfaces 196, 111347. doi:10.1016/j.colsurfb.2020.111347
Nong, W., Wu, J., Ghiladi, R. A., and Guan, Y. (2021). The structural appeal of metal–organic frameworks in antimicrobial applications. Coord. Chem. Rev. 442, 214007. doi:10.1016/j.ccr.2021.214007
Oladoye, P. O., Bamigboye, M. O., Ogunbiyi, O. D., and Akano, M. T. (2022). Toxicity and decontamination strategies of Congo red dye. Groundw. Sustain. Dev. 19, 100844. doi:10.1016/j.gsd.2022.100844
Osterrieth, J. W., and Fairen-Jimenez, D. (2021). Metal–organic framework composites for theragnostics and drug delivery applications. Biotechnol. J. 16, 2000005. doi:10.1002/biot.202000005
Punnakkal, V. S., and Anila, E. (2023). Polypyrrole/silver/graphene ternary nanocomposite synthesis and study on photocatalytic property in degrading Congo red dye under visible light. Surfaces Interfaces 42, 103342. doi:10.1016/j.surfin.2023.103342
Qasem, N. A., Mohammed, R. H., and Lawal, D. U. (2021). Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean. Water 4, 36–15. doi:10.1038/s41545-021-00127-0
Robert, B., Chenthamara, D., and Subramaniam, S. (2022). Fabrication and biomedical applications of Arabinoxylan, Pectin, Chitosan, soy protein, and silk fibroin hydrogels via laccase-Ferulic acid redox chemistry. Int. J. Biol. Macromol. 201, 539–556. doi:10.1016/j.ijbiomac.2021.12.103
Saadh, M. J., Jafar, N. N., Altalbawy, F. M., Sharma, P., Kumar, A., Alamir, H. T. A., et al. (2024). Microwave-assisted synthesis, characterization, and in vitro biological evaluation of a novel nanocomposite using molybdenum and [2, 2′-bipyridine]-4, 4′-dicarboxylic acid. RSC Adv. 14, 24473–24482. doi:10.1039/d4ra03758h
Salama, H. E., and Aziz, M. S. A. (2020). Novel biocompatible and antimicrobial supramolecular O-carboxymethyl chitosan biguanidine/zinc physical hydrogels. Int. J. Biol. Macromol. 163, 649–656. doi:10.1016/j.ijbiomac.2020.07.029
Shehabeldin, A., Hussey, C., Aggad, R., and Truong, L. (2023). Increased diagnostic specificity of Congo red stain for amyloid: the potential role of Texas red–filtered fluorescence microscopy. Archives Pathology and Laboratory Med. 147, 907–915. doi:10.5858/arpa.2021-0512-oa
Skanda, S., Bharadwaj, P., Kar, S., Sai Muthukumar, V., and Vijayakumar, B. (2023). Bioremoval capacity of recalcitrant azo dye Congo red by soil fungus Aspergillus arcoverdensis SSSIHL-01. Bioremediation J. 27, 32–43. doi:10.1080/10889868.2021.1984198
Starko, I., Tatarchuk, T., Naushad, M., and Danyliuk, N. (2024). Enhanced activity of La-substituted nickel–cobalt ferrites in Congo red dye removal and hydrogen peroxide decomposition. Water, Air, and Soil Pollut. 235, 527. doi:10.1007/s11270-024-07329-5
Suksatan, W., Kazemzadeh, P., Afzali, D., Moghaddam-Manesh, M., Chauhan, N. P. S., and Sargazi, G. (2022). A controllable study on ultrasound assisted synthesis of a novel Ni/Zn based hybrid MOF nanostructures for Dextranase immobilization. Inorg. Chem. Commun. 139, 109410. doi:10.1016/j.inoche.2022.109410
Tkaczyk, A., Mitrowska, K., and Posyniak, A. (2020). Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: a review. Sci. total Environ. 717, 137222. doi:10.1016/j.scitotenv.2020.137222
Uddin, M. J., Ampiaw, R. E., and Lee, W. (2021). Adsorptive removal of dyes from wastewater using a metal-organic framework: a review. Chemosphere 284, 131314. doi:10.1016/j.chemosphere.2021.131314
Wang, W., Xue, C., and Mao, X. (2020). Chitosan: structural modification, biological activity and application. Int. J. Biol. Macromol. 164, 4532–4546. doi:10.1016/j.ijbiomac.2020.09.042
Yang, F., Dong, J., Li, Z., and Wang, Z. (2023). Metal–Organic frameworks (MOF)-Assisted sonodynamic therapy in anticancer applications. ACS nano 17, 4102–4133. doi:10.1021/acsnano.2c10251
Yao, T., Zeng, X., Tao, X., and Xu, H. (2024). Recent progress of MOF-based antibacterial hydrogels. Chem. Eng. J. 487, 150641. doi:10.1016/j.cej.2024.150641
Yu, K. L., Lee, X. J., Ong, H. C., Chen, W.-H., Chang, J.-S., Lin, C.-S., et al. (2021). Adsorptive removal of cationic methylene blue and anionic Congo red dyes using wet-torrefied microalgal biochar: equilibrium, kinetic and mechanism modeling. Environ. Pollut. 272, 115986. doi:10.1016/j.envpol.2020.115986
Zhang, X., Chen, Z., Liu, X., Hanna, S. L., Wang, X., Taheri-Ledari, R., et al. (2020). A historical overview of the activation and porosity of metal–organic frameworks. Chem. Soc. Rev. 49, 7406–7427. doi:10.1039/d0cs00997k
Zhao, H., Zhang, Y., Zhou, C., Zhang, C., and Liu, B. (2023). Engineering pH responsive carboxyethyl chitosan and oxidized pectin-based hydrogels with self-healing, biodegradable and antibacterial properties for wound healing. Int. J. Biol. Macromol. 253, 127364. doi:10.1016/j.ijbiomac.2023.127364
Zheng, Q., Li, Q., Tao, Y., Gong, J., Shi, J., Yan, Y., et al. (2023). Efficient removal of copper and silver ions in electroplating wastewater by magnetic-MOF-based hydrogel and a reuse case for photocatalytic application. Chemosphere 340, 139885. doi:10.1016/j.chemosphere.2023.139885
Zohud, A., and Alam, L. (2022). A review of groundwater contamination in West Bank, Palestine: quality, sources, risks, and management. Water 14, 3417. doi:10.3390/w14213417
Keywords: wastewater treatment, Congo red absorption, wastewater microbial strain, metal-organic frameworks, hydrogel, molybdenum
Citation: Ghnim ZS, Adhab AH, Altalbawy FMA, Salih Mahdi M, Salah Mansoor A, Radi UK, Saadoun Abd N, Abdul-Reda Hussein U, Hussain Hamza H, Muzammil K and Alkhayyat A (2025) Novel pectin/chitosan Mo-MOF hydrogel for dye adsorption and pathogenic bacteria inhibition. Front. Mater. 12:1535825. doi: 10.3389/fmats.2025.1535825
Received: 27 November 2024; Accepted: 10 March 2025;
Published: 26 March 2025.
Edited by:
Ahmed A. Abdala, Texas A&M University at Qatar, QatarReviewed by:
Feng Xue, Analog Devices Inc., United StatesCopyright © 2025 Ghnim, Adhab, Altalbawy, Salih Mahdi, Salah Mansoor, Radi, Saadoun Abd, Abdul-Reda Hussein, Hussain Hamza, Muzammil and Alkhayyat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmad Alkhayyat, YWhtZWRhbGtoYXl5YXQ4NUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.