- 1IMT Nord Europe, Centre for Materials and Processes, Institut Mines-Télécom, Univ. Lille, Lille, France
- 2ULR 4515 – LGCgE, Laboratoire de Génie Civil et Géo-Environnement, Institut Mines-Télécom, Université de Lille, Univ. Artois, Junia, Lille, France
Bio-based plastics represent an opportunity to reduce the impact of petroleum-based plastics on the environment, leading to harmful effects on both terrestrial and marine ecosystems. Nevertheless, the plant origin of bio-based plastics does not necessarily imply better management of their end of life. However, when recycling is impossible, the biological degradation of bio-based plastics would be an effective method to reduce their environmental impact. Polylactic acid (PLA) is one of the most produced biopolymers currently among the bio-based plastics already developed for several years. Thus, the objective of this article is to provide a state of the art on the biodegradation of bio-based plastics based on PLA. In particular, the microorganisms catalyzing the different biochemical reactions and the main biodegradation mechanisms are reviewed according to aerobic and anaerobic conditions. Moreover, different microorganisms involved in the degradation of PLA are summarized. Furthermore, a special attention is paid to the analytical methods to evaluate the biodegradation of polylactic acid and to the different existing biodegradation test methods, because this subject has rarely been reviewed in the literature. In the end, several promising topics for the future research are proposed, such as enzyme engineering technology as a recently emerging method for PLA degradation and a new common testing method to collect as much data as possible on the biodegradability to compare different studies.
1 Introduction
The widespread use of petroleum-based plastics usually named “plastics” leads to some major environmental issues. Indeed, their accumulation in the environment constitutes a major source of pollution since plastics take a lot of years to degrade and thus affect the terrestrial and marine ecosystems. Geyer et al. (2017) estimated that amongst the 8.3 × 109 tons of plastics that have been produced, 5 × 109 tons have been released into the environment. However, the accumulation of plastics which continues to increase due to the difficulty of their end-of-life treatment drastically reduces the use of plastics. Thus, if there is no reduction in the production of the latter and improvement in the management of plastic waste, it is estimated that in 2030, the quantity of these plastics entering the aquatic ecosystem will reach 90 × 106 tons per year (Borrelle et al., 2020). Moreover, micro- and nanoplastics (MNPs) pose a risk to human health because they accumulate in the human brain and the concentrations are rising over time (Campen et al., 2024). Several studies have shown a relationship among the increase of MNP concentrations in the environment, the presence of MNP concentrations in the human brain and the increase of the global rate of Alzheimer’s disease (van Bussel et al., 2017; Zhu et al., 2023).
Consequently, one of the challenges is to develop a better management of plastics from their production to their end of life (Hopewell et al., 2009). As the recycling of plastics is very limited given the cost generated, one of the solutions is to produce plastics that are more rapidly biodegraded by adding additives to improve the oxidation of the polymer at the end of their life. For example, pro-oxidants allow the more rapid cleavage of macromolecules induced by different environmental factors, such as oxygen, heat, visible light or ultraviolet (Sivan, 2011). Both fragmentation and biodegradation would reduce the persistence of plastics in the environment. Another attractive alternative to these petroleum-based plastics is to produce bio-based plastics from agricultural resources, such as starch, cellulose or molasses. These bio-based plastics are already used in the manufacturing of many cosmetic and industrial products and could limit the environmental consequences linked to the overconsumption of plastics. Nevertheless, only a part of bio-based plastics is biodegradable. A biodegradable plastic can be degraded by microorganisms (bacteria, fungi, algae, etc.) which are present in the environment. These microorganisms have the ability to use biodegradable plastics as a source of carbon and energy, which leads to the production of organic carbon (biomass) and inorganic carbon (CO2). Amongst biodegradable bio-based plastics, polylactic acid (PLA) that is one of the most produced bio-based plastics, is particularly interesting.
The objective of this article is to provide a state of the art on the biodegradation of bio-based plastics containing PLA. The research in the field is constantly evolving to the development of more biodegradable bioplastics. For example, Tosakul et al. (2024) developed packaging films by incorporating Para rubber (NR) and thermoplastic starch (TPS) into PLA to improve their mechanical properties (tensile toughness) and to significantly increase their degradation rate in a home-compost environment. There have been many review papers on the degradation of PLA in the literature (Anderson and Shive, 1997; Elsawy et al., 2017; Kervran et al., 2022; Zaaba and Jaafar, 2020; Pesaranhajiabbas et al., 2023; Rosli et al., 2021; Shalem et al., 2024). Different mechanisms of (bio) degradation of PLA have been described in these references. In the current work, all the biodegradation mechanisms are classified into two categories according to the degradation conditions, namely, aerobic and anaerobic conditions. In addition, the experimental methods for the quantitative assessment of biodegradation are presented with their advantages and disadvantages.
2 Origins and properties of bio-based plastics
A plastic material consists of polymer which has a structural unit repeating many times. The repeating units are monomers which are bonded together via covalent bonds to form a high molecular weight polymer consisting of long carbon chains. These polymers have varied structures, sizes and compositions, leading to specific properties. Owing to their bio-based nature, bio-based plastics can reduce the carbon footprint during their production, whereas the origin of most of plastics is petroleum, which significantly increases energy consumption and greenhouse gas emission during the materials production. Bio-based plastics can have different origins: a direct extraction from biomass (starch, cellulose, etc.) or a chemical synthesis from bio-based monomers, such as polylactic acid (PLA), polyethylene (bio-PE), polyethylene terephthalate (bio-PET) and polypropylene (bio-PP). Alternatively, they can be produced by microorganisms, as is the case of polyhydroxyalkanoates (PHAs). Bio-based plastics can have the same properties as their petroleum-based counterparts and contribute to reduce the dependence on fossil resources, since they are partially produced from renewable carbon. Nevertheless, some bio-based plastics are non-biodegradable (bio-PE, bio-PET, bio-PP), whereas others are biodegradable (mixtures of starch, PLA and PHAs). PHAs belong to the family of aliphatic polyesters with, as the most common compounds, poly-βhydroxybutyrate (PHB) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) (Bugnicourt et al., 2014). Biodegradable bio-based plastics have the benefit of reducing the carbon footprint and energy consumption during the whole life cycle.
3 Origins and properties of polylactic acid
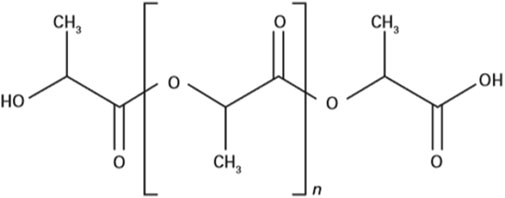
PLA is a linear aliphatic polyester synthesized by polycondensation of lactic acid produced from renewable resources by fermentation using facultative anaerobic bacteria (Lactococcus, Lactobacillus) from agricultural co-products rich in carbohydrates, such as starch, corn, wheat, sugar cane, beet, cellulose or molasses (Murariu and Dubois, 2016). So, it can be used without concern of its depletion. It consists of L and/or D monomeric units of lactic acid (C3H4O2) (Figure 1). The natural isomer is L-lactic acid, while D-lactic acid is a synthetic isomer (Penu and Helou, 2017). L-lactic acid can dimerize to form lactide (a cyclic dimer of lactic acid). Thus, PLA can be obtained by the direct polycondensation of L- or D-lactic acid or by the “ring opening polymerization” reaction of lactide. The “ring opening polymerization” reaction produces high molecular mass PLA (>100,000 Da) with different ratios of L-lactide or D-lactide (Boey et al., 2021). Lim et al. (2008) demonstrated that polymers consisting of more than 8% of D-lactide isomer tend to remain amorphous and that the crystallinity of the polymer tends to decrease even more as the stereoisomeric purity of the polymer decreases. It is also possible to obtain a high molecular weight PLA polymer directly by the condensation reaction of lactic acid following dehydration.
PLA is a compostable polymer which has excellent mechanical, structural, thermal and physical properties (Binti Taib et al., 2023). It is one of the best-selling bio-based plastics in the world, since it represents a very good alternative to polypropylene and polystyrene. The possibility of being extruded, injection molded and thermoformed allows it to be used for the manufacture of various products used in the agriculture, packaging and automotive industries. Ideally, PLA packaging is durable during its service life to optimize its application and has biodegradation properties at the end of life. PLA is also used in the medical industry for the manufacture of resorbable threads owing to its biocompatibility properties (da Silva et al., 2018). In fact, it has no toxic or carcinogenic effects on the human body (Abd Alsaheb et al., 2015). Another advantage of using PLA-based plastics is that its production requires less energy (25%–55%) than conventional petroleum-based plastics (Farah et al., 2016).
As PLA have some drawbacks (such as low elongation) that limit their applications, many researches have been conducted to develop a new material by blending PLA with other polymers, such as polybutylene adipate-co-terephtalate (PBAT) (Gonçalves et al., 2017). Various kinds of inorganic and organic fillers, such as mineral clays and natural fibers, have been used in PLA/PBAT biocomposite as reinforcement to improve their performance. The addition of nanofilers can improve the rheological and mechanical performances of these materials. Thanks to new scientific data in the synthesis and properties of these bioplastics, PBAT and PLA represent nearly 35% of the total production capacity of biodegradable polymers in 2023, with PLA having the highest production (31%) (Table 1) (Bioplastic Market Development update 2023, 2024). Given their growing use in different industrial applications and manufacturing technologies (e.g., injection molding, extrusion, film blowing, 3D printing, etc.), especially in the packaging industry, PBAT, PLA and their blends will remain among the most demanded biodegradable polymers in the market. According to the European Bioplastic, it was estimated that in 2028, the production of biodegradable bioplastics would increase from 52% to 62% to the detriment of biobased/non-biodegradable bioplastics (Table 1) (Bioplastic Market Development update 2023, 2024). Among biodegradable bioplastics, PLA remains the most produced biopolymer and its use is increasing significantly (44%) (Table 1) (Bioplastic Market Development update 2023, 2024).
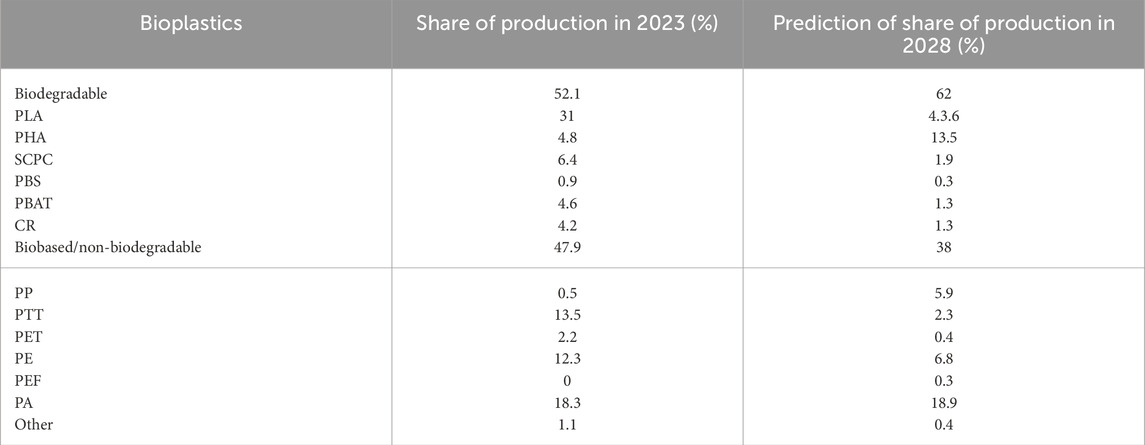
Table 1. Share of different kinds of bioplastics in 2023 and prediction in 2028 according to European Bioplastics in percent (Bioplastic Market Development update 2023, 2024).
4 Assessment of biodegradation of bio-based plastics
Bio-based plastics degrade in various environments, such as aerobic compost, soils or marine sediments (Kalita et al., 2019; Kalita et al., 2021a; Delacuvellerie et al., 2021; Weng et al., 2013). Currently, industrial composts constitute the main treatment for bio-based plastics. However, the mechanisms involved are not fully understood even in assays or tests performed in controlled conditions, since biodegradation is strongly linked to the environmental conditions. The microorganisms used in biodegradation assays are mainly collection microorganisms isolated from different environments. In most cases, they come from domestic or industrial composts (Sakai et al., 2001; Sedničkova et al., 2018), activated sludge from wastewater treatment plants recovering municipal or industrial wastes as well as digestates from anaerobic digestion plants (Pattanasuttichonlakul et al., 2018; Yagi et al., 2009). Some studies have also been carried out using soil samples (Karamanlioglu et al., 2014), sea water (Curto et al., 2021) or river water (Chiellini et al., 2007).
Standards have been developed by various international standardization organizations (e.g., ASTM International, JIS, ECN, OECD) (Eubeler et al., 2009), in order to assess the impact of the environmental conditions, such as soil, fresh water or sea water, domestic or industrial compost, digester (Table 2). These standards are based on reliable, repeatable and reproducible standardized tests (in solid and liquid phases), in relation to the microbial inoculum and the conditions (temperature, relative humidity) to carry out the tests. Analytical and testing standards define the methodology used to measure biodegradation. Conversely, specification standards set the biodegradation threshold to be achieved for a defined duration depending on the environment. The biodegradation yield must be greater than or equal to 90% in 6, 12 or 24 months for an industrial compost, a domestic compost or a soil, respectively.
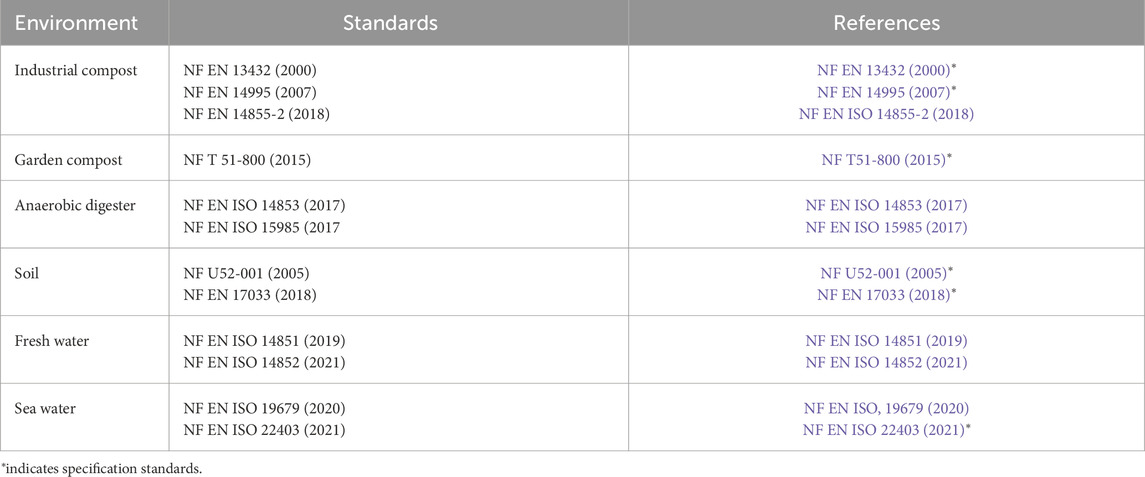
Table 2. Main European standards to assess the biodegradation of plastics in different environments.
5 Biodegradation mechanism of PLA-based polymers
The biodegradation of PLA-based polymers is a complex mechanism involving successive or concomitant physical, chemical and biological steps. The degradation of PLA-based polymers can occur by abiotic phenomena (photo-degradation, thermolysis, hydrolysis, oxidation) and/or biotic (enzymatic, biochemical) phenomena. Then, when the assimilation of degraded PLA-based polymers occurs, it corresponds to the integration by microorganisms of low molecular mass degradation residues (Figure 2). These mechanisms can be carried out not only in aerobic conditions, but also in anaerobic conditions with transformation of the polymer into biomass, water, and CO2 and CH4.
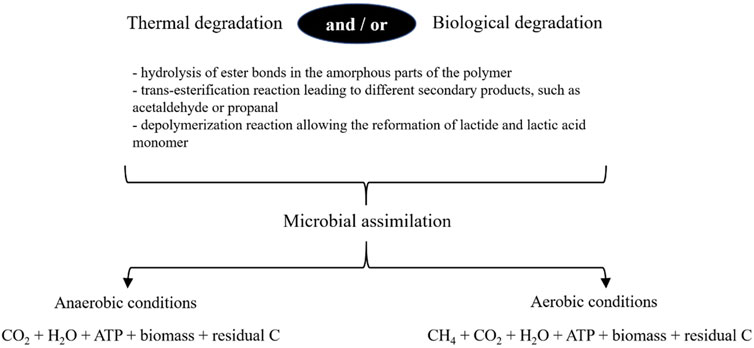
Figure 2. Mechanisms involved in the biodegradation of PLA-based polymers. CO2, carbon dioxide; CH4, methane; H2O, water; ATP, adenosine triphosphate; residual C, residual carbon.
5.1 Mechanisms of biodegradation under aerobic conditions
PLA shows various biodegradation rates depending on the environment. In ambient conditions encountered in soil or sewage, its biodegradation rate is low (Kale et al., 2007a). It is the same case in marine environments. Conversely, high biodegradation rate was observed in more severe conditions (temperature around 58°C–60°C) such as in industrial composting (Farah et al., 2016).
Biodegradation of PLA-based polymers under aerobic conditions takes place in two successive stages:
- A thermal degradation stage (abiotic) which does not require microorganisms, allowing the PLA to be degraded by hydrolysis;
- A stage of biological degradation, followed by microbial assimilation.
The first abiotic step corresponds to a hydrolysis reaction releasing oligomers, dimers and monomers from PLA. This step is most often carried out between 50°C and 70°C (thermal degradation) by three main reactions (Penu and Helou, 2017). Two reactions occur randomly at the polymer chain: a hydrolysis of ester bonds (R-COO-R′) of PLA induced by the diffusion of water molecules in the amorphous parts of the polymer, and the trans-esterification reaction leading to different secondary products, such as acetaldehyde or propanal. The third reaction that takes place only at the end of the chain, is a depolymerization reaction allowing the reformation of lactide and lactic acid monomers which are more easily degraded by microorganisms. Then, biological degradation extends the degradation of PLA with similar chemical reactions. Indeed, some enzymes secreted by microorganisms can also cut the ester bonds between lactic acid monomers. PLA-degrading enzymes from microorganisms mainly are proteases (serine proteases), and a few of them are lipases, esterases and cutinases (Qi et al., 2017; Xu et al., 2022a). Compared to proteases, lipases and cutinases are not enantio-selective. Some enzymes randomly cut the chain, with the release of polymers having a lower molecular mass. It is mainly proteinase K that causes the breakage of the ester bonds within the polymer. Other enzymes hydrolyse only the ester bonds present at the end of the chain. Usually, optimal conditions for the enzymatic activity are an alkaline pH and a temperature of 37°C. However, Cui et al. (2022a) showed that depolymerization activity was maintained at temperatures up to 50°C, resulting in a high thermal stability of enzymes as it is Proteinase K. The residues obtained have a lower molecular mass and can be assimilated by microorganisms once they have been transported into bacterial cells. Consequently, high molecular weight PLA-based polymers have a lower degradation rate than low molecular weight polymers (Tokiwa et al., 2009). Indeed, the higher the molecular mass of the polymer, the more difficult it will be to degrade it into small compounds easily assimilated by microorganisms.
In addition, the rate of degradation of a polymer depends on the structure of its chains and its percentage of crystallinity (Richert and Dąbrowska, 2021). In fact, water molecules diffuse more easily into the amorphous part of the polymer, which will then be more easily degraded than the crystalline part. Since water is an essential element for the microbial growth, the water absorption by the polymer increases the microbial degrading activity and the microbial attack in the amorphous phase. Thus, the crystalline part becomes more predominant as biodegradation progresses. Therefore, the degradation rate of PLA increases with its low crystallinity (Tsuji and Miyauchi, 2001). The rate of biodegradation also depends on the shape and size of the polymer samples, which determines the surface area of the sample. Thus, thin polymer strips have faster biodegradation kinetics than thicker discs made with the same polymer (Al Hosni et al., 2019). Temperature is also a very important factor for the degradation phenomenon in conjunction with the glass transition temperature of polymer. It corresponds to the transition temperature from a glassy state to a rubbery state resulting from the breakdown of “weak bonds,” such as hydrogen bridges or van der Walls bonds between the different polymer chains (Auras et al., 2004). Temperatures close to the glass transition temperature make degradation easier, since the ester bonds can break more easily. In the case of PLA-based polymers, the glass transition temperature is 58°C for amorphous PLA and 62°C for semi-crystalline PLA. Thus, thermophilic conditions improve the PLA biodegradation rate compared to lower temperatures around 37°C (Itävaara et al., 2002). Durairaju et al. (2024) have developed a method for assessing the biodegradation of PLA in vitro based on clear zone method (Shah et al., 2014) that was improved by complementary analyses. Agar plates were emulsified with PLA oligomers and then inoculated by Bacillus licheniformis (spores and vegetative cells). PLA biodegradation was determined by the dosage of lactic acid and by the monomerization of the residual PLA oligomers obtained by a chemical hydrolyzation (Durairaju et al., 2024). The absence of free lactic acid monomers in all the clarified plates demonstrated that they were completely consumed by Bacillus licheniformis. The results indicated that PLA oligomers were biodegraded up to 70% by weight in 30 days at 37°C.
It has been reported that PLA-based polymers can be completely degraded when the compost reaches 60°C (Pranamuda and Tokiwa, 1999). Several conditions were tested, including aerobic conditions that lead to 90% transformation of PLA into CO2 in compost at 58°C ± 2°C after 70 days, according to the standard ISO 14855–2 biodegradation test (Funabashi et al., 2009). A complete degradation of PLA beverage cups was observed after 15 days in soil with dairy wastewater sludge under thermophilic conditions when PLA was initially subjected to an ultraviolet (UV) treatment (Pattanasuttichonlakul et al., 2018). The addition of Pseudomonas geniculata WS3 significantly increased the PLA biodegradation, indicating a synergetic action of a microbial consortium in the soil mixture and this bacterial strain. The same observations were carried out when a consortium of PLA-degrading microorganisms was added (Qi et al., 2017). The addition of soybean combined with P. geniculata WS3, manure extract or wastewater sludge extract as the indigenous microbial consortium, significantly increased PLA weight loss percentage and content into lactic acid (Boonluksiri et al., 2021). Thus, soybean is an excellent source of nitrogen making it possible to increase the activity of PLA-degrading bacteria and the biodegradation rate of PLA in soil. The beneficial impact of a source nitrogen is also observed when directly added into the biopolymer. Kalita et al. (2021b) showed that the algal biomass containing high elementary nitrogen content accelerated the degradation process of PLA/algae bio-plastics (5-wt% of algae biomass in PLA) under thermophilic composting conditions (58°C ± 2°C). Plants can also be involved in the biodegradation of PLA. Miscanthus, in contaminated soils by plastic waste, accelerated the biodegradation of PLA and PET (polyethylene terephthalate) resulting partly from a higher quantity of microorganisms at the level of the rhizosphere (Janczak et al., 2018).
The biodegradation of PLA-based polymers can also be amplified in the presence of a source of carbon such as glucose (Jang et al., 2002). This compound constitutes an additional source of carbon that is easily assimilated by microorganisms providing energy for the growth of microorganisms. A significant increase in the degradation rate of bio-based plastics has already been observed with a glucose concentration of 0.1% (wt) (Jang et al., 2002). Nevertheless, there exists a threshold glucose concentration as glucose concentration of 5% (wt) is ineffective (Jang et al., 2002). Glucose in starch, which is an extremely efficient carbon source, enhanced the biodegradability of the PLA/starch blends (Lv et al., 2017). The same tendency was obtained by adding gelatin. Guzman-Sielicka et al. (2013) developed a new blend based on PLA/starch/gelatin that could be used for the manufacture of short-shelf-life packaging materials. The effect of the source of carbon will mainly depend on the microflora of the environment in which biodegradation occurs. For example, it was reported that the addition of gelatin in the soil did not improve the biodegradability of the PLA in the specific soil used in that study (Munoz et al., 2024). The addition of plasticizers to improve the flexibility of PLA, such as polyethylene glycol (PEG), also increases the degradation rate of PLA (Xue et al., 2013). Indeed, low molecular weight hydrophilic plasticizers readily dissolve into the medium from the blend, increasing the accessible volume and allowing the entry of water and microorganisms.
PLA/PBAT-based materials, newly introduced in the market, also have the potential to biodegrade in a compost environment (Tabasi and Ajji, 2015). However, the incorporation of other additives into PLA/PBAT blends may affect biodegradability of blends by different mechanisms. In particular, the incorporation of compatibilizers could significantly decrease the biodegradation rate of PLA/PBAT-based materials by generating species with a higher molecular weight, which can make a barrier against water diffusion and hydrolysis of the material (Freitas et al., 2017). Given the limited data available in this field, further research is needed to study the effect of compatibilizers, fillers and other additives used in these blends on their biodegradation in different environments.
5.2 Mechanisms of biodegradation under anaerobic conditions
Anaerobic digestion is a very promising alternative for managing biodegradable plastic wastes. Currently, research about the degradation of biodegradable plastics in anaerobic conditions has mainly been carried out at laboratory scale with very little data at pilot or industrial scales (Ruggero et al., 2019). Anaerobic digestion is a biological process, during which organic matter can be transformed into biogas (CO2 and CH4) in the absence of oxygen. This biogas can then be valorised by producing heat and electricity using a cogeneration system. Methane can also be used directly as a biofuel (Sahota et al., 2018). This bioprocess can be used to treat different types of organic waste, such as agricultural and agri-food wastes, activated sludges, and organic municipal wastes (Meyer-Kohlstock et al., 2016). Moreover, anaerobic digestion produces a digestate rich in nutrients that can be mineralized under aerobic conditions. The product obtained can then be used as fertilizer in agriculture (Sheets et al., 2015). It is therefore important to ensure that the digestate can be spread on soil, by evaluating the potential toxicity of (micro)plastic residues on terrestrial and/or marine organisms (Zimmermann et al., 2020; Fojt et al., 2020).
Anaerobic digestion mainly takes place at two temperature ranges: 35°C–38°C and 55°C–58°C. Several strategies can improve the digestion kinetics, such as the use of a pre-treatment, the incorporation of additives into polymers (fibers, enzymes, calcium carbonate) or the acclimation of the microbial inoculum (Calabro et al., 2020; Ryan et al., 2018). Indeed, pretreatment, in particular at 90°C for 48 h at pH 10, allows to reduce the molecular mass of PLA by hydrolysis leading to a better efficiency of the degrading microorganisms (Benn and Zitomer, 2018). Digestion at high temperature (55°C–58°C) has shown better conversion of PLA to methane: 60, 80% and 90% of PLA were biodegraded in 30, 40 and 60 days, respectively (Yagi et al., 2009). A significant biodegradation of PLA (86%–100%) was also observed in commercial PLA-based disposable cups and plates under thermophilic conditions at different inoculum–substrate ratio and material sizes (Bracciale et al., 2024). In mesophilic conditions (35°C–38°C), PLA is little degraded or even undegraded. For example, PLA conversion to biomethane was not detected in mesophilic anaerobic digester after 170 days (Kolstad et al., 2012). Anaerobic degradability tests on commercial bioplastic bags of polybutylene adipate-co-terephtalate (PBAT) or PLA/PBAT carried out under mesophilic conditions (Alvarez-Mendez et al., 2023) also showed that the bags were hardly biodegradable after anaerobic digestion. Although biodegradation was detected mostly in the PLA fraction, none of bags met the NF EN 13432 standard (NF EN 13432, 2000). Finally, a moderate biomethane production was reported by Yagi et al. (2009, 2014) (Yagi et al., 2009; Yagi et al., 2014): 12% at 77 days, 23% at 182 days, and up to 49% after 277 days. Thus, the duration of the experiment is an important factor to consider as along with the C/N ratio of the substrate which influences the performance of anaerobic digestion. To obtain optimal anaerobic digestion, it should lie between 20/1 and 30/1. However, for food waste and urban sludge which are rich in protein, this ratio should be between 6/1 and 16/1. The addition of biodegradable bioplastics, containing more carbon than nitrogen, can therefore increase the C/N ratio, allowing a more stable process in the long term (Benn and Zitomer, 2018).
6 Microorganisms degrading polylactic acid
The biodegradation of PLA has been studied mainly in industrial compost under aerobic conditions (Kale et al., 2007b; Castro-Aguirre et al., 2018; Stloukal et al., 2015) or under anaerobic (Bracciale et al., 2024; Gómez and Michel, 2013) conditions at mesophilic or thermophilic temperatures. Most of the isolated microbial strains, capable of degrading PLA, were obtained from consortia from soils, composts, sludge from wastewater treatment plants and/or methanizers (Table 3). They mainly belong to bacteria of the Actinomycetes class (filamentous bacteria), but some fungal strains also have this capacity. The first strain of Actinomycetes isolated from soil and capable of degrading PLA is Amycolatopsis HT-32 (Pranamuda et al., 1997). Identification based on 16S rRNA sequencing revealed the presence of other genera belonging to the Pseudonocardiaceae family, such as Saccharothrix, Lentzea, Kibdelosporangium and Streptoalloteichus (Jarerat et al., 2002). Then, the development of molecular biology techniques made it possible to reference other strains of Actinomycetes degrading PLA, such as Streptomyces, Laceyella, Pseudonocardia (Table 3). Other bacterial genera, such as Geobacillus, Bacillus, Pseudomonas and Stenotrophomonas, can also be involved in the degradation of PLA under aerobic conditions (Table 3). Moreover, some bacteria are involved in the digestion of PLA under anaerobic conditions (Table 3): Xanthomonadaceae bacterium, Mesorhizobium sp. and Ureibacillus sp. (Yagi et al., 2014). The importance of the genus Tepidimicrobium, and more particularly Tepidimicrobium xylanilyticum, was reported in the digestion of PLA (Tseng et al., 2020). In fact, several bacterial strains of the genus Tepidimicrobium were involved in the anaerobic digestion (at high temperatures) of coffee capsules made of biodegradable polymers (Cazaudehore et al., 2021). As these capsules contain a mixture of polymers, genus Tepidimicrobium is probably able to biodegrade polymers other than PLA. Tepidimicrobium is therefore the dominant genus capable of degrading PLA under anaerobic conditions. On the other hand, the degradation of PLA by fungal strains has been reported in the literature. Some researchers explored the fungal degradation of PLA in soil and compost under aerobic conditions and isolated fungal strains belonging to the genera Aspergillus, Thermomyces, Cladosporium, Penicillium and Rhodotorula (Karamanlioglu et al., 2014; Nair et al., 2016). Strains degrading PLA are mainly effective at a temperature range between 50°C and 70°C, but some strains can be active at lower temperatures close to 30°C (Table 3).
Microbial communities develop on the surface of PLA-based biopolymers, mostly in the form of a biofilm. The latter is a viscous layer made up of microorganisms coated with extracellular polymeric substances (EPS), excreted by microbial cells (Walczak et al., 2015). The composition of the microbial communities evolves with the time of immersion of the biopolymer (De Tender et al., 2017). It also depends on the environmental conditions in which the biopolymer samples are placed. In the marine environment, the microbial communities of the biofilm present on floating plastics are made up of a much higher proportion of Rhodobacteraceae and Actinomycetaceae compared to those that are developed on the surface of sedimented plastics. On the other hand, the communities of the biofilm at the surface of sedimented plastics contain a greater proportion of Pseudoalteromonadaceae and Shewanellaceae (Delacuvellerie et al., 2021). Some specific tests have been performed in order to accelerate the biodegradation of PLA or to improve the rate of PLA biodegradation (Castro-Aguirre et al., 2018). For example, the compost containing PLA coupons (1 × 1 cm2) was inoculated with one or more specific microbial strains capable of degrading PLA. This requires re-culturing the pure strains in a liquid nutrient medium (such as R2B medium) under the optimal conditions for growth of these bacteria (stirring at 58°C), in order to obtain a bacterial culture at the end of growth phase (beginning of the plateau of the number of bacterial cells after the exponential growth phase). This culture is then used to inoculate the bioreactor containing the compost with the PLA films, by adding bacterial cells equivalent to 1% of the total bacterial community (108 bacterial cells/g of compost) present in the bioreactor. The results indicated that the biodegradation of PLA films occurred in times comparable to organic waste.
7 Analytical methods to assess the biodegradation of PLA
7.1 Morphological analyses by macroscopic or microscopic observations
The biodegradation process induces some modifications of both optical and mechanical properties of PLA. For example, cracks or discoloration may be visible at the surface of PLA-based polymer samples (Janczak et al., 2020). Moreover, amorphous PLA and crystalline PLA are likely to biodegrade differently leading to specific patterns of degradation (Pantani and Sorrentino, 2013). Amorphous PLA had a spongy appearance while crystalline PLA had a more compact appearance whereas the initial PLA had a smooth surface (Pantani and Sorrentino, 2013; Leejarkpai et al., 2011). These observations support the fact that amorphous PLA is more easily degraded than crystalline PLA.
7.2 Mass loss measurements
Mass loss between samples before and after biodegradation is a simple measure to evaluate the degree of polymer biodegradation as it does not require specific equipment (Apinya et al., 2015). The polymer samples are weighed before and after the biodegradation test. Nevertheless, a sample drying step is necessary to avoid biasing the results. The percent mass loss (ML) of the polymer is calculated using the following equation (Equation 1):
with, mi the initial mass of the polymer, mf the final mass after biodegradation.
7.3 Respirometry in aerobic conditions
During the biodegradation of polymers in aerobic conditions (with enough oxygen available in the environment), the polymers are transformed into CO2 and H2O by microorganisms following a succession of biochemical reactions using oxygen as an electron acceptor. OxiTop (WTW, Weilheim, Germany) heads inserted at the opening of the reactors allow to continuously monitor O2 consumption, resulting in a reduction in pressure inside the reactor. This is only possible as the released CO2 is trapped in a solution of sodium or potassium hydroxide (NaOH or KOH) contained in a beaker suspended in the reactor. The amount of produced CO2 is measured using a solution of hydrochloric acid in the presence of phenolphthalein at the end of the experiment (Kolstad et al., 2012). It can be noticed that a nitrification inhibitor must be added to nitrogen-rich environment, in order to inhibit the oxidation of nitrogen compounds.
This method makes it possible to monitor the biochemical oxygen demand (BOD) of microorganisms over time (Walczak et al., 2015). It allows to determine the percentage of biodegradation using the quantity of CO2 released (Pattanasuttichonlakul et al., 2018).
The aerobic biodegradation rate (BR) is calculated with respect to a control (reactor not containing a sample) according to Equation 2 (Kale et al., 2007b):
where CO2 is the quantity of carbon dioxide released (g), CO2b the quantity of carbon dioxide released in the control (g), m the mass of the sample (g) and C the organic carbon content (%) of the sample.
The concentration of CO2 released is determined according to Equation 3 (Kolstad et al., 2012):
where V is the volume of HCl (mL) and C the concentration of the HCl solution (g/L).
7.4 Respirometry in anaerobic conditions
The biodegradability of polymers under anaerobic conditions results in the transformation of complex molecules into simple molecules (organic acids such as acetic, butyric and propionic acids) which leads to the formation of methane (CH4) and carbon dioxide (CO2). The biodegradation of a polymer sample can be evaluated in the laboratory by monitoring the production of methane, in order to evaluate its methanogenic potential (BMP) (Cazaudehore et al., 2022). For these laboratory tests, the substrate is first mixed with a culture of anaerobic bacteria, generally from an anaerobic digester. Then, the mixture is stirred in a sealed bottle at a given temperature. The amount of methane generated from the substrate is then measured and the biochemical methane potential of the sample can be determined in g CH4/g of added volatiles. Several devices can be used to monitor the methane produced: volume displacement device, pressure gauge or pressure transducer (Filer et al., 2019). The generation of methane can also be measured by analytical techniques, such as gas chromatography. The anaerobic biodegradation rate (ABR) of a PLA-based polymer is calculated using Equation 4:
where EMP is the experimental methane production and TMP the theoretical methane production.
For an organic compound of formula CxHyOzNnSs, the equivalent theoretical methane production (TMP) is calculated using Equation 5:
7.5 Size exclusion chromatography
Size exclusion chromatography (SEC) is used to determine the molecular weight of PLA-based polymer samples before and after biodegradation. SEC is based on a separation of compounds according to their size during their passage through an exclusion gel. Smaller molecules can penetrate the pores of the exclusion gel particles, whereas large molecules cannnot. Thus, the smaller molecules will be retained on the exclusion gel and the larger ones will be quickly carried away by the mobile phase. This will therefore induce a difference in the elution time of molecules depending on their molecular weight. The device used is a liquid chromatograph equipped with a refractive index (RI) detector, with tetrahydrofuran (THF) as mobile phase (containing 2% by weight of triethylamine). A polystyrene standard (0.5–2,480 kDa) is generally used (Nair et al., 2016).
7.6 Infrared spectrometry
Infrared spectroscopy consists of measuring the light absorbed by a solid, a liquid or a gas sample, at several wavelengths contained in the infrared spectrum. In the case of PLA biodegradation, infrared spectroscopy allows to monitor the evolution of the chemical groups present in the polymer. Indeed, as presented previously, PLA polymers are made up of polylactic acid monomers linked by ester bonds. During the hydrolysis of these bonds, the formed hydroxyl groups (-OH) can be detected by the appearance of the peak characterizing these groups at 3,340 cm−1 (Kalita et al., 2019). The surface spectra of PLA samples can be obtained by Fourier Transform InfraRed (FTIR) spectroscopy using the Attenuated Total Reflectance (ATR) technique.
7.7 Differential scanning calorimetry
Differential scanning calorimetry (DSC) is a thermal analysis, which allows monitoring the glass transition and crystallinization. These parameters are determined by the difference in heat exchange between the sample to be tested and a reference compound (indium standard). The sample to be analyzed is heated up to 200°C with a heating rate of 10°C/min under a nitrogen atmosphere. DSC enables us to determine the melting temperature (Tm), the glass transition temperature (Tg), the crystallization temperature (Tc), the enthalpy of fusion (ΔHm) and the enthalpy of crystallization (ΔHc) (Delacuvellerie et al., 2021).
Carrying out DSC experiments on the PLA-based polymer sample before and after biodegradation gives valuable information on the impact of biodegradation on the crystallinity and melting temperature. If the PLA-based polymer is degraded, Tg decreases whereas crystallinity increases. This result is mainly the consequence of the quicker degradation of the amorphous PLA than the crystalline PLA. The percentage of crystallinity of the polymer can be determined using Equation 6 (Benali et al., 2015):
where
8 Perspectives and future directions
Composting is one of the most efficient biodegradation methods for biopolymers. However, its efficiency depends on the environmental conditions (temperature, humidity, etc.), because these parameters strongly impact the microbial activity into the compost. So, the relevant parameters must be controlled to avoid a variability in the biodegradation yield. Moreover, the composition of compost also plays an important role in the biodegradation process, in particular its content in degrading microorganisms and the presence of carbon sources easily assimilated by microorganisms. Therefore, further research should be conducted to determine the composition of the compost and the environmental conditions that allow for optimized biodegradation of a given bioplastic.
Much research has been conducted to develop PLA-based biopolymers by mixing them with other constituents to improve their specific properties and increase their biodegradation rate. However, few studies have collected data on the effects of these additives to explain the improvement in the biodegradation rate. To progress in the development of new eco-friendly plastics, it is necessary to better understand the mechanisms involved by studying in more detail the morphology, water absorption, hydrophily and structural properties of PLA blends to predict their biodegradation in a given environment.
Significant advances have been made on the biodegradation of PLA, highlighting the vital role of enzymes from microorganisms in biodegradation of PLA. The use of enzymes instead of microbial cells would allow to avoid the assimilation phase of lactic acid products by microorganisms. The released lactic acid could be directed towards repolymerization to manufacture new PLA or towards other applications, thus ensuring a circular economy (Acosta and Alper, 2023). The use of enzymes for PLA degradation is still emerging. Enzyme engineering technology would improve the performance of enzymatic degradation. In addition, the isotopic tracing and fluorescent labelling method would allow new advances to better understand the catalytic mechanism.
9 Conclusion
Bio-based plastics offer many environmental benefits as a replacement for petroleum-based plastics. However, the sole reduction of greenhouse gas emissions (mainly carbon dioxide) is not the major characteristic for putting an end to the accumulation of plastics in ecosystems leading to major pollution of the nature. Indeed, plastics of biological origin should have a much higher rate of biodegradation than that of plastics from petroleum. Plastics based on PLA, which represents around a quarter of the production of bio-based plastics, have this property of biodegradability. The reported bibliographic review dedicated to PLA-based plastics highlights that there is a good understanding of the mechanisms involved in their biodegradation both in aerobic and anaerobic environments. Thus, using PLA-degrading enzymes is very effective process in order to increase the biodegradation rate. Moreover, several analytic techniques make it possible to properly characterize the effect of biodegradation on their properties. It has been demonstrated that the biodegradation rate appears to be higher for the amorphous part of the polymer compared to the crystalline part. Blends of PLA with other polymers or sources of carbon also impact the biodegradation rate. Generally, a source of carbon easily assimilated by microorganisms will amplify the biodegradation rate whereas the incorporation of compatibilizers could significantly decrease the biodegradation rate in the case of PLA/PBAT-based materials.
In addition, there is a whole normative context which makes it possible to frame validation tests according to the environment in which bio-based plastics could be found. It has thus been demonstrated that PLA-based plastics have good biodegradability in industrial compost under aerobic conditions but that biodegradation in a marine environment or in domestic compost is less effective. In this regard, the biodegradation of PLA in marine or domestic environments should be the focus on the future research, as microplastics in such environments are a big issue to address nowadays. On the other hand, anaerobic digestion, i.e., a thermophilic and anaerobic process, also seems to be a promising alternative to the biodegradation of bio-based plastics leading to the production of biogas that can be converted into energy. However, a deeper knowledge of the PLA-degrading microorganisms found in different environments and of the corresponding biodegradation mechanisms involved is still necessary to develop even more effective strategies leading to the reduction of the duration of the biodegradation process. Nevertheless, despite the existence of standards for plastic degradation testing, a common testing procedure should be developed to collect as much data as possible on the biodegradability of bioplastics and to make cross-comparisons between studies.
Author contributions
CL: Conceptualization, Formal Analysis, Writing–original draft, Writing–review and editing. PL: Methodology, Writing–original draft. CP: Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd Alsaheb, R. A., Aladdin, A., Othman, N., Malek, R., Leng, O. M., Aziz, R., et al. (2015). Recent applications of polylactic acid in pharmaceutical and medical industries. J. Chem. Pharm. Res. 7 (12), 51–63. doi:10.3390/polym14091874
Acosta, D. J., and Alper, H. S. (2023). Advances in enzymatic and organismal technologies for the recycling and upcycling of petroleumderived plastic waste. Curr. Opin. Biotechnol. 84, 103021–103029. doi:10.1016/j.copbio.2023.103021
Akutsu-Shigeno Yukie, Y., Teeraphatpornchai, T., Teamtisong, K., Nomura, N., Uchiyama, H., Nakahara, T., et al. (2003). Cloning and sequencing of a poly(DL-lactic acid) depolymerase gene from Paenibacillus amylolyticus Strain TB-13 and its functional expression in Escherichia coli. Appl. Environ. Microbiol. 69, 2498–2504. doi:10.1128/AEM.69.5.2498–2504.2003
Al Hosni, A. S., Pittman, J. K., and Robson, G. D. (2019). Microbial degradation of four biodegradable polymers in soil and compost demonstrating polycaprolactone as an ideal compostable plastic. Waste Manag. 97, 105–114. doi:10.1016/j.wasman.2019.07.042
Alvarez-Mendez, S. J., Ramos-Suarez, J. L., Ritter, A., Gonzalez, J. M., and Perez, A. C. (2023). Anaerobic digestion of commercial PLA and PBAT biodegradable plastic bags: potential biogas production and 1H NMR and ATR-FTIR assessed biodegradation. Heliyon 9, e16691. doi:10.1016/j.heliyon.2023.e16691
Anderson, J. M., and Shive, M. S. (1997). Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 28 (1), 5–24. doi:10.1016/S0169-409X(97)00048-3
Apinya, T., Sombatsompop, N., and Prapagdee, B. (2015). Selection of a Pseudonocardia sp. RM423 that accelerates the biodegradation of poly(lactic) acid in submerged cultures and in soil microcosms. Int. Biodeterior. Biodegr. 99, 23–30. doi:10.1016/j.ibiod.2015.01.001
Arena, M., Abbate, C., Fukushima, K., and Gennari, M. (2011). Degradation of poly(lactic acid) and nanocomposites by Bacillus licheniformis. Environ. Sci. Pollut. Res. 18, 865–870. doi:10.1007/s11356-011-0443-2
Auras, R., Harte, B., and Selke, S. (2004). An Overview of polylactides as packaging materials. Macromol. Biosci. 4, 835–864. doi:10.1002/mabi.200400043
Benali, S., Aouadi, S., Dechief, A.-L., Murariu, M., and Dubois, P. (2015). Key factors for tuning hydrolytic degradation of polylactide/zinc oxide nanocomposites. Nanocomposites 1, 51–61. doi:10.1179/2055033214Y.0000000007
Benn, N., and Zitomer, D. (2018). Pretreatment and anaerobic co-digestion of selected PHB and PLA bioplastics. Front. Environ. Sci. 5, 93. doi:10.3389/fenvs.2017.00093
Binti Taib, N.-A. A., Rahman, Md R., Huda, D., Kuok, K., Hamdan, S., Bin Bakri, M. K., et al. (2023). A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 80, 1179–1213. doi:10.1007/s00289-022-04160-y
Bioplastic Market Development update 2023 (2024). European bioplastics. Available at: https://www.european-bioplastics.org/bioplastics-market-development-update-2023-2/ (Accessed October 20, 2024).
Boey, J. Y., Mohamad, L., Khok, Y. S., Tay, G. S., and Baidurah, S. (2021). A review of the applications and biodegradation of polyhydroxyalkanoates and poly(lactic acid) and its Composites. Polymers 13, 1544. doi:10.3390/polym13101544
Boonluksiri, Y., Prapagdee, B., and Sombatsompop, N. (2021). Promotion of polylactic acid biodegradation by a combined addition of PLA-degrading bacterium and nitrogen source under submerged and soil burial conditions. Polym. Degrad. Stab. 109562, 109562. doi:10.1016/j.polymdegradstab.2021.109562
Borrelle, S. B., Ringma, J., Law, K. L., Monnahan, C. C., Lebreton, L., McGivern, A., et al. (2020). Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 369, 1515–1518. doi:10.1126/science.aba3656
Bracciale, M. P., De Gionnis, G., Falzarano, M., Muntoni, A., Polettini, A., Pomi, R., et al. (2024). Anaerobic biodegradation of disposable PLA-based products: assessing the correlation with physical, chemical and microstructural properties. J. Hazard. Mater. 452, 131244. doi:10.1016/j.jhazmat.2023.131244
Bubpachat, T., Sombatsompop, N., and Prapagdee, B. (2018). Isolation and role of polylactic acid-degrading bacteria on degrading enzymes productions and PLA biodegradability at mesophilic conditions. Polym. Degrad. Stab. 152, 75–85. doi:10.1016/j.polymdegradstab.2018.03.023
Bugnicourt, E., Cinelli, P., Lazzeri, A., and Alvarez, V. (2014). Polyhydroxyalkanoate (PHA): review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 8 (11), 791–808. doi:10.3144/expresspolymlett.2014.82
Calabro, P. S., Folino, A., Fazzino, F., and Komilis, D. (2020). Preliminary evaluation of the anaerobic biodegradability of three biobased materials used for the production of disposable plastics. J. Hazard. Mater. 390, 121653. doi:10.1016/j.jhazmat.2019.121653
Campen, M., Nihart, A., Garcia, M., Liu, R., Olewine, M., Castillo, E., et al. (2024). Bioaccumulation of microplastics in decedent human brains assessed by pyrolysis gas chromatography-mass spectrometry. Res. Square. doi:10.21203/rs.3.rs-4345687/v1
Castro-Aguirre, E., R. Auras, R., Selke, S. M., Rubino, M., and Marsh, T. (2018). Enhancing the biodegradation rate of poly(lactic acid) films and PLA bio-nanocomposites in simulated composting through bioaugmentation. Polym. Degrad. Stab. 154, 46–54. doi:10.1016/j.polymdegradstab.2018.05.017
Cazaudehore, G., Guyoneaud, R., Evon, P., Martin-Closas, L., Pelacho, A. M., Raynaud, C., et al. (2022). Can anaerobic digestion be a suitable end-of-life scenario for biodegradable plastics? A critical review of the current situation, hurdles, and challenges. Biotechnol. Adv. 56, 107916. doi:10.1016/j.biotechadv.2022.107916
Cazaudehore, G., Monlau, F., Gassie, C., Lallement, A., and Guyoneaud, R. (2021). Methane production and active microbial communities during anaerobic digestion of three commercial biodegradable coffee capsules under mesophilic and thermophilic conditions. Sci. Total Environ. 784, 146972. doi:10.1016/j.scitotenv.2021.146972
Chiellini, E., Corti, A., and D’Antone, S. (2007). Oxo-biodegradable full carbon backbone polymers - biodegradation behaviour of thermally oxidized polyethylene in an aqueous medium. Polym. Degrad. Stab. 92, 1378–1383. doi:10.1016/j.polymdegradstab.2007.03.007
Cui, L., Wang, X., Szarka, G., Hegyesi, N., Wang, Y., Sui, X., et al. (2022a). Quantitative analysis of factors determining the enzymatic degradation of poly(lactic acid). Int. J. Biol. Macromol. 209, 1703–1709. doi:10.1016/j.ijbiomac.2022.04.121
Curto, M., M., Le Gall, M., Catarino, A. I., Niu, Z., Davies, P., Everaert, G., et al. (2021). Long-term durability and ecotoxicity of biocomposites in marine environments: a review. RSC Adv. 11, 32917–32941. doi:10.1039/d1ra03023j
da Silva, D., Kaduri, M., Poley, M., Adir, O., Krinsky, N., Shainsky-Roitman, J., et al. (2018). Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 340, 9–14. doi:10.1016/j.cej.2018.01.010
Delacuvellerie, A., Benali, S., Cyriaque, V., Moins, S., Raquez, J.-M., Gobert, S., et al. (2021). Microbial biofilm composition and polymer degradation of compostable and non-compostable plastics immersed in the marine environment. J. Hazard. Mater. 419, 126526. doi:10.1016/j.jhazmat.2021.126526
De Tender, C., Schlundt, C., Devriese, L. I., Mincer, T. J., Zettler, E. R., and Amaral-Zettler, L. A. (2017). A review of microscopy and comparative molecular-based methods to characterize “Plastisphere” communities. Anal. Methods 9, 2132–2143. doi:10.1039/c7ay00260b
Durairaju, P., Bouarab, L., Cottaz, A., Planchon, S., Oulahal, N., and Joly, C. (2024). Method for assessing the biodegradation of poly(lactic acid) in vitro (on agar plates): application using PLA oligomers and Bacillus licheniformis vegetative cells or spores. Polym. Test. 132, 108345. doi:10.1016/j.polymertesting.2024.108345
Elsawy, M. A., Kim, K. H., Park, J. W., and Deep, A. (2017). Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 79, 1346–1352. doi:10.1016/j.rser.2017.05.143
Eubeler, J. P., Zok, S., M. Bernhard, M., and Knepper, T. P. (2009). Environmental biodegradation of synthetic polymers I. Test methodologies and procedures. Trac. Trends Anal. Chem. 28, 1057–1072. doi:10.1016/j.trac.2009.06.007
Farah, S., Anderson, D. G., and Langer, R. (2016). Physical and mechanical properties of PLA, and their functions in widespread applications - a comprehensive review. Adv. Drug. Deliv. Rev. 107, 367–392. doi:10.1016/j.addr.2016.06.012
Filer, J., Ding, H. H., and Chang, S. (2019). Biochemical methane potential (BMP) assay method for anaerobic digestion research. Water 11, 921. doi:10.3390/w11050921
Fojt, J., David, J., Přikryl, R., Řezáčová, V., and Kučerík, J. (2020). A critical review of the overlooked challenge of determining micro-bioplastics in soil. Sci. Total Environ. 745, 140975. doi:10.1016/j.scitotenv.2020.140975
Freitas, A. L. P. D. L., Tonini Filho, L. R., Calvao, P. S., and de Souza, A. M. C. (2017). Effect of montmorillonite and chain extender on rheological, morphological and biodegradation behavior of PLA/PBAT blends. Polym. Test. 62, 189–195. doi:10.1016/j.polymertesting.2017.06.030
Funabashi, M., Ninomiya, F., and Kunioka, M. (2009). Biodegradability evaluation of polymers by ISO 14855-2. Int. J. Mol. Sci. 10, 3635–3654. doi:10.3390/ijms10083635
Geyer, R., Jambeck, J. R., and Law, K. L. (2017). Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782. doi:10.1126/sciadv.1700782
Gómez, E. F., and Michel, F. C. (2013). Biodegradability of conventional and bio-based plastics and natural fiber composites during composting, anaerobic digestion and long-term soil incubation. Polym. Degrad. Stab. 98, 2583–2591. doi:10.1016/j.polymdegradstab.2013.09.018
Gonçalves, C., Gonçalves, I. C., Magalhães, F. D., and Pinto, A. M. (2017). Poly(lactic acid) composites containing carbon-based nanomaterials: a review. Polym. (Basel) 9 (7), 269–337. doi:10.3390/polym9070269
Guzman-Sielicka, A., Janik, H., and Sielicki, P. (2013). Proposal of new starch-blends composition quickly degradable in marine environment. J. Polym. Environ. 21 (3), 802–806. doi:10.1007/s10924-012-0558-7
Hanphakphoom, S., Maneewong, N., Sukkhum, S., Tokuyama, S., and Kitpreechavanich, V. (2014). Characterization of poly(L-lactide)-degrading enzyme produced by thermophilic filamentous bacteria Laceyella sacchari LP175. J. Gen. Appl. Microbiol. 60, 13–22. doi:10.2323/jgam.60.13
Hopewell, J., Dvorak, R., and Kosior, E. (2009). Plastics recycling: challenges and opportunities. Philos. Trans. R. Soc. B 364, 2115–2126. doi:10.1098/rstb.2008.0311
Hoshino, A., and Isono, Y. (2002). Degradation of aliphatic polyester films by commercially available lipases with special reference to rapid and complete degradation of poly(L-lactide) film by lipase PL derived from Alcaligenes sp. Biodegradation 13, 141–147. doi:10.1023/a:1020450326301
Itävaara, M., Karjomaa, S., and Selin, J.-F. (2002). Biodegradation of polylactide in aerobic and anaerobic thermophilic conditions. Chemosphere 46, 879–885. doi:10.1016/S0045-6535(01)00163-1
Janczak, K., Dąbrowska, G. B., Raszkowska-Kaczor, A., Kaczor, D., Hrynkiewicz, K., and Richert, A. (2020). Biodegradation of the plastics PLA and PET in cultivated soil with the participation of microorganisms and plants. Int. Biodeterior. Biodegr. 155, 105087. doi:10.1016/j.ibiod.2020.105087
Janczak, K., Hrynkiewicz, K., Znajewska, Z., and Dąbrowska, G. (2018). Use of rhizosphere microorganisms in the biodegradation of PLA and PET polymers in compost soil. Int. Biodeterior. Biodegr. 130, 65–75. doi:10.1016/j.ibiod.2018.03.017
Jang, J.-C., Shin, P.-K., Yoon, J.-S., Lee, I.-M., Lee, H.-S., and Kim, M.-N. (2002). Glucose effect on the biodegradation of plastics by compost from food garbage. Polym. Degrad. Stab. 76, 155–159. doi:10.1016/s0141-3910(02)00011-3
Jarerat, A., Pranamuda, H., and Tokiwa, Y. (2002). Poly(L-lactide)-degrading activity in various Actinomycetes. Macromol. Biosci. 2, 420–428. doi:10.1002/mabi.200290001
Jeon, H. J., and Kim, M. N. (2013). Biodegradation of poly(L-lactide) (PLA) exposed to UV irradiation by a mesophilic bacterium. Int. Biodeterior. Biodegr. 85, 289–293. doi:10.1016/j.ibiod.2013.08.013
Kale, G., Auras, R., and Singh, S. P. (2007a). Comparison of the degradability of poly (lactide) packages in composting and ambient exposure conditions. Packag. Technol. Sci. Int. J. 20 (1), 49–70. doi:10.1002/pts.742
Kale, G., Auras, R., Singh, S. P., and Narayan, R. (2007b). Biodegradability of polylactide bottles in real and simulated composting conditions. Polym. Test. 26, 1049–1061. doi:10.1016/j.polymertesting.2007.07.006
Kalita, N. K., Hazarika, D., Kalamdhad, A., and Katiyar, V. (2021a). Biodegradation of biopolymeric composites and blends under different environmental conditions: approach towards end-of-life panacea for crop sustainability. Bioresour. Technol. Rep. 15, 100705. doi:10.1016/j.biteb.2021.100705
Kalita, N. K., Damare, N. A., Hazarika, D., Bhagabati, P., Kalamdhad, A., and Katiyar, V. (2021b). Biodegradation and characterization study of compostable PLA bioplastic containing algae biomass as potential degradation accelerator. Environ. Chall. 3, 100067. doi:10.1016/j.envc.2021.100067
Kalita, N. K., Nagar, M. K., Mudenur, C., Kalamdhad, A., and Katiyar, V. (2019). Biodegradation of modified poly(lactic acid) based biocomposite films under thermophilic composting conditions. Polym. Test. 76, 522–536. doi:10.1016/j.polymertesting.2019.02.021
Karamanlioglu, M., Houlden, A., and Robson, G. D. (2014). Isolation and characterisation of fungal communities associated with degradation and growth on the surface of poly(lactic) acid (PLA) in soil and compost. Int. Biodeterior. Biodegr. 95, 301–310. doi:10.1016/j.ibiod.2014.09.006
Kervran, M., Vagner, C., Cochez, M., Ponçot, M., Saeb, M. R., and Vahabi, H. (2022). Thermal degradation of polylactic acid (PLA)/polyhydroxybutyrate (PHB) blends: a systematic review. Degrad. Stab. 201, 109995. doi:10.1016/j.polymdegradstab.2022.109995
Kim, M. Y., Kim, C., Moon, J., Heo, J., Jung, S. P., and Kim, J. R. (2017). Polymer film-based screening and isolation of polylactic acid (PLA)-degrading microorganisms. J. Microbiol. Biotechnol. 27, 342–349. doi:10.4014/jmb.1610.10015
Kolstad, J. J., Vink, E. T. H., De Wilde, B., and Debeer, L. (2012). Assessment of anaerobic degradation of Ingeo ™ polylactides under accelerated landfill conditions. Polym. Degrad. Stab. 97, 1131–1141. doi:10.1016/j.polymdegradstab.2012.04.003
Konkit, M., Jarerat, A., Khanongnuch, C., Lumyong, S., and Pathom-aree, W. (2012). Poly(lactide) degradation by Pseudonocardia alni AS4.1531T. Chiang Mai J. Sci. 39 (1), 128–132.
Leejarkpai, T., Suwanmanee, U., Rudeekit, Y., and Mungcharoen, T. (2011). Biodegradable kinetics of plastics under controlled composting conditions. Waste Manag. 31, 1153–1161. doi:10.1016/j.wasman.2010.12.011
Li, F., Wang, S., Liu, W., and Chen, G. (2008). Purification and characterization of poly(L-lactic acid)-degrading enzymes from Amycolatopsis orientalis ssp. orientalis. FEMS Microbiol. Lett. 282, 52–58. doi:10.1111/j.1574-6968.2008.01109.x
Liang, T.-W., Jen, S.-N., Nguyen, A., and Wang, S.-L. (2016). Application of chitinous materials in production and purification of a poly(L-lactic acid) depolymerase from Pseudomonas tamsuii TKU015. Polymers 8, 98. doi:10.3390/polym8030098
Lim, L.-T., Auras, R., and Rubino, M. (2008). Processing technologies for poly(lactic acid). Prog. Polym. Sci. 33, 820–852. doi:10.1016/j.progpolymsci.2008.05.004
Lv, S., Zhang, Y., Gu, J., and Tan, H. (2017). Biodegradation behavior and modelling of soil burial effect on degradation rate of PLA blended with starch and wood flour. Colloids Surf. B Biointerfaces 159, 800–808. doi:10.1016/j.colsurfb.2017.08.056
Meyer-Kohlstock, D., Haupt, T., Heldt, E., Heldt, N., and Kraft, E. (2016). Biochar as additive in biogas-production from bio-waste. Energies 9, 247. doi:10.3390/en9040247
Mistry, A. N., Kachenchart, B., Wongthanaroj, A., Somwangthanaroj, E., and Luepromchai, E. (2022). Rapid biodegradation of high molecular weight semi-crystalline polylactic acid at ambient temperature via enzymatic and alkaline hydrolysis by a defined bacterial consortium. Polym. Degrad. Stab. 202, 110051. doi:10.1016/j.polymdegradstab.2022.110051
Munoz, R. C., Castillo, H. S. V., Concha, J. L. H., and Duque, J. F. S. (2024). Aerobic biodegradation of poly(lactic acid) (PLA) in thermoplastic starch (TPS) blends in soil induced by gelatin. Int. Biodeterior. Biodegr. 193, 105831. doi:10.1016/j.ibiod.2024.105831
Murariu, M., and Dubois, P. (2016). PLA composites: from production to properties. Adv. Drug Deliv. Rev. 107, 17–46. doi:10.1016/j.addr.2016.04.003
Nair, N. R., Sekhar, V. C., and Nampoothiri, K. M. (2016). Augmentation of a microbial consortium for enhanced polylactide (PLA) degradation. Indian J. Microbiol. 56, 59–63. doi:10.1007/s12088-015-0559-z
Nakamura, K., Tomita, T., Abe, N., and Kamio, Y. (2001). Purification and characterization of an extracellular Poly(L-lactic acid) depolymerase from a soil isolate, Amycolatopsis sp. Strain K104-1. Appl. Environ. Microbiol. 67, 345–353. doi:10.1128/AEM.67.1.345–353.2001
NF EN 13432 (2000). Emballage - Exigences relatives aux emballages valorisables par compostage et biodégradation - Programme d'essai et critères d'évaluation de l'acceptation finale des emballages, Norme AFNOR.
NF EN 14995 (2007). Matières plastiques - Evaluation de la compostabilité - Programme d'essais et spécifications, Norme AFNOR.
NF EN 17033 (2018). Plastiques - Films de paillage biodégradables thermoplastiques pour utilisation en agriculture et horticulture - Exigences et méthodes d'essai, norme AFNOR.
NF EN ISO 14851 (2019). Evaluation de la biodégradabilité aérobie ultime des matériaux plastiques en milieu aqueux - Méthode par détermination de la demande en oxygène dans un respiromètre fermé, norme AFNOR. Available at: https://www.iso.org/standard/
NF EN ISO 14852 (2021). Evaluation de la biodégradabilité aérobie ultime des matériaux plastiques en milieu aqueux - Méthode par analyse du dioxyde de carbone libéré, norme AFNOR. Available at: https://www.iso.org/standard/
NF EN ISO 14853 (2017). Plastiques - Evaluation de la biodégradabilité anaérobie ultime des matériaux plastiques en milieu aqueux - Méthode par détermination de la production de biogaz, norme AFNOR. Available at: https://www.iso.org/standard/
NF EN ISO 14855-2 (2018). Détermination de la biodégradabilité aérobie ultime des matériaux plastiques dans des conditions contrôlées de compostage - Méthode par analyse du dioxyde de carbone libéré - Partie 2: mesurage gravimétrique du dioxyde de carbone libéré lors d'un essai de laboratoire. Norme AFNOR. Available at: https://www.iso.org/standard/
NF EN ISO 15985 (2017). Plastiques - Evaluation de la biodégradation anaérobie ultime dans des conditions de digestion anaérobie à teneur élevée en solides - Méthode par analyse du biogaz libéré, norme AFNOR. Available at: https://www.iso.org/standard/
NF EN ISO 19679 (2020). Plastiques - Détermination de la biodégradation aérobie des matières plastiques non-flottantes dans une interface eau de mer/sédiments - Méthode par analyse du dioxyde de carbone libéré, norme AFNOR.
NF EN ISO 22403 (2021). Plastiques - Evaluation de la biodégradabilité aérobie inhérente et de la sécurité environnementale des matériaux non flottants exposés à des inocula marins dans des conditions de laboratoire et mésophiles - Méthodes d'essai et exigences, norme AFNOR. Available at: https://www.iso.org/standard/
NF T51-800 (2015). Plastiques - Spécifications pour les plastiques aptes au compostage domestique, norme AFNOR.
NF U52-001 (2005). Matériaux biodégradables pour l'agriculture et l'horticulture - Produits de paillage - Exigences et méthodes d'essai, norme AFNOR.
Pantani, R., and Sorrentino, A. (2013). Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stab. 98, 1089–1096. doi:10.1016/j.polymdegradstab.2013.01.005
Pattanasuttichonlakul, W., Sombatsompop, N., and B. Prapagdee, B. (2018). Accelerating biodegradation of PLA using microbial consortium from dairy wastewater sludge combined with PLA-degrading bacterium. Int. Biodeterior. Biodegr. 132, 74–83. doi:10.1016/j.ibiod.2018.05.014
Penkhrue, W., Khanongnuch, C., Masaki, K., Pathom-aree, W., Punyodom, W., and Lumyong, S. (2015). Isolation and screening of biopolymer-degrading microorganisms from northern Thailand. World J. Microbiol. Biotechnol. 31, 1431–1442. doi:10.1007/s11274-015-1895-1
Penu, C., and Helou, M. (2017). Acide polylactique (PLA). Tech. l'Ingénieur AM3317V. doi:10.51257/a-v1-am3317
Pesaranhajiabbas, E., Manjusri Misra, M., Amar, K., and Mohanty, A. K. (2023). Recent progress on biodegradable polylactic acid based blends and their biocomposites: a comprehensive review. Inter. J. Biol. Macromol. 253, 126231. doi:10.1016/j.ijbiomac.2023.126231
Pranamuda, H., and Tokiwa, Y. (1999). Degradation of poly(L-lactide) by strains belonging to genus Amycolatopsis. Biotechnol. Lett. 21, 901–905. doi:10.1023/a:1005547326434
Pranamuda, H., Tokiwa, Y., and Tanaka, H. (1997). Polylactide degradation by an Amycolatopsis sp. Appl. Environ. Microbiol. 63, 1637–1640. doi:10.1128/aem.63.4.1637-1640.1997
Qi, X., Ren, Y., and Wang, X. (2017). New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegr. 117, 215–223. doi:10.1016/j.ibiod.2017.01.010
Richert, A., and Dąbrowska, G. B. (2021). Enzymatic degradation and biofilm formation during biodegradation of polylactide and polycaprolactone polymers in various environments. Int. J. Biol. Macromol. 176, 226–232. doi:10.1016/j.ijbiomac.2021.01.202
Rosli, N. A., Karamanlioglu, M., Kargarzadeh, H., and Ahmad, I. (2021). Comprehensive exploration of natural degradation of poly(lactic acid) blends in various degradation media: a review. Int. J. Biol. Macromol. 187, 732–741. doi:10.1016/j.ijbiomac.2021.07.196
Ruggero, F., Gori, R., and Lubello, C. (2019). Methodologies to assess biodegradation of bioplastics during aerobic composting and anaerobic digestion: a review. Waste Manag. Res. 37, 959–975. doi:10.1177/0734242X19854127
Ryan, C. A., Billington, S. L., and Criddle, C. S. (2018). Biocomposite fiber-matrix treatments that Enhance in-service performance can also accelerate end-of-life fragmentation and anaerobic biodegradation to methane. J. Polym. Environ. 26, 1715–1726. doi:10.1007/s10924-017-1068-4
Sahota, S., Shah, G., Ghosh, P., Kapoor, R., Sengupta, S., Singh, P., et al. (2018). Review of trends in biogas upgradation technologies and future perspectives. Bioresour. Technol. Rep. 1, 79–88. doi:10.1016/j.biteb.2018.01.002
Sakai, K., Kawano, H., Iwami, A., Nakamura, M., and Moriguchi, M. (2001). Isolation of a thermophilic poly-L-lactide degrading bacterium from compost and its enzymatic characterization. J. Biosci. Bioeng. 92, 298–300. doi:10.1016/s1389-1723(01)80266-8
Satti, S. M., Shah, A. A., Auras, R., and Marsh, T. L. (2017). Isolation and characterization of bacteria capable of degrading poly(lactic acid) at ambient temperature. Polym. Degrad. Stab. 144, 392–400. doi:10.1016/j.polymdegradstab.2017.08.023
Sedničkova, M., Pekařová, S., Kucharczyk, P., Bočkaj, J., Janigová, I., Kleinová, A., et al. (2018). Changes of physical properties of PLA-based blends during early stage of biodegradation in compost. Int. J. Biol. Macromol. 113, 434–442. doi:10.1016/j.ijbiomac.2018.02.078
Shah, A. A., Kato, S., Shintani, N., Kamini, N. R., and Nakajima-Kambe, T. (2014). Microbial degradation of aliphatic and aliphatic-aromatic co-polyesters. Appl. Microbiol. Biotechnol. 98, 3437–3447. doi:10.1007/s00253-014-5558-1
Shalem, A., Yehezkeli, O., and Fishman, A. (2024). Enzymatic degradation of polylactic acid (PLA). Appl. Microbiol.Biotechnol. 108, 413. doi:10.1007/s00253-024-13212-4
Sheets, J. P., Yang, L., Ge, X., Wang, Z., and Li, Y. (2015). Beyond land application: emerging technologies for the treatment and reuse of anaerobically digested agricultural and food waste. Waste Manag. 44, 94–115. doi:10.1016/j.wasman.2015.07.037
Sivan, A. (2011). New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 22, 422–426. doi:10.1016/j.copbio.2011.01.013
Stloukal, P., Pekar, S., Kalendova, A., Mattausch, H., Stephan Laske, S., Holzer, C., et al. (2015). Kinetics and mechanism of the biodegradation of PLA/clay nanocomposites during thermophilic phase of composting process. Waste Manag. 42, 31–40. doi:10.1016/j.wasman.2015.04.006
Sukkhum, S., Tokuyama, S., and Kitpreechavanich, V. (2009). Development of fermentation process for PLA-degrading enzyme production by a new thermophilic Actinomadura sp. T16-1. Biotechnol. Bioprocess Eng. 14, 302–306. doi:10.1007/s12257-008-0207-0
Tabasi, R. Y., and Ajji, A. (2015). Selective degradation of biodegradable blends in simulated laboratory composting. Polym. Degrad. Stab. 120, 435–442. doi:10.1016/j.polymdegradstab.2015.07.020
Tokiwa, Y., Calabia, B., Ugwu, C., and Aiba, S. (2009). Biodegradability of plastics. Int. J. Mol. Sci. 10, 3722–3742. doi:10.3390/ijms10093722
Tomita, K., Kuroki, Y., and Nagai, K. (1999). Isolation of thermophiles degrading poly(L-lactic acid). J. Biosci. Bioeng. 87, 752–755. doi:10.1016/s1389-1723(99)80148-0
Tomita, K., Nakajima, T., Kikuchi, Y., and Miwa, N. (2004). Degradation of poly(L-lactic acid) by a newly isolated thermophile. Polym. Degrad. Stab. 84, 433–438. doi:10.1016/j.polymdegradstab.2003.12.006
Tomita, K., Tsuji, H., Nakajima, T., Kikuchi, Y., Ikarashi, K., and Ikeda, N. (2003). Degradation of poly(D-lactic acid) by a thermophile. Polym. Degrad. Stab. 81, 167–171. doi:10.1016/s0141-3910(03)00086-7
Tosakul, T., Chanthot, P., and Pattamaprom, C. (2024). High toughness and fast home-compost biodegradable packaging films derived from polylactic acid/thermoplastic starch/para-rubber ternary blends. Nature 14, 18603. doi:10.1038/s41598-024-69508-y
Tseng, H.-C., Fujimoto, N., and Ohnishi, A. (2020). Characteristics of Tepidimicrobium xylanilyticum as a lactate-utilising bacterium in polylactic acid decomposition during thermophilic anaerobic digestion. Bioresour. Technol. Rep. 12, 100596. doi:10.1016/j.biteb.2020.100596
Tsuji, H., and Miyauchi, S. (2001). Poly(l-lactide): VI effects of crystallinity on enzymatic hydrolysis of poly(l-lactide) without free amorphous region. Polym. Degrad. Stab. 71 (3), 415–424. doi:10.1016/S0141-3910(00)00191-9
van Bussel, E. F., Richard, E., Arts, D. L., Nooyens, A. C. J., Coloma, P. M., de Waal, M. W. M., et al. (2017). Dementia incidence trend over 1992-2014 in The Netherlands: analysis of primary care data. PLoS Med. 14, e1002235. doi:10.1371/journal.pmed.1002235
Walczak, M., Swiontek Brzezinska, M., Sionkowska, A., Michalska, M., Jankiewicz, U., and Deja-Sikora, E. (2015). Biofilm formation on the surface of polylactide during its biodegradation in different environments. Colloids Surf. B Biointerfaces 136, 340–345. doi:10.1016/j.colsurfb.2015.09.036
Wang, Z., Wang, Y., Guo, Z., Li, F., and Chen, S. (2011). Purification and characterization of poly(L-lactic acid) depolymerase from Pseudomonas sp. strain DS04-T. Polym. Eng. Sci. 51, 454–459. doi:10.1002/pen.21857
Weng, Y.-X., Jin, Y.-J., Meng, Q.-Y., Wang, L., Zhang, M., and Wang, Y.-Z. (2013). Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 32, 918–926. doi:10.1016/j.polymertesting.2013.05.001
Xu, B., Chen, Y., He, J., Cao, S., Liu, J., Xue, R., et al. (2022a). New insights into the biodegradation of polylactic acid: from degradation to upcycling. Environ. Rev. 30, 30–38. doi:10.1139/er-2020-0117
Xue, P., Wang, K., Jia, M., and Yang, M. (2013). Biodegradation and mechanical property of polylactic acid/thermoplastic starch blends with poly (ethylene glycol). J. Wuhan. Univ. Technol.-Mat. Sci. Ed. 28 (1), 157–162. doi:10.1007/s11595-013-0658-9
Yagi, H., Ninomiya, F., Funabashi, M., and Kunioka, M. (2009). Anaerobic biodegradation tests of poly(lactic acid) under mesophilic and thermophilic conditions using a new evaluation system for methane fermentation in anaerobic sludge. Int. J. Mol. Sci. 10, 3824–3835. doi:10.3390/ijms10093824
Yagi, H., Ninomiya, F., Funabashi, M., and Kunioka, M. (2013). Thermophilic anaerobic biodegradation test and analysis of eubacteria involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym. Degrad. Stab. 98, 1182–1187. doi:10.1016/j.polymdegradstab.2013.03.010
Yagi, H., Ninomiya, F., Funabashi, M., and Kunioka, M. (2014). Mesophilic anaerobic biodegradation test and analysis of eubacteria and archaea involved in anaerobic biodegradation of four specified biodegradable polyesters. Polym. Degrad. Stab. 110, 278–283. doi:10.1016/j.polymdegradstab.2014.08.031
Yottakot, S., and Leelavatcharamas, V. (2019). Isolation and optimisation of polylactic acid (PLA)-packaging-degrading Actinomycete for PLA-packaging degradation, polylactic acid. Pertanika J. Trop. Agric. Sci. 42 (3), 1111–1129.
Zaaba, N. F., and Jaafar, M. (2020). A review on degradation mechanisms of polylactic acid: hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 60/9, 2061–2075. doi:10.1002/pen.25511
Zhu, Z., Zheng, Z., Zhou, C., Cao, L., and Zhao, G. (2023). Trends in prevalence and disability-adjusted life-years of alzheimer's disease and other dementias in China from 1990 to 2019. Neuroepidemiology 57, 206–217. doi:10.1159/000530593
Keywords: polylactic acid (PLA), biodegradation, bio-based polymers, aerobic and anaerobic conditions, microorganisms, bacteria, fungi
Citation: Lors C, Leleux P and Park CH (2025) State of the art on biodegradability of bio-based plastics containing polylactic acid. Front. Mater. 11:1476484. doi: 10.3389/fmats.2024.1476484
Received: 07 August 2024; Accepted: 11 December 2024;
Published: 07 January 2025.
Edited by:
Ilaria Cacciotti, University Niccolò Cusano, ItalyReviewed by:
Shunli Wang, Chinese Academy of Agricultural Sciences, ChinaTan Suet May Amelia, Chang Gung University, Taiwan
Copyright © 2025 Lors, Leleux and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine Lors, Y2hyaXN0aW5lLmxvcnNAaW10LW5vcmQtZXVyb3BlLmZy
 Christine Lors
Christine Lors Pauline Leleux1
Pauline Leleux1 Chung Hae Park
Chung Hae Park